Introduction
In recent years, the rapid development of genetic
technologies, proteomics, and tumor immunology has identified
several new biomarkers for liver cancer (1). Golgi protein 73 (GP73) is one of the
most promising serum markers for liver cancer diagnosis. GP73, also
named Golgi phosphoprotein 2 (GOLPH2) and Golgi membrane protein 1
(GOLM1), is termed GP73 due to its molecular mass of 73 kDa in
SDS-PAGE (2,3). It is a transmembrane protein in the
Golgi apparatus and highly expressed in liver cancer tissues and
patient serum. It has been predicted that GP73 may become a marker
for early diagnosis of liver cancer with sensitivity superior to
α-fetoprotein (AFP) (4,5).
Currently, the mechanism underlying high GP73
expression in liver cancer tissues remains unclear. Kladney et
al (6) demonstrated that GP73
protein expression in liver cancer cells was significantly elevated
during hepatitis B virus (HBV) replication, indicating that HBV
replication may activate GP73 protein expression. Currently, the
mechanism of HBV-induced GP73 protein overexpression is not clear.
Because hepatitis B virus protein X (HBx), hypoxia-inducible factor
(HIF)-1α and HIF-2α are important factors promoting liver cancer
occurrence and development, it was postulated that upregulation of
GP73 expression may be related to these three factors. Therefore,
the present study investigated the role of HBx, HIF-1α and HIF-2α
in inducing GP73 expression in liver cancer tissues.
Materials and methods
Clinicopathological patient data
All human studies were approved by the Human Ethics
Committee of the Center Hospital of Huanggang (Huanggang, China).
All hepatocellular carcinoma (HCC) patients included in the present
study tested negative for hepatitis C virus and human
immunodeficiency virus. All tissue samples were collected from
patients prior to medical treatment. HBV-positive HCC tissues and
their paired peritumoral tissues (n=52), and HBV-negative HCC
tissues (n=10) were sectioned for immunohistochemical analysis.
Tissues were collected from the Center Hospital of Huanggang
(Huanggang, China) between June 2008 and June 2011. Peritumoral
tissues were obtained at least 2 cm away from the primary tumor
site. Clinical data and tumor characteristics are presented in
Table I.
 | Table I.Clinicopathological features of 52
patients with hepatocellular carcinoma. |
Table I.
Clinicopathological features of 52
patients with hepatocellular carcinoma.
| Variable | Patients |
|---|
| Mean age (range) | 42 (25–67) |
| Gender |
|
| Male | 45 (86.5%) |
|
Female | 7
(13.5%) |
| Cirrhosis |
|
|
Presence | 31 (59.6%) |
|
Absence | 21 (40.4%) |
| Tumor size |
|
| <5
cm | 36 (69.2%) |
| ≥5
cm | 16 (30.8%) |
| Vascular
invasion |
|
|
Presence | 6
(11.5%) |
|
Absence | 46 (88.5%) |
| Tumor number |
|
|
Single | 44 (84.6%) |
|
Multiple | 8
(15.4%) |
| Tumor
differentiation |
|
| Well | 11 (21.2%) |
|
Moderate | 28 (53.8%) |
| Poor | 13 (25.0%) |
Reagents and cell lines
Mouse anti-human GP73 monoclonal antibodies
(sc-365817), mouse anti-human HIF-1α monoclonal antibodies (cat.
no. sc-53546), mouse anti-human HIF-2α monoclonal antibodies (cat.
no. s-13596), and mouse monoclonal antibodies raised against
baculovirus-expressed recombinant HepBx (cat. no. sc-57760) were
purchased from Santa Cruz Biotechnology, Inc., (Dallas, TX, USA).
The human hepatoblastoma cell line HepG2 was purchased from
American Type Culture Collection (Manassas, VA, USA). HepG2.2.15
cell line was kindly provided by Professor Yinping Lu (Department
of Infectious Disease, Union Hospital, Tongji Medical College,
Huazhong University of Science and Technology). Cells were grown in
6-well plates with Dulbecco's modified Eagle's medium (DMEM; GE
Healthcare Life Sciences, Logan, UT, USA) containing 10% fetal
bovine serum (FBS), 2 mmol/l L-glutamine (all GE Healthcare Life
Sciences) at 37°C in 5% CO2. HIF-1α-overexpressing
plasmid, HIF-2α-overexpressing plasmid, and the corresponding
control plasmid (pcDNA3.1), as well as HBx-overexpression plasmid
and its control plasmid (pEGFP-N1) were purchased from Shanghai
GeneChem Co., Ltd. (Shanghai, China). HIF-2α small interfering
(si)RNA (cat. no. sc-35316) and negative control siRNA (cat. no.
sc-37007) were purchased from Santa Cruz Biotechnology, Inc.
HepG2 cells at 60–70% confluence were transfected
with 2.0 µg plasmids or 100 pmol siRNA using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), according to the manufacturer's protocol.
Transfected cells were incubated at 37°C for 6 h, and were then
cultured for a further 16 h with fresh DMEM medium containing 10%
FBS (all GE Healthcare Life Sciences).
Immunohistochemistry
Immunohistochemical analysis was performed as
previously described (7,8). The levels of GP73 protein were scored
according to the number of cells exhibiting cytoplasmic staining
using the classification system published by Sai et al
(8). Cytoplasmic staining in <25%
of tumor cells was considered low expression, while staining in
>25% of tumor cells was considered high expression (8). The levels of HIF-2α protein were scored
according to the number of cells exhibiting cytoplasmic and nuclear
staining using the classification system published by Yang et
al (7). Nuclear or cytoplasmic
staining in <50% of tumor cells was considered low expression,
while staining in >50% of tumor cells was considered high
expression (7). Liver fibrosis was
scored on a 0–4 scale according to the METAVIR scoring system
(9).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and western blotting
The methods of Yang et al (10) were followed for these experiments.
TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA)
was used to isolate total RNA from 150–200 mg of 52 freshly frozen
HCC tumors and their adjacent liver tissues. RNA extraction, cDNA
synthesis, qPCR reactions, and western blotting were performed as
previously reported (10). The primer
sequences used in the current study are listed in Table II.
 | Table II.Polymerase chain reaction primers and
conditions. |
Table II.
Polymerase chain reaction primers and
conditions.
| Gene | Primer sequence
(5′-3′) | Temperature (°C) | Product size
(bp) |
|---|
| GP73 |
GTGGCCTGCATCATCGTCTT | 60.7 | 167 |
|
|
CTGCTTCTCCAGCTCTCCCT |
|
|
| HIF-1α |
CATCTCCATCTCCTACCCACA | 58.3 | 105 |
|
|
CTTTTCCTGCTCTGTTTGGTG |
|
|
| HIF-2α |
TCATGCGACTGGCAATCAGC | 61.3 | 141 |
|
|
GTCACCACGGCAATGAAACC |
|
|
| HBx |
CGTCCTTTGTCTACGTCCCG | 59.4 | 408 |
|
|
AAGTTGCATGGTGCTGGTGA |
|
|
| β-actin |
AGTTGCGTTACACCCTTTCTTGAC | 63.9 | 171 |
|
|
GCTCGCTCCAACCGACTGC |
|
|
Promoter analysis
The TRANSFAC software (http://www.gene-regulation.com/pub/programs.html#match)
was used to identify potential HIF-2α binding sites in the promoter
region of GP73 by following the software's instructions, and as
previously reported (10).
Statistical analysis
Paired t-test statistical analyses were performed
using SPSS software (version 20.0; IBM Corp., Armonk, NY, USA).
Results are expressed as mean ± standard deviation of three
independent experiments. Linear associations were evaluated using
Spearman's correlation coefficients. P<0.05 was considered to
indicate a statistically significant difference.
Results
GP73 is overexpressed in HCC
tissues
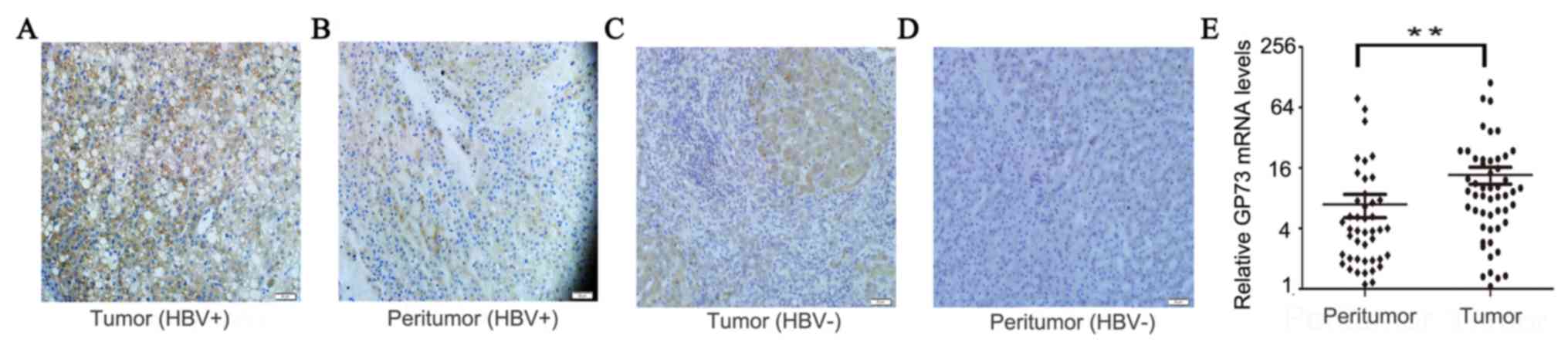
GP73 was overexpressed in HBV-positive HCC tissues,
as evidenced by high expression of GP73 in 73.1% (38/52) of tumor
tissues but only 36.5% (19/52) of peritumoral tissues (Fig. 1A and B). In HBV-negative HCC, GP73 was
overexpressed in 40.0% (4/10) of tumor tissues, which was lower
compared with HBV-positive HCC tissues (P=0.04; Fig. 1C and D). In peritumoral tissues, the
GP73 level in patients of F2-4 groups which with significant
fibrosis was significantly higher (54.5%; 12/22) compared with
patients of F0-1 groups with no/minimal fibrosis (23.3%; 7/30),
which was consistent with results from previously published studies
(data not shown) (11). The positive
GP73 staining was located in the cytoplasm (Fig. 1). In addition, the mRNA expression
levels of GP73 were also demonstrated to be increased in tumor
tissues compared with peritumoral normal tissues (Fig. 1E). In summary, these results
demonstrated that both mRNA and protein expression levels of GP73
were increased in HBV-positive HCC tissues.
HBV enhances the expression of
GP73
To investigate the mechanisms that lead to the high
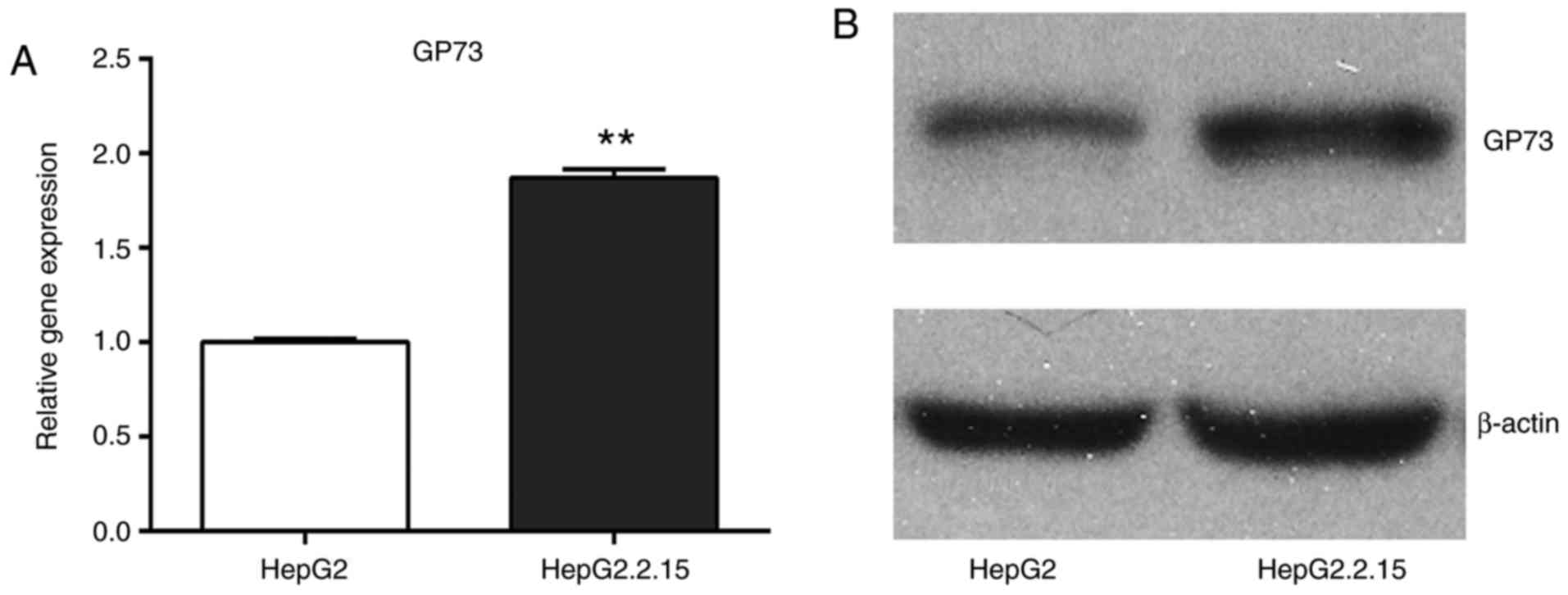
expression of GP73 in HCC tissues, first the effect of HBV on GP73
expression was examined because HBV is a common cause of liver
cancer. The mRNA and protein expression levels of GP73 were
compared between the HepG2 cells, which are negative for HBV, and
the HepG2.2.15 cells, which were stably transfected with a complete
HBV genome. The results demonstrated that GP73 mRNA and protein
levels were significantly higher in the HepG2.2.15 cells compared
with the HepG2 cells (Fig. 2).
Therefore, it was hypothesized that HBV may be a positive regulator
of GP73.
HIF-2α is involved in HBV-induced
upregulation of GP73
To further investigate the mechanism of HBV-mediated
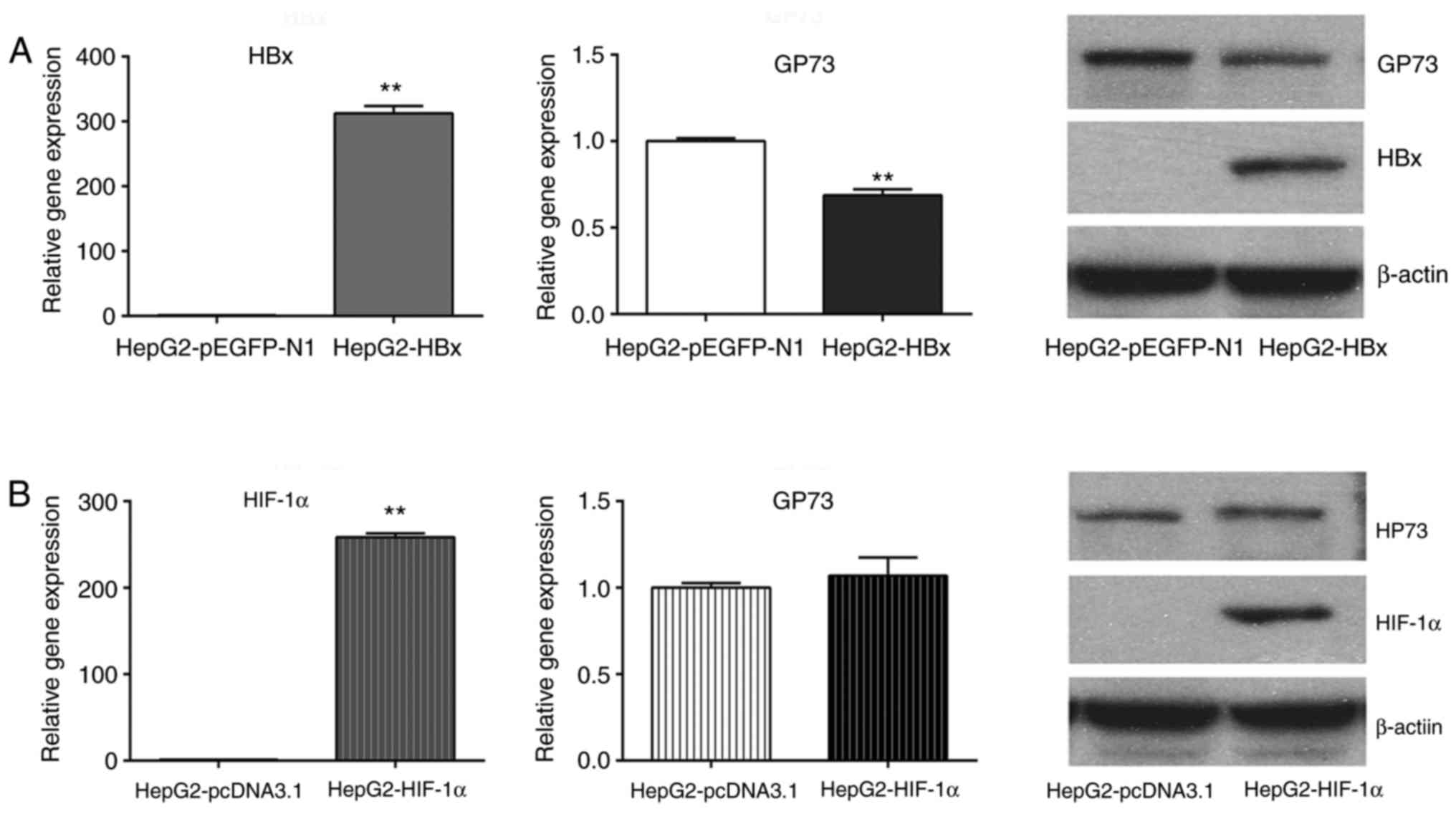
upregulation of GP73, the effect of HBx, HIF-1α and HIF-2α was
examined on GP73 expression. The results demonstrated that
transfection of HepG2 cells with a HBx-overexpressing plasmid
reduced the mRNA and protein levels of GP73 (Fig. 3A), while transfection with a
HIF-1α-overexpressing plasmid did not alter GP73 mRNA and protein
levels (Fig. 3B). By contrast,
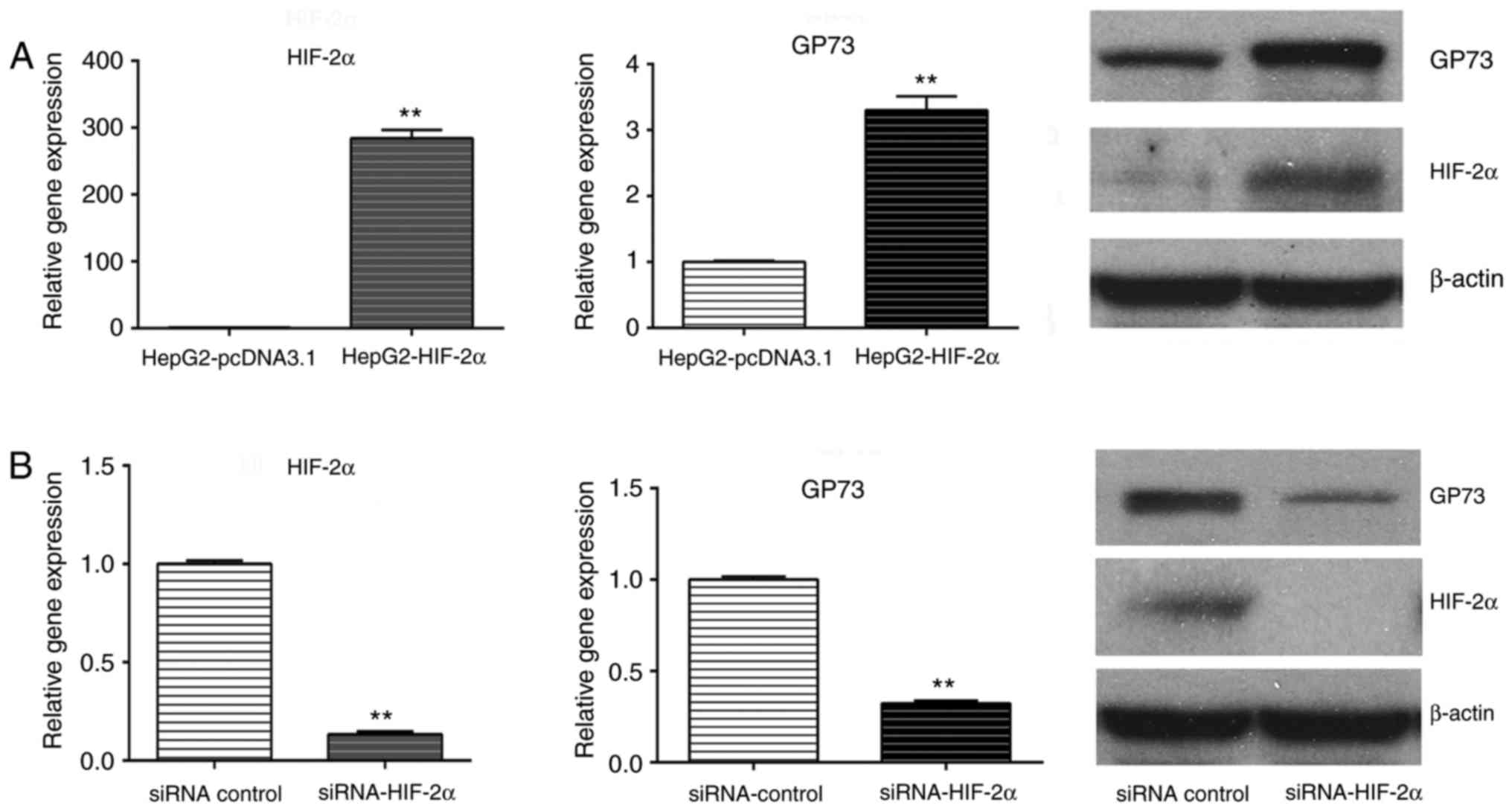
transfection with the HIF-2α-overexpressing plasmid increased the
levels of GP73 mRNA and protein (Fig.
4A). Similarly, GP73 mRNA and protein expression was
downregulated following HIF-2α siRNA silencing (Fig. 4B). Using the TRANSFAC software, two
potential HIF-2α binding sites were identified in the promoter
region of GP73 (Fig. 5). HIF-2α may
enhance the expression of GP73 through binding with the hypoxia
response elements (CGTG) in its promoter region. Our previous
research demonstrated that HBV could activate HIF-2α signaling
(12). The present results indicate
that HIF-2α may be involved in HBV-induced upregulation of
GP73.
HIF-2α and GP73 expression are
positively correlated in HCC tissues
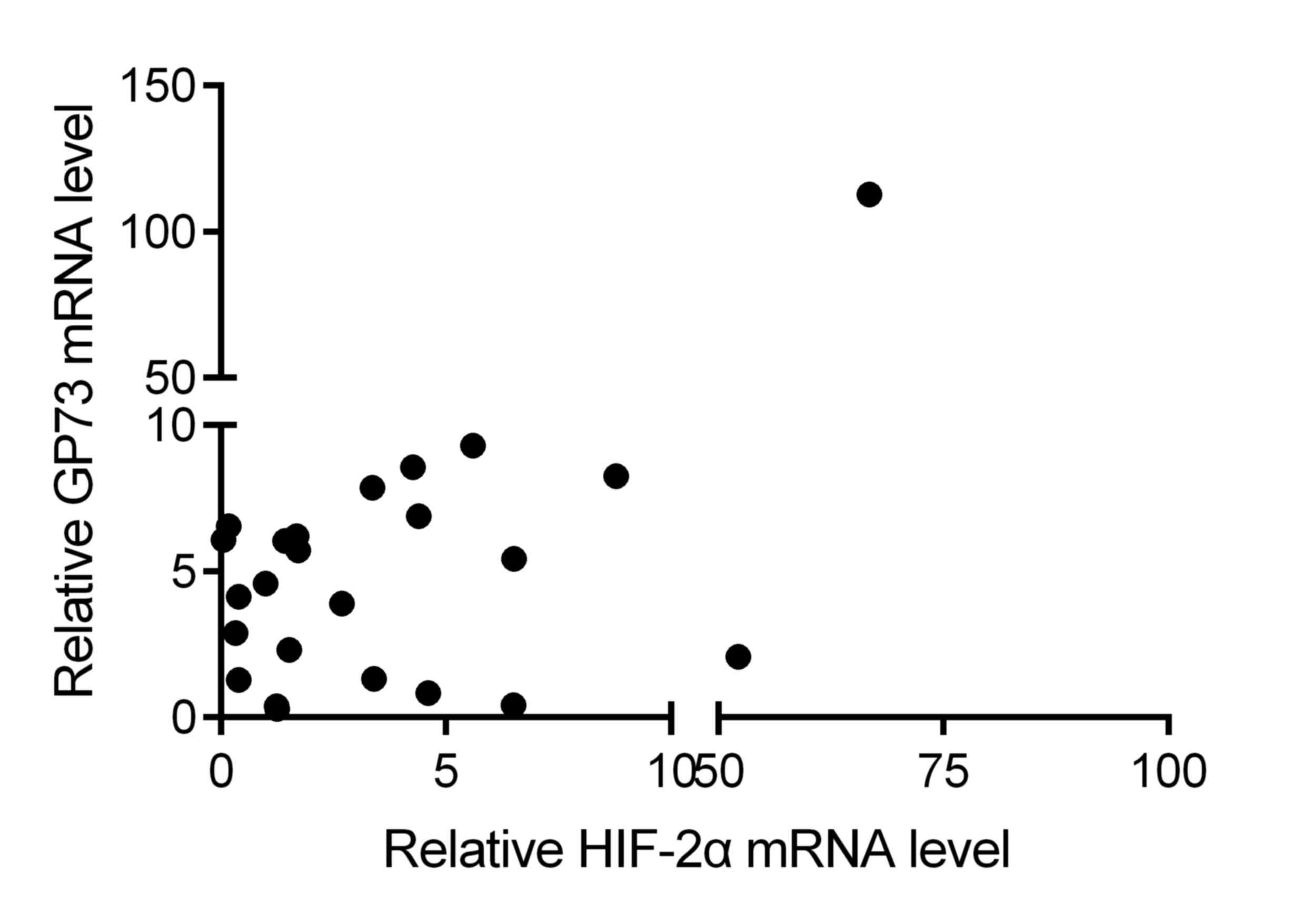
Further, the correlation between the expression
levels of HIF-2α and GP73 was determined in HCC tissues. The
results demonstrated that HIF-2α mRNA expression was positively
correlated with GP73 mRNA expression in HCC tissues (r=0.427;
P<0.001 by Spearman's correlation; Fig. 6). These data further indicate that
HIF-2α may be a factor that contributes to the upregulation of GP73
in liver cancer.
Discussion
In 2000, Kladney et al (13) discovered the Golgi apparatus protein
GP73 while studying the pathogenesis of human giant-cell hepatitis.
Subsequent studies demonstrated that GP73 expression is closely
related to liver diseases. The majority of liver cells in normal
tissues do not express GP73, and only a small number of liver cells
express GP73 at low levels. However, GP73 expression is
significantly increased in hepatitis and hepatocirrhosis tissues,
with the highest expression in liver cancer tissues (13). In the present study, it was
demonstrated that the expression of GP73 in liver cancer tissues
was significantly higher compared with adjacent normal tissues,
which is consistent with previous reports.
GP73 is an integral membrane protein in the cis
Golgi capsule. Under pathogenic states, GP73 can be released from
the cis Golgi capsule and localize to the cytoplasm and cell
surface (2,6). The 55th amino acid in GP73 can be
enzymatically digested by proprotein convertases, including furin
protease, transported through endosomes, and released into the
extracellular space and the blood stream as sGP73 (2,6). It has
been discovered that sGP73 is highly expressed in the serum from
patients with specific types of tumors, including liver cancer,
cholangiocarcinoma, and lung adenocarcinoma. Thus, sGP73 can be
regarded as a serum marker for early liver cancer diagnosis
(2,6).
Currently, a definitive mechanism for the high
expression of GP73 in hepatitis, hepatocirrhosis, and liver cancer
remains unclear. The ex vivo experiments have confirmed the
upregulation of GP73 following HBV infection in liver cancer cells
(6). Therefore, it was hypothesized
that HBV infection, the main inducer of hepatitis, hepatocirrhosis
and liver cancer, may have a role in inducing GP73 expression.
HBx is a major HBV coding protein and serves a
significant role in HBV-induced hepatocarcinogenesis and increased
serum AFP level in liver cancer patients (14–18).
However, in the present study, founder results demonstrated that
overepxression of HBx decreased rather than increased GP73
expression in liver cancer cells. The GP73 mRNA and protein levels
in the HBx-transfected HepG2 cells were 69±2 and 51±5% of the
levels in the control group, respectively, indicating that
HBV-mediated GP73 upregulation did not occur through the HBx
pathway.
Next, the potential roles of HIF-1α and HIF-2α in
inducing GP73 expression were explored. HIF-1α and HIF-2α are
important transcription factors in HCC (19–22). Under
normoxic conditions, the HIF-α subunits are hydroxylated in key
proline residues by the von Hippel-Lindau (VHL) protein complex,
followed by proteasome degradation. Under hypoxic conditions, the
low level of oxygen inhibits the activity of hydroxylase, leading
to the stabilization of HIF-α subunits (19–22).
Accumulated HIF-α subunits translocate to nuclei and dimerize with
HIF-1β to form a functional transcription factor capable of DNA
binding at the hypoxia response elements (HREs) and the
transcriptional activation of target genes that are involved in
cell survival, tumor angiogenesis, metastasis, and resistance to
radiation and chemotherapy (19–22). In
addition to the hypoxic microenvironment, the stability of HIF-α
proteins is modulated by HBV, as our previous study demonstrated
that HBV induced HIF-2α expression by its encoded protein HBx
though binding to pVHL and activating the nuclear factor (NF)-κB
signaling pathway (12). Although
HIF-1α and HIF-2α have similar structure and common HREs, their
target genes are different (23–25). HIF-1
preferentially induces genes that encode glycolytic enzymes, such
as phosphofructokinase and lactate dehydrogenase A. By contrast,
HIF-2 induces genes that are involved in invasion, including the
matrix metalloproteinase (MMP) 2 and 13, and the stem cell factor
OCT-3/4 (23–25). In the present study, it was
demonstrated that HIF-2α, but not HIF-1α, induced the expression of
GP73 in liver cancer cells. GP73 expression was upregulated
following HIF-2α overexpression and was downregulated following
HIF-2α silencing. In addition, two potential HIF-2α binding sites
were identified in the promoter region of GP73 by bioinformatics
analysis. In human HCC tissues, the expression of HIF-2α was
positively correlated with GP73 expression. These results indicated
that HIF-2α can activate GP3 expression in liver cancer cells.
The present study identified that HBV induced GP73
expression in liver cancer cells through HIF-2α signaling
activation. HIF-2α may upregulate GP73 expression in liver cancer
cells by directly binding to and activating its promoter. These
results provided a possible mechanism explaining GP73 upregulation
in liver cancers. More detailed investigations on the molecular
mechanisms of GP73 expression in the future will contribute to
understanding its functional implication in diseases and evaluating
its role as a novel biomarker for liver cancer.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81402041) and the
2016–2017 Special Fund for the Medical Colleges of Health and
Family Planning of Hubei Province (grant no. WJ2016-YZ-10).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tunissiolli NM, Castanhole-Nunes MMU,
Biselli-Chicote PM, Pavarino EC, da Silva RF, da Silva RC and
Goloni-Bertollo EM: Hepatocellular carcinoma: A comprehensive
review of biomarkers, clinical aspects, and therapy. Asian Pac J
Cancer Prev. 18:863–872. 2017.PubMed/NCBI
|
|
2
|
Gao G, Dong F, Xu X, Hu A and Hu Y:
Diagnostic value of serum Golgi protein 73 for HBV-related primary
hepatic carcinoma. Int J Clin Exp Pathol. 8:11379–11385.
2015.PubMed/NCBI
|
|
3
|
Ismail MM, Morsi HK, Abdulateef NA, Noaman
MK and Abou El-Ella GA: Evaluation of prothrombin induced by
vitamin K absence, macrophage migration inhibitory factor and Golgi
protein-73 versus alpha fetoprotein for hepatocellular carcinoma
diagnosis and surveillance. Scand J Clin Lab Invest. 77:175–183.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dai M, Chen X, Liu X, Peng Z, Meng J and
Dai S: Diagnostic value of the combination of Golgi protein 73 and
alpha-fetoprotein in hepatocellular carcinoma: A meta-analysis.
PLoS One. 10:e01400672015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waidely E, Al-Yuobi AR, Bashammakh AS,
El-Shahawi MS and Leblanc RM: Serum protein biomarkers relevant to
hepatocellular carcinoma and their detection. Analyst. 141:36–44.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kladney RD, Cui X, Bulla GA, Brunt EM and
Fimmel CJ: Expression of GP73, a resident Golgi membrane protein,
in viral and nonviral liver disease. Hepatology. 35:1431–1440.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang SL, Liu LP, Niu L, Sun YF, Yang XR,
Fan J, Ren JW, Chen GG and Lai PB: Downregulation and pro-apoptotic
effect of hypoxia-inducible factor 2 alpha in hepatocellular
carcinoma. Oncotarget. 7:34571–34581. 2016.PubMed/NCBI
|
|
8
|
Sai W, Wang L, Zheng W, Yang J, Pan L, Cai
Y, Qiu L, Zhang H, Wu W and Yao D: Abnormal expression of Golgi
protein 73 in clinical values and their role in HBV-related
hepatocellular carcinoma diagnosis and prognosis. Hepat Mon.
15:e329182015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bedossa P and Poynard T: An algorithm for
the grading of activity in chronic hepatitis C. The METAVIR
cooperative study group. Hepatology. 24:289–293. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang SL, Ren QG, Zhang T, Pan X, Wen L, Hu
JL, Yu C and He QJ: Hepatitis B virus X protein and
hypoxiainducible factor-1α stimulate Notch gene expression in liver
cancer cells. Oncol Rep. 37:348–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei H, Li B, Zhang R, Hao X, Huang Y, Qiao
Y, Hou J and Li X and Li X: Serum GP73,a marker for evaluating
progression in patients with chronic HBV infections. PLoS One.
8:e538622013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hu JL, Liu LP, Yang SL, Fang X, Wen L, Ren
QG and Yu C: Hepatitis B virus induces hypoxia-inducible factor-2α
expression through hepatitis B virus X protein. Oncol Rep.
35:1443–1448. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kladney RD, Bulla GA, Guo L, Mason AL,
Tollefson AE, Simon DJ, Koutoubi Z and Fimmel CJ: GP73, a novel
Golgi-localized protein upregulated by viral infection. Gene.
249:53–65. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kong F, You H, Tang R and Zheng K: The
regulation of proteins associated with the cytoskeleton by
hepatitis B virus X protein during hepatocarcinogenesis. Oncol
Lett. 13:2514–2520. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hou Z, Xu X, Fu X, Tao S, Zhou J, Liu S
and Tan D: HBx-related long non-coding RNA MALAT1 promotes cell
metastasis via up-regulating LTBP3 in hepatocellular carcinoma. Am
J Cancer Res. 7:845–856. 2017.PubMed/NCBI
|
|
16
|
Chen S, Dong Z, Yang P, Wang X, Jin G, Yu
H, Chen L, Li L, Tang L, Bai S, et al: Hepatitis B virus X protein
stimulates high mobility group box 1 secretion and enhances
hepatocellular carcinoma metastasis. Cancer Lett. 394:22–32. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu M, Li W, Lu Y, Dong X, Lin B, Chen Y,
Zhang X, Guo J and Li M: HBx drives alpha fetoprotein expression to
promote initiation of liver cancer stem cells through activating
PI3K/AKT signal pathway. Int J Cancer. 140:1346–1355. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu M, Lu Y, Li W, Guo J, Dong X, Lin B,
Chen Y, Xie X and Li M: Hepatitis B virus X protein driven alpha
fetoprotein expression to promote malignant behaviors of normal
liver cells and hepatoma cells. J Cancer. 7:935–946. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Geis T, Döring C, Popp R, Grossmann N,
Fleming I, Hansmann ML, Dehne N and Brüne B: HIF-2alpha-dependent
PAI-1 induction contributes to angiogenesis in hepatocellular
carcinoma. Exp Cell Res. 331:46–57. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo XF, Wang AY and Liu J:
HIFs-MiR-33a-Twsit1 axis can regulate invasiveness of
hepatocellular cancer cells. Eur Rev Med Pharmacol Sci.
20:3011–3016. 2016.PubMed/NCBI
|
|
21
|
Yuan P, Cao W, Zang Q, Li G, Guo X and Fan
J: The HIF-2α-MALAT1-miR-216b axis regulates multi-drug resistance
of hepatocellular carcinoma cells via modulating autophagy. Biochem
Biophys Res Commun. 478:1067–1073. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee JH, Hur W, Hong SW, Kim JH, Kim SM,
Lee EB and Yoon SK: ELK3 promotes the migration and invasion of
liver cancer stem cells by targeting HIF-1α. Oncol Rep. 37:813–822.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu T, Tang B and Sun X: Development of
inhibitors targeting hypoxia-inducible factor 1 and 2 for cancer
therapy. Yonsei Med J. 58:489–496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin D and Wu J: Hypoxia inducible factor
in hepatocellular carcinoma: A therapeutic target. World J
Gastroenterol. 21:12171–12178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Keith B, Johnson RS and Simon MC: HIF1α
and HIF2α: Sibling rivalry in hypoxic tumour growth and
progression. Nat Rev Cancer. 12:9–22. 2011. View Article : Google Scholar : PubMed/NCBI
|