Introduction
Monopolar spindle-one-binder proteins (MOBs) are
highly conserved from yeast to mammals. MOBs function as signal
transducers in signaling pathways via their interactions with the
nuclear Dbf2-related (NDR)/large tumor suppressor (LATS) family of
kinases (1–3). To date, at least six different human MOB
genes (MOB1A, MOB1B, MOB2, MOB3A, MOB3B and MOB3C) have been
identified (1). Among them, MOB1A/B
may interact directly with NDR1/2 and LATS1/2 and enhance their
activity via the Hippo signaling pathway (1,2). By
contrast, MOB2 interacts specifically with NDR1/2 kinases, but not
with LATS1/2 kinases in mammalian cells (4–6).
Specifically, MOB2 and MOB1 may compete for binding with the same
NDR1/2 N-terminal regulatory domain, where MOB1 binds to NDR1/2 to
promote the kinase activity of NDR1/2 and MOB2 interacts with
NDR1/2 to interfere with the activity of NDR1/2 (4–6). Although
MOB2 has been potentially linked to cell cycle progression and the
DNA damage response in the context of NDR kinase signaling
(1,4,7), the
biological role of MOB2 has not yet been fully clarified.
An inhibitory effect of MOB2 on the migration and
invasion of human hepatocellular carcinoma (HCC) cell lines
SMMC-7721 and HepG2 has been previously described (8). However, the underlying molecular
mechanism remains unclarified. In the present study, the effects of
MOB2 on the activation of NDR/LATS kinases and the molecular
mechanism through which MOB2 regulates LATS/yes-associated protein
(YAP) activation were investigated.
Materials and methods
Cell lines and culture conditions
Human HCC cell line SMMC-7721 and human 293T cells,
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China), were cultured in Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100 µg/ml streptomycin and
100 U/ml penicillin, and maintained in a humidified incubator with
5% CO2 at 37°C.
Construction and lentiviral
infection
The lentiviral vectors were prepared, and the
lentiviruses encoding MOB2 (LV-MOB2) and control lentiviruses
(LV-C) were generated and purified. Viral titers were determined by
the Shanghai GeneChem Co., Ltd. (Shanghai, China). Following
lentiviral infection, 1.0 µg/ml puromycin (cat. no. sc-205821;
Santa Cruz Biotechnology, Inc., Dalla, TX, USA) was subsequently
used to select stably transduced cell lines for two weeks. The cell
lines that express a stable expression of control or MOB2 were
established and screened by western blotting as previously
described (8).
For clustered regularly interspaced short
palindromic repeats (CRISPR)/CRISPR associated protein 9
(Cas9)-mediated MOB2 gene knockout, the single-guide RNA (sgRNA)
targeting MOB2 was generated using the online CRISPR Design Tool
(http://crispr.mit.edu/), and the sgRNA-MOB2
sequence is 5′-AGAAGCCCGCTGCGGAGGAG-3′. The lentiCRISPRv2 vector
(Addgene, Inc., Cambridge, MA, USA) harboring a puromycin
resistance cassette was digested using BsmBI and ligated
using annealing oligonucleotides (forward,
5′-CACCGAGAAGCCCGCTGCGGAGGAG-3′ and reverse,
5′-AAACCTCCTCCGCAGCGGGCTTCTC-3′). Once the sequence was verified by
sequencing, the constructs were transfected into 293T cells, which
were grown to 70–80% confluence in a 10 cm dish, using EndoFectin
Lenti reagent (GeneCopoeia, Inc., Rockville, MD, USA) together with
the lentiviral packaging vectors pSPAX2 and pCMV-VSV-G (all from
Addgene, Inc.). After transfection for 48 h, the viral particles
were harvested and purified, and 1.5×106 SMMC-7721 cells
were seeded in a 10 cm dish were infected with the indicated
lentiviruses in the presence of polybrene (5 µg/ml; Shanghai
GeneChem Co., Ltd.) for 14 h at 37°C. The infected SMMC-7721 cells
were selected using puromycin 6 days following successful
lentiviral transduction, followed by monoclonalization. The
knockout of MOB2 expression was screened using western blotting. To
construct the vector that express short hairpin RNA (shRNA) against
human yes-associated protein (YAP) (shYAP), the primers: Forward,
5′-GATCCGCTGGTCAGAGATACTTCTTAATTCAAGAGATTAAGAAGTATCTCTGACCAGCTTTTTTA-3′
and reverse,
5′-CGCGTAAAAAAGCTGGTCAGAGATACTTCTTAATCTCTTGAATTAAGAAGTATCTCTGACCAGCG-3′
were synthesized and annealed, and followed by cloning into the
BamH I and MluI sites of the pLent-U6-GFP-Puro vector
(ViGene Biosciences Inc., Rockville, MD, USA), and the
non-silencing control shRNA vector (shNC) was also generated. The
MOB2 knockout SMMC-7721 cells were transfected with shYAP or with
shNC for 72 h using Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. The knockdown of YAP expression was screened by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blotting.
Wound-healing assay
In total, 5.0×105 of the SMMC-7721 cells
that overexpressed MOB2 (LV-MOB2), MOB2-knocked out cells
(LV-sgMOB2) and the corresponding vector controls (LV-C and LV-sgC)
were seeded onto 6-well culture plates and serum-starved overnight
at 37°C. The cell monolayers were wounded by scratching with a
sterile 200 µl plastic pipette tip and gently washed three times
with phosphate-buffered saline (PBS) and captured under a
phase-contrast microscope at ×100 magnification and marked as 0 h.
The cells were further cultured with DMEM supplemented with 1% FBS
at 37°C for 48 h, and wound closure was observed and captured under
an inverted microscope at a magnification of ×100. The relative
migration of cells was calculated. All experiments were performed
in triplicate and repeated three times.
Transwell assay
Transwell migration and invasion assays were
performed using Boyden chambers (diameter, 6.5 mm; pore size, 8.0
µm; Corning Incorporated, Corning, NY, USA) as described previously
(8). The migrated or invaded cells on
the lower surface of the inserts were fixed with methanol for 15
min at room temperature, stained with 0.1% crystal violet for 20
min at room temperature and counted from six random fields using a
phase-contrast microscope at a magnification of ×100 per insert
from triplicate wells. A total of three separate experiments were
performed.
RNA extraction and RT-qPCR
Total RNA was isolated and purified from the
SMMC-7721 cells that overexpressed MOB2 (LV-MOB2), MOB2-knocked out
cells (LV-sgMOB2) and the corresponding vector controls (LV-C and
LV-sgC) using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). cDNA was obtained using the HiScript First
Strand cDNA Synthesis kit (Vazyme, Piscataway, NJ, USA) according
to the manufacturer's protocol. qPCR was performed using the SYBR
Green qPCR system (Takara Biotechnology, Co., Ltd., Dalian, China)
according to the manufacturer's protocol, and the qPCR
thermocycling conditions were 95°C for 5 min followed by 40 cycles
of 95°C for 10 sec and 60°C for 34 sec. Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) served as an internal control. The primer
sequences were as follows: Connective tissue growth factor
(CTGF) forward, 5′-AGGAGTGGGTGTGTGACGA-3′ and reverse,
5′-CCAGGCAGTTGGCTCTAATC-3′; cysteine-rich angiogenic inducer 61
(CYR61) forward, 5′-AGCCTCGCATCCTATACAACC-3′ and reverse,
5′-TTCTTTCACAAGGCGGCACTC-3′; YAP forward,
5′-CTCGAACCCCAGATGACTTC-3′ and reverse, 5′-CCAGGAATGGCTTCAAGGTA-3′;
and GAPDH forward, 5′-GCACCGTCAAGGCTGAGAAC-3′ and reverse,
5′-TGGTGAAGACGCCAGTGGA-3′. The relative expression of the indicated
mRNAs (normalized to GAPDH expression) was calculated using the
2−ΔΔCq method as described (9). All experiments were performed in
triplicate and repeated three times.
Western blot analysis
Protein extraction and subsequent western blotting
were performed following standard methods as described previously
(8). In brief, the cells were lysed
using RIPA buffer (Beyotime Institute of Biotechnology, Haimen,
China) supplemented with protease inhibitors for 30 min at 4°C, and
proteins were quantified using the Bradford method, and
approximately 40 µg cellular proteins were separated using 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and then transferred onto polyvinylidene fluoride
membranes (EMD Millipore, Billerica, MA, USA). The membranes were
blocked with 5% non-fat dried milk in tris-buffered saline with
Tween-20 for 2 h at room temperature, and incubated with primary
antibodies at 4°C overnight followed by incubation for 2 h at room
temperature with horseradish peroxidase (HRP)-conjugated secondary
antibodies. The bands were detected using the Pierce ECL Plus
western blotting substrate (Thermo Fisher Scientific, Inc.). GAPDH
served as the loading control, and quantification of the western
blotting results was performed using ImageJ 2× software (National
Institutes of Health, Bethesda, MD, USA). A total of three
independent experiments were performed. The antibodies used were as
follows: Rabbit polyclonal anti-MOB2 (cat. no. SAB1301138; 1:500;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany); rabbit polyclonal
anti-NDR1/2 (cat. no. sc-271703; 1:200; Santa Cruz Biotechnology,
Inc., Dalla, TX, USA); rabbit monoclonal anti-YAP (cat. no. 14074;
1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), rabbit
monoclonal anti-pS127YAP (cat. no. 13008; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit monoclonal
anti-pS397YAP (cat. no. 13619; 1:1,000; Cell Signaling Technology,
Inc., Danvers, MA, USA), rabbit monoclonal anti-LATS1 (cat. no.
3477; 1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA),
rabbit monoclonal anti-pY1079LATS1 (cat. no. 8654; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), rabbit monoclonal
anti-pS909 LATS1 (cat. no. 9157; 1:1,000; Cell Signaling
Technology, Inc., Danvers, MA, USA), rabbit monoclonal anti-MOB1
(cat. no. 13730; 1:1,000; Cell Signaling Technology, Inc., Danvers,
MA, USA), rabbit monoclonal anti-pY35 MOB1 (cat. no. 8699; 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA), HRP-conjugated
anti-mouse IgG and HRP-linked anti-rabbit IgG (cat. no. 7076 and
7074, respectively; 1:2,000; Cell Signaling Technology, Inc.,
Danvers, MA, USA); mouse monoclonal anti-GAPDH (cat. no. KC-5G4;
1:1,000; KangChen Bio-tech Co., Ltd., Shanghai, China). The rabbit
polyclonal antibody against NDR1/2 (pThr444/442) was generated as
described previously (10,11). The peptides (KDWVFINYT(PO4)YKRFEG)
were synthesized and conjugated to keyhole limpet hemocyanin in the
laboratory. The rabbit injections and bleed collection were
performed by DGpeptidesCo., Ltd (Hangzhou, China), and the antisera
were purified and extensively characterized in the laboratory.
Immunoprecipitation
Immunoprecipitation was performed using the Pierce
Classic IP kit (cat. no. 26146; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. Briefly, the cells were
washed with PBS three times and lysed at 4°C for 10 min in IP
lysis/wash buffer (pH 7.4) containing 0.025M Tris, 0.15M NaCl,
0.001M EDTA, 1% NP-40 and 5% glycerol, and incubated for 20 min at
4°C. The samples were mixed periodically. Subsequent to
centrifugation at 13,000 × g and 4°C for 15 min to pellet the cell
debris, the protein concentration of the supernatant was determined
using the Bradford method, and equivalent quantities of protein
(800 µg) were pre-cleared using control agarose resin (component of
the Pierce Classic IP kit). The pre-cleared cell lysates were
incubated with the rabbit polyclonal anti-NDR1/2 (3 µg per sample)
or rabbit monoclonal anti-LATS1 (dilution, 1:100) antibodies
overnight at 4°C to form the immune complex followed by incubation
at 4°C with 20 µl Protein A/G Plus Agarose beads (component of the
Pierce Classic IP kit) for 1 h. Following four washes with the IP
lysis/wash buffer, the beads were washed once with conditioning
buffer and heated at 100°C for 10 min in Lane Marker Sample buffer
(both buffers are components of the Pierce Classic IP kit)
supplemented with dithiotheritol to a final concentration of 20 mM.
The samples were then subjected to SDS-PAGE and western blotting as
aforementioned.
Statistical analysis
All data were expressed as the mean ± standard
deviation. Statistical analysis was performed using SPSS 19.0
software (SPSS, Inc., Chicago, IL, USA). The analyses were
performed using unpaired Student's t-test or a one-way analysis of
variance followed by Tukey's post-hoc test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Knockout of MOB2 positively regulates
the migration and invasion of SMMC-7721 cells
The inhibitory effects of MOB2 on the motility of
the HCC cell lines SMMC-7721 and HepG2 were previously reported,
where migration and invasion were markedly suppressed in
MOB2-overexpressing cells and cell motility was decreased in
MOB2-knocked down cells (8). In the
present study, the aim was to further confirm this role of MOB2 in
the regulation of migration and invasion of HCC cells.
MOB2-knockout SMMC-7721 cells were generated by infecting the cells
with lentiCRISPRv2 viruses that encode the Cas9 nuclease and
single-guide RNA (sgRNA) that targets MOB2. Gene knockout was
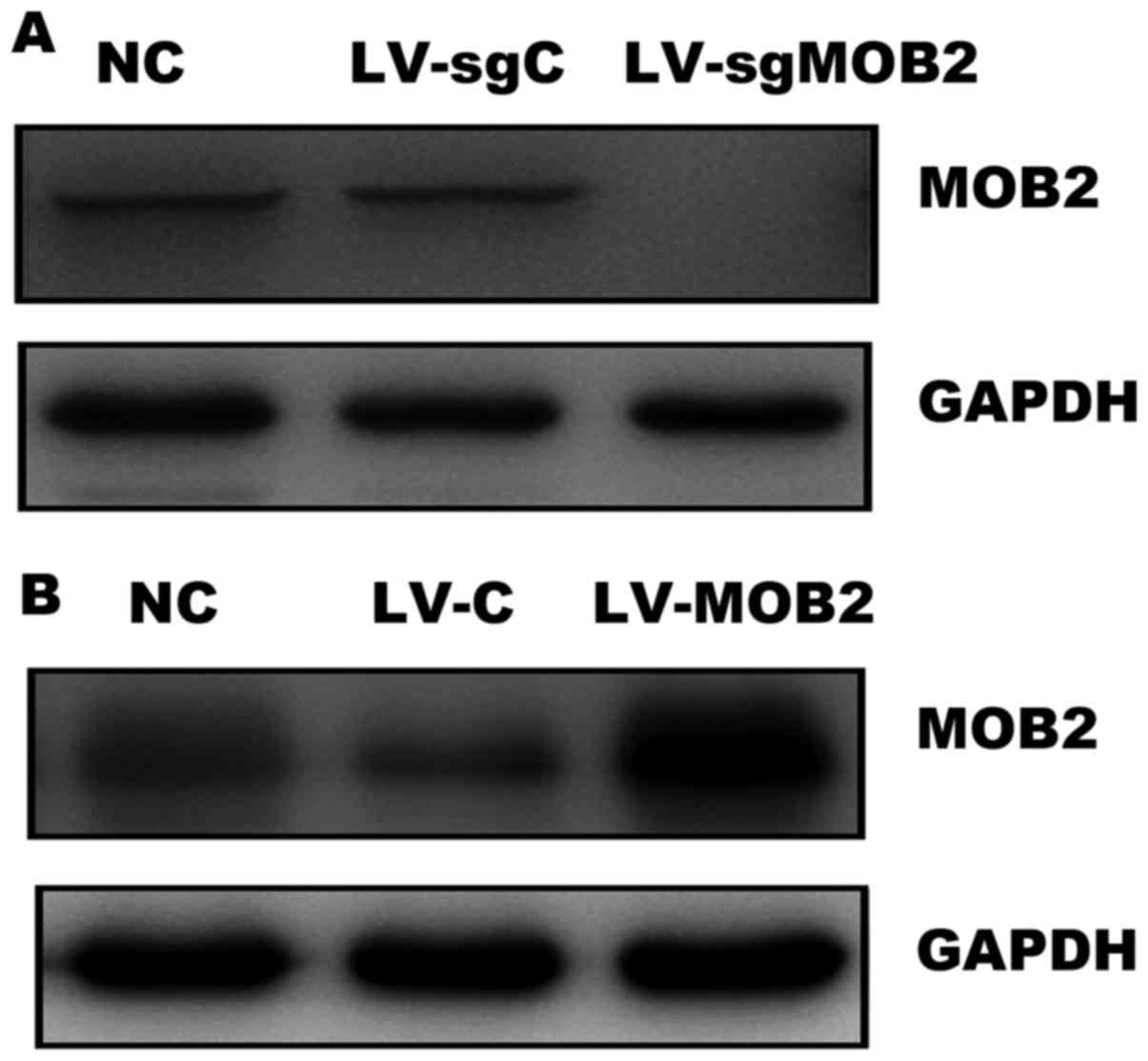
confirmed by western blotting. As presented in Fig. 1A, the expression of MOB2 was
undetectable in cells that stably express MOB2-targeted sgRNA
(LV-sgMOB2) when compared with the blank vector-transduced cells
(LV-sgC) and the normal control cells (NC). In addition, the
efficiency of MOB2 overexpression was also confirmed in
LV-MOB2-transduced SMMC-772 cells by western blotting (Fig. 1B). Therefore, SMMC-7721 cells that
stably overexpress MOB2, SMMC-7721 MOB2 knockout cells and their
corresponding blank vector-transduced cells (LV-sgC and LV-C) were
successfully established in order to perform subsequent
experiments.
Subsequently, wound-healing and Transwell assays
were performed in order to evaluate the effect of the knockout or
overexpression of MOB2 on the migration and invasion of SMMC-7721
cells. The results of the wound-healing assay revealed that MOB2
knockout cells were able to repair the wound areas significantly
faster compared with LV-sgC-transduced cells while
MOB2-overexpressing SMMC-7721 cells exhibited a decreased
wound-healing capacity compared with LV-C-transduced cells
(P<0.05; Fig. 2). The effect of
MOB2 on the migratory and invasive capacities of SMMC-7721 cells
was also assessed using Transwell assays. Compared with LV-sgC, the
knockout of MOB2 significantly increased the number of cells that
migrated and invaded, whereas opposite results were observed in
MOB2-overexpressing cells when compared with LV-C (Fig. 2). Therefore, these results clearly
demonstrate that silencing MOB2 significantly promoted cell
migration and invasion. By contrast, the overexpression of MOB2
significantly decreased the motility of SMMC-7721 cells, which is
consistent with the results of a previous study (8), thus providing further confirmation that
MOB2 regulates the migratory and invasive abilities of HCC
cells.
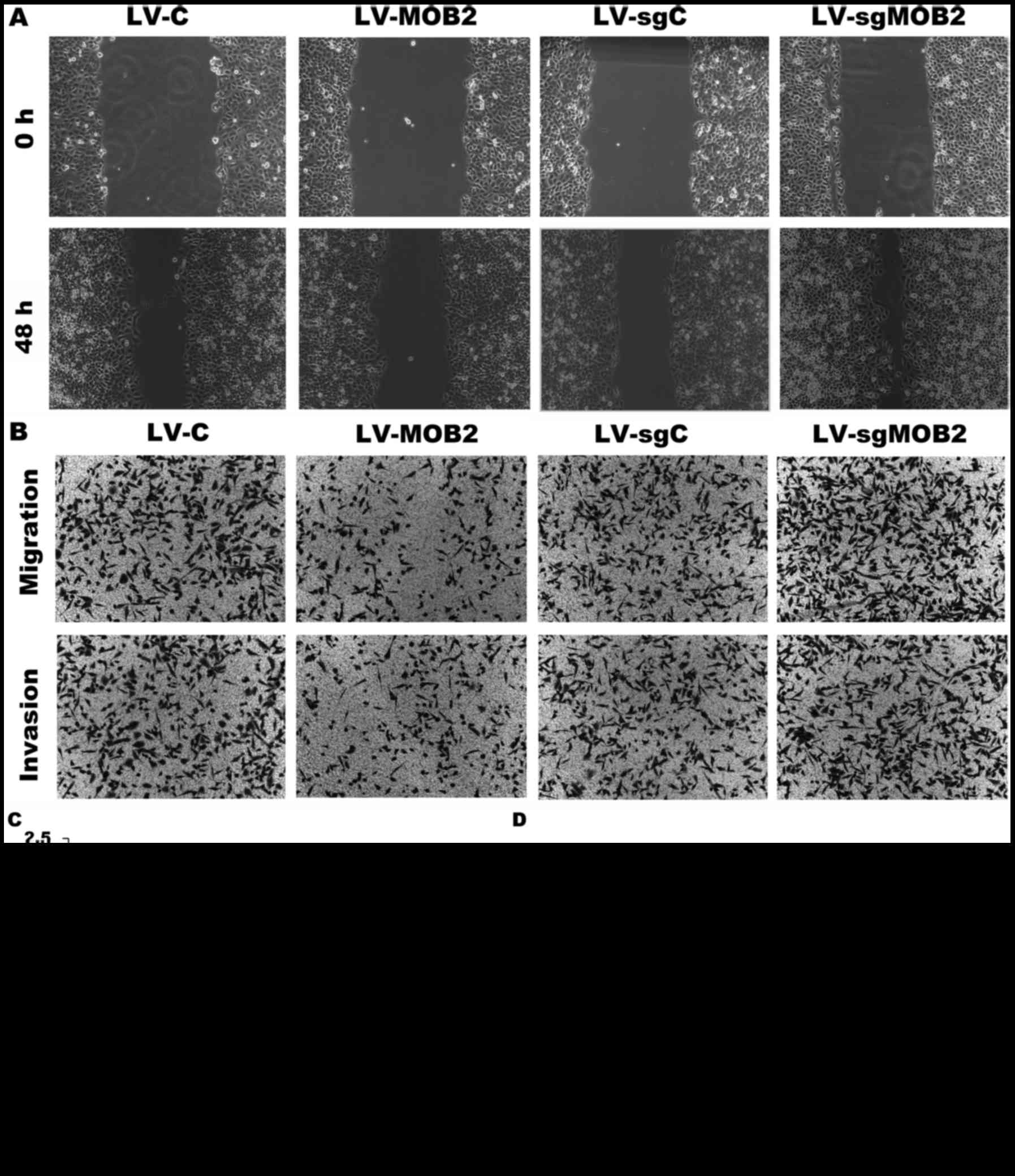 | Figure 2.Effect of MOB2 knockout or
overexpression on the migration and invasion of SMMC-7721 cells.
(A) Wound-healing assay was performed to assess the migration of
SMMC7721 cells that overexpress MOB2 (LV-MOB2), MOB2-knocked out
cells (LV-sgMOB2) and the corresponding vector controls (LV-C and
LV-sgC, respectively) (magnification, ×100). (B) Representative
Transwell cell migration and invasion assays in SMMC-7721 cells
that overexpress MOB2, MOB2-knocked down cells (LV-sgMOB2) and the
corresponding vector controls (LV-C and LV-sgC, respectively)
(magnification, ×100) (C) Quantification of relative wound healing.
(D) Quantification of relative numbers of migrated and invaded
cells. All experiments were performed independently three times,
and the data were presented as the mean ± standard deviation.
*P<0.05 vs. the corresponding blank vector control (LV-C or
LV-sgC). LV-C, control lentivirus; LV-sgMOB2, MOB2-knockout cells;
LV-MOB2, MOB2-overexpressing cells; MOB2, monopolar
spindle-one-binder protein 2; sgRNA, single-guide RNA. |
MOB2-induced YAP phosphorylation is
independent of NDR1/2 activation
Accumulating evidence has suggested that MOB2 may
perform its functions by competing with MOB1 for interaction with
NDR1/2, where the binding of MOB2 to NDR1/2 blocks the activity of
NDR kinase, and the binding of MOB1 to NDR1/2 leads to increased
activation of NDR1/2 kinases (1–4). NDR1/2
and LATS1/2 function as YAP kinases and may be considered as
members of the Hippo core cassette (4,12,13). The phosphorylation of YAP leads to
decreased nuclear YAP transcriptional activity, which serves
critical roles in cell motility (14–17).
Therefore, in the present study, the expression of
NDR1/2 and YAP in SMMC-7721 cells that stably overexpress MOB2,
MOB2 knockout-SMMC-7721 cells and their corresponding vector
control cells were examined. It was revealed that there was a
significant decrease in the level of Thr444/442 phosphorylation
(pT444/442) at the hydrophobic motif of NDR1/2 in
MOB2-overexpressing cells compared with their corresponding vector
control cells (P<0.05; Fig. 3A and
B). There was also a significant increase in the level of YAP
phosphorylation at S127 (pS127YAP) and S397 (pS397YAP) sites in
MOB2-overexpressing cells compared with their corresponding control
(P<0.05; Fig. 3C). By contrast,
the knockout of MOB2 resulted in the significant upregulation of
pT444/442 proteins and the downregulation of pS127YAP and pS397YAP
compared with the corresponding vector controls (P<0.05;
Fig. 3A-C). There were no significant
differences observed between the empty vector-infected cells and
the NC cells (data not shown). It is widely accepted that the
phosphorylation of YAP results in its cytoplasmic retention and
reduced nuclear YAP and transcriptional activity of TEA domain
family (14–16). Therefore, RT-qPCR was performed in
order to analyze the expression of CTGF and CYR61, two
well-characterized YAP target genes (18,19), in
SMMC-7721 cells MOB2 overexpressing-cells, MOB2 knockout cells and
their corresponding vector control cells. It was revealed that the
overexpression of MOB2 significantly decreased the expression of
CTGF and CYR61, while the knockout of MOB2 significantly promoted
the transcription of CTGF and CYR61 compared with the levels in
their corresponding vector control cells (P<0.05; Fig. 3D and E).
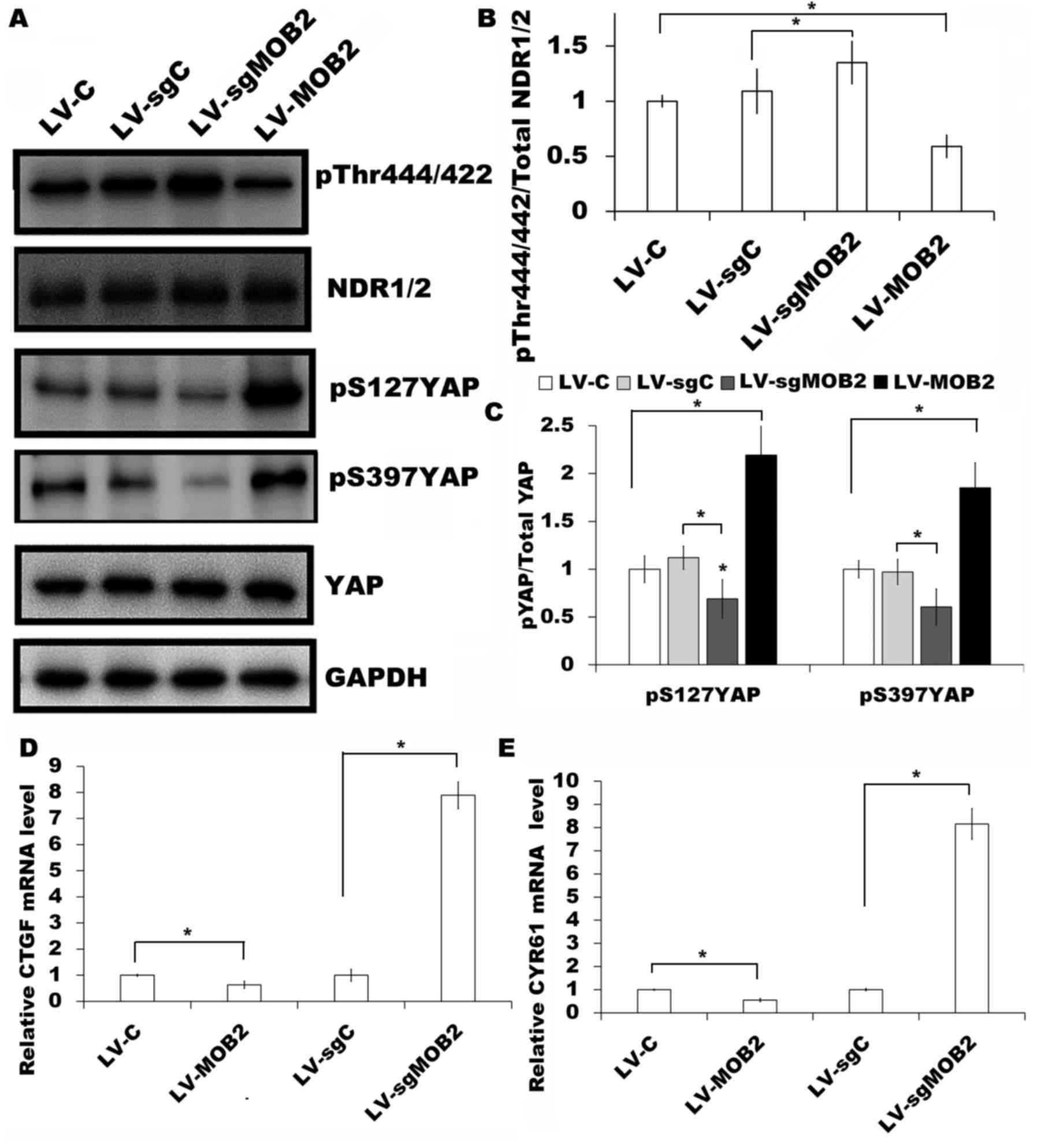 | Figure 3.Effect of MOB2 knockout and
overexpression on the expression of NDR1/2 and YAP in SMMC-7721
cells. (A) Protein expression of NDR1/2 pT444/442, NDR1/2, YAP
pS127, YAP pS397 YAP and YAP were analyzed in SMMC7721 cells that
overexpress MOB2 (LV-MOB2), MOB2-knocked out cells (LV-sgMOB2) and
the corresponding vector controls (LV-C and LV-sgC, respectively)
by western blotting. GAPDH was used as a loading control (n=3).
Densitometric analysis of the fold expression levels of (B)
pT444/442 of NDR1/2 compared with total NDR1/2 and (C) pS127YAP and
pS397YAP compared with total YAP. Data represents the mean ± SD of
three independent experiments. *P<0.05 vs. the corresponding
blank vector control (LV-C or LV-sgC). mRNA levels of (D) CTGF and
(E) CYR61, two well-characterized YAP target genes, were analyzed
from the indicated cells using reverse transcription-quantitative
polymerase chain reaction and normalized to GAPDH expression. Data
represents the mean ± SD (n=5). *P<0.05 vs. the corresponding
blank vector control (LV-C or LV-sgC). MOB2, monopolar
spindle-one-binder protein 2; NDR, nuclear-Dbf2-related kinase;
YAP, yes-associated protein; p, phosphorylated; CTGF, connective
tissue growth factor; CYR61, cysteine rich angiogenic inducer 61;
LV-C, control lentivirus; LV-sgMOB2, MOB2-knockout cells; LV-MOB2,
MOB2-overexpressing cells; LV-sgC, sgRNA control cells; sgRNA,
single-guide RNA; SD, standard deviation. |
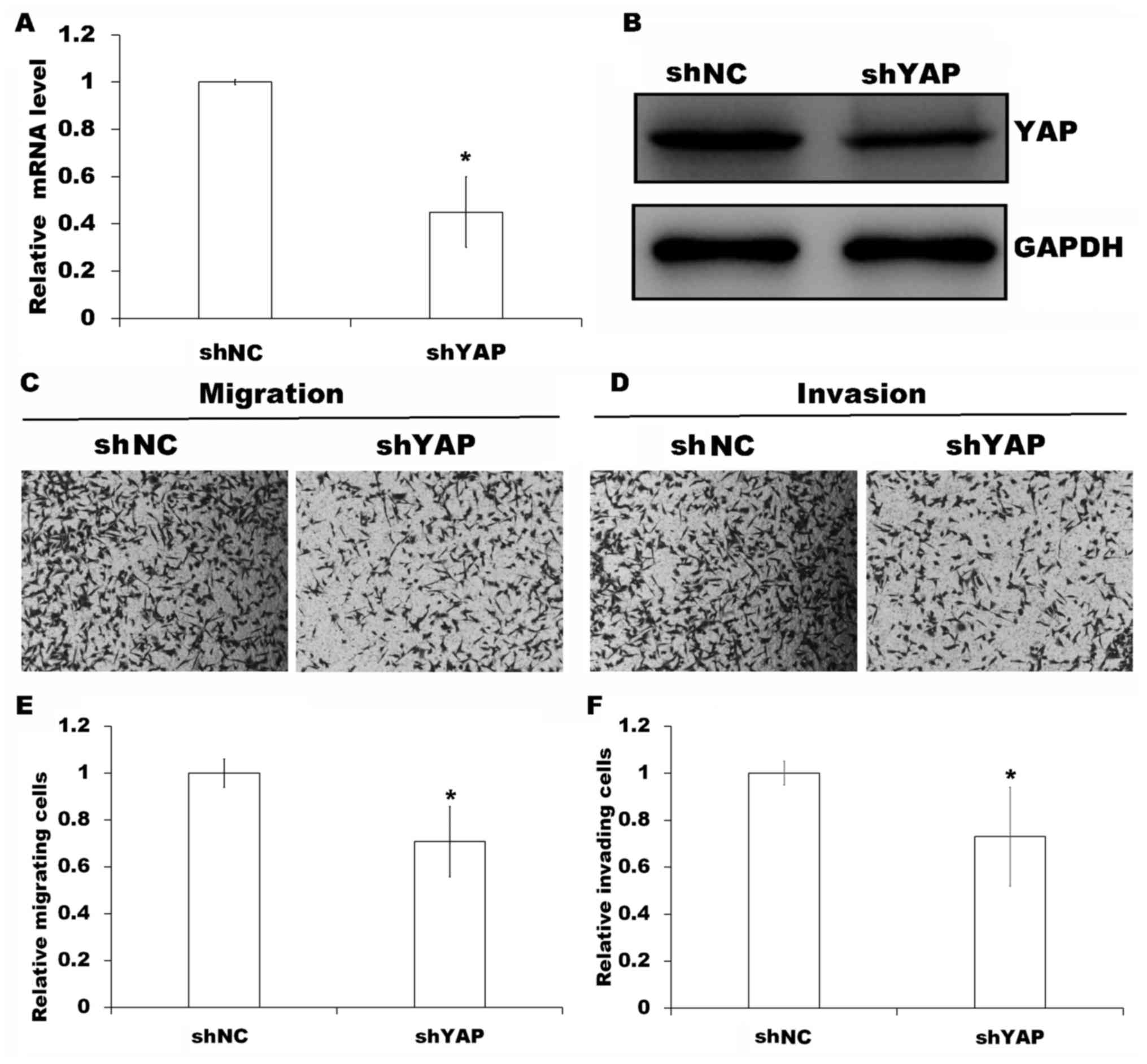
To evaluate the requirement of YAP for
MOB2-regulated cell motility, YAP was knocked down in MOB2 knockout
cells as verified by RT-qPCR and western blotting (P<0.05;
Fig. 4A and B) followed by Transwell
cell migration and invasion assays. Compared with the MOB2-silenced
cells that were transfected with non-targeting shRNA vector (shNC),
the silencing of YAP in MOB2 knockout cells significantly decreased
cell migration and invasion (P<0.05; Fig. 4C-F), suggesting that YAP is involved
in the modulation of the motility of SMMC-7721 cells via MOB2.
Although NDR1/2 kinases have been reported to function as the
upstream kinases of YAP and directly phosphorylate YAP (12), the results of the present study
suggest that MOB2-mediated regulation of YAP phosphorylation
appears to be independent of NDR1/2 activation.
MOB2-induced YAP activation may be
mediated via activating MOB1-LATS signaling
A key function of the Hippo tumor suppressor pathway
is to inhibit the activity of YAP transcriptional co-activators by
MOB1-LATS signaling through LATS-mediated direct phosphorylation of
YAP (20,21). Therefore, the present study aimed to
investigate the effect of MOB2 on the expression of MOB1 and LATS1
in SMMC-7721 cells. It was revealed that the overexpression of MOB2
significantly upregulated the levels of MOB1 phosphorylation
(pMOB1) and LATS1 phosphorylation at Y1079 (pY1079LATS1) and S909
(pS909LATS1) sites compared with their corresponding controls
(Fig. 5A-C). By contrast, the
knockout of MOB2 significantly decreased the levels of pMOB1 and
pLATS1 (pY1079LATS1 and pS909LATS1) compared with the corresponding
vector control (P<0.05; Fig.
5A-C). Together, these results indicated that MOB2-induced YAP
activation may be mediated through activating MOB1-LATS
signaling.
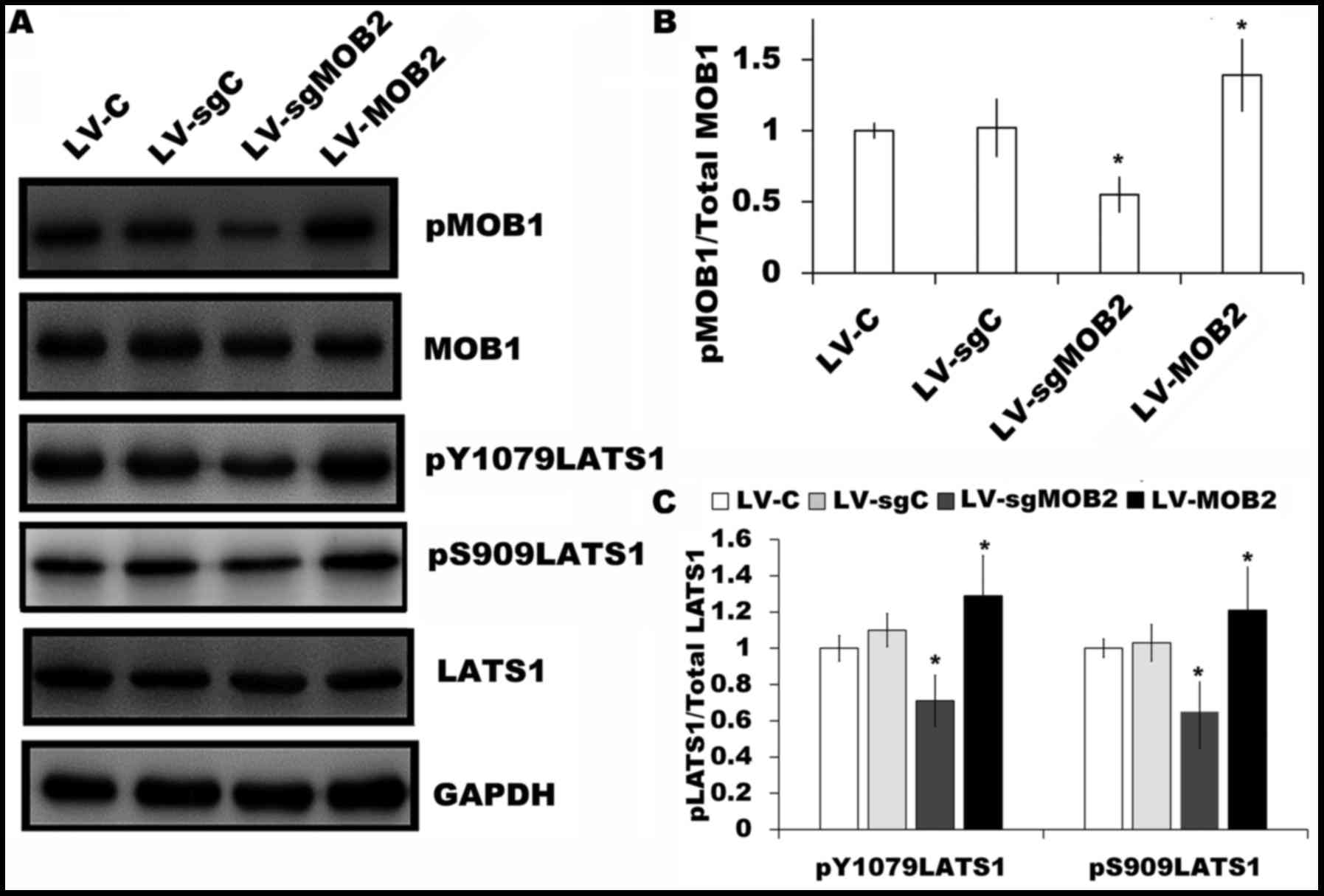 | Figure 5.Effect of MOB2 knockout and
overexpression on the expression of MOB1 and LATS1 in SMMC-7721
cells. (A) The levels of pMOB1, MOB1, pY1079LATS1, pS909LATS1 and
LATS1 protein expression were determined in SMMC-7721 cells that
stably express MOB2, MOB2-knocked out cells and the corresponding
vector controls by western blotting. GAPDH served as the loading
control (n=3). The fold expression of (B) pMOB1 relative to total
MOB1, and (C) pY1079LATS1 and pS909LATS1 relative to total LATS1 as
determined by densitometric analysis. Data are presented as the
mean ± standard deviation of three independent experiments.
*P<0.05 vs. the corresponding vector control. MOB1, monopolar
spindle-one-binder protein 1; MOB2, monopolar spindle-one-binder
protein 2; LATS1, large tumor suppressor kinase 1; p-,
phosphorylated; LV-C, control lentivirus; LV-sgMOB2, MOB2-knockout
cells; LV-MOB2, MOB2-overexpressing cells; sgRNA, single-guide
RNA. |
MOB2 regulates the alternative
interaction of MOB1 with LATS and NDR kinases
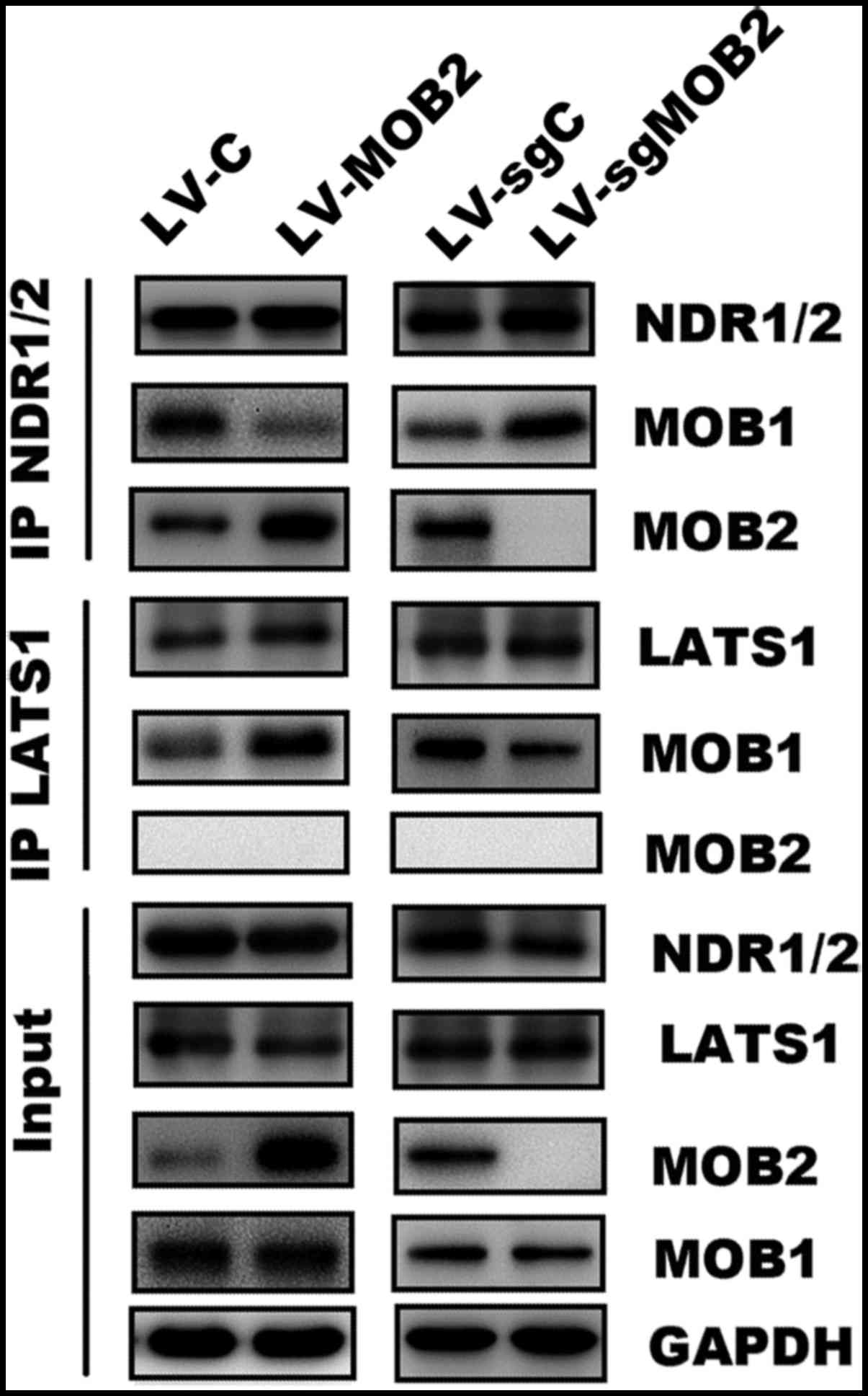
Mammalian NDR kinases are the only reported binding
partners of MOB2, whereas MOB1 may associate with NDR and LATS
kinases (1,5). To further dissect the molecular
mechanism of MOB2 in LATS/YAP activation, immunoprecipitation assay
was performed followed by western blotting. As hypothesized, it was
confirmed that MOB2 may interact with NDR but not LATS, while MOB1
may bind to NDR1/2 and LATS1 (Fig.
6). It was revealed that MOB1 was enriched in LATS1
precipitates but reduced in NDR1/2 precipitates in
MOB2-overexpressing cells. Furthermore, the knockout of MOB2
resulted in accumulation of MOB1 in NDR1/2 precipitates and
reduction of MOB1 in LATS1 precipitates (Fig. 6). These observations demonstrate a
role of MOB2 in regulating the alternative interaction of MOB1 with
LATS1 and NDR1/2.
Discussion
In the present study, it was demonstrated that the
knockout of MOB2 by CRISPR/Cas9 promoted the cell migration and
invasion, induced NDR1/2 phosphorylation and decreased YAP
phosphorylation in the SMMC-7721 HCC cell line. By contrast, the
overexpression of MOB2 resulted in the opposite effect. It was
additionally demonstrated that MOB2 exerts its regulation on cell
motility at least in part via regulating the phosphorylation of YAP
and the activity of YAP in the Hippo signaling pathway. The results
of the present study further indicated a role of MOB2 in regulating
the alternative interaction of MOB1 with LATS1 and NDR1/2, which
result in increased phosphorylation of LATS1 and MOB1 and
consequently the inactivation of YAP and regulation of cell
motility. These results supported the hypothesis that MOB2 serves a
positive role in the activation of LATS/YAP by activating the Hippo
signaling pathway.
Previous studies have linked endogenous MOB2 to cell
survival, cell cycle progression and DNA damage responses in the
context of NDR kinase signaling (4,7). MOB2
directly phosphorylates YAP, and the inactivation of YAP by
cytoplasmic retention is linked with the regulation of cell
motility and proliferation (12,16–18,22–24),
thereby establishing an association between NDR1/2 kinases with the
Hippo signaling pathway on a cellular level. A previous study by
the present authors has demonstrated that MOB2 serves an inhibitory
role in the motility of the HCC cell lines SMMC-7721 and HepG2
(8). However, the details of the
roles and underlying mechanisms involved in these processes remain
unclarified. Hence, in the present study, the effect of MOB2
knockout by CRISPR/Cas9 and MOB2 overexpression on the migration
and invasion of SMMC-7721 cells was investigated by wound-healing
and Transwell assays. The data are consistent with the results of
the previous study, where the overexpression of MOB2 decreased cell
migration and invasion. However, the knockout of MOB2 decreased
cell motility and markedly promoted the migration and invasion of
SMMC-7721 cells. These results further demonstrated the role of
MOB2 in regulating the migration and invasion of HCC cells.
NDR1/2 kinases and not LATS1/2 were reported to be
the binding partners of MOB2. NDR1/2 kinases may function as the
upstream kinases of YAP, which is the main downstream effector of
the Hippo signaling pathway (1,4–7,12,18). The present study investigated whether
MOB2 regulates the activation of NDR1/2 kinases and subsequently
modulates the phosphorylation of YAP. It was revealed that
phosphorylation at Thr444/442 of NDR1/2 was decreased in
MOB2-overexpressing SMMC-7721 cells but increased in MOB2 knockout
cells, suggesting that MOB2 may negatively regulate the activity of
NDR1/2. Notably, it was additionally revealed that the
overexpression of MOB2 resulted in the upregulation of YAP
phosphorylation, while the knockout of MOB2 resulted in the
downregulation of YAP phosphorylation, which is inconsistent with
the function of NDR1/2 as YAP kinases (12). In addition, the functional
significance of MOB2 expression on YAP activity was further
evaluated. As hypothesized, there was a strong negative association
between MOB2 expression and the expression of two YAP target genes,
CYR61 and CTGF (P<0.05). Furthermore, in order to evaluate
whether YAP is involved in MOB2-regulated cell motility, YAP was
silenced in the MOB2 knockout cells. Compared with the
MOB2-silenced cells that were transfected with non-targeting shRNA
vector (shNC), the silencing of YAP in MOB2 knockout cells
significantly decreased cell migration and invasion. Taken
together, these results suggest that MOB2-mediated regulation of
YAP phosphorylation appears to be independent of NDR1/2
activation.
Conventionally, the activated Hippo signaling
pathway inhibits the transcriptional co-activator functions of YAP
through the MOB1/LATS-mediated phosphorylation of YAP (1,25,26). Therefore, whether MOB2 expression
exerts an effect on the expression of MOB1 and LATS in SMMC-7721
cells was examined. It was revealed that the overexpression of MOB2
increased the expression levels of pMOB1 and pLATS1. By contrast,
the knockout of MOB2 expression decreased the expression levels of
pMOB1 and pLATS1, thereby inhibiting YAP phosphorylation as
aforementioned.
LATS kinases are activated by MOB1. MOB2 exerts its
function upstream of NDR1/2 and functions as an inhibitor of
MOB1-mediated NDR1/2 activation (27). An immunoprecipitation assay was
performed in order to investigate whether the immunoprecipitation
of MOB1 by NDR1/2 and LATS1 was affected by the overexpression or
knockout of MOB2. It was revealed that the overexpression of MOB2
resulted in a notable reduction in the quantity of MOB1 that was
immunoprecipitated with NDR1/2 but a substantial increase in the
quantity of MOB1 that was immunoprecipitated with LATS1. By
contrast, the knockout of MOB2 failed to accumulate the
interactions of MOB1 with LATS1/2, and a more efficient interaction
was displayed between MOB1 and NDR1/2, although further research is
required. Taken together, these results demonstrate that MOB2 may
compete with MOB1 for interaction with NDR1/2 and partially
displace MOB1 from NDR1/2, which promotes the interaction of MOB1
with LATS1/2 and activates the Hippo signaling pathway.
In conclusion, the findings of the present study
demonstrated that MOB2 regulates the alternative interaction of
MOB1 with NDR1/2 and LATS1, which results in increased
phosphorylation/activity of LATS1 and MOB1. This leads to the
inactivation of YAP and consequently the inhibition of cell
motility. Further study of the underlying mechanism as to how MOB2
knockout coordinates the Hippo signaling could be validated.
However, these findings may reinforce MOB2 as a potential
diagnostic or therapeutic target for HCC.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant no. 81672336), the
Training Program of Innovation and Entrepreneurship for College
Students in Jiangsu (grant nos. 201511117045Z and 201611117041Z)
and the Open Research Fund Program of the Jiangsu Key Laboratory of
Integrated Traditional Chinese and Western Medicine for Prevention
and Treatment of Senile Diseases.
References
|
1
|
Hergovich A: MOB control: Reviewing a
conserved family of kinase regulators. Cell Signal. 23:1433–1440.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hergovich A: Regulation and functions of
mammalian LATS/NDR kinases: Looking beyond canonical Hippo
signalling. Cell Biosci. 3:322013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hergovich A: The roles of NDR protein
kinases in Hippo signalling. Genes (Basel). 7:E212016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gundogdu R and Hergovich A: The possible
crosstalk of MOB2 with NDR1/2 kinases in cell cycle and DNA damage
signaling. J Cell Signal. 1:1252016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kohler RS, Schmitz D, Cornils H, Hemmings
BA and Hergovich A: Differential NDR/LATS interactions with the
human MOB family reveal a negative role for human MOB2 in the
regulation of human NDR kinases. Mol Cell Biol. 30:4507–4520. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bothos J, Tuttle RL, Ottey M, Luca FC and
Halazonetis TD: Human LATS1 is a mitotic exit network kinase.
Cancer Res. 65:6568–6575. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gomez V, Gundogdu R, Gomez M, Hoa L,
Panchal N, O'Driscoll M and Hergovich A: Regulation of DNA damage
responses and cell cycle progression by hMOB2. Cell Signal.
27:326–339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu W, Zhang X, Qin H, Peng W, Xue Q, Lv H,
Zhang H, Qiu Y, Cheng H, Zhang Y, et al: Modulation of tumor cell
migration, invasion and cell-matrix adhesion by human monopolar
spindle-one-binder 2. Oncol Rep. 33:2495–2503. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tamaskovic R, Bichsel SJ, Rogniaux H,
Stegert MR and Hemmings BA: Mechanism of Ca2+-mediated
regulation of NDR protein kinase through autophosphorylation and
phosphorylation by an upstream kinase. J Biol Chem. 278:6710–6718.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vichalkovski A, Gresko E, Cornils H,
Hergovich A, Schmitz D and Hemmings BA: NDR kinase is activated by
RASSF1A/MST1 in response to Fas receptor stimulation and promotes
apoptosis. Curr Biol. 18:1889–1895. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Tang F, Terracciano L, Hynx D,
Kohler R, Bichet S, Hess D, Cron P, Hemmings BA, Hergovich A and
Schmitz-Rohmer D: NDR functions as a physiological YAP1 kinase in
the intestinal epithelium. Curr Biol. 25:296–305. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang L, Xue G, Grzmil M, Yang Z,
Hergovich A, Hollaender GA, Stein JV, Hemmings BA and Matthias P:
The kinases NDR1/2 act downstream of the Hippo homolog MST1 to
mediate both egress ofthymocytes from the thymus and lymphocyte
motility. Sci Signal. 8:ra1002015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, George J, Deb S, Degoutin JL,
Takano EA, Fox SB; AOCS Study group; Bowtell DD and Harvey KF: The
Hippo pathway transcriptional co-activator, YAP, is an ovarian
cancer oncogene. Oncogene. 30:2810–2822. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Li J, Li P, Wang Y, Liang Z, Jiang
Y, Li J, Feng C, Wang R, Chen H, et al: Loss of DLG5 promotes
breast cancer malignancy by inhibiting the Hippo signaling pathway.
Sci Rep. 7:421252017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu FX, Zhang Y, Park HW, Jewell JL, Chen
Q, Deng Y, Pan D, Taylor SS, Lai ZC and Guan KL: Protein kinase A
activates the Hippo pathway to modulate cell proliferation and
differentiation. Genes Dev. 27:1223–1232. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang S, Zhang L, Purohit V, Shukla SK,
Chen X, Yu F, Fu K, Chen Y, Solheim J, Singh PK, et al: Active YAP
promotes pancreatic cancer cell motility, invasion and
tumorigenesis in a mitotic phosphorylation-dependent manner through
LPAR3. Oncotarget. 6:36019–36031. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Moroishi T, Park HW, Qin B, Chen Q, Meng
Z, Plouffe SW, Taniguchi K, Yu FX, Karin M, Pan D and Guan KL: A
YAP/TAZ-induced feedback mechanism regulates Hippo pathway
homeostasis. Genes Dev. 29:1271–1284. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hayashi H, Higashi T, Yokoyama N, Kaida T,
Sakamoto K, Fukushima Y, Ishimoto T, Kuroki H, Nitta H, Hashimoto
D, et al: An imbalance in TAZ and YAP expression in hepatocellular
carcinoma confers cancer stem cell-like behaviors contributing to
disease progression. Cancer Res. 75:4985–4997. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mo JS, Park HW and Guan KL: The Hippo
signaling pathway in stem cell biology and cancer. EMBO Rep.
15:642–656. 2014.PubMed/NCBI
|
|
21
|
Mo JS, Yu FX, Gong R, Brown JH and Guan
KL: Regulation of the Hippo-YAP pathway by protease-activated
receptors (PARs). Genes Dev. 26:2138–2143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harvey KF, Zhang X and Thomas DM: The
Hippo pathway and human cancer. Nat Rev Cancer. 13:246–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Haskins JW, Nguyen DX and Stern DF:
Neuregulin 1-activated ERBB4 interacts with YAP to induce Hippo
pathway target genes and promote cell migration. Sci Signal.
7:ra1162014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huelter-Hassler D, Tomakidi P, Steinberg T
and Jung BA: Orthodontic strain affects the Hippo-pathway effector
YAP concomitant with proliferation in human periodontal ligament
fibroblasts. Eur J Orthod. 39:251–257. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oh H and Irvine KD: Yorkie: The final
destination of Hippo signaling. Trends Cell Biol. 20:410–417. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vahid S, Thaper D, Gibson KF, Bishop JL
and Zoubeidi A: Molecular chaperone Hsp27 regulates the Hippo tumor
suppressor pathway in cancer. Sci Rep. 6:318422016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hergovich A, Schmitz D and Hemmings BA:
The human tumour suppressor LATS1 is activated by human MOB1 at the
membrane. Biochem Biophys Res Commun. 345:50–58. 2006. View Article : Google Scholar : PubMed/NCBI
|