Introduction
Malignant melanoma is a type of malignancy produced
by the melanocytes of the skin and other organs (1). Although the occurrence rate of malignant
melanoma is low, metastasis occurs is early and the mortality is
high (2); therefore, it is necessary
to identify a novel treatment strategy for malignant melanoma.
Cdk5 regulatory subunit-associated protein 1
(CDK5RAP1), with homology to the bacterial MiaB protein, is a
radical S-Adenosyl methionine (SAM) enzyme (3,4). As
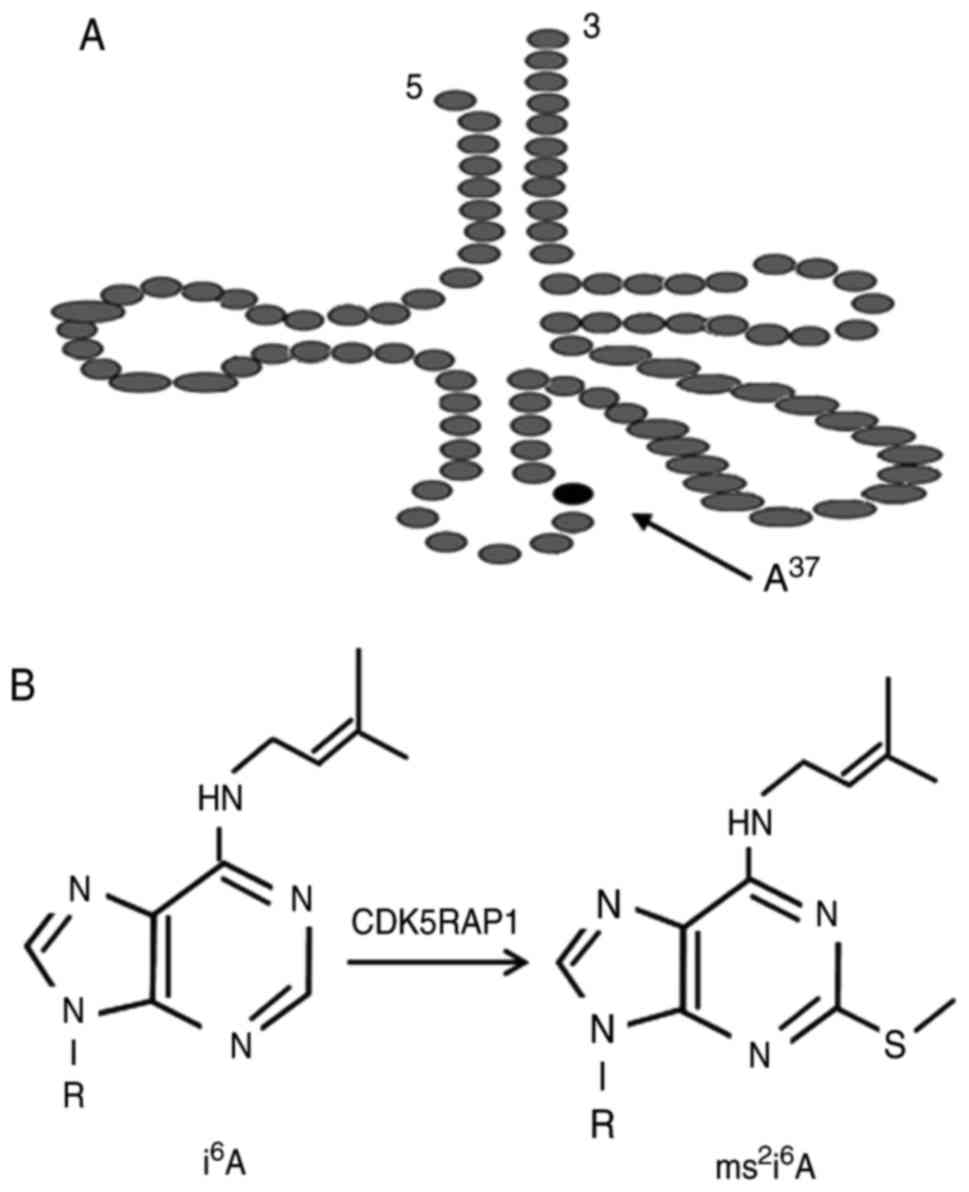
presented in Fig. 1, CDK5RAP1
catalyzes the 2-methylthio (ms2) modification of
mitochondrial transfer (t)RNAs at amino acid 37 (5). Deficiencies in the ms2
modification of 2-methylthio-N6-isopentenyladenosine
(ms2i6A) at amino acid 34 or 37 impair
reading frame maintenance in bacteria and cause defective
mitochondrial protein synthesis, which leads to a reduction of
respiratory activity and increase in ROS (6). The ms2 modifications, which
initiate mitochondrial responses, are considered to be the major
pathway for apoptosis (7). It has
been reported that, CDK5RAP1 deficiency, induces cancer cell
apoptosis via the phospho-c-Jun N-terminal kinase (p-JNK) signaling
pathway (8).
Apoptosis refers to specific programmed cell death,
which is a critical mechanism for cancer therapy. Ca2+
influx, mediated by extracellular signal regulated kinase (ERK1/2)
pathway, contributes an important role in early apoptotic cells by
activating downstream ROS generation (9). The reactive oxygen species (ROS)
mediates cell dysfunction, contributes to the development of cell
damage (10), and is responsible for
cancer cell apoptosis (11). Nuclear
factor-κB (NF-κB) pathway is commonly involved in numerous cellular
responses (12), and is activated by
mitochondrial-generated ROS (13) to
induce cell apoptosis (14). The
phosphorylation of NF-κB is associated with the expression of
pro-apoptosis B-cell lymphoma-2 (Bcl-2) family, including
Bcl-2/Bcl-xl-associated death promoter (Bad), and anti-apoptosis
Bcl-2 family, including B-cell lymphoma-xl (Bcl-xl) and Bcl-2
(15). Since targeting mitochondrial
modifications is a new field for the treatment of cancer, and
apoptosis is linked with NF-κB signaling pathway (16), the aim of the present study was to
investigate the relationship between CDK5RAP1 deficiency and NF-κB
signaling pathway during the apoptosis process in human malignant
melanoma (A375) cells.
In the current study, it was demonstrated that
CDK5RAP1 deficiency induces cell apoptosis in malignant melanoma
A375 cells via the ROS and NF-κB signaling pathway. This study
indicated a unique candidate of anti-skin cancer.
Materials and methods
Cell culture
The human malignant melanoma A375 cell line (A375-P)
was purchased from American Type Culture Collection (Manassas, VA,
USA). In accordance with experimental guidelines and ethical
approval of Harbin Medical University (Harbin, China), the study
was performed in Harbin Medical University. A375 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented
with 10% fetal bovine serum, 100 U/ml penicillin and 100 mg/ml
streptomycin (all from Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) with 5% CO2 at 37°C in a humidified incubator
(Sanyo Electric Co., Ltd., Tokyo, Japan).
Small interfering RNA (siRNA)
transfection
Control siRNA and the CDK5RAP1 siRNA were purchased
from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). A375 cells
were seeded at a density of 1×105 cells/well onto
six-well plates. After cells obtained 60–80% confluency, siRNAs
were transfected into A375 cells according to the manufacturer's
protocol. A375 cells were incubated for another 48 h prior to use
in subsequent experiments. The transfection efficiency was analyzed
by reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), according to the protocol outlined below.
Measurement of cytoplasmic
Ca2+ influx
Ca2+ influx was performed as described
previously (17). Briefly, A375 cells
at a density of 2×106 cells/ml were loaded with 1 µM
calcium-sensitive Fura 2-AM in Ca2+-free buffer (Hank's
balanced salt solution containing 20 mM HEPES and 1% bovine serum
albumin, pH 7.4; Sigma-Aldrich; Merck KGaA) at 37°C for 30 min.
Adenosine triphosphate (10 µM) was added to the cell suspension
directly. An F-2500 calcium imaging system (Hitachi, Tokyo, Japan)
was used to record within FL Solutions. The ratio of fluorescent
signals was measured under wavelengths of 340 nm, 380 nm
(excitation) and wavelength of 510 nm (emission). The free Fura2
and the Ca2+-bound Fura2 were measured using excitation
wavelengths of 380 and 340 nm, respectively. The fluorescent
activities of F1 at 340/500 nm and F2 at 380/500 nm, as well as the
ratio of F1 to F2 were recorded using the spectrophotometer at 5
sec intervals. The Ca2+ concentration was finally
calculated using the formula: 224× R, where 224 is the Kd
number.
RT-qPCR
Total RNA was extracted from A375 cells by
TRIzol® reagent (Life Technologies; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) at 48 h post-transfection of
CDK5RAP1 siRNA and control siRNA. Reverse transcription was
performed using a Transcriptor First Strand cDNA Synthesis kit
(Roche Applied Science, Madison, WI, USA). A total of 200 ng RNA
was used for the RT reaction, and 2 µl input cDNA from the RT
product was used for the qPCR. LightCycler® 480 SYBR
Green I Master (Roche Diagnostics, Basel, Switzerland) was used and
the qPCR was performed using the ABI 7300 Fast real-time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) with the
following primers: CDK5RAP1 forward, 5′-ATGGCTGCCAGATGAATGTGA-3′
and reverse, 5′-CTCTTGGAGGTTACTGGTCCG-3′; 18s forward,
5′-GTAACCCGTTGAACCCCATT-3′ and reverse, 5′-CCATCCAATCGGTAGTAGCG-3′.
PCR was performed using SYBR Premix Ex Taq II (Takara Bio, Inc.,
Otsu, Japan). The thermocycling conditions were: Pre-denaturation
at 95°C for 30 sec, denaturation at 95°C for 3 sec for 40 cycles,
annealing at 60°C for 31 sec, elongation at 72°C for 60 sec and
re-elongation at 72°C for 5 min. CDK5RAP1 mRNA expression was
normalized to 18s mRNA expression. 2−ΔΔCq method was
used as the quantification method for the gene of interest, as
described previously (18).
MTT assay
The viability of A375 cells, which were transfected
with control siRNA and CDK5RAP1 siRNA with or without pyrrolidine
dithiocarbamate (PDTC; 100 µmol) treatment, was determined by a
colorimetric MTT assay as described previously (19). Cell viability was determined by
measuring the absorbance at 550 nm on a microplate reader.
Absorbance at 690 nm was also measured as the reference wavelength.
The measured absorbance under 550 nm was read as the optical
density was used to reflect the number of viable cells.
Nuclear staining with Hoechst 33342
for morphological evaluation
A375 cells were plated in six-well plates at a
density of 1×105 cells/well and pretreated with or
without PDTC (100 µmol). Cells were washed with PBS after
transfection with CDK5RAP1 siRNA or control siRNA for 48 h. Cells
were then fixed in 4% paraformaldehyde (BIOSS, Beijing, China) for
30 min at 22°C followed by staining with Hoechst 33342 (20 mg/ml)
for 15 min at room temperature in the dark. Cells were then imaged
by using fluorescence microscopy (C1-T-SM; Nikon Corporation,
Tokyo, Japan) at a magnification, ×100.
Detection of intracellular ROS
Intracellular accumulation of ROS was estimated
using the fluorescent dye H2-DCFDA (Thermo Fisher
Scientific, Inc.) as described previously (20). The A375 cells were seeded at a density
of 1×105 cells/well, in a six-well plate, with or
without PDTC (100 µmol) pretreatment. Following transfection with
CDK5RAP1 siRNA or control siRNA for 48 h, A375 cells were washed
with serum-free DMEM medium (Sigma-Aldrich; Merck KGaA) and
incubated in H2-DCFDA (5 µM) for 60 min at 37°C. The
cells were then examined under a fluorescence microscope (C1-T-SM;
Nikon Corporation, Tokyo, Japan). Finally, cells were collected and
subjected to fluorescence Spectrophotometer (F-2500; Hitachi, Ltd.,
Tokyo, Japan) to detect the fluorescence of DCF inside cells
(excitation, 488 nm; emission, 521 nm).
Western blot analysis
Total proteins were extracted from cells. Cells were
lysed by lysis buffer (1 M Tris-HCl, pH 7.4; 1 M NaCl; 20% Triton
X100; 10% SDS; and 0.5 M EDTA; Sigma-Aldrich; Merck KGaA) and
centrifuged at 3,300 × g for 3 min at 22°C. Electrophoresis was
performed using a vertical slab gel with 12% polyacrylamide
content. A total of 20 µg of protein was loaded per gel lane and
the transfer of proteins was performed electrophoretically
according to the method previously described (21,22).
Subsequent to treatment with Block Ace™ (4%) for 30 min
at 22°C, the polyvinylidene fluoride membranes (Thermo Fisher
Scientific, Inc.) were probed with rabbit IgG primary antibodies
against NF-κB (cat. no., SAB4502609; 1:500; Sigma-Aldrich; Merck
KGaA), Bcl-xl (cat. no., SAB4502623; 1:500; Sigma-Aldrich; Merck
KGaA) or Bcl-2 (cat. no., SAB4500003; 1:500; Sigma-Aldrich; Merck
KGaA) in PBS containing 0.03% Tween-20 (PBST) for 1 h at 22°C.
Following three washes with PBST, the second reaction was performed
using horseradish peroxidase-conjugated anti-rabbit goat IgG (cat.
no., A0545; 20 ng/ml; Sigma-Aldrich; Merck KGaA) secondary antibody
for 30 min at 22°C. Following three washes with PBST, the membrane
was incubated with ECL Plus Western Blotting Detection
system™ (GE Healthcare Life Sciences, Beijing, China)
followed by detection. ImageJ (version 1.38e; National Institutes
of Health, Bethesda, MD, USA) was used for the quantification of
western blots. Histone H1.4 (cat. no., H7665; 2 µg/ml;
Sigma-Aldrich; Merck KGaA) and β-actin (cat. no., A5441; 1:5,000;
Sigma-Aldrich; Merck KGaA) were used for normalization.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Analyses were performed using SPSS (v.19.0; IBM SPSS, Armonk, NY,
USA). Each experiment was repeated at least three times. One-way
analysis of variance and Dunnett's test was used and P<0.05 was
considered to indicate a statistically significant difference.
Results
CDK5RAP1 deficiency suppresses cell
proliferation of A375 cells
In order to investigate the potential effect of
CDK5RAP1, prior to subsequent experiments, human malignant melanoma
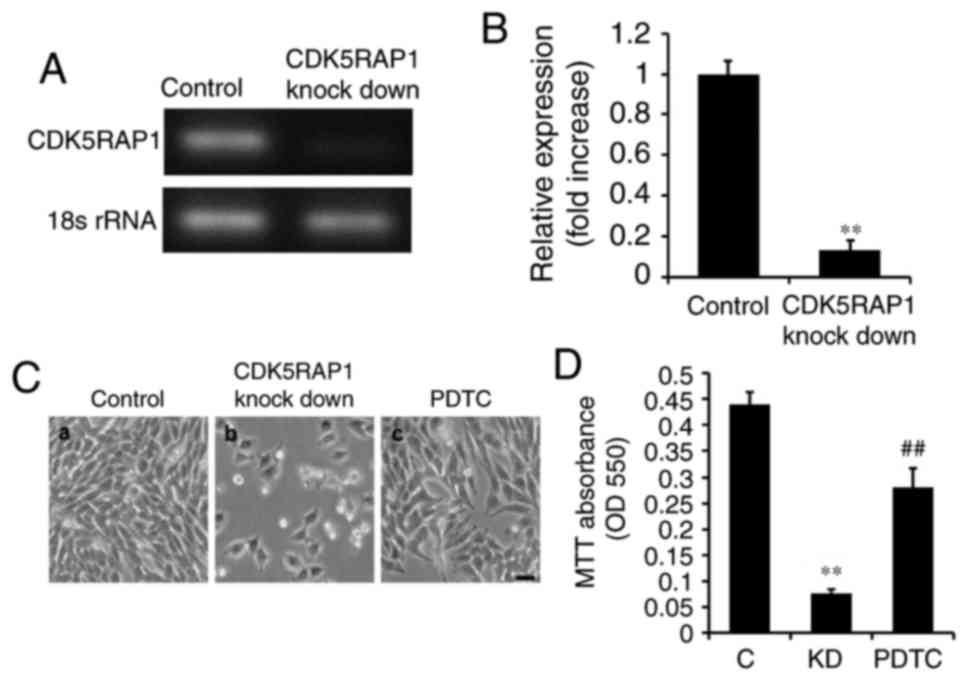
A375 cells were transfected with siRNA for 48 h. It was confirmed
by PCR that CDK5RAP1 knockdown was evident following transfection
with CDK5RAP1 siRNA compared with control siRNA in A375 cells
(Fig. 2A and B). In addition, the
effects of CDK5RAP1 deficiency on the proliferation of A375 cells,
was investigated. At 48 h post-transfection with CDK5RAP1 or
control siRNA, the viability of A375 cells was determined by a
colorimetric MTT assay. It was demonstrated that CDK5RAP1
deficiency suppressed the proliferation of A375 cells significantly
compared with control cells (Fig. 2C a, b
and D; C vs. KD; P<0.01).
CDK5RAP1 deficiency induces the cell
apoptosis in A375 cells
To determine whether CDK5RAP1 knockdown affects the
potential apoptosis effect of A375 cells, a series of apoptosis
assays were performed. Firstly, Ca2+ influx was measured
to estimate the early stage of apoptosis. It was demonstrated that
CDK5RAP1 deficiency significantly inhibited Ca2+ influx
in A375 cells (Fig. 3A; P<0.01).
This indicates that A375 cells lost the normal ability of
Ca2+ handling, which renders cells to the possibility of
apoptosis. Furthermore, apoptosis in cells was examined by nuclear
staining with Hoechst 33342 and fluorescence microscopy. CDK5RAP1
deficiency significantly induced A375 cell apoptosis compared with
that of control cells (Fig. 3B a, b and
C; C vs. KD; P<0.01). To further determine apoptosis in
CDK5RAP1 deficient cells, apoptotic markers of the Bad/Bcl
signaling pathway were detected by western blot analysis. It was
demonstrated that Bad protein was upregulated and its inhibitors,
Bcl-xl and Bcl-2, were downregulated in CDK5RAP1 deficient cells
(Fig. 3D and E). Taken together,
these results indicated a consistent conclusion that CDK5RAP1
deficiency induced apoptosis of A375 cells.
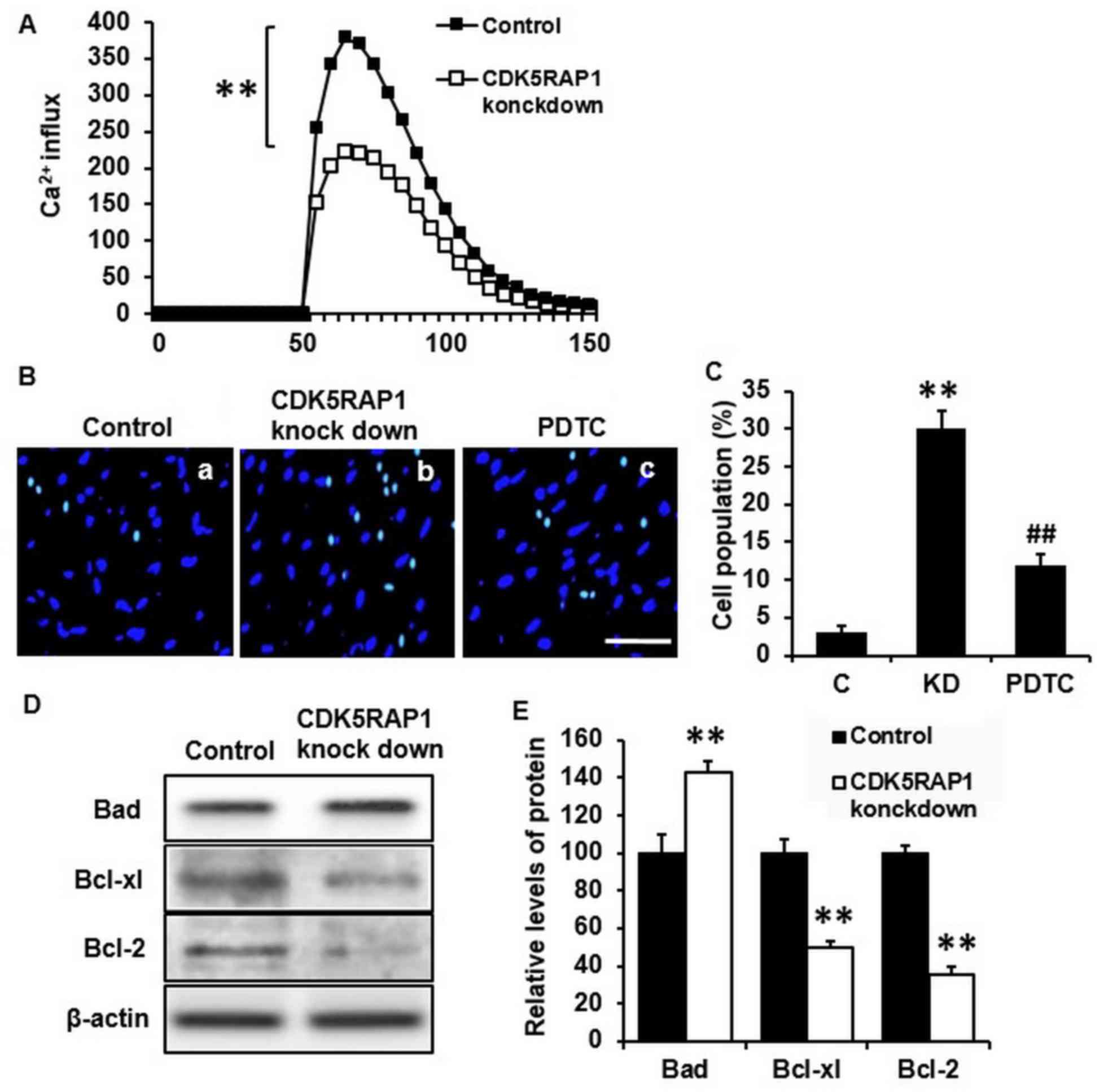 | Figure 3.(A) Representative recording image of
Ca2+ influx in A375 cells transfected by CDK5RAP1 siRNA
and control siRNA. CDK5RAP1 deficiency significantly inhibited the
Ca2+ influx in A375 cells. (B) Representative image of
apoptotic A3735 cells transfected by CDK5RAP1 siRNA with or without
PDTC pretreatment (×400 magnification; Scale bar=20 µm.) and (C)
quantification of apoptotic cells percentage. Pretreatment with
PDTC prevented the apoptosis induced by CDK5RAP1 deficiency in A375
cells significantly. (D) Representative western blot of
phosphorylation of Bad, Bcl-xl and Bcl-2 in A375 cells transfected
by CDK5RAP1 siRNA and (E) quantification. CDK5RAP1 deficiency
significantly upregulated the phosphorylation of Bad, and
downregulated the phosphorylation of Bcl-xl and Bcl-2. β-actin was
used as the normalization respectively. Data are expressed as the
mean ± SD (n=3). **P<0.01, CDK5RAP1 knockdown vs. control;
##P<0.01, PDTC vs. CDK5RAP1 knockdown. C, control;
KD, CDK5RAP1 knockdown; siRNA, small interfering RNA; CDK5RAP1,
Cdk5 regulatory subunit-associated protein 1; Bad,
Bcl-xl-associated death promoter; Bcl-xl, B-cell lymphoma-xl;
Bcl-2, B-cell lymphoma; PDTC, pyrrolidine dithiocarbamate. |
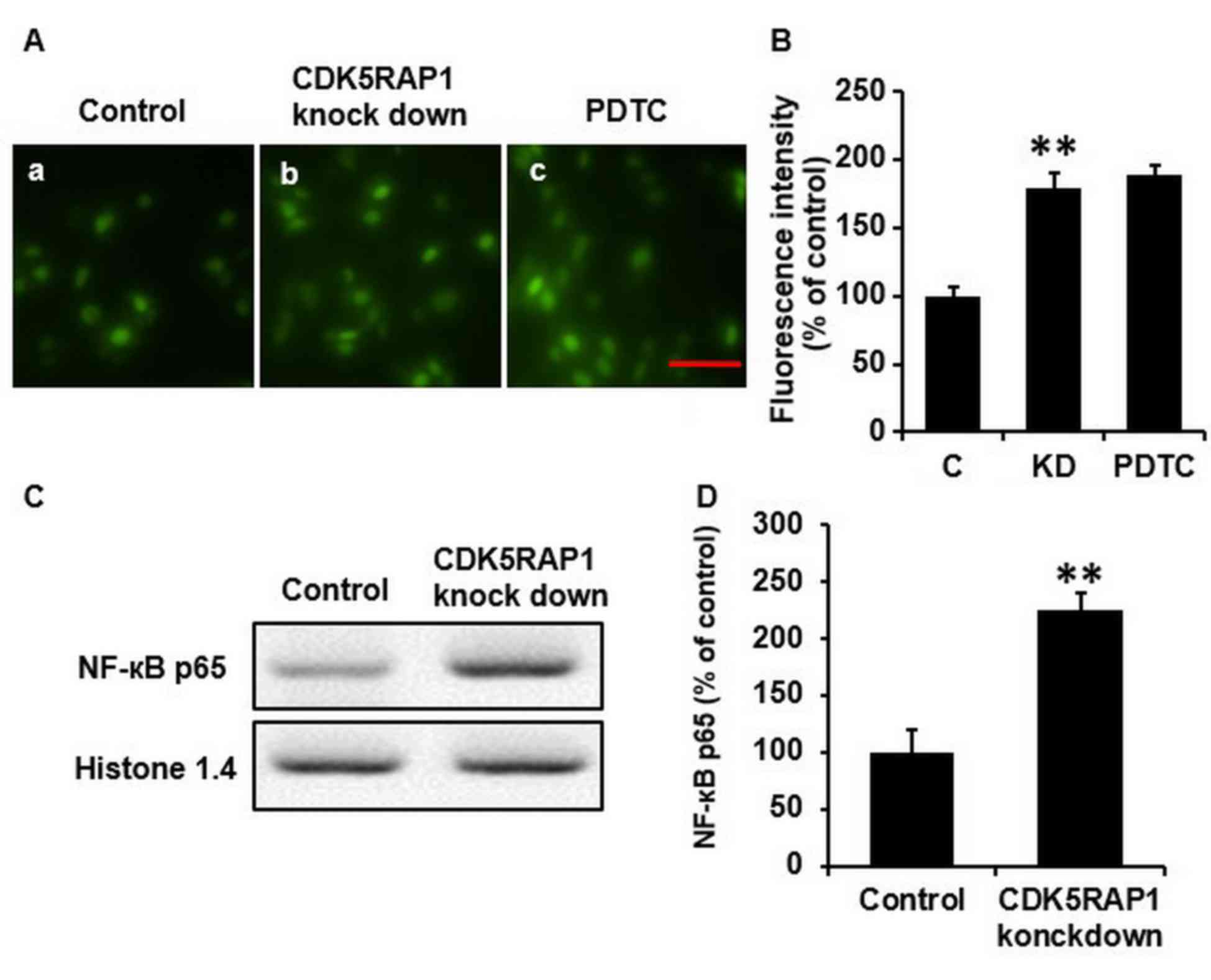
CDK5RAP1 deficiency induces ROS
generation in A375 cells
To explore the mechanism of this aforementioned
apoptotic effect in A375 cells, ROS was further investigated, as
ROS is considered to induce, and accompanies, cancer cell
apoptosis. Thus, the intracellular generation of ROS was detected
using the fluorescent dye H2-DCFDA. CDK5RAP1 deficiency
induced ROS generation and accumulation in A375 cells significantly
(Fig. 4A a, b and B; C vs. KD;
P<0.01).
CDK5RAP1 deficiency upregulates the
phosphorylation of NF-κB
To further study the mechanisms of this beneficial
effect of CDK5RAP1 deficiency, western blot analysis for NF-κB was
performed. It was revealed that the phosphorylation of NF-κB in
A375 cells was significantly upregulated in CDK5RAP1-deficient
cells compared with the control cells. Histone 1.4 and β-actin were
used for normalization (Fig. 4C and
D; P<0.01).
Pretreatment with PDTC prevents the
decrease in proliferation and increases apoptosis induced by
CDK5RAP1 deficiency in A375 cells
As it was demonstrated that CDK5RAP1 deficiency
induced NF-κB expression, it was imperative to further explore if
the NF-κB pathway was sufficient to explain the effects of CDK5RAP1
deficiency. To determine this, NF-κB was inhibited using PDTC (100
µmol) prior to transfection with CDK5RAP1 siRNA. Firstly, the
viability of CDK5RAP1-deficient A375 cells pretreated with PDTC was
measured using a colorimetric MTT assay. It was revealed that NF-κB
inhibition, by PDTC, prevented the decrease in proliferation
induced by CDK5RAP1 deficiency in A375 cells (Fig. 2C b, c and D; KD vs. PDTC; P<0.01).
In addition, NF-κB inhibition, by PDTC, prevented the apoptosis
induced by CDK5RAP1 deficiency in A375 cells (Fig. 3B b, c and C; KD a vs. PDT; P<0.01).
However, notably, CDK5RAP1 deficiency induced-ROS was not
significantly inhibited by pretreatment with PDTC (Fig. 4A b, c and B; KD vs. PDTC). Taken
together, the results from the present study indicated that NF-κB
may contribute in the process of CDK5RAP1 deficiency-induced
apoptosis, and in contrast, it is possible that NF-κB was not
upstream of ROS production.
Discussion
To the best of our knowledge, the present study is
the first to report that CDK5RAP1 deficiency promoted apoptosis in
the malignant melanoma cell line, A375. This favorable effect on
A375 cells was induced by ROS generation and NF-κB activation,
which was necessary to explain the pathway of CDK5RAP1 deficiency
induced A375 cells apoptosis.
CDK5RAP1, with homology to the bacterial MiaB
protein, is a radical SAM enzyme (3,4). CDK5RAP1
catalyzes 2-methylthio (ms2) modification of
mitochondrial tRNAs (i6A into
ms2i6A) at A37 (5). The ms2 modifications optimize
mitochondrial translation (23,24).
CDK5RAP1 established the biochemical association between the
enzymatic modification and cell apoptosis (6). Deficiency in ms2 modification
has been demonstrated to markedly impaired mitochondrial protein
synthesis, which results in a number of diseases, including
vitiligo and type 2 diabetes (6,25). It has
been suggested that deficiency in ms2 modification
induced by CDK5RAP1 deficiency induces cancer cell apoptosis via
the p-JNK signaling pathway (8).
Apoptosis is an essential mechanism. Numerous
chemotherapeutic agents inhibit tumor growth through suppressing
apoptosis of cancer cells (26).
Ca2+ influx serves an important function and is
associated with the phosphatide-conjugated protein, Annexin V,
which has presented a high binding affinity to phosphatidylserine
of apoptotic cells in early stages (9). Apoptosis is also considered to be
associated with mitochondria-initiated responses. As CDK5RAP1
deficiency-induced deficiency in ms2 modification is
closely associated with cancer cell apoptosis, CDK5RAP1 deficiency
may have a beneficial effect on inhibiting cancer process.
According to these theories, the present study demonstrated that
CDK5RAP1 deficiency inhibited the Ca2+ influx in human
malignant melanoma A375 cells, and suppressed the proliferation and
induced apoptosis of A375 cells.
ROS, the normal cellular oxidative process
byproduct, has been reported in regulating apoptotic initiation
signaling and is associated with several oncogenic pathways
(27). Thus, the beneficial role to
suppress ROS generation and accumulation is evident. There is
compelling evidence that only ROS, which overcomes cellular
antioxidant defenses, may trigger apoptosis and facilitate cancer
cells more sensitive to ROS compared with normal cells (10). Basal ROS levels elevate oncogenic
transformation significantly, thus apoptotic programming in cancer
cells is easily triggered by further acute increases in ROS
(11). Redundant ROS initiates
cytotoxicity, which is also considered to be an important mechanism
in the anticancer mechanism (28).
Consistently, the results of the current study demonstrated that
CDK5RAP1 deficiency significantly promoted ROS generation and
accumulation in A375 cells, suggesting that apoptosis induced by
CDK5RAP1 deficiency in A375 cells is promoted through increased ROS
production.
ROS generated by mitochondria has been demonstrated
to activate NF-κB (13). NF-κB
activation through the ROS-dependent pathway induces cell apoptosis
(14). NF-κB, a transcription factor,
usually exists as an inactive form through binding to its
inhibitory protein, IκB in the cytoplasm (12). The nuclear NF-κB family is well known
in regulating inflammatory responses, which are also important for
cancer cell apoptosis (29). It has
also been reported that apoptosis is associated with NF-κB
(16) and the activation of NF-κB
plays pivotal roles in apoptosis (30). It is well documented that NF-κB has
bidirectional modulatory effects on cell apoptosis (31,32). The
phosphorylation of NF-κB is associated with the pro-apoptosis Bcl-2
family, including Bad, and anti-apoptosis Bcl-2 family, including
Bcl-xl and Bcl-2 (15). As
demonstrated in the present study, CDK5RAP1 deficiency
significantly induced the phosphorylation of NF-κB and Bad in human
malignant melanoma A375 cells, confirming that the NF-κB signaling
pathway was targeted by mitochondria modification. Notably, the
phosphorylation of Bcl-xl and Bcl-2 was downregulated by CDK5RAP1
deficiency.
To better understand the signaling pathway in the
apoptosis process, PDTC, an inhibitor of NF-κB, was used.
Pretreatment with PDTC prevented the decrease in proliferation and
apoptosis induced by CDK5RAP1 deficiency in A375 cells. However,
pretreatment with PDTC did not affect the generation of ROS in A375
cells, indicating that the ROS signaling pathway is upstream of the
NF-κB signaling pathway during the apoptosis process.
CDK5RAP1 is associated with mitochondrial
modification, thus CDK5RAP1 deficiency leads to mitochondrial
dysfunction. However, as a limitation, CDK5RAP1 does not exist only
in cancer cells, but also in all normal cells. This limits the use
of CDK5RAP1 deficiency for therapy. In order for CDK5RAP1
deficiency to be used in gene therapy, it is important to develop a
means of specifically targeting CDK5RAP1 in cancer cells.
To the best of our knowledge, this is the first
study to demonstrate that CDK5RAP1 deficiency induces apoptosis in
human malignant melanoma A375 cells via the ROS/NF-κB signaling
pathway. Although the present data provides evidence that cancer
progression was remarkably inhibited in CDK5RAP1 deficient cells,
practical application still needs to be further investigated. The
potential effects of CDK5RAP1 deficiency in cancer cells is
expected to provide important insight for developing a novel
clinical cancer therapy.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hatanaka M, Higashi Y, Kawai K, Su J, Zeng
W, Chen X and Kanekura T: CD147-targeted siRNA in A375 malignant
melanoma cells induces the phosphorylation of EGFR and
downregulates cdc25C and MEK phosphorylation. Oncol Lett.
11:2424–2428. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostheimer C, Bormann C, Fiedler E, Marsch
W and Vordermark D: Malignant melanoma brain metastases: Treatment
results and prognostic factors-a single-center retrospective study.
Int J Oncol. 46:2439–2448. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Reiter V, Matschkal DM, Wagner M, Globisch
D, Kneuttinger AC, Müller M and Carell T: The CDK5 repressor
CDK5RAP1 is a methylthiotransferase acting on nuclear and
mitochondrial RNA. Nucleic Acids Res. 40:6235–6240. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zou X, Ji C, Jin F, Liu J, Wu M, Zheng H,
Wang Y, Li X, Xu J, Gu S, et al: Cloning, characterization and
expression of CDK5RAP1_v3 and CDK5RAP1_v4, two novel splice
variants of human CDK5RAP1. Genes Genet Syst. 79:177–182. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pierrel F, Douki T, Fontecave M and Atta
M: MiaB protein is a bifunctional radical-S-adenosylmethionine
enzyme involved in thiolation and methylation of tRNA. J Biol Chem.
279:47555–47563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei FY, Zhou B, Suzuki T, Miyata K,
Ujihara Y, Horiguchi H, Takahashi N, Xie P, Michiue H, Fujimura A,
et al: Cdk5rap1-mediated 2-methylthio modification of mitochondrial
tRNAs governs protein translation and contributes to myopathy in
mice and humans. Cell Metab. 21:428–442. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang H, Wei L, Li C, Zhou J and Li Z:
CDK5RAP1 deficiency induces cell cycle arrest and apoptosis in
human breast cancer cell line by the ROS/JNK signaling pathway.
Oncol Rep. 33:1089–1096. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brody JG, Rudel RA, Michels KB, Moysich
KB, Bernstein L, Attfield KR and Gray S: Environmental pollutants,
diet, physical activity, body size, and breast cancer: Where do we
stand in research to identify opportunities for prevention? Cancer.
109 12 Suppl:S2627–S2634. 2007. View Article : Google Scholar
|
|
9
|
Li X, Zhao H, Wang Q, Liang H and Jiang X:
Fucoidan protects ARPE-19 cells from oxidative stress via
normalization of reactive oxygen species generation through the
Ca2+-dependent ERK signaling pathway. Mol Med Rep.
11:3746–3752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Myatt SS, Brosens JJ and Lam EW: Sense and
sensitivity: FOXO and ROS in cancer development and treatment.
Antioxid Redox Signal. 14:675–687. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang H, Zhang T, Sun W, Wang Z, Zuo D,
Zhou Z, Li S, Xu J, Yin F, Hua Y and Cai Z: Erianin induces
G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK
signaling pathway in human osteosarcoma cells in vitro and in vivo.
Cell Death Dis. 7:e22472016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kurihara Y and Furue M: Interferon-γ
enhances phorbol myristate acetate-induced cell attachment and
tumor necrosis factor production via the NF-κB pathway in THP-1
human monocytic cells. Mol Med Rep. 7:1739–1744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li F, Ambrosini G, Chu EY, Plescia J,
Tognin S, Marchisio PC and Altieri DC: Control of apoptosis and
mitotic spindle checkpoint by survivin. Nature. 396:580–584. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chandel NS, Trzyna WC, McClintock DS and
Schumacker PT: Role of oxidants in NF-κB activation and TNF-alpha
gene transcription induced by hypoxia and endotoxin. J Immunol.
165:1013–1021. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Turillazzi E, Neri M, Cerretani D,
Cantatore S, Frati P, Moltoni L, Busardò FP, Pomara C, Riezzo I and
Fineschi V: Lipid peroxidation and apoptotic response in rat brain
areas induced by long-term administration of nandrolone: The mutual
crosstalk between ROS and NF-kB. J Cell Mol Med. 20:601–612. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lou L, Zhou J, Liu Y, Wei YI, Zhao J, Deng
J, Dong B, Zhu L, Wu A, Yang Y and Chai L: Chlorogenic acid induces
apoptosis to inhibit inflammatory proliferation of IL-6-induced
fibroblast-like synoviocytes through modulating the activation of
JAK/STAT and NF-κB signaling pathways. Exp Ther Med. 11:2054–2060.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nishiura H, Tokita K, Li Y, Harada K,
Woodruff TM, Taylor SM, Nsiama TK, Nishino N and Yamamoto T: The
role of the ribosomal protein S19 C-terminus in Gi
protein-dependent alternative activation of p38 MAP kinase via the
C5a receptor in HMC-1 cells. Apoptosis. 15:966–981. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang L, Shu T, Liang Y, Gu W, Wang C, Song
X, Fan C and Wang W: GDC-0152 attenuates the malignant progression
of osteosarcoma promoted by ANGPTL2 via PI3K/AKT but not p38MAPK
signaling pathway. Int J Oncol. 46:1651–1658. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li X, Zhao H, Wang Q, Liang H and Jiang X:
Fucoidan protects ARPE-19 cells from oxidative stress via
normalization of reactive oxygen species generation through the
Ca2+-dependent ERK signaling pathway. Mol Med Rep.
11:3746–3752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu Y, Tian Z and Xie P: Targeting
complement anaphylatoxin C5a receptor in hyperoxic lung injury in
mice. Mol Med Rep. 10:1786–1792. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang X, Yu J, Ma Z, Zhang H and Xie F:
Effects of fucoidan on insulin stimulation and pancreatic
protection via the cAMP signaling pathway in vivo and in vitro. Mol
Med Rep. 12:4501–4507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moncini S, Bevilacqua A, Venturin M,
Fallini C, Ratti A, Nicolin A and Riva P: The 3′untranslated region
of human Cyclin-Dependent kinase 5 regulatory subunit 1 contains
regulatory elements affecting transcript stability. BMC Mol Biol.
8:1112007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jenner LB, Demeshkina N, Yusupova G and
Yusupov M: Structural aspects of messenger RNA reading frame
maintenance by the ribosome. Nat Struct Mol Biol. 17:555–560. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shin MK, Uhm YK, Lee JH, Kim SK, Chung JH
and Lee MH: Association between CDK5RAP1 polymorphisms and
susceptibility to vitiligo in the Korean population. Eur J
Dermatol. 22:495–499. 2012.PubMed/NCBI
|
|
26
|
Matsumoto T, Jimi S, Migita K, Takamatsu Y
and Hara S: Inhibition of glucose transporter 1 induces apoptosis
and sensitizes multiple myeloma cells to conventional
chemotherapeutic agents. Leuk Res. 41:103–110. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dewaele M, Maes H and Agostinis P:
ROS-mediated mechanisms of autophagy stimulation and their
relevance in cancer therapy. Autophagy. 6:838–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zou P, Zhang J, Xia Y, Kanchana K, Guo G,
Chen W, Huang Y, Wang Z, Yang S and Liang G: ROS generation
mediates the anti-cancer effects of WZ35 via activating JNK and ER
stress apoptotic pathways in gastric cancer. Oncotarget.
6:5860–5876. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Padua MB and Hansen PJ: Changes in
expression of cell-cycle-related genes in PC-3 prostate cancer
cells caused by ovine uterine serpin. J Cell Biochem.
107:1182–1188. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chu H, Yu H, Ren D, Zhu K and Huang H:
Plumbagin exerts protective effects in nucleus pulposus cells by
attenuating hydrogen peroxide-induced oxidative stress,
inflammation and apoptosis through NF-κB and Nrf-2. Int J Mol Med.
37:1669–1676. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Baldwin AS: Control of oncogenesis and
cancer therapy resistance by the transcription factor NF-kappaB. J
Clin Invest. 107:241–246. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang G, Xiao X, Rosen DG, Cheng X, Wu X,
Chang B, Liu G, Xue F, Mercado-Uribe I, Chiao P, et al: The
biphasic role of NF-kappaB in progression and chemoresistance of
ovarian cancer. Clin Cancer Res. 17:2181–2194. 2011. View Article : Google Scholar : PubMed/NCBI
|