Introduction
Glioblastoma multiforme (GBM) is the most common and
malignant brain tumor and has a median survival rate of less than
two years (1,2). Current therapies, including surgery,
radiotherapy and chemotherapy, are generally ineffective at
controlling the progression and development of GBM, and recurrence
is practically inevitable (3).
Therefore, it is important to understand the molecular mechanisms
that are responsible for the abnormal features of GBM and identify
new therapeutic targets.
The Warburg effect, also known as aerobic
glycolysis, is an emerging hallmark of tumor cell metabolism
(4–6).
Lactate dehydrogenase A (LDHA) is a key enzyme in aerobic
glycolysis that increases glucose uptake and lactate production in
tumor cells; LDHA has attracted increasing attention in recent
years due to its critical role in tumor progression (7–9). In breast
cancer, LDHA knockdown suppresses tumor progression by inducing
mitochondrial pathway apoptosis (10). In oesophageal squamous cell carcinoma
(ESCC), knocking down LDHA inhibited cell growth and migration
in vitro, as well as tumorigenesis in vivo (11). In hepatocellular carcinoma (HCC), LDHA
knockdown resulted in a significant reduction in metastatic
potential in a xenograft mouse model (12). In pancreatic cancer, when LDHA was
overexpressed, tumor growth was increased (13). In renal cell carcinoma, knocking down
LDHA resulted in potential anticancer effects (14). Although the abnormal expression of
LDHA has been reported in several cancers (10–15),
little is known about the expression pattern of LDHA and its
underlying roles in GBM. Previous reports using data from the
Oncomine database showed that the mRNA levels of LDHA were
up-regulated in GBM compared to matched tissues (16); these finding prompted us to examine
the underlying role of LDHA in glioma malignancy.
In the present study, we investigated the
relationship between LDHA expression levels and GBM malignancy; we
also explored the effects of LDHA knockdown on proliferation and
apoptosis and its underlying mechanisms, and we further determined
whether the inhibition of LDHA could be used to increase the
chemosensitivity of glioma cells to temozolomide (TMZ).
Materials and methods
Patient glioma samples
Human glioma samples were obtained from patients of
the Department of Neurosurgery, Affiliated Hospital of Hebei
University, and the study was approved by the Ethics Committee of
the Affiliated Hospital of Hebei University. The human glioma
tissue samples used in this study were from 10 patients with grade
II GBM, 7 patients with grade III GBM and 9 patients with grade IV
GBM; these patients were derived according to the World Health
Organization (WHO) classification.
Cells and reagents
The human glioma cell lines U87 and U251 were
obtained from Peking Union Medical College Cell Library (Beijing,
China) and were maintained in Dulbecco's modified Eagles medium
(DMEM) medium supplemented with 10% heat inactivated foetal bovine
serum (FBS), 100 units/ml penicillin, and 100 mg/ml streptomycin at
37°C in a humidified atmosphere containing 5% CO2. All
media and sera were purchased from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA).
Antibodies against LDHA (3582, 1:1,000), Bax (5023,
1:1,000), Bcl-2 (3498, 1:1,000), PARP (9532, 1:1,000), cyclin D1
(2978, 1:1,000), VE-cadherin (2500, 1:1,000) and GAPDH (5174,
1:1,000) were from Cell Signalling Technology, Inc. (Danvers, MA,
USA). Antibodies against MMP-2 (sc-53630, 1:500), MMP-9 (sc-21736,
1:500) and VEGF (sc-365578, 1:500) were from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA).
siRNA transfection
The sequence of siRNA targeting LDHA (GenePharma,
Shanghai, China) has been described previously:
5′-CGAACTGGGCAGTATAAAC-3′; negative control (NC),
5′-UUCUCCGAACGUGUCACGUTT-3′14. U87 and U251 cells were transfected
with specific siRNAs using the Lipofectamine 2000 reagent in
antibiotic-free Opti-MEM medium (both from Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
protocol.
Western blotting
U87 and U251 cells were added to
radioimmunoprecipitation assay (RIPA) lysis buffer (CW Biotech,
Beijing, China) containing a protease inhibitor cocktail (Roche
Diagnostics, Basel, Switzerland) and incubated for 30 min at 4°C.
After sodium dodecyl sulfate polyacrylamide gel electrophoresis
(SDS-PAGE), the proteins were transferred onto nitrocellulose
membranes and blocked with 5% milk in TBST buffer (20 mM Tris-HCl,
pH 7.4; 137 mM NaCl; and 0.1% Tween-20) for 1 h at 37°C. Then, the
cells were incubated overnight at 4°C with primary antibodies
against the target proteins. The membranes were washed five times
and incubated with the appropriate horseradish peroxidase
(HRP)-conjugated secondary antibody for 90 min at room temperature;
the membranes were then developed using ECL reagents (EMD
Millipore, Billerica, MA, USA).
Quantitative PCR (qPCR)
Total RNA was extracted from cultured U87 and U251
cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and cDNA was synthesized using the RevertAid First-Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc.). qRT-PCR was
performed using a MasterCycler system (Eppendorf AG, Hamburg,
Germany) and the FastStart Universal SYBR Green Master kit (Roche
Diagnostics, Indianapolis, IN, USA). Primer sequences were as
follows: LDHA forward, 5′-CTCCTGTGCAAAATGGCAAC-3′ and reverse,
5′-CCTAGAGCTCACTAGTCACAG-3′; β-actin forward,
5′-CGTGACATTAAGGAGAAGCTG-3′ and reverse,
5′-CTAGAAGCATTTGCGGTGGAC-3′. The relative expression of LDHA was
calculated by using the Δ-Ct of the target gene and normalized to
the expression of β-actin.
Cell viability assay
U87 or U251 cells transfected with si-NC or si-LDHA
were seeded in 96-well plates at 5,000 cells/well. After the
samples were incubated with
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT)
dissolved in 150 µl of dimethyl sulfoxide (DMSO) for 4–6 h at 37°C,
the absorbance was measured at 490 nm using a microplate
reader.
Cell cycle analysis
Cell cycle analysis was performed using propidium
iodide (PI) stain (BD Biosciences, Franklin Lakes, NJ, USA)
according to the manufacturer's instructions. Briefly, U87 and U251
cells (5×105) transfected with si-NC or si-LDHA were
harvested, fixed in 5 ml of ice-cold 70% ethanol overnight, and
incubated with 50 µg/ml PI at 4°C; then, the DNA content was
determined using a FACSVerse flow cytometer (BD Biosciences). The
percentages of cells in G0/G1, S, and G2/M phases are presented
quantitatively.
Apoptosis assay
Apoptosis assays were performed using an Annexin
V-FITC apoptosis detection kit (BD Biosciences) according to the
manufacturer's instructions. Briefly, U87 and U251 cells were
harvested and stained with Annexin V-FITC and PI in the dark for 15
min at room temperature. Apoptotic cells were quantitatively
analysed using a FACSVerse flow cytometer and FACSuite analysis
software (BD Biosciences). The total percentages of Annexin
V-positive cells are presented quantitatively.
Statistical analysis
Statistical analyses were performed using SPSS
Statistics software (SPSS, Inc., Chicago, IL, USA). One-way ANOVA
analysis was used to analyze the significance of the data between
the two groups. Mann-Whitney U test was performed to analyze the
relationship between LDHA and GBM malignancy. Differences with
P-values <0.05 were considered to be statistically
significant.
Results
The prognostic significance of LDHA
expression levels in GBM
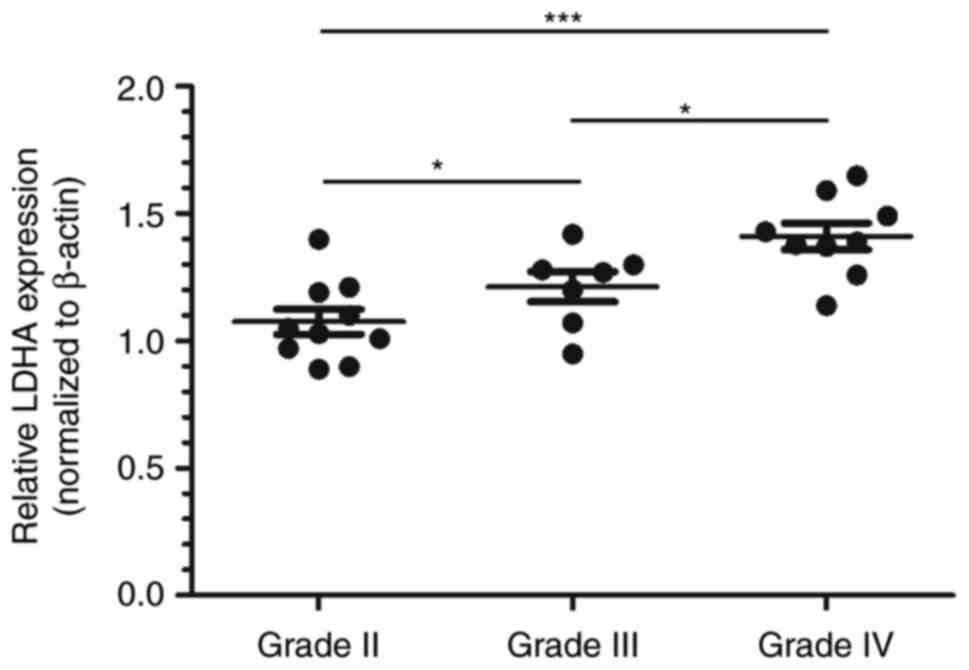
To investigate the association between LDHA
expression levels and GBM malignancy, the expression of LDHA was
examined by real-time PCR analysis. We categorized all glioma
samples as grade II, grade III or grade IV according to the WHO
classification guidelines. We found that the expression levels of
LDHA in the high-grade samples (grade III and grade IV) were
significantly higher than those in grade II tumors (Fig. 1). These results further confirm that
LDHA might play an oncogenic role in the progression of GBM.
LDHA knockdown inhibits glioma cell
growth and epithelial-mesenchymal transition (EMT)
To further assess the potential oncogenic roles of
LDHA in GBM, we used siRNA to knock down the expression of LDHA in
two GBM cell lines: U87 and U251. We treated the cells with LDHA
siRNA (si-LDHA) for 72 h and used scrambled siRNA as a negative
control (si-NC); we then determined protein and mRNA expression
levels via western blotting (Fig. 2A)
and PCR (Fig. 2B). The data indicated
that LDHA siRNA treatment successfully knocked down LDHA at both
the protein and mRNA levels (Fig. 2A and
B). Moreover, MTT assays showed that treatment of U87 and U251
cells with LDHA siRNA for 72 h led to an approximately 30 or 25%
inhibition of cell growth compared to the results of si-NC
treatment (Fig. 2C). Furthermore, we
analysed the effects of LDHA knockdown on apoptosis and
proliferation by using flow cytometry. As shown in Fig. 2D, according to the Annexin V-FITC/PI
staining results, we found that treating U87 or U251 cells with
si-LDHA led to a marked increase in the percentage of apoptotic
cells compared to that of the si-NC group (Fig. 2D). In addition, cell cycle analyses
showed that si-LDHA treatment significantly induced cell cycle
arrest at the G0/G1 phase and inhibited S phase transition
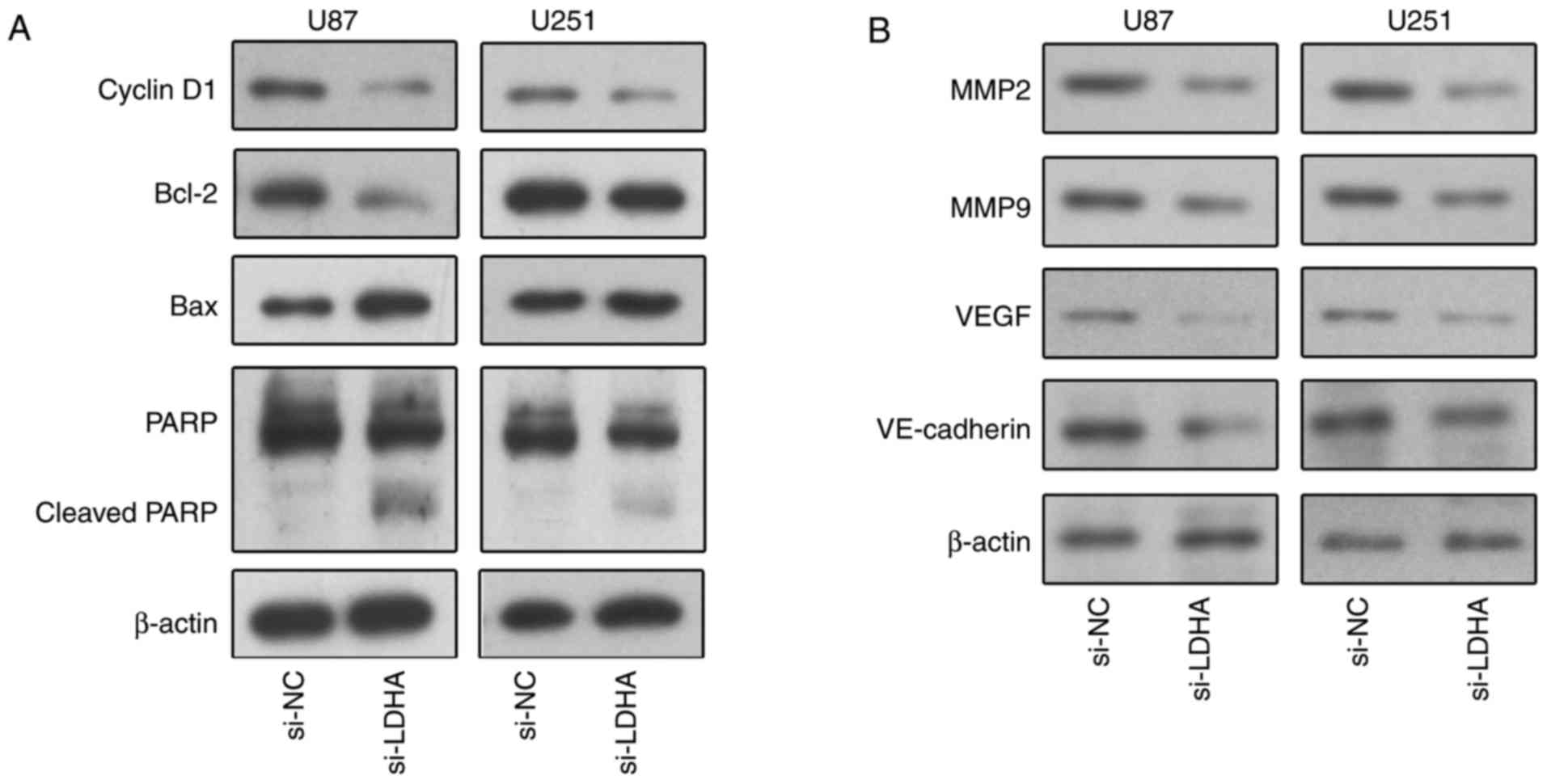
(Fig. 2E). Mechanistically, to
further determine the impact of LDHA knockdown on cell
growth-associated proteins, we treated the cells with si-LDHA for
48 h and analysed protein levels by western blotting (Fig. 3A). We found that LDHA siRNA treatment
was related to decreased cyclin D1 expression and increased PARP
cleavage; moreover, we found that the expression of Bcl-2 was
decreased, whereas the expression of Bax was increased, indicating
an enhancement in the apoptotic potential of LDHA-deficient U87 and
U251 cells (Fig. 3A). These results
indicated that LDHA knockdown inhibits the growth of glioma
cells.
Furthermore, to determine the effects of LDHA on
tumor progression-related proteins, we treated U87 and U251 cells
with si-LDHA for 48 h and analysed protein levels by western
blotting. As shown in Fig. 3B, we
found that the levels of MMP-2, MMP-9, VE-Cadherin and VEGF were
reduced in U87 and U251 cells after LDHA knockdown (Fig. 3B). These findings indicated that the
knockdown of LDHA might inhibit EMT in GBM.
LDHA knockdown enhances the
chemosensitivity of glioma cells to TMZ
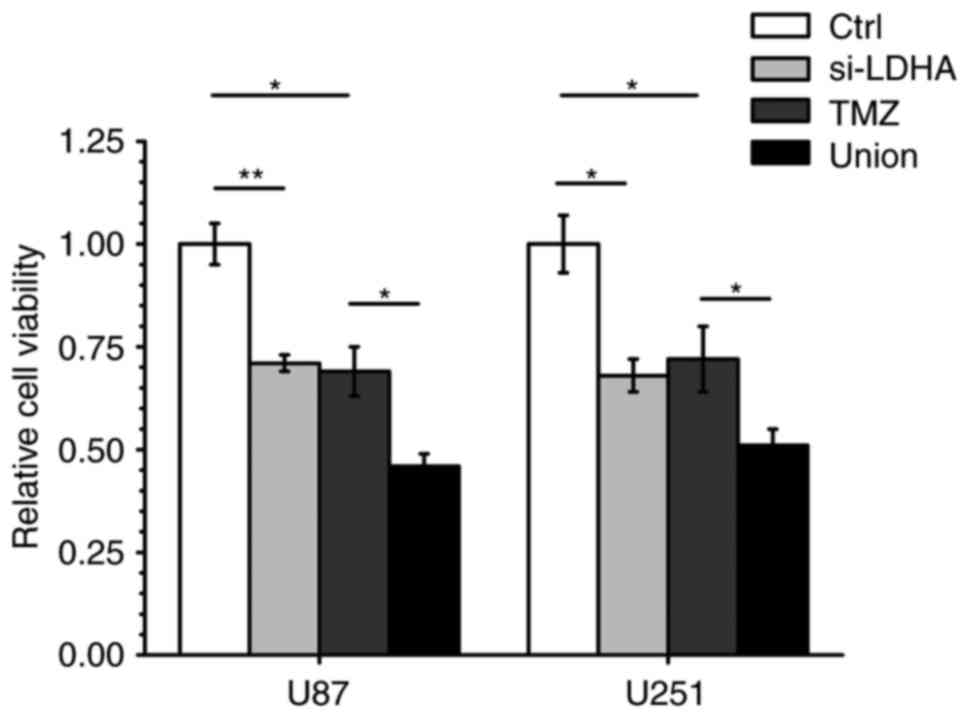
Since it is known that TMZ chemotherapy is widely
used for the treatment of advanced GBM, we determined whether
knocking down LDHA would increase glioma cell sensitivity to TMZ.
We transfected U87 and U251 cells with LDHA siRNA for 24 h; then,
we treated the cells with TMZ at its IC50 (57.58 µM) for
72 h and determined the cell viability by MTT assay. We found that
under these experimental conditions, pre-treatment with LDHA siRNA
followed by TMZ exposure (Union group) led to an approximately 40%
inhibition of cell viability compared to the results of TMZ
treatment alone (TMZ group) (Fig. 4).
These results indicate that knocking down LDHA enhances the
chemosensitivity of glioma cells to TMZ.
Discussion
In the present study, we demonstrated that there is
a significant positive correlation between LDHA expression levels
and GBM development. LDHA catalyzes the conversion of pyruvate to
lactate and is considered to be a key checkpoint of anaerobic
glycolysis (9). Increased levels of
LDHA have been reported in several types of tumors (11,13–15), and
its expression levels have been correlated with clinical stages in
breast cancer, renal cell carcinoma and prostate cancer; these
reports further support that therapies targeting LDHA may provide
useful strategies for controlling cancer progression. Moreover,
previous reports using data from the Oncomine database showed that
the mRNA levels of LDHA were up-regulated in GBM compared to those
in matched normal tissues; these results prompted us to determine
the correlation between LDHA expression levels and glioma
malignancy (16). Consistent with
previous studies, our results showed that there was a significant
positive correlation between LDHA expression levels and GBM
clinical stages. We also found that LDHA knockdown via siRNA
inhibited cell proliferation and induced cell apoptosis. Upon
examining the molecular mechanism, we found that LDHA siRNA
treatment was related to decreased cyclin D1 expression, increased
cleavage of PARP, and altered Bcl-2 and Bax expression, which
contributes to growth arrest; in addition, LDHA knockdown leads to
marked downregulation of the expression of matrix
metalloproteinases (MMPs), VE-Cadherin and VEGF; this
downregulation results in decreased cell migration and invasion
in vitro. Thus, we provide evidence that LDHA may be
involved in multiple mechanisms that modulate GBM cell viability,
apoptosis and angiogenesis; however, the exact molecular mechanisms
that modulate protease expression and angiogenesis need further
examination.
Another interesting and important finding of this
study was the effects of LDHA knockdown on chemosensitization to
TMZ treatment. The current standard of prognosis, even after
radiation therapy plus adjuvant TMZ, remains poor, with a median
survival of 14.6 months (3). It is
important to explore novel strategies instead of studying
conventional therapies to enhance the cytotoxicity of
chemotherapeutic drugs. Our study showed that the combination of
LDHA knockdown with TMZ treatment significantly reduced cell
viability. Thus, targeting LDHA combined with TMZ treatment could
be an effective therapeutic strategy for suppressing the growth of
glioma. Although siRNA-based therapeutics are still in their
initial stages of development, our findings are encouraging and
suggest the potential of using LDHA as a diagnostic/prognostic
marker and targeting LDHA with siRNA as a novel treatment for
glioma in the future.
In conclusion, the current results strongly
demonstrate that LDHA, an oncogene that contributes to the Warburg
effect, has dramatic implications on glioma development via
inhibiting cell proliferation, apoptosis, EMT and chemosensitivity
to TMZ therapy. Our studies further confirm that the expression
levels of LDHA should be of high clinical relevance in the
treatment of GBM; these findings may open new avenues for
developing novel treatments.
Acknowledgements
The present study was funded by the Project of Hebei
Province Science and Technology Program (162777254); the Medical
Science Research Key Program of Hebei Province (20160379); the
Clinical Talent Cultivation and Basic Research Project of Hebei
Province (361007); the Foundation for High-level Talents in Higher
Education of Hebei Province (CY201601); and the 2017 Science and
Technology Research and Development Guidance Project of Baoding
(17ZF003).
Glossary
Abbreviations
Abbreviations:
|
LDHA
|
lactate dehydrogenase A
|
|
GBM
|
glioblastoma
|
|
TMZ
|
Temozolomide
|
|
PCR
|
polymerase chain reaction
|
References
|
1
|
Ellis HP, Greenslade M, Powell B, Spiteri
I, Sottoriva A and Kurian KM: Current challenges in glioblastoma:
Intratumour heterogeneity, residual disease, and models to predict
disease recurrence. Front Oncol. 5:2512015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jordan JT, Gerstner ER, Batchelor TT,
Cahill DP and Plotkin SR: Glioblastoma care in the elderly. Cancer.
122:189–197. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khosla D: Concurrent therapy to enhance
radiotherapeutic outcomes in glioblastoma. Ann Transl Med.
4:542016.PubMed/NCBI
|
|
4
|
Razungles J, Cavaillès V, Jalaguier S and
Teyssier C: The warburg effect: From theory to therapeutic
applications in cancer. Med Sci (Paris). 29:1026–1033. 2013.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Courtnay R, Ngo DC, Malik N, Ververis K,
Tortorella SM and Karagiannis TC: Cancer metabolism and the warburg
effect: The role of HIF-1 and PI3K. Mol Biol Rep. 42:841–851. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Upadhyay M, Samal J, Kandpal M, Singh OV
and Vivekanandan P: The warburg effect: Insights from the past
decade. Pharmacol Ther. 137:318–330. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Teng Y, Zhang Y, Qu K, Yang X, Fu J, Chen
W and Li X: MicroRNA-29b (mir-29b) regulates the warburg effect in
ovarian cancer by targeting AKT2 and AKT3. Oncotarget.
6:40799–40814. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Valvona CJ, Fillmore HL, Nunn PB and
Pilkington GJ: The regulation and function of lactate dehydrogenase
A: Therapeutic potential in brain tumor. Brain Pathol. 26:3–17.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Miao P, Sheng S, Sun X, Liu J and Huang G:
Lactate dehydrogenase A in cancer: A promising target for diagnosis
and therapy. IUBMB Life. 65:904–910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang ZY, Loo TY, Shen JG, Wang N, Wang DM,
Yang DP, Mo SL, Guan XY and Chen JP: LDH-A silencing suppresses
breast cancer tumorigenicity through induction of oxidative stress
mediated mitochondrial pathway apoptosis. Breast Cancer Res Treat.
131:791–800. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yao F, Zhao T, Zhong C, Zhu J and Zhao H:
LDHA is necessary for the tumorigenicity of esophageal squamous
cell carcinoma. Tumour Biol. 34:25–31. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheng SL, Liu JJ, Dai YH, Sun XG, Xiong XP
and Huang G: Knockdown of lactate dehydrogenase A suppresses tumor
growth and metastasis of human hepatocellular carcinoma. FEBS J.
279:3898–3910. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rong Y, Wu W, Ni X, Kuang T, Jin D, Wang D
and Lou W: Lactate dehydrogenase A is overexpressed in pancreatic
cancer and promotes the growth of pancreatic cancer cells. Tumour
Biol. 34:1523–1530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang X, Xu L, Wu Q, Liu M, Tang F, Cai Y,
Fan W, Huang H and Gu X: Inhibition of LDHA deliver potential
anticancer performance in renal cell carcinoma. Urol Int.
99:237–244. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xian ZY, Liu JM, Chen QK, Chen HZ, Ye CJ,
Xue J, Yang HQ, Li JL, Liu XF and Kuang SJ: Inhibition of LDHA
suppresses tumor progression in prostate cancer. Tumour Biol.
36:8093–8100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li J, Zhu S, Tong J, Hao H, Yang J, Liu Z
and Wang Y: Suppression of lactate dehydrogenase A compromises
tumor progression by downregulation of the warburg effect in
glioblastoma. Neuroreport. 27:110–115. 2016. View Article : Google Scholar : PubMed/NCBI
|