Introduction
Lung cancer has been the most common cancer
worldwide, and the leading cause of cancer-associated mortality,
since 1985 (1). The 5-year survival
rate for lung carcinoma overall is poor, at 16–17% (2). Surgery is not suitable for the majority
of patients with lung cancer, as diagnoses are often obtained at
advanced disease stages. The standard of care for advanced
non-small cell lung cancer (NSCLC) is cisplatin in combination with
1 to 3 of the following drugs: Paclitaxel, gemcitabine and
docetaxel (3). The efficacy of
chemotherapy is limited due to its side effects and the lack of
response in certain sub-populations of patients. Research efforts
have focused on identifying molecular targets and developing
molecular-targeted therapies based on the understanding of the
molecular abnormalities associated with lung cancer (4–6).
Insight into the pathobiology of NSCLC has
facilitated the development of targeted molecular therapies that
target specific mutations that serve critical roles in the
progression to aggressive disease. Mutations in the epidermal
growth factor receptor (EGFR), KRAS and anaplastic lymphoma
kinase (ALK) genes are mutually exclusive in patients with
NSCLC, and targeted therapy may be affected due to the existence of
one mutation in lieu of another. Therefore, the detection of these
mutations and the subsequent tailoring of therapy accordingly are
widely accepted as standard practice (7,8).
EGFR is expressed on the cell surface of a substantial
proportion of NSCLC tumors. EGFR overexpression is observed
in 50–80% of patients with NSCLC, and 65% of these individuals also
exhibit an EGFR gene copy number amplification (9,10).
EGFR is a member of the tyrosine kinase growth
factor receptor family. Its ligands, EGF and transforming growth
factor-α, bind to the ectodomain of EGFR to elicit biological
effects through the RAS/RAF/mitogen-activated protein kinase kinase
1/extracellular signal-related kinase pathway, the phosphoinositide
3-kinase/AKT pathway, the phospholipase-Cγ/protein kinase C
pathway, the SRC/signal transducer and activator of transcription
pathway and the corresponding crosstalk, leading to the regulation
of cellular proliferation, survival, apoptosis, invasion and
metastasis (11,12). The overexpression of EGFR usually
indicates rapid progress, resistance to chemoradiotherapy and a
poor prognosis for patients with lung cancer (13,14).
Various small-molecule tyrosine kinase inhibitors (TKIs) and
anti-EGFR antibodies targeting EGFR have been developed over the
previous 30 years. EGFR TKIs directly bind to the kinase domain and
block its kinase activity, including the first-generation EGFR TKIs
Erlotinib and Gefitinib, second-generation Aftinib and
third-generation AZD9191. Conversely, EGFR-targeting antibodies
such as Cetuximab, Panitumumab and nimotuzumab bind
extracellularly, blocking ligand binding and preventing receptor
dimerization (15).
Nimotuzumab, also known as h-R3, is a humanized IgG1
isotype monoclonal antibody against EGFR (16,17), which
is different from Cetuximab and Panituzumab as it demonstrates
different pharmacokinetic traits, including a prolonged half-life,
and elevated area under the curve compared with Cetuximab at the
equivalent dose level (6). Safety
data has demonstrated that nimotuzumab rarely causes severe
dermatological toxicity, which is the most common adverse event
resulting from Cetuximab and Panitumumab treatment; therefore,
nimotuzumab is expected to improve the quality of life for patients
receiving EGFR inhibition therapy (18). Randomized studies have demonstrated
that nimotuzumab, combined with irradiation or chemoradiotherapy,
may provide an improvement in prognosis to patients with head and
neck cancer (18), gliomas (19) and esophagus squamous cell carcinoma
(20).
Considering that patients with NSCLC diagnosed in
the advanced stage usually exhibit a poor survival rate, EGFR
inhibition alone, or combined with other options, in the treatment
of NSCLC has become an attractive strategy validated in numerous
pre-clinical and preliminary clinical trials (21–23). This
has led to a further phase III randomized study to evaluate the
efficacy of the combination of nimotuzumab with other chemotherapy
drugs as a first line treatment for stage IIIB-IV NSCLC (24). A431 cells, a vulvar epidermoid
carcinoma cell line with high EGFR expression demonstrated less
receptor activation upon ligand binding when treated with
nimotuzumab (25). Nimotuzumab is
equally successful as an EGFR inhibitor in normal and mutant
backgrounds (26). Xenograft models
have confirmed this antitumor effect in A431 cells in mice in
vivo. In addition, in vivo data indicated significant
antitumor effects of nimotuzumab in xenografts utilizing the NSCLC
H460, Ma-1 and H292 cell lines (26).
In the present study, the inhibitory effect of
nimotuzumab combined with cisplatin against lung cancer cell growth
was investigated.
Materials and methods
Cell culture
The A549 human lung adenocarcinoma epithelial cell
line (supplied by the Central Laboratory of Qinhuangdao No. 1
People's Hospital) was cultured in Dulbecco's modified Eagle's
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Zhejiang Tianhang
Biotechnology Co., Ltd., Huzhou, China), 1 U/ml penicillin and 1
mg/ml streptomycin at 37°C, with 5% CO2 and 95%
humidity.
Reagents
Cisplatin was purchased from Qilu Pharmaceutical,
Co., Ltd. (Shandong, China). Nimotuzumab was purchased from Biotech
Pharmaceutical Co., Ltd. (Beijing, China).
Cell proliferation assays
Cell viability was measured using the MTT
colorimetric assay, as described previously (27). Briefly, 100 µl cell suspension was
inoculated in each well of 96-well plates at the density of
3×104 cells/well. At 12 h, the medium was removed by
aspiration and replaced with 100 µl culture medium without serum,
as serum may alter the effects of the drugs, and cultured for a
further 12 h. The medium was then removed and replaced by the
experimental medium containing 1% FBS.
A series of MTT assays were then performed to
evaluate the effects of nimotuzumab combined with cisplatin on A549
cells at different time-points for 5 treatment groups: Group A, 200
µg/ml nimotuzumab; group B, 2 µg/ml cisplatin; group C, 200 µg/ml
nimotuzumab for 24 h, then the supernatant was removed and replaced
with 2 µg/ml cisplatin; group D, treatment with cisplatin and
nimotuzumab simultaneously; group E, treatment with fresh culture
medium containing 1% FBS only. The treatments were performed for
24, 48 and 72 h. Following incubation, MTT solution (20 µl, 5
mg/ml) was added to each well, and the plates were incubated in the
dark for 4 h at 37°C, followed by the removal of the culture medium
and addition of 100 µl dimethyl sulfoxide. The absorbance was
measured at 492 with 655 nm as the reference wavelength. A total of
6 parallel wells were used for each group, and all experiments were
performed in triplicate. The inhibitory rate was calculated as
follows: Cell growth inhibitory rate (%) = (OD value in blank group
- OD value in experiment group)/OD value in blank group × 100%.
Cell cycle analysis
The cell cycle distribution was evaluated by flow
cytometry using Vindelov's method (28). Briefly, A549 cells were harvested
following treatment with either nimotuzumab or nimotuzumab combined
with cisplatin, as aforementioned. Then, cells were treated with
0.25% trypsin to prepare single cell suspensions. The cells were
transferred into 10 ml centrifuge tubes and centrifuged at 4°C at
580 × g for 5 min. The precipitate was collected, washed once with
1X PBS and processed for cell cycle analysis by flow cytometry. The
cells were fixed in 75% ethanol and incubated at 4°C overnight. The
fixed cells were centrifuged at 4°C at 145 × g for 5 min and washed
twice with cold PBS. RNase A (20 µg/ml) and propidium iodide
staining solution (50 µg/ml) were added to the cells and incubated
for 30 min at 37°C in the dark. The percentage of cells in each
phase of the cell cycle was determined with ModFit LT 4.0 software
(Verity Software House, Inc., Topsham, ME, USA). Experiments were
performed at least twice.
Western blot analysis
The cell culture supernatant was removed, and the
cells were washed in 4 ml PBS, then centrifuged at 4°C at 900 × g
for 5 min. The supernatant was discarded and the cells were washed
repeatedly with PBS. A total of 200 µl RIPA lysis buffer (cat. no.
P0013B; Beyotime Institute of Biotechnology, Shanghai, China) was
added, the cells were lysed by shaking the flask for 40 min and
cell lysate was transferred to 1.5 ml centrifuge tube. The cell
lysates were centrifuged at 4°C at 10,000 × g for 15 min and the
supernatant was transferred to a new centrifuge tube; tubes were
stored at −80°C until subsequent use.
Protein was quantified using the Enhanced BCA
Protein Assay kit (cat. no. P0009; Beyotime Institute of
Biotechnology). Whole cell extracts were subjected to SDS-PAGE
(with a 5% stacking gel and a 12% separating gel) and transferred
to a nitrocellulose membrane for western blot analysis, with a
loading quantity of 35 µg protein/lane. Subsequently, 5% skimmed
milk powder was used for blocking at room temperature for 1 h.
Blots were incubated with monoclonal anti-cyclin D1 antibody
produced in mice (1:200 dilution; cat. no. C7464; Sigma-Aldrich;
Merck KGaA, Darmstadt, Germany), and anti-β-actin antibody (1:2,000
dilution; cat. no. A1978; Sigma-Aldrich; Merck KGaA) overnight at
4°C, washed with TBS-T, and incubated at room temperature for 1 h
with horseradish peroxidase-labeled goat-anti-mouse IgG (1:200
dilution; cat. no. A0216; Beyotime Institute of Biotechnology). The
protein bands were detected using 3,3′-diaminobenzidine
tetrahydrochloride (DAB Horseradish Peroxidase Color Development
kit; cat. no. P0202; Beyotime Institute of Biotechnology). β-actin
served as a reference gene for the normalization of cyclin D1
expression. Densitometric analysis was performed using ImageJ 1.48
software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
Statistical analyses of the experimental data were
performed by two-way analysis of variance followed by Bonferroni's
multiple comparison test with the SPSS, version 16.0 statistical
software package (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Cell proliferation
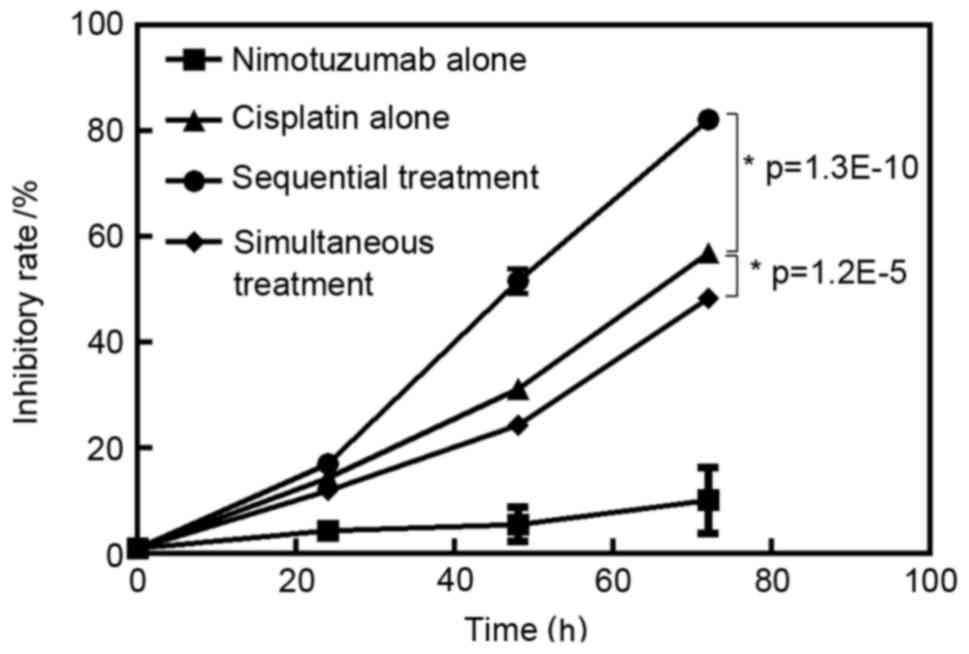
The inhibitory rate for each group is presented in
Fig. 1. Nimotuzumab alone and
cisplatin alone inhibited A549 cell growth in a time-dependent
manner (P<0.05). The inhibitory effect in the sequential
treatment group was higher compared with the cisplatin treatment
alone group (P<0.05). However, the inhibitory effect in the
simultaneous treatment group was lower compared with that in
cisplatin treatment alone group (P<0.05). The inhibitory rate in
the sequential treatment group after 72 h treatment was 82.17±1.62%
(Fig. 1).
Cell cycle analysis
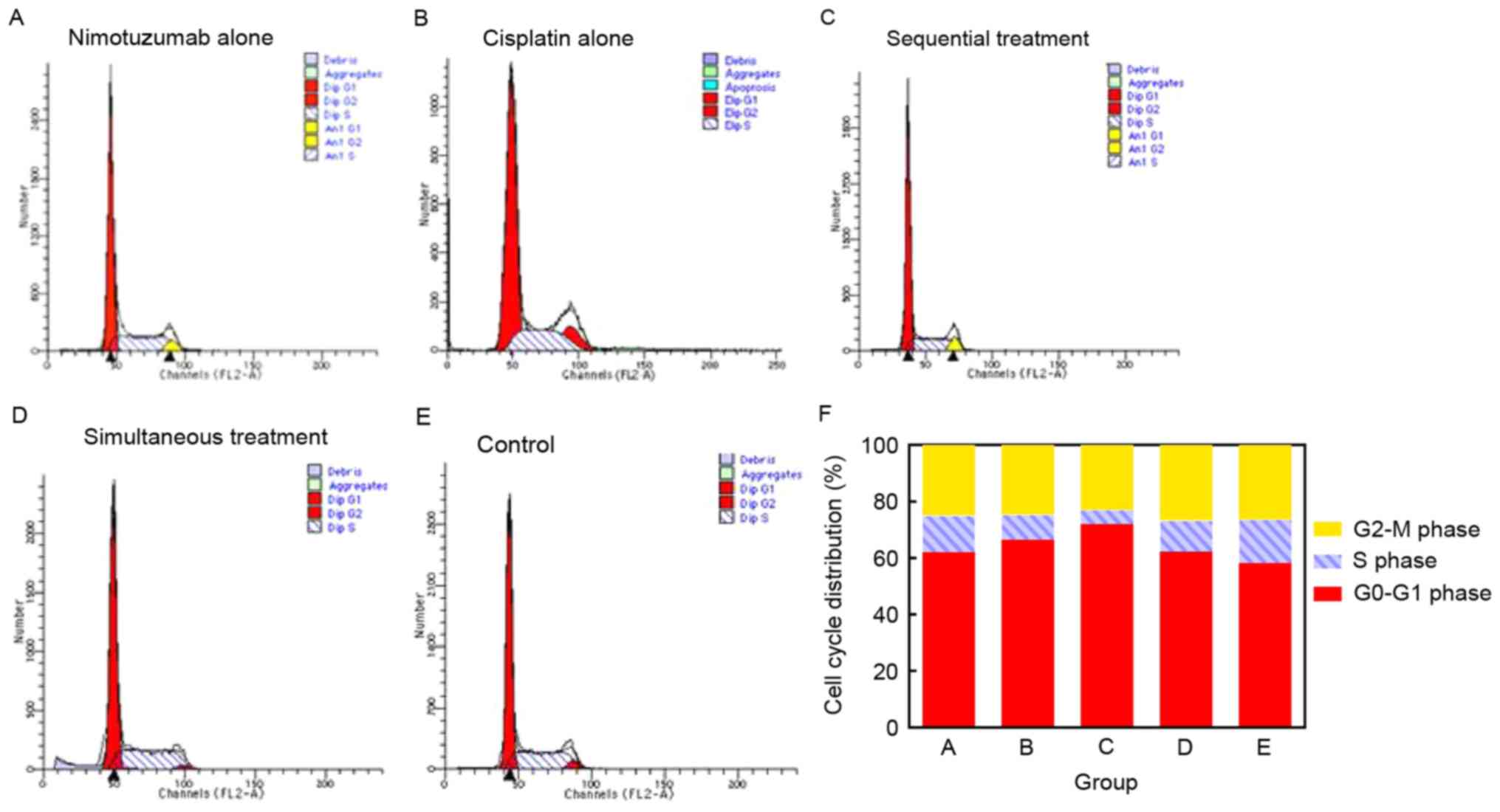
To study the growth inhibitory effect of nimotuzumab
in combination with cisplatin, the cell cycle distribution was
analyzed with propidium iodide staining and flow cytometry. A
typical G0/G1 arrest pattern in groups A-D
was observed, particularly in the sequential treatment group
(Fig. 2; Table I).
 | Table I.Cell cycle distribution in each group
following treatment for 48 h. |
Table I.
Cell cycle distribution in each group
following treatment for 48 h.
|
| Treatment group,
% |
|---|
|
|
|
|---|
| Cell cycle phase | A, Nimotuzumab | B, Cisplatin | C, Sequential
treatment | D, Simultaneous
treatment | E, Untreated
control |
|---|
|
G0/G1 | 61.72±1.68 | 66.40±2.31 | 72.23±2.07 | 62.28±1.68 | 58.25±1.07 |
| S | 12.32±1.32 | 8.78±0.91 | 5.87±0.78 | 11.01±1.37 | 15.35±0.54 |
| G2-M | 24.91±0.62 | 24.48±2.80 | 24.16±1.86 | 26.71±1.31 | 26.40±0.53 |
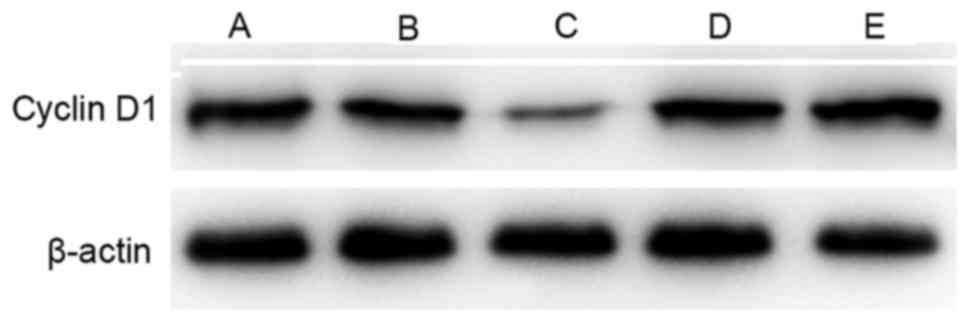
Cyclin D1 expression level
Cyclin D1 is a critical component of the cell cycle
machinery. A number of types of human tumor have been identified to
exhibit abnormally high levels of cyclin D1 (29). Compared with the control group, the
expression of cyclin D1 was decreased in groups A-D following
treatment, particularly in the sequential treatment group. However,
cyclin D1 expression was higher in the simultaneous treatment group
compared with that in the cisplatin alone group (Fig. 3).
Discussion
Nimotuzumab is well-tolerated, and efficacious
against a number of tumor types (18,30–32).
Numerous studies have demonstrated that for patients with advanced
carcinoma, nimotuzumab combined with chemotherapy, radiotherapy or
chemoradiotherapy was effective, and that it was well-tolerated and
safe for conditions such as glioblastoma multiforme (GBM) and
esophageal squamous cell cancer (ESCC); in combination with
radiation therapy, nimotuzumab demonstrated few side effects
(19) and improved the disease
control rate in patients with GBM compared with radiation therapy
alone (33). An open, uncontrolled
phase II study indicated that the combination of nimotuzumab with
concurrent chemoradiation was tolerated reasonably well in patients
with advanced or metastatic ESCC, and increased the efficacy of
treatment (34). Ramos-Suzarte et
al (32) evaluated the adverse
events of nimotuzumab combined with chemotherapy, radiotherapy or
chemoradiotherapy in 835 patients pathologically diagnosed with
malignant tumors in stage II–IV with metastasis or advanced
carcinomas, and indicated that nimotuzumab with chemoradiotherapy
was tolerated.
The present study demonstrated that A549 cell growth
was inhibited somewhat by nimotuzumab alone, and was inhibited to a
greater extent by cisplatin alone, consistent with one previous
study (35). A549 cells pretreated
with nimotuzumab for 24 h prior to cisplatin treatment exhibited a
higher inhibitory rate compared with cells treated with cisplatin
alone (P<0.05). A possible mechanism for the arrest at
G0/G1 (Fig. 2;
Table I) may have been the
downregulation of cyclin D1 (Fig. 3).
It is hypothesized that the antitumor effects of EGFR-specific
monoclonal antibodies are produced by blocking the binding of
ligands to EGFR. This may affect the activity of EGFR and its
downstream signaling pathways, therefore inhibiting the
proliferation of tumor cells, inducing cell cycle arrest and
apoptosis.
However, a degree of antagonism was identified when
A549 cells were treated with nimotuzumab and cisplatin
simultaneously. Previous studies have reported similar conclusions;
Ji et al (35) identified that
nimotuzumab exhibited a weaker inhibition effect on human ESCC EC1
cells when administered in combination with other chemotherapeutic
agents, although the extent was not significant, and Du et
al (36) indicated that the
combination of nimotuzumab with S-1-cisplatin provided no
additional benefit compared with chemotherapy alone in the
first-line treatment of unresectable or metastatic gastric cancer.
Further studies investigating the molecular mechanisms of this
potential antagonistic effect are required.
The present study provides evidence that the A549
human lung adenocarcinoma epithelial cell line was inhibited to a
greater extent by treatment with cisplatin subsequent to
pretreatment with molecularly targeted therapy. Further studies are
required to assess the antitumor activity of nimotuzumab in
combination with cisplatin in vivo, and its clinical
significance.
References
|
1
|
Provencio M, Isla D, Sánchez A and Cantos
B: Inoperable stage III non-small cell lung cancer: Current
treatment and role of vinorelbine. J Thorac Dis. 3:197–204.
2011.PubMed/NCBI
|
|
2
|
Zeng H, Zheng R, Guo Y, Zhang S, Zou X,
Wang N, Zhang L, Tang J, Chen J, Wei K, et al: Cancer survival in
China, 2003–2005: A population-based study. Int J Cancer.
136:1921–1930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Non-Small Cell Lung Cancer Treatment
(PDQ®): National Cancer Institute (US). 2013, https://www.cancer.gov/types/lung/patient/non-small-cell-lung-treatment-pdq
|
|
4
|
Giaccone G, Herbst RS, Manegold C,
Scagliotti G, Rosell R, Miller V, Natale RB, Schiller JH, Von Pawel
J, Pluzanska A, et al: Gefitinib in combination with gemcitabine
and cisplatin in advanced non-small-cell lung cancer: A phase III
trial-INTACT 1. J Clin Oncol. 22:777–784. 2004. View Article : Google Scholar
|
|
5
|
Ciardiello F, Caputo R, Bianco R, Damiano
V, Pomatico G, De Placido S, Bianco AR and Tortora G: Antitumor
effect and potentiation of cytotoxic drugs activity in human cancer
cells by ZD-1839 (Iressa), an epidermal growth factor
receptor-selective tyrosine kinase inhibitor. Clin Cancer Res.
6:2053–2063. 2000.
|
|
6
|
Crombet T, Torres L, Neninger E, Catalá M,
Solano ME, Perera A, Torres O, Iznaga N, Torres F, Pérez R and Lage
A: Pharmacological evaluation of humanized anti-epidermal growth
factor receptor, monoclonal antibody h-R3, in patients with
advanced epithelial-derived cancer. J Immunother. 26:139–148. 2003.
View Article : Google Scholar
|
|
7
|
Keedy VL, Temin S, Somerfield MR, Beasley
MB, Johnson DH, McShane LM, Milton DT, Strawn JR, Wakelee HA and
Giaccone G: American Society of Clinical Oncology provisional
clinical opinion: Epidermal growth factor receptor (EGFR) mutation
testing for patients with advanced non-small-cell lung cancer
considering first-line EGFR tyrosine kinase inhibitor therapy. J
Clin Oncol. 29:2121–2127. 2011. View Article : Google Scholar
|
|
8
|
Stella GM, Scabini R, Inghilleri S, Cemmi
F, Corso S, Pozzi E, Morbini P, Valentini A, Dore R, Ferrari S, et
al: EGFR and KRAS mutational profiling in fresh non-small cell lung
cancer (NSCLC) cells. J Cancer Res Clin Oncol. 139:1327–1335. 2013.
View Article : Google Scholar
|
|
9
|
Raben D, Helfrich B and Bunn PA Jr:
Targeted therapies for non-small-cell lung cancer: Biology,
rationale, and preclinical results from a radiation oncology
perspective. Int J Radiat Oncol Biol Phys. 59 Suppl 2:S27–S38.
2004. View Article : Google Scholar
|
|
10
|
Cappuzzo F, Hirsch FR, Rossi E, Bartolini
S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini
I, et al: Epidermal growth factor receptor gene and protein and
gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer
Inst. 97:643–655. 2005. View Article : Google Scholar
|
|
11
|
Mendelsohn J and Baselga J: Status of
epidermal growth factor receptor antagonists in the biology and
treatment of cancer. J Clin Oncol. 21:2787–2799. 2003. View Article : Google Scholar
|
|
12
|
Yoshida T, Zhang G and Haura EB: Targeting
epidermal growth factor receptor: Central signaling kinase in lung
cancer. Biochem Pharmacol. 80:613–623. 2010. View Article : Google Scholar
|
|
13
|
Xu N, Fang W, Mu L, Tang Y, Gao L, Ren S,
Cao D, Zhou L, Zhang A, Liu D, et al: Overexpression of wildtype
EGFR is tumorigenic and denotes a therapeutic target in non-small
cell lung cancer. Oncotarget. 7:3884–3896. 2016.
|
|
14
|
Sabattini S, Mancini FR, Marconato L,
Bacci B, Rossi F, Vignoli M and Bettini G: EGFR overexpression in
canine primary lung cancer: Pathogenetic implications and impact on
survival. Vet Comp Oncol. 12:237–248. 2014. View Article : Google Scholar
|
|
15
|
Tebbutt N, Pedersen MW and Johns TG:
Targeting the ERBB family in cancer: Couples therapy. Nat Rev
Cancer. 13:663–673. 2013. View
Article : Google Scholar
|
|
16
|
Bebb G, Smith C, Rorke S, Boland W,
Nicacio L, Sukhoo R and Brade A: Phase I clinical trial of the
anti-EGFR monoclonal antibody nimotuzumab with concurrent external
thoracic radiotherapy in Canadian patients diagnosed with stage
IIb, III or IV non-small cell lung cancer unsuitable for radical
therapy. Cancer Chemother Pharmacol. 67:837–845. 2011. View Article : Google Scholar
|
|
17
|
Rojo F, Gracias E, Villena N, Cruz T,
Corominas JM, Corradino I, Cedeño M, Campas C, Osorio M, Iznaga N,
et al: Pharmacodynamic trial of nimotuzumab in unresectable
squamous cell carcinoma of the head and neck: A SENDO Foundation
study. Clin Cancer Res. 16:2474–2482. 2010. View Article : Google Scholar
|
|
18
|
Crombet T, Osorio M, Cruz T, Roca C, del
Castillo R, Mon R, Iznaga-Escobar N, Figueredo R, Koropatnick J,
Renginfo E, et al: Use of the humanized anti-epidermal growth
factor receptor monoclonal antibody h-R3 in combination with
radiotherapy in the treatment of locally advanced head and neck
cancer patients. J Clin Oncol. 22:1646–1654. 2004. View Article : Google Scholar
|
|
19
|
Ramos TC, Figueredo J, Catala M, González
S, Selva JC, Cruz TM, Toledo C, Silva S, Pestano Y, Ramos M, et al:
Treatment of high-grade glioma patients with the humanized
anti-epidermal growth factor receptor (EGFR) antibody h-R3: Report
from a phase I/II trial. Cancer Biol Ther. 5:375–379. 2006.
View Article : Google Scholar
|
|
20
|
Ma NY, Cai XW, Fu XL, Li Y, Zhou XY, Wu
XH, Hu XC, Fan M, Xiang JQ, Zhang YW, et al: Safety and efficacy of
nimotuzumab in combination with radiotherapy for patients with
squamous cell carcinoma of the esophagus. Int J Clin Oncol.
19:297–302. 2014. View Article : Google Scholar
|
|
21
|
Herbst RS and Bunn PA Jr: Targeting the
epidermal growth factor receptor in non-small cell lung cancer.
Clin Cancer Res. 9:5813–5824. 2003.
|
|
22
|
Raben D, Helfrich B, Chan DC, Ciardiello
F, Zhao L, Franklin W, Barón AE, Zeng C, Johnson TK and Bunn PA Jr:
The effects of cetuximab alone and in combination with radiation
and/or chemotherapy in lung cancer. Clin Cancer Res. 11:795–805.
2005.
|
|
23
|
Cascone T, Morelli MP and Ciardiello F:
Small molecule epidermal growth factor receptor (EGFR) tyrosine
kinase inhibitors in non-small cell lung cancer. Ann Oncol. 17
Suppl 2:ii46–ii48. 2006. View Article : Google Scholar
|
|
24
|
Babu KG, Prabhash K, Vaid AK, Sirohi B,
Diwakar RB, Rao R, Kar M, Malhotra H, Nag S, Goswami C, et al:
Nimotuzumab plus chemotherapy versus chemotherapy alone in advanced
non-small-cell lung cancer: A multicenter, randomized, open-label
Phase II study. Onco Targets Ther. 7:1051–1060. 2014. View Article : Google Scholar
|
|
25
|
Diaz Miqueli A, Blanco R, Garcia B, Badia
T, Batista AE, Alonso R and Montero E: Biological activity in vitro
of anti-epidermal growth factor receptor monoclonal antibodies with
different affinities. Hybridoma (Larchmt). 26:423–431. 2007.
View Article : Google Scholar
|
|
26
|
Akashi Y, Okamoto I, Iwasa T, Yoshida T,
Suzuki M, Hatashita E, Yamada Y, Satoh T, Fukuoka M, Ono K and
Nakagawa K: Enhancement of the antitumor activity of ionising
radiation by nimotuzumab, a humanised monoclonal antibody to the
epidermal growth factor receptor, in non-small cell lung cancer
cell lines of differing epidermal growth factor receptor status. Br
J Cancer. 98:749–755. 2008. View Article : Google Scholar
|
|
27
|
Maioli E, Torricelli C, Fortino V,
Carlucci F, Tommassini V and Pacini A: Critical appraisal of the
MTT assay in the presence of rottlerin and uncouplers. Biol Proced
Online. 11:227–240. 2009. View Article : Google Scholar
|
|
28
|
Vindeløv LL, Christensen IJ and Nissen NI:
A detergent-trypsin method for the preparation of nuclei for flow
cytometric DNA analysis. Cytometry. 3:323–327. 1983. View Article : Google Scholar
|
|
29
|
Casimiro MC, Velasco-Velázquez M,
Aguirre-Alvarado C and Pestell RG: Overview of cyclins D1 function
in cancer and the CDK inhibitor landscape: Past and present. Expert
Opin Investig Drugs. 23:295–304. 2014. View Article : Google Scholar
|
|
30
|
Ramakrishnan MS, Eswaraiah A, Crombet T,
Piedra P, Saurez G, Iyer H and Arvind AS: Nimotuzumab, a promising
therapeutic monoclonal for treatment of tumors of epithelial
origin. MAbs. 1:41–48. 2009. View Article : Google Scholar
|
|
31
|
Massimino M, Bode U, Biassoni V and
Fleischhack G: Nimotuzumab for pediatric diffuse intrinsic pontine
gliomas. Expert Opin Biol Ther. 11:247–256. 2011. View Article : Google Scholar
|
|
32
|
Ramos-Suzarte M, Lorenzo-Luaces P, Lazo
NG, Perez ML, Soriano JL, Gonzalez CE, Hernadez IM, Albuerne YÁ,
Moreno BP, Alvarez ES, et al: Treatment of malignant,
non-resectable, epithelial origin esophageal tumours with the
humanized anti-epidermal growth factor antibody nimotuzumab
combined with radiation therapy and chemotherapy. Cancer Biol Ther.
13:600–605. 2012. View Article : Google Scholar
|
|
33
|
Wang Y, Pan L, Sheng XF, Chen S and Dai
JZ: Nimotuzumab, a humanized monoclonal antibody specific for the
EGFR, in combination with temozolomide and radiation therapy for
newly diagnosed glioblastoma multiforme: First results in Chinese
patients. Asia Pac J Clin Oncol. 12:e23–e29. 2016. View Article : Google Scholar
|
|
34
|
Zhao KL, Hu XC, Wu XH, Fu XL, Fan M and
Jiang GL: A phase I dose escalation study of nimotuzumab in
combination with concurrent chemoradiation for patients with
locally advanced squamous cell carcinoma of esophagus. Invest New
Drugs. 30:1585–1590. 2012. View Article : Google Scholar
|
|
35
|
Ji YH, Yang XY, Wu JQ, Huo XQ, Li WW, Li
GJ, Mu YL and Lu P: Nimotuzumab with cisplatin or fluorouracil on
human esophageal squamous cell carcinoma EC1 cells. Eur Rev Med
Pharmacol Sci. 19:586–591. 2015.
|
|
36
|
Du F, Zheng Z, Shi S, Jiang Z, Qu T, Yuan
X, Sun Y, Song Y, Yang L, Zhao J, et al: S-1 and cisplatin with or
without nimotuzumab for patients with untreated unresectable or
metastatic gastric cancer: A randomized, open-label phase 2 trial.
Medicine (Baltimore). 94:e9582015. View Article : Google Scholar
|