Introduction
Coal tar pitch (CTP) has been determined to be a
potent risk factor to human health. This material represents the
collective by-products of the combustion of organic materials that
are widely used in everyday life (1–3).
Epidemiological studies and animal experiments have demonstrated
that CTP is carcinogenic and leads to the development of lung
cancer, often considered to be an occupational tumor (4–6). Exposure
to aerosols and dust from CTP is toxic to the human respiratory,
gastrointestinal and urinary tracts, as well as the skin (7,8). Thus,
although CTP serves multiple functions in modern life, its
carcinogenicity has attracted attention.
Oxidative damage is one factor that contributes to
the development of cancer, by leading to mutations in DNA,
including point mutations, deletions, insertions, chromosomal
translocations, crosslinks and other modifications (9). Direct DNA damage or genomic instability
coupled with altered gene expression and alterations in protein
conformation occur simultaneously in cancer development (10,11). The
anti-oxidative signaling pathway regulated by NF-E2-related factor
2 (Nrf2) serves an important function in resisting the oxidative
damage of external stimuli (12). The
antioxidant response element (ARE) is an upstream sequence that
regulates the expression of the enzymes that provide resistance to
toxins or oxidative stress (13).
Nrf2 is a factor required for binding to ARE, along with other
nuclear factors, in order to initiate the transcription of
downstream genes, including NAD(P)H:Quinone oxidoreductase 1 (NQO1)
(14,15). NQO1 functions as an important enzyme
for protection against the reactive forms of oxygen and inhibition
of neoplasia via a number of functions: i) Catalytic activity
toward quinones, which are toxic to cells; ii) maintenance of the
reduced and active forms of the endogenous lipid-soluble
antioxidants, α-tocopherol-hydroquinone and ubiquinol; iii)
requirement for the stabilization of P53 (16,17).
Therefore, it is important to understand whether the
anti-oxidative signaling pathway regulated by Nrf2 serves a
function in resistance to the adverse impact induced by CTP. In
order to investigate its function in cells exposed to CTP,
immortalized human bronchial epithelial cells (BEAS-2B cells) were
cultured with CTP extract, and the expression of Nrf2 was knocked
down. Subsequently, malignant transformation of BEAS-2B and the
expression of Nrf2 and NQO1 at the mRNA and protein levels were
monitored. The results indicated that Nrf2 was a quick response
factor to CTP exposure and was associated with the malignant
transformation. The present study shed some light on carcinogenic
mechanism and cancer prevention.
Materials and methods
Preparation of the CTP extract
Raw CTP powder (Henan Branch China Aluminum Co.,
Ltd., Zhengzhou, China) was heated to 400°C, and the volatile gas
was collected for 100 min with a common sampling-head. The
collected CTP gas was dissolved in 50 ml ethyl acetate, agitated
for 10 min with ultrasonication and filtered with a sand core
funnel to remove the debris. Subsequently, the extracts were
collected and dried at 45°C, with the residue then dissolved in
dimethyl sulfoxide (DMSO) to a concentration of 2 mg/ml.
Cell culture and survival within
CTP
BEAS-2B cells, provided by Professor Weidong Wu
(Zhengzhou University, Zhengzhou, China) were maintained in
RPMI-1640 (Beijing Solarbio Science and Technology Co., Ltd.,
Beijing, China) supplemented with 10% fetal bovine serum (Hangzhou
Sijiqing Biological Engineering Materials Co., Ltd., Hangzhou,
China). The cells (1×106) were cultured at 37°C
overnight in a 6-well plate and then co-cultured for 24 h at 37°C
with CTP at the indicated concentrations (1, 2.5, 5, 10, 20, 40 and
80 µg/ml) along with a blank control, a 5 µg/ml benzo(a)pyrene
[B(a)P]-treated positive control and a DMSO vehicle control. Each
exposure condition was performed in triplicate. Subsequently, the
cells were detached using trypsin, suspended at 2,000 cells/ml and
incubated with 0.4% trypan blue solution (v/v=1:1) at room
temperature for 3 min. The cells were evaluated using inverted
microscope (cat no. IX71; Olympus, Corporation, Tokyo, Japan) with
which the blue-stained and unstained cells were counted at
magnification, ×100. The half-inhibitory concentration
(IC50) of CTP for these cells was calculated using the
Probit regression model.
RNA interference for Nrf2
expression
An oligonucleotide encoding short hairpin (sh)RNA
targeting the exon region of Nrf2 (177233320–17723340 in chromosome
2, GRCh38.p7) (18) was designed and
synthesized according to the nucleotide sequence of human Nrf2. Its
nucleotide sequence, as well as those of the negative and positive
control [sh-negative control (shNC) and shGAPDH, respectively], are
listed in Table I. These sequences
were inserted into the U6/green fluorescent protein (GFP)/Neo
plasmid (Shanghai GenePharma Co., Ltd., Shanghai, China), and
transfections were carried out using 40 µl RNAi-Mate Transfection
reagent (Shanghai GenePharma Co., Ltd.), according to the
manufacturer's protocol. A total of 48 h later, stable cell lines
were screened by administration of G418 (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
 | Table I.Sequences designed for RNA
interference to silence Nrf2 expression. |
Table I.
Sequences designed for RNA
interference to silence Nrf2 expression.
| Item | Sequence |
|---|
| Nrf2 |
GGTTGCCCACATTCCCAAATC |
| shNC |
GTTCTCCGAACGTGTCACGT |
| shGAPDH |
GTATGACAACAGCCTCAAG |
Soft agar assay for colony formation
and tumor-bearing mouse model
A cell suspension was prepared by digesting cells
with 0.3% trypsin solution and diluting with culture medium
(RPMI-1640 medium supplemented with 20% fetal bovine serum) to a
concentration of 1×106 cells/ml. Cell suspensions were
mixed with a penicillin and streptomycin-containing 0.6% agarose
solution at a ratio of 1:1 (v/v). The mixture was then added to
6-well microplates containing solidified 0.6% agarose gel with 100
U/ml penicillin and 0.1 mg/ml streptomycin. Once the agarose gel
had solidified at room temperature, 2 ml culture medium was added.
Subsequently, the cells were incubated at 37°C in 5% CO2
for 2 weeks. Aggregates consisting of >50 cells were counted as
colonies by observation using an inverted microscope (IX71,
Olympus, Japan) at magnification, ×100.
Subsequently, cells from the blank control, vehicle
DMSO control and CTP groups at the 20 and 30th passages were
transferred via an intradermal injection to the back of the necks
of 18 male BALB/C mice (4 weeks old; average weight ~15 g). Mice
were free of specific pathogens and had been purchased from Hunan
Slack King Laboratory Animal Co., Ltd. (Changsha, China). All the
mice lived in the environment free of specific pathogens (25°C;
50–70% humidity) with a 12 h light/dark cycle and were fed a
standard full-nutrient diet for medical experimental animals in
China (19). The mice were treated
humanely and with regard for alleviation of suffering; clean water,
sufficient food and sufficient and comfortable room for rest were
provided; mice were also housed with companions. The growth of the
cells was observed and the tumors, if any developed, were excised
for further examination after 30 days. The tumor volume was
calculated by the following equation: V=(long diameter × short
diameter × short diameter)/2.
All animal experiments were performed in accordance
with international guidelines for the care and use of laboratory
animals, as well as local and national regulations. The mice were
sacrificed by inhaling ether 30 days following intradermal
injection. The humane endpoint was defined when the tumor reached
1,500 mm3. If the tumor reached this volume, the mouse
would be sacrificed ahead of time to relieve pain via inhalation of
ether only, even if the experiment was incomplete. However, no
mouse was sacrificed in advance due to such large tumor volume
during the experiment. The lab in which the animal experiments were
performed was certified by the Science and Technology Department of
Henan Province (Henan, China; SYXK2012-007). The experimental
protocol was approved by the Life Sciences Institutional Review
Board of Zhengzhou University (Henan, China).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analyses of Nrf2 and NQO1
At the indicated time points (3, 6, 12 and 24 h),
total RNA was extracted using RNAiso Plus (Takara Biotechnology
Co., Ltd., Dalian, China). The concentration of 2 µl RNA was
calculated (µg/ml=OD260 × 40 × dilution factor), whereas
the integrity of 5 µl of RNA was visualized under ultraviolet light
as two bands at 28S and 18S following 1.5% agarose gel
electrophoresis. The total RNA was then reverse transcribed to cDNA
by Prime Script™ RT reagent kit (Takara Bio, Inc., Otsu Japan)
according to the manufacturer's protocols. Then mRNA transcription
levels of Nrf2 and NQO1 were assessed by Mx3000P qPCR
System (Stratagene; Agilent Technologies, Inc., Santa Clara, CA,
USA) with the SYBR R Premix Ex Taq™ (Tli RNaseH Plus) kit (Takara
Bio, Inc.) and genes specific primers (Nrf2: Forward,
5′-GACGGTATGCAACAGGACATTGAG-3′ and reverse,
5′-AACTTCTGTCAGTTTGGCTTCTGGA-3′. NQO1: Forward,
5′-GGATTGGACCGAGCTGGAA-3′ and reverse,
5′-AATTGCAGTGAAGATGAAGGCAAC-3′). GAPDH was used as an
internal control (Forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′). qPCR was performed with the SYBR R
Premix Ex Taq™ (Tli RNaseH Plus) kit with the following
thermocycling conditions: 95°C for 30 sec as initial denaturing
followed by between 40 and 45 cycles of 95°C for 5 sec and 55°C for
15 sec, and a final extension at 72°C for 10 sec. A melting curve
analysis was automatically performed following amplification in
qPCR instrument and MxPro qPCR software version 4.10 (cat no.
MX3000P; Stratagene; Agilent Technologies, Inc.). The results of
the expression of the target genes were quantified using the
2−∆∆Cq method (20).
Western blotting of Nrf2 and NQO1
BEAS-2B cells (106 cells/ml) were
cultured overnight at 37°C and with 5% CO2, and then the
RPMI-1640 medium was replaced with fresh medium containing the
different concentrations of CTP (1, 2.5, 5, 10, 20, 40 and 80
µg/ml). The total cells were lysed with RIPA Lysis Buffer
(CWBiotech, Beijing, China) at various time points (3, 6, 12 and 24
h), and then the lysates were collected. These protein samples (30
µg) were quantified using the standard curve method, resolved using
SDS-PAGE (30% polyacrylamide) and transferred to polyvinylidene
difluoride membranes. The protein-bound membrane was blocked in TBS
with Tween 20 buffer containing 10% milk for 2 h at room
temperature, immunoblotted with a primary rabbit anti-human
polyclonal antibody (1:500; Nrf2, cat no. ab62352; NQO1, cat no.
ab34173; Abcam, Cambridge, UK) overnight at 4°C, and then probed
with a biotinylated secondary goat anti-rabbit antibody (1:1,000;
cat no. ab205718; Abcam, Cambridge, UK). The immunoblots were
visualized using a diaminobenzidine kit (Beijing Zhongqiao Jinqiao
Biotechnology Co., Ltd., Beijing, China) according to the
manufacturer's protocol and quantified using GeneSnap 7.2 Software
(Syngene, Cambridge, UK). β-actin served as an internal control to
verify that equal amounts of protein were loaded into each well of
the SDS-PAGE gel (Rabbit anti-human polyclonal β-actin antibody,
cat no. ab8227; Abcam, Cambridge, UK; 1:1,000 dilution in 20 mM TBS
buffer containing 10% milk and 0.1% Tween 20).
Statistical analysis
The data are presented as mean ± standard deviation.
The difference between groups for Nrf2 expression level was
analyzed via one-way analysis of variance (ANOVA) and
Student-Newman-Keuls post-hoc test, using Nrf2-NC group as control
(Table II). The difference for
colony formation was also tested by one-way ANOVA and
Student-Newman-Keuls post-hoc test (Table III). All of the statistical analysis
was performed using SPSS software (version 21.0; IBM Corp., Armonk,
NY, USA). P<0.05 was considered to indicate a statistically
significant difference.
 | Table II.Expression levels of Nrf2 in the test
group and the controls. |
Table II.
Expression levels of Nrf2 in the test
group and the controls.
| Group | Level of
Nrf2a | F | P-value |
|---|
| shNC | 1.001±0.107 | 149.399 | <0.001 |
| shGAPDH |
0.144±0.015b |
|
|
| Nrf2 |
0.150±0.009b |
|
|
 | Table III.Results of the soft agar assay for
colony formation. |
Table III.
Results of the soft agar assay for
colony formation.
| Passage | Groups | Cells | Coloniesa | Ratio, %a | F | P-value |
|---|
| 10th | DMSO | 1×104 | 21.25±2.63 | 2.13±0.26 | 0.928 | 0.457 |
|
| B(a)P | 1×104 | 21.75±1.71 | 2.18±0.17 |
|
|
|
| CTP | 1×104 | 22.00±2.16 | 2.20±0.22 |
|
|
|
| RNAi | 1×104 | 23.50±1.29 | 2.35±0.13 |
|
|
| 20th | DMSO | 1×104 | 21.75±2.22 | 2.18±0.22 | 794.790 | <0.001 |
|
| B(a)P | 1×104 | 92.75±3.30 |
9.28±0.33b |
|
|
|
| CTP | 1×104 | 97.25±1.71 |
9.73±0.17b |
|
|
|
| RNAi | 1×104 | 126.25±4.57 |
12.63±0.46b,c,d |
|
|
| 30th | DMSO |
1×104 | 30.25±2.75 | 3.03±0.28 | 783.068 | <0.001 |
|
| B(a)P |
1×104 | 182.50±9.75 |
18.25±0.98b,d |
|
|
|
| CTP |
1×104 | 211.75±6.40 |
21.18±0.64b,c |
|
|
|
| RNAi |
1×104 | 243.50±6.25 |
24.35±0.63b,c,d |
|
|
Results
Cytotoxic effect of CTP on cell
viability
The regression of cell survival rate alongside the
toxicity of CTP was calculated using Probit regression. This was
evaluated using the regression equation: Y (cell
survival)=−1.25+1.375 log (exposure dose).
The IC50 for the effect of CTP on the
cell survival was 8.11 µg/ml. The final concentration applied in
the subsequent experiment was 30% of the IC50, or 1
µg/ml.
Effect of RNA interference
The optimization results identified that the optimal
concentration of G418 was 400 µg/ml. When cells were cultured in
medium containing 400 µg/ml G418, no abnormal morphology was
observed on the first day. By the 7th day, the cell concentration
was <50% and the cells stopped growing by the 13th day.
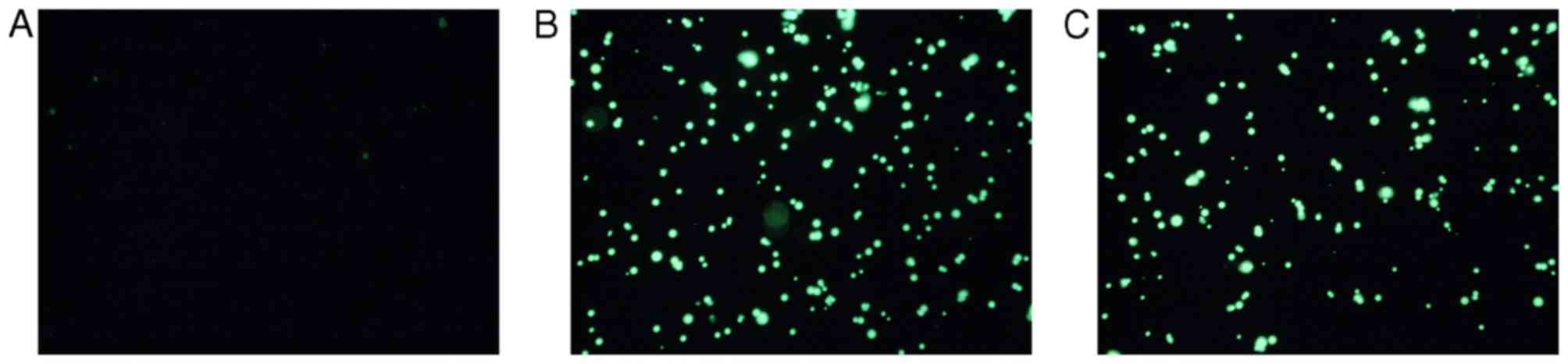
The expression of GFP indicated satisfactory
transfection efficiency (Fig. 1). The
cells were cultured for 24 h following transfection and observed
using a fluorescence microscope. The expression of GFP was observed
in >40% of the cells.
The expression of Nrf2 was compared between the test
group and controls, and the results are presented in Table II. It was notable that the expression
of Nrf2 in the interference group was decreased compared with that
in the negative control (shNC; P<0.001). However, there was no
significant difference indicated between the interference group and
the positive control (shGAPDH). Therefore, these results suggested
that the interference group would be suitable for further
investigation.
Soft agar assay for colony formation
and the tumor-bearing mouse model
In this assay, four groups were designed to
investigate the malignant transformation of BEAS-2B cells exposed
to CTP. In these groups, the cells were treated with CTP, CTP and
RNA interference (marked as RNAi), B(a)P and DMSO, respectively.
The results indicated that when the BEAS-2B cells were subcultured
for 20 passages, there was a significant increase in colonies in
the RNAi group compared with the CTP and B(a)P groups (P<0.001).
Furthermore, there was no notable difference between the CTP and
B(a)P groups (P>0.066). Additionally, in the 30th passage cells,
the numbers of colonies in descending order were; RNAi, CTP, B(a)P
and DMSO (Table III), and the
differences between any two groups were statistically significant.
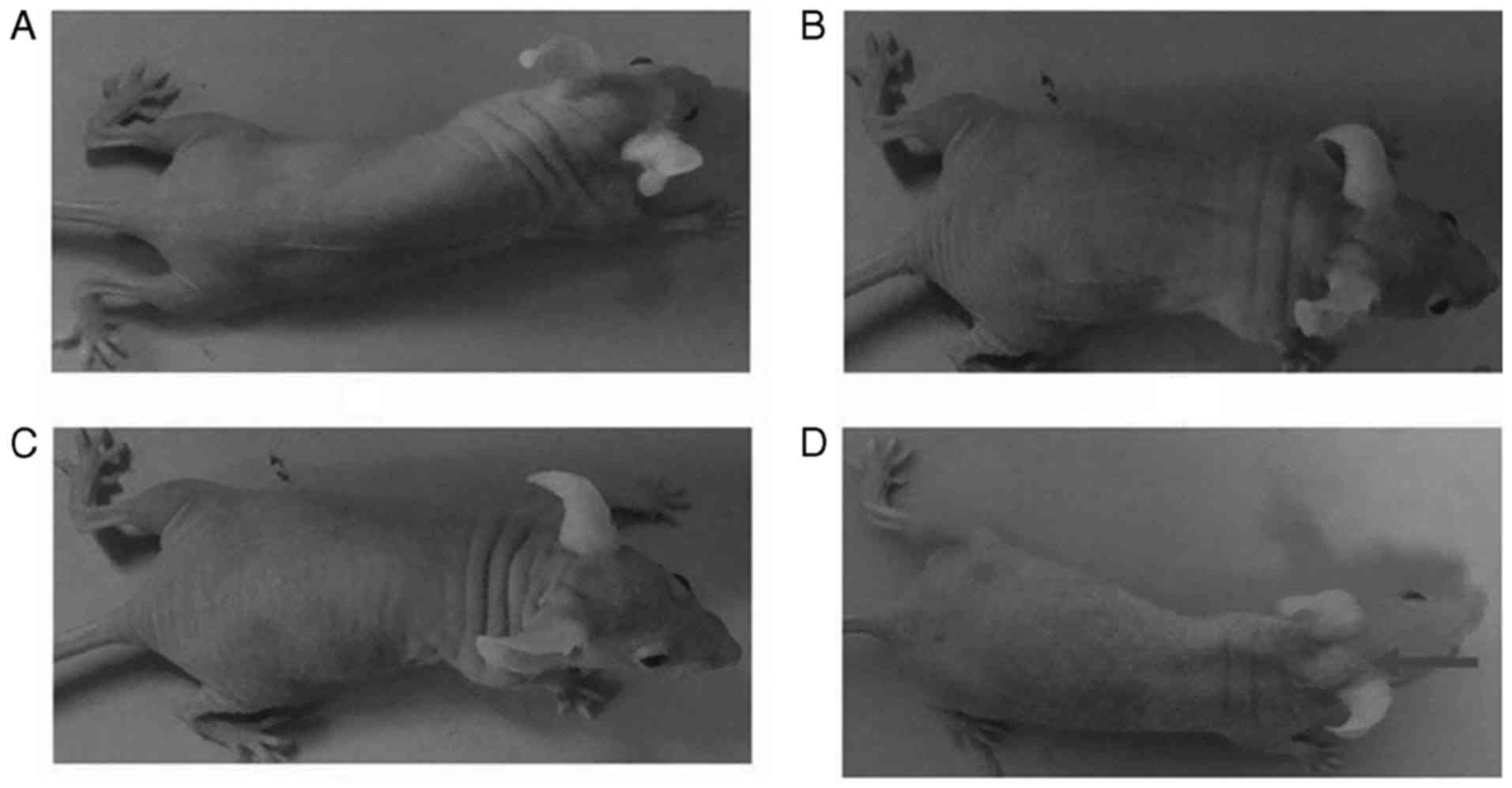
Furthermore, the results of the tumor-bearing mouse model indicated
that cells of the 30th passage treated with CTP were able to grow
into tumors (Fig. 2). Therefore,
these results suggested that CTP was able to induce considerable
toxicity to BEAS-2B cells, which may lead to malignant
transformation, and Nrf2 served a key function in this process.
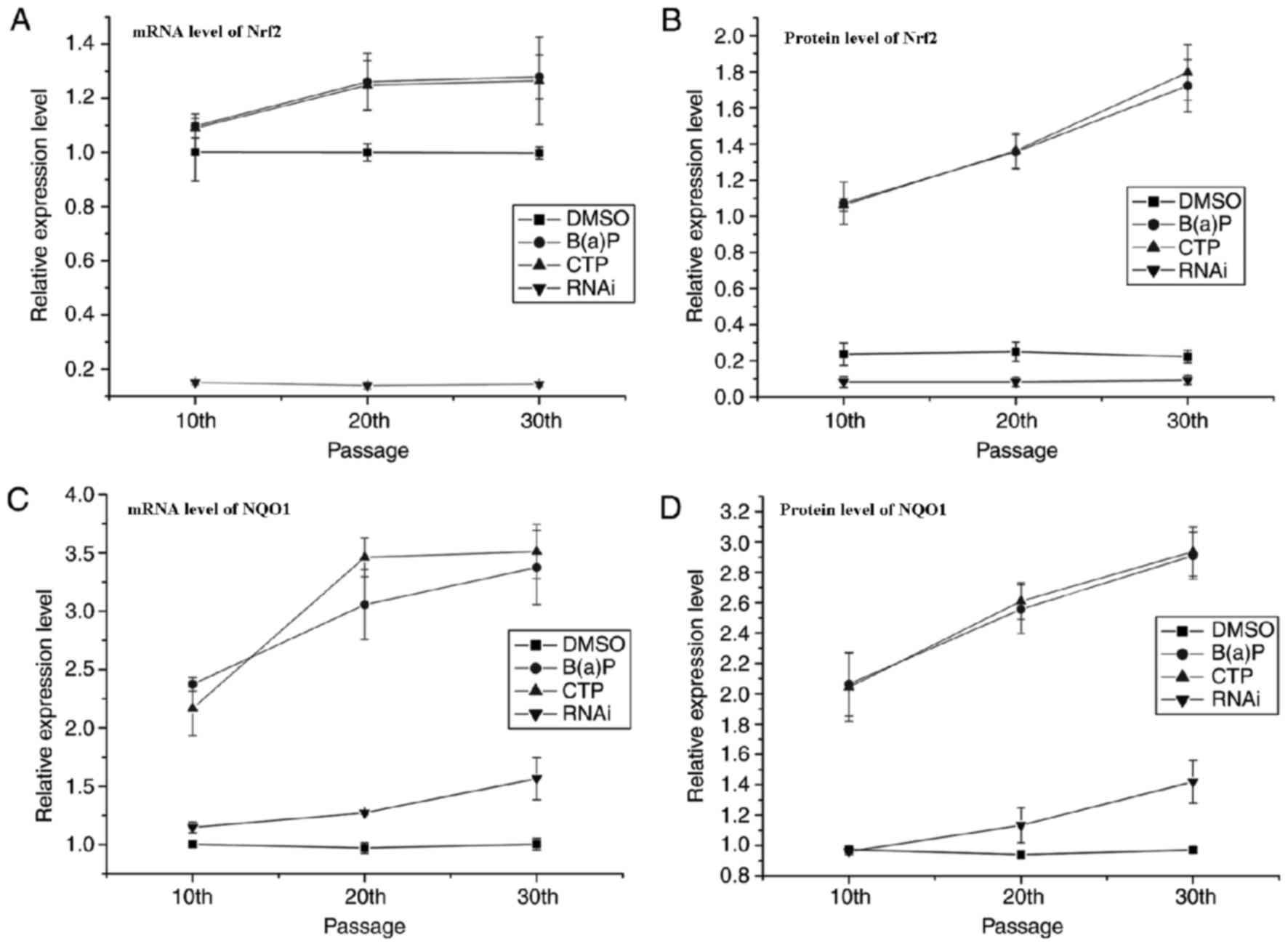
Expression of Nrf2 and NQO1 was
determined by RT-qPCR and western blot analysis
The expression of Nrf2 and NQO1 was determined at
the mRNA level using RT-qPCR and at the protein level using western
blotting. The results of the qPCR are presented in Fig. 3. These data revealed that there was a
notable positive linear correlation between the expression of NQO1
and Nrf2 at the mRNA and protein levels in CTP and B(a)P groups
(Table IV). The expression of Nrf2
at the mRNA and protein levels remained at a low level in the RNAi
group whereas the levels were markedly increased in the CTP and
B(a)P groups. However, the expression of NQO1 in the RNAi group
decreased when the expression of Nrf2 was silenced. Thus, it was
notable that the anti-oxidative system was triggered in BEAS-2B
cells exposed to CTP and that Nrf2 was a required factor for the
expression of anti-oxidative genes.
 | Table IV.Results of the correlation analysis
between the expression levels of NQO1 and Nrf2. |
Table IV.
Results of the correlation analysis
between the expression levels of NQO1 and Nrf2.
|
| mRNA level | Protein level |
|---|
|
|
|
|
|---|
| Analysis | B(a)P | CTP | B(a)P | CTP |
|---|
| Correlation
coefficient | 0.9757 | 0.9987 | 0.9929 | 0.9667 |
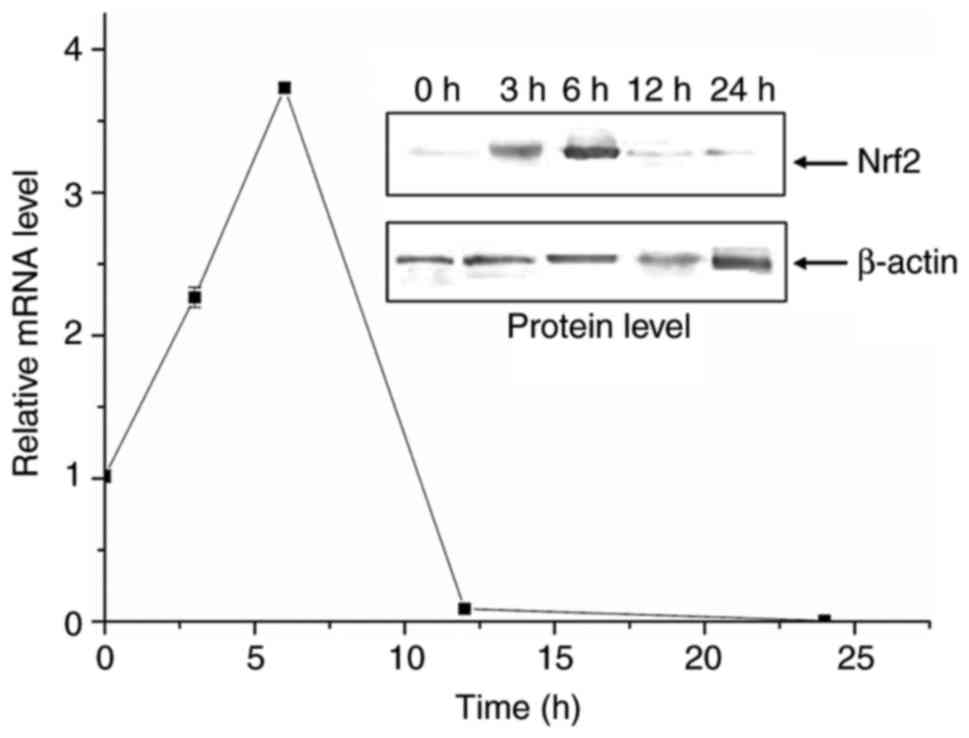
CTP-inducible rapid expression of
Nrf2
The instantaneous response of Nrf2 was investigated
when BEAS-2B cells were cultured with CTP. The expression of Nrf2
was monitored at time points of 3, 6, 12 and 24 h following
treatment with CTP (Fig. 4). The
results revealed that the expression of Nrf2 increased at the mRNA
and protein levels once the cells were treated with CTP. The
expression reached maximal values at 6 h and then returned to the
basal level by 12 h. This indicated that Nrf2 was a rapid response
factor that was sensitive to CTP exposure.
Discussion
The results of the soft agar assay for colony
formation demonstrated that malignant transformation occurred in
cells at the 20th passage, and a greater degree of transformation
was observed in cells at the 30th passage. Therefore, CTP may have
the potential to induce malignant transformation in BEAS-2B cells.
The expression level of Nrf2 and NQO1 increased at the mRNA and
protein levels during the extended culture procedure. Thus, the
anti-oxidative system may take part in the malignant transformation
of BEAS-2B cells exposed by CTP.
It has been reported that Kelch-like ECH-associated
protein 1 retains Nrf2 in the cytoplasm and targets it to the
ubiquitin-proteasome degradation signaling pathway under normal
homeostatic conditions. Upon oxidative stress, Nrf2 translocates to
the nucleus and heterodimerizes with a small Maf protein, allowing
the complex to bind to ARE and activate the expression of NQO1 for
protection against oxidative stress (11).
Nrf2 is a required factor for the expression of
NQO1. Thus, the expression of NQO1 is dependent on the level of
Nrf2 (21). This was observed in the
CTP and B(a)P groups in the present study. The level of NQO1
increased in parallel with increases in the level of Nrf2. There
was a notable positive correlation between the expression of NQO1
and Nrf2 in the normal cells exposed to CTP. When the expression of
Nrf2 was blocked, the expression of NQO1 decreased accordingly.
Thus, it is hypothesized that CTP is able to trigger the
Nrf2-regulated anti-oxidative system during malignant
transformation.
Gas chromatography-mass spectrometry analysis was
combined with the NIST library (https://www.nist.gov) to identify the chemical
composition of the CTP extracts, and the relative content of each
component was determined by a normalized method (22). The compounds of the CTP extracts are
divided into four categories according to their benzene ring
structure: i) Polycyclic aromatic hydrocarbons (PAHs); ii)
heterocyclic hydrocarbons; iii) single-ring aromatic hydrocarbons;
and iv) cycloaliphatic hydrocarbons. These four components account
for 90.981, 7.745, 1.058 and 0.216% of the extracts, respectively.
This suggested that the PAHs may be the main trigger of the
signaling pathway regulated by Nrf2.
It has been reported that oxidative damage caused by
PAHs may be one factor that leads to cancer (23). PAHs may be metabolized to reactive
metabolites, which may produce DNA adducts that result in DNA
mutations, altered gene expression profiles and tumors. In the
present study, the results of a tumor-bearing mouse model indicated
that the cells treated with CTP for between 20 and 30 passages were
able to grow into tumors.
These data suggest that Nrf2 may be a factor
associated with the development of lung cancer induced by CTP.
Therefore, Nrf2 may be a potential intervention target for
prevention of CTP-induced lung cancer.
In conclusion, extended culture with CTP was able to
induce malignant transformation of BEAS-2B cells. The
anti-oxidative signaling pathway was activated in the BEAS-2B cells
exposed to CTP. Nrf2 is a factor that responded rapidly to the
exposure of CTP, regulating the downstream expression of phase II
detoxification enzyme NQO1 to relieve cell damage. This action
continued throughout the extended exposure process. In addition,
the malignant transformation process was accelerated when Nrf2
expression was inhibited. These results indicated that Nrf2 may be
a factor associated with the development of CTP-induced lung
cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81573203, 81402721
and 81402712).
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ARE
|
antioxidant response element
|
|
CTP
|
coal tar pitch
|
|
DMSO
|
dimethyl sulfoxide
|
|
NQO1
|
NAD(P)H:quinone oxidoreductase 1
|
|
Nrf2
|
NF-E2-related factor 2
|
|
PAHs
|
polycyclic aromatic hydrocarbons
|
|
RNAi
|
RNA interference
|
References
|
1
|
Gibbs GW and Labrèche F: Cancer risks in
aluminum reduction plant workers: A review. J Occup Environ Med. 56
5 Suppl:S40–S59. 2014. View Article : Google Scholar
|
|
2
|
Kim SY, Lee J, Kim BH, Kim YJ, Yang KS and
Park MS: Facile synthesis of carbon-coated silicon/graphite
spherical composites for high-performance lithium-ion batteries.
ACS Appl Mater Interfaces. 8:12109–12117. 2016. View Article : Google Scholar
|
|
3
|
Yamaoka M, Asami SS, Funaki N, Kimura S,
Yingjie L, Fukuda T and Yamashita M: Preparation of organic
light-emitting diode using coal tar pitch, a low-cost material, for
printable devices. PLoS One. 8:e629032013. View Article : Google Scholar
|
|
4
|
Koganti A, Singh R, Rozett K, Modi N,
Goldstein LS, Roy TA, Zhang FJ, Harvey RG and Weyand EH:
7H-benzo[c]fluorene: A major DNA adduct-forming component of coal
tar. Carcinogenesis. 21:1601–1609. 2000. View Article : Google Scholar
|
|
5
|
Lavoué J, Gérin M, Côté J and Lapointe R:
Mortality and cancer experience of Quebec aluminum reduction plant
workers. Part I: The reduction plants and coal tar pitch volatile
(CTPV) exposure assessment. J Occup Environ Med. 49:997–1008. 2007.
View Article : Google Scholar
|
|
6
|
Spinelli JJ, Demers PA, Le ND, Friesen MD,
Lorenzi MF, Fang R and Gallagher RP: Cancer risk in aluminum
reduction plant workers (Canada). Cancer Causes Control.
17:939–948. 2006. View Article : Google Scholar
|
|
7
|
Bolliet C, Juery C and Thiebaut B: Impact
of oxidation process on polycyclic aromatic hydrocarbon (PAH)
content in bitumen. J Occup Environ Hyg. 10:435–445. 2013.
View Article : Google Scholar
|
|
8
|
Feng F, Wu Y, Zhang S, Liu Y, Qin L, Wu Y,
Yan Z and Wu W: Macrophages facilitate coal tar pitch
extract-induced tumorigenic transformation of human bronchial
epithelial cells mediated by NF-kB. PLoS One. 7:e516902012.
View Article : Google Scholar
|
|
9
|
Valko M, Leibfritz D, Moncol J, Cronin MT,
Mazur M and Telser J: Free radicals and antioxidants in normal
physiological functions and human disease. Int J Biochem Cell Biol.
39:44–84. 2007. View Article : Google Scholar
|
|
10
|
Pilger A and Rudiger HW:
8-Hydroxy-2′-deoxyguanosine as a marker of oxidative DNA damage
related to occupational and environmental exposures. Int Arch Occup
Environ Health. 80:1–15. 2006. View Article : Google Scholar
|
|
11
|
Taguchi K, Motohashi H and Yamamoto M:
Molecular mechanisms of the Keap1-Nrf2 pathway in stress response
and cancer evolution. Genes Cells. 16:123–140. 2011. View Article : Google Scholar
|
|
12
|
Biswas C, Shah N, Muthu M, La P, Fernando
AP, Sengupta S, Yang G and Dennery PA: Nuclear heme oxygenase-1
(HO-1) modulates subcellular distribution and activation of Nrf2,
impacting metabolic and anti-oxidant defenses. J Biol Chem.
289:26882–26894. 2014. View Article : Google Scholar
|
|
13
|
Ma Q: Role of nrf2 in oxidative stress and
toxicity. Annu Rev Pharmacol Toxicol. 53:401–426. 2013. View Article : Google Scholar
|
|
14
|
Li LR, Dong H, Song E, Xu X, Liu L and
Song Y: Nrf2/ARE pathway activation, HO-1 and NQ01 induction by
polychlorinated biphenyl quinone is associated with reactive oxygen
species and PI3K/AKT signaling. Chem Biol Interact. 209:56–67.
2014. View Article : Google Scholar
|
|
15
|
Anwar-Mohamed A, Elshenawy OH, Soshilov
AA, Denison MS, Chris Le X, Klotz LO and El-Kadi AO: Methylated
pentavalent arsenic metabolites are bifunctional inducers, as they
induce cytochrome P450 1A1 and NAD(P)H:quinone oxidoreductase
through AhR- and Nrf2-dependent mechanisms. Free Radic Biol Med.
67:171–187. 2014. View Article : Google Scholar
|
|
16
|
Nioi P and Hayes JD: Contribution of
NAD(P)H:quinone oxidoreductase 1 to protection against
carcinogenesis, and regulation of its gene by the Nrf2 basic-region
leucine zipper and the arylhydrocarbon receptor basic
helix-loop-helix transcription factors. Mutat Res. 555:149–171.
2004. View Article : Google Scholar
|
|
17
|
Oh ET and Park HJ: Implications of NQO1 in
cancer therapy. BMB Rep. 48:609–617. 2015. View Article : Google Scholar
|
|
18
|
National Center for Biotechnology
Information. https://www.ncbi.nlm.nih.govJanuary. 2018
|
|
19
|
Feed ingredients for laboratory animal,
National standard in China, GB 14924.3. 2010
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(T)(-Delta Delta C) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
21
|
Yu R, Chen C, Mo YY, Hebbar V, Owuor ED,
Tan TH and Kong AN: Activation of mitogen-activated protein kinase
pathways induces antioxidant response element-mediated gene
expression via a Nrf2-dependent mechanism. J Biol Chem.
275:39907–39913. 2000. View Article : Google Scholar
|
|
22
|
Feng FF, Qin LJ, Wu YJ, Li YQ and Wu YM:
GC-MS analysis of coal tar pitch fume exract. Ind Health Occup Dis.
37:198–203. 2011.(In Chinese).
|
|
23
|
Moorthy B, Chu C and Carlin DJ: Polycyclic
aromatic hydrocarbons: From metabolism to lung cancer. Toxicol Sci.
145:5–15. 2015. View Article : Google Scholar
|