Introduction
Lung cancer is a malignant tumor that threatens
human health and life, and exhibited the highest global occurrence
rate and lethality among all types of cancer in 2012 (1). Its morbidity is increasing in multiple
countries (1). At present, the
treatment options for patients with lung cancer include surgery,
radiotherapy, and chemotherapy. In non-small cell lung cancer
(NSCLC), the typical first choice of therapy is operative treatment
but, once NSCLC has been definitively diagnosed, surgery is not
possible in 75% of patients since lesions have already developed,
or the state of their health following surgery would potentially be
poorer compared with that prior to surgery (2). Therefore, the patient may opt for
chemotherapy. Though chemotherapy may decrease the recurrence rate
of NSCLC in patients, only ~30% of patients with NSCLC undergo
effective treatment with platinum-based first-line chemotherapy,
and chemotherapy resistance is common (3). Furthermore, the 5-year survival rate for
patients with NSCLC is only 15% (4).
Previous research has indicated that, although novel
chemotherapeutics have improved the curative effect in patients
with transfer NSCLC, the associated median survival time remains
only 8–9 months (5). Therefore, it is
crucial to understand the occurrence and development of lung
cancer, identify the molecular mechanisms underlying metastasis,
and develop effective treatments for patients with malignant lung
tumors.
The epidermal growth factor receptor (EGFR) family,
also known as the Erb-b2 receptor tyrosine kinases (ERBB), includes
four main members: EGFR, ERBB2, ERBB3 and ERBB4. The EGFR family
members are allosteric enzymes located at the cell surface
(3). The members may be divided into
three areas: A ligand-coupling domain that protrudes out of the
cytomembrane, an intramembrane tyrosine kinase-activated functional
domain and a transmembrane domain. The EGFR family is associated
with tumorigenesis and tumor development (6). The coding products of the EGFR family
exhibit phosphatase functions, and participate in cytoskeletal
protein recombination, thereby facilitating signal transduction,
proliferation, apoptosis regulation and malignant transformation in
cells (7). Increased EGFR expression
has been reported in multiple tumors, including colon, esophageal,
breast, lung, ovarian, cervical and pancreatic cancer, and glioma
(8). Abnormal EGFR expression is
associated with malignant cell proliferation, adhesion,
vascularization, metastasis and radiosensitivity (8). EGFR represents one of the most promising
therapeutic targets in the study of antineoplastic molecules
(9). In previous years, anti-EGFR
molecular targeted drugs have received increasing attention
(10). A series of antineoplastic
drugs targeting EGFR have previously been studied. Cetuximab and
gefitinib have undergone clinical trials and acquired improved
curative effects (10,11). There are >10 EGFR-targeting
inhibitors, and they have acquired antineoplastic curative effects
by being used to treat different types of human tumor (2). Therefore, the present study evaluated
the function of EGFR and its molecular targets in NSCLC.
Materials and methods
Lung cancer specimens
From July 2015 to January 2016, 12 patients (male,
56.5±6.2 years age) with lung cancer who underwent surgery were
enrolled in the present study at the First Affiliated Hospital of
Xi'an Jiaotong University (Xi'an, China). Normal adjacent tissues
specimens were used as controls. The study was approved by the
Ethics Committee of the First Affiliated Hospital of Xi'an Jiaotong
University and all patients provided written informed consent.
Immunohistochemical analysis
Cancer tissue samples were fixed with 4%
paraformaldehyde for 24 h at room temperature, paraffin-embedded,
and tumor tissue was cut into 4-µm-thick tissue samples. The
sections were deparaffinized, then washed with PBS, and immersed in
3% hydrogen peroxide to block endogenous peroxidase activity at
room temperature for 10 min. Sections were incubated with anti-ERGF
(cat. no. sc-367974; 1:100; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) for 1 h at room temperature. Sections were incubated with
goat anti-rabbit IgG, horseradish peroxidase-conjugated secondary
antibody (cat. no. 7074; 1:500; Cell Signaling Technology, Inc.)
for 1 h at room temperature. Tissue samples were observed with a
Nikon E200 light microscope (Nikon Corporation, Tokyo, Japan) at
magnification, ×20.
Cell culture
The bronchial epithelioid cell line BEAS-2B, and the
human lung cancer SW-900 and A549 cell lines were purchased from
the Shanghai Cell Bank of the Chinese Academy of Sciences
(Shanghai, China) and cultured in Dulbecco's modified Eagle's
medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% heat-inactivated fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.), 50 U/ml penicillin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and 50 µg/ml
streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C in a humidified
atmosphere of 5% CO2.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the lung cancer tissue
samples or BEAS-2B, SW-900 and A549 cell lines using TRIzol™
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). RT was
performed at 94°C for 5 min and 42°C for 30 min with the
PrimeScript RT reagent kit (Takara Biotechnology Co., Ltd., Dalian,
China). The RT-qPCR exponential phase occurred for 40 cycles to
permit quantitative comparison of the complementary DNAs amplified
from identical reactions using SYBR Premix Taq (Takara
Biotechnology Co., Ltd.). The primer sequences used were as
follows: EGFR forward, 5′-TGGAGCTACGGGGTGACCGT-3′ and reverse,
5′-GGTTCAGAGGCTGATTGTGAT-3′; GAPDH forward,
5′-ACCTGACCTGCCGTCTAGAA-3′ and reverse, 5′-TCCACCACCCTGTTGCTGTA-3′;
microRNA (miR)200a forward, 5′-GCTCACCCTTGCAGGTCTCC-3′ and reverse,
5′-CCCGAAACCCAGCCGCATC-3′; U6 forward, 5′-CGCTTCGGCAGCACATATACTA-3′
and reverse, 5′-CGCTTCACGAATTTGCGTGTCA-3′. Each cycle was as
follows: 94°C for 5 min, followed by 30 cycles of 94°C for 10 sec,
60°C for 30 sec and 72°C for 30 sec. Data analysis was performed
using the 2−∆∆Cq method (12). Experiments were repeated in
triplicate.
miR transfection
si-miR200a (5′-ACAUCGUUACCAGACAGUGUUA-3′) and
miR-negative control (NC; 5′-CAGAUUUUGUGUAGUACAA-3′) were designed
and synthesized by Zimmer Company (Shanghai, China). Anti-miR200a
(100 ng) and miR-NC (100 ng) were transfected into the A549 cells
(1×105) using Lipofectamine 2000® reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) for 6 h in DMEM, and
then the medium was changed to DMEM, according to the
manufacturer's protocol.
MTT assay
Following transfection with anti-miR200a and miR-NC
for 48 h, A549 cells (1×103) were seeded onto a 96-well
plate and cultured in DMEM. Subsequently, 20 µl MTT (5 µg/ml) was
added to each well and the cells were cultured for 4 h at 37°C. The
medium was then discarded and 150 µl dimethyl sulfoxide was added
(Invitrogen; Thermo Fisher Scientific, Inc.). Absorbance was
measured using a multi-well spectrophotometer (BioTek Instruments,
Inc., Winooski, VT, USA) at 490 nm.
Flow cytometry analysis and apoptosis
assay
Following transfection with anti-miR200a and miR-NC
for 48 h, the A549 cells (1×106) were seeded onto a
6-well plate and cultured in DMEM. An Annexin V-Fluorescein
Isothiocyanate (FITC) Apoptosis Detection kit (BD Biosciences, San
Jose, CA, USA) was used according to the manufacturer's protocol to
detect apoptosis. Annexin V-FITC (10 µl) was added to the cells for
15 min. Subsequently, a further 5 µl Annexin V-FITC was added to
the cells for 5 min. The apoptosis rate was assessed using a
FACSCalibur™ flow cytometer (BD Biosciences) Data was analyzed
using FlowJo 7.6.1 (FlowJo LLC, Ashland, OR, USA).
Analysis of caspase (CASP)3/9
activity
Following transfection with anti-miR200a and miR-NC
for 48 h, the A549 cells were seeded onto a 6-well plate
(1×106) and cultured in DMEM. Ac-DEVD-pNA from a Caspase
3 Activity Assay kit, or Ac-LEHD-pNA from a Caspase 9 Activity
Assay kit (both from Beyotime Institute of Biotechnology, Haimen,
China) were added to each well and the cells were cultured for 1 h
at 37°C. CASP3/9 activity was measured using a multi-well
spectrophotometer (BioTek Instruments, Inc.) at 405 nm.
Western blot analysis
Following transfection with anti-miR200a and miR-NC
for 48 h, the A549 cells were seeded onto a 6-well plate. Total
protein from the A549 cells was subsequently extracted using RIPA
lysis buffer (Beyotime Institute of Biotechnology) supplemented
with protease inhibitors (Sigma-Aldrich; Merck KGaA). Protein
content was quantified using the bicinchoninic acid method
(Beyotime Institute of Biotechnology). Protein (50 µg/lane) was
fractionated using SDS-PAGE on a 10% gel and subsequently
transferred to polyvinylidene fluoride (PVDF) membranes (EMD
Millipore, Billerica, MA, USA). The PVDF membranes were blocked
using 0.5% non-fat milk in TBS containing 0.1% Tween-20 at 37°C for
1 h. The membranes were incubated with anti-ERGF (cat. no.
sc-367974; 1:500, Santa Cruz Biotechnology, Inc.),
anti-phosphorylated (p)-extracellular signal-regulated kinase (ERK;
cat. no. sc-23759-R; 1:500, Santa Cruz Biotechnology, Inc.) and
anti-GAPDH (sc-25778, 1:2,000, Santa Cruz Biotechnology, Inc.)
antibodies overnight at 4°C. The membrane was washed three times
for 10 min each, with TBS containing 0.1% Tween at room temperature
and incubated with goat anti-rabbit IgG, horseradish
peroxidase-conjugated secondary antibody (cat. no. 7074; 1:5,000,
Cell Signaling Technology, Inc.) for 1 h at room temperature.
Protein expression was visualized using an enhanced
chemiluminescent kit (GE Healthcare, Chicago, IL, USA) and analyzed
using Bio-Rad Laboratories Quantity One software 3.0 (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
Data were presented as the mean ± standard error
using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Statistical
analysis was performed using the Student's t-test or, when
comparing multiple groups, one-way analysis of variance with
Bonferroni's multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference.
Results
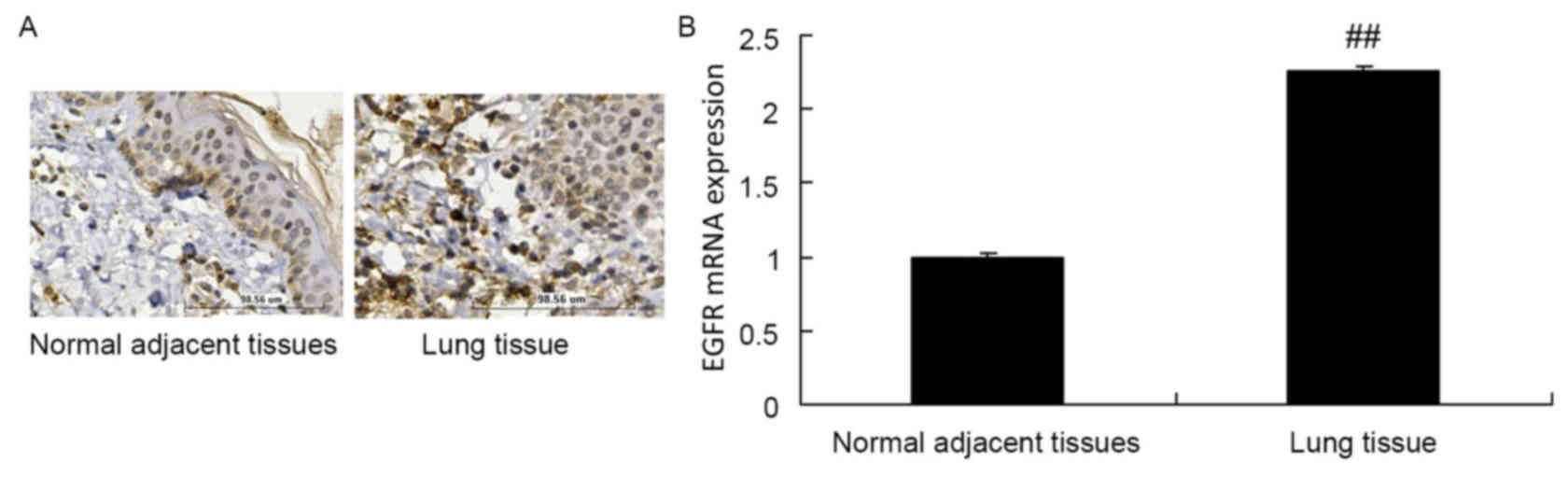
EGFR expression is upregulated in lung
cancer tissue
The protein expression of EGFR in normal adjacent
tissue specimens was decreased compared with that in lung cancer
tissue samples (Fig. 1A).
Furthermore, the RT-qPCR results of the present study revealed that
the mRNA expression of EGFR in normal adjacent tissue specimens was
decreased compared with that in lung cancer tissue samples
(Fig. 1B).
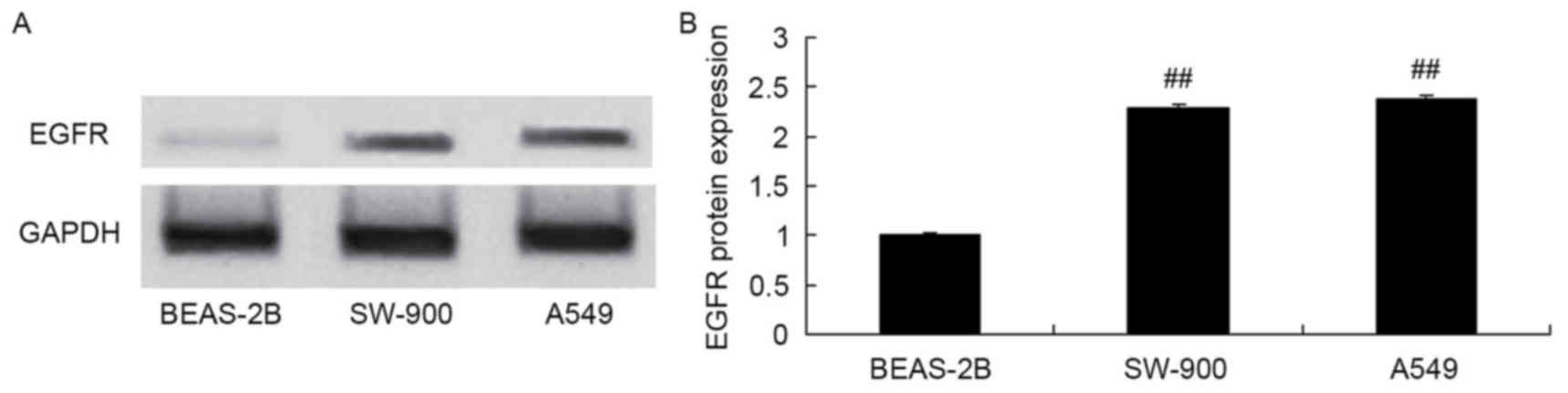
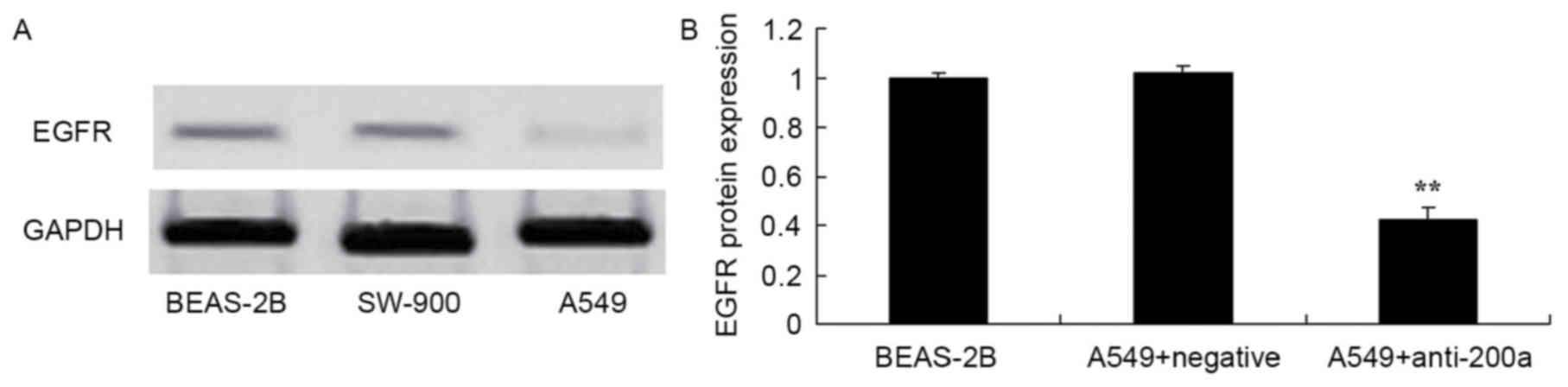
Expression of EGFR protein in A549
cells
EGFR protein expression was measured in the BEAS-2B,
SW-900 and A549 cell lines using western blot analysis. EGFR
protein expression in the lung cancer cell lines SW-900 and A549
was increased compared with that in the bronchial epithelioid cell
line BEAS-2B (Fig. 2).
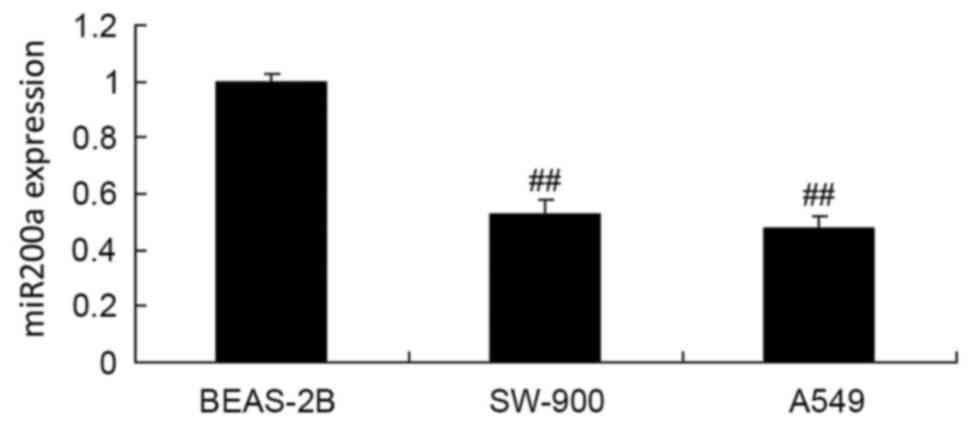
Expression of miR200a in A549
cells
RT-qPCR was used to assess miR200a expression in the
BEAS-2B, SW-900 and A549 cell lines. The expression of miR200a in
the BEAS-2B cells was significantly decreased compared with that in
the SW-900 and A549 cells (Fig.
3).
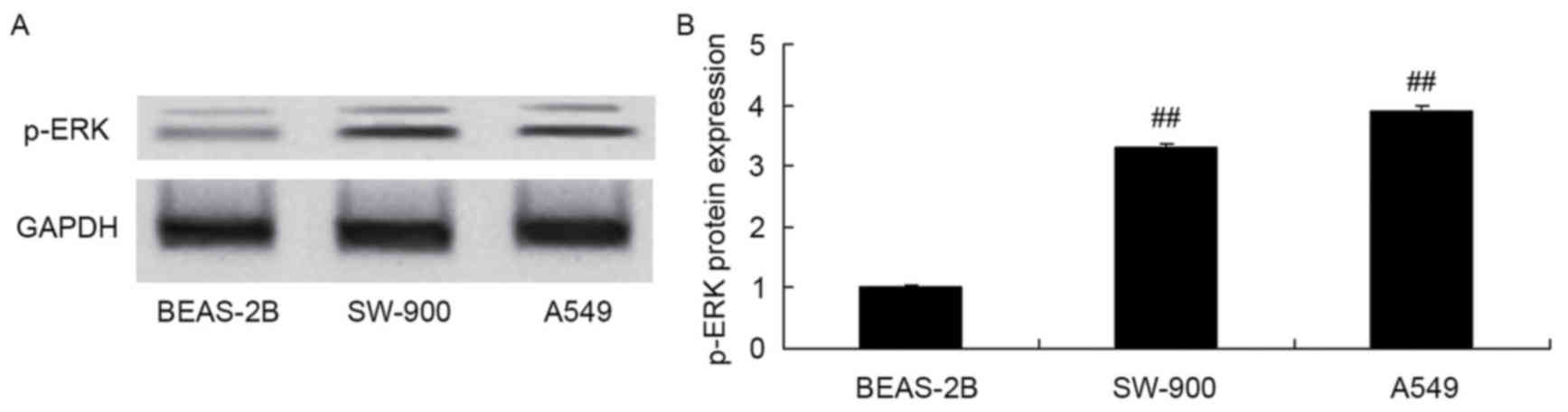
Expression of ERK protein in A549
cells
p-ERK protein expression was measured in the
BEAS-2B, SW-900 and A549 cells using western blot analysis. Protein
expression of p-ERK in SW-900 and A549 cells was significantly
increased compared with that in BEAS-2B cells (Fig. 4).
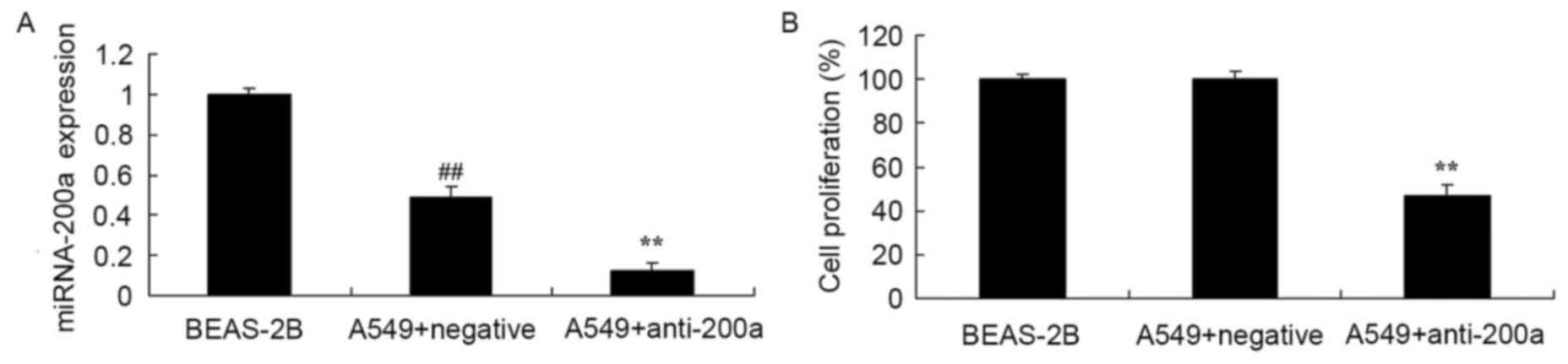
Effect of downregulating miR200a
expression on A549 cell proliferation
To evaluate the effect of downregulating miR200a
expression on A549 cell proliferation, anti-miR200a and miR-NC were
transfected into A549 cells. miR200a expression in A549 cells was
significantly decreased compared with that in BEAS-2B cells, and
miR200a expression in A549 cells transfected with anti-miR200a was
significantly suppressed compared with that in A549 cells
transfected with miR-NC (Fig. 5A).
Proliferation was significantly suppressed in A549 cells in which
miR200a was downregulated compared with that in miR-NC-treated A549
cells (Fig. 5B).
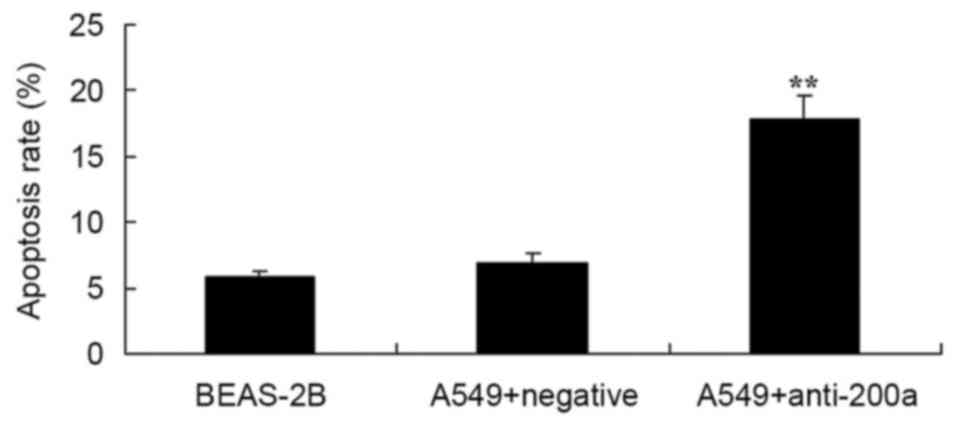
Effect of downregulating miR200a
expression on A549 cell apoptosis
Flow cytometry analysis was used to assess the
effect of downregulating miR200a on the apoptosis of A549 cells.
The apoptosis rate of A549 cells in which miR200a was downregulated
was significantly promoted compared with that of miR-NC-treated
A549 cells (Fig. 6).
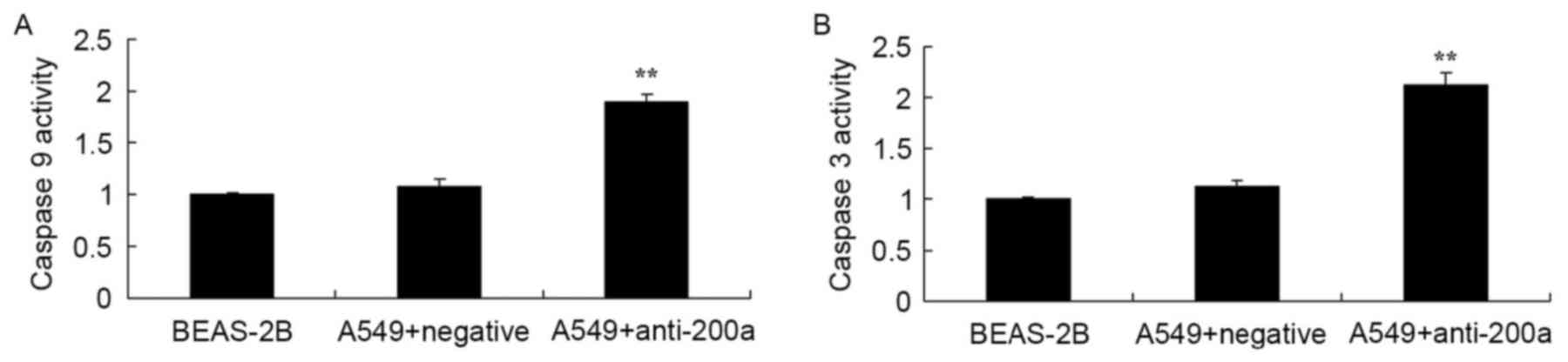
Effect of downregulating miR200a
expression on CASP3/9 activity in A549 cells
The present study evaluated the effect of
downregulating miR200a expression on CASP3/9 activity in A549
cells. CASP3 and CASP9 activity was significantly increased in A549
cells treated with anti-miR200a compared with that in
miR-NC-treated A549 cells (Fig.
7).
Effect of downregulating miR200a
expression on EGFR protein expression in A549 cells
The present study assessed the effect of
downregulating miR200a expression on the protein expression of EGFR
in A549 cells. EGFR protein expression was significantly inhibited
in A549 cells in which miR200a was downregulated compared with that
in miR-NC-treated A549 cells (Fig.
8).
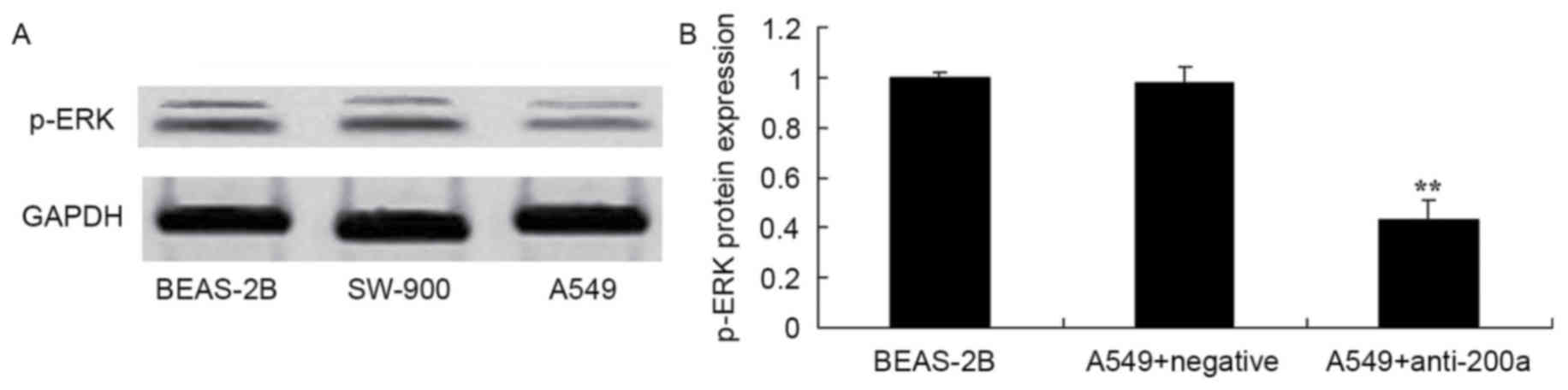
Effect of downregulating miR200a
expression on the protein expression of ERK in A549 cells
To assess the effect of downregulating miR200a
expression on the protein expression of ERK in A549 cells, p-ERK
protein expression in SW-900 and A549 cells was measured using
western blot analysis. The protein expression of p-ERK was
significantly suppressed in A549 cells in which miR200a was
downregulated compared with that in miR-NC-treated A549 cells
(Fig. 9).
Discussion
Lung cancer was the most common malignant tumor
globally in 2012 (13). There are
~1.8 million incident cases (14),
and lung cancer-associated mortality occurred in >1.5 million
people globally, with the majority of these mortalities being male.
NSCLC accounts for >80% of patients with lung cancer. Numerous
patients exhibit NSCLC in the middle or advanced stage at the time
of diagnosis. By this stage, the 5-year survival rate has decreased
(13). Therefore, diagnosing NSCLC
early is crucial. At present, imagological examination is a major
diagnostic tool in oncology, but increased expense and risks of
radiation exposure, among other factors, render it inappropriate
for large-scale screening, so it is crucial to be able to
accurately screen using inspection, decreased expenses and trauma
(15). The importance of miRs has
become apparent in oncology (16).
Furthermore, as the molecular diagnosis marker of NSCLC, EGFR has
received increased attention (15).
The present study revealed that mRNA expression of EGFR in normal
adjacent tissue specimens was decreased compared with that in lung
cancer tissue samples.
miRs are single-stranded, highly conserved,
noncoding nucleotide molecules that are 19–25 nt in length. By
degrading or inhibiting mRNA targets, miRs participate in multiple
important biological processes, including growth, differentiation,
proliferation, apoptosis, hormone secretion and neoplasia in animal
and plant cells (14). miRs are also
associated with sensitivity to tumor drugs. By downregulating
miR200C expression, sensitivity to adriamycin is decreased in
breast cancer cells (17). In acute
promyelocytic leukemia, increased expression of miR125b is involved
in the action of current therapeutics (18). In colorectal cancer, upregulating
miR224 may increase resistance to methylamine (19). A previous study revealed that NSCLC
tissues often possess a deficiency of mixed miR128b (20). miR128B may regulate EGFR gene and
protein expression (20). The present
study demonstrated that the expression of miR200a in BEAS-2B cells
was increased compared with that in SW-900 and A549 cells.
EGFR has attracted attention since it was discovered
in 1962 (8). EGFR may combine with
ligands to phosphorylate the isogeneous second area of the SRC
proto-oncogene protein, induce normal mitosis, and promote
homeostasis, cell differentiation and migration; abnormal
expression is associated with the proliferation, adhesion,
vascularization and metastasis of malignant cells (21). EGFR has gained attention in the study
of antineoplastic molecular targeted therapy. The present study
demonstrated that downregulating miR200a significantly inhibited
EGFR protein expression in A549 cells. Zhen et al (22) reported that overexpressing miR200a
significantly downregulated EGFR expression in NSCLC cells.
ERK is a protein kinase that is located in the
terminal position in the signaling pathway of mitogen-activated
protein kinase (MAPK), and forms p-ERK via phosphorylation
(23). p-ERK may influence the
transcriptional expression of associated genes, is associated with
cell proliferation and serves a crucial function in malignant
transformation. Among the multiple components of the MAPK signaling
pathway, ERK is crucial (24). ERK
helps to regulate cell proliferation and serves a function in
multiple physiological and pathological processes, including cell
differentiation, period circular regulation and intercellular
functional synchronization (24). A
previous study revealed that the ERK1/2 signaling pathway is
activated in NSCLC; ERK1/2 exhibited increased expression and
phosphorylation (25). On entering
the nucleus, p-ERK1/2 affects the expression of jun proto-oncogene,
nuclear factor κB subunit 1, ELK1, fos proto-oncogene, MYC
proto-oncogene, and transcription factors, phosphorylates multiple
substrates of nuclear transcription factors, regulates the
transcription of associated genes, and serves a key function in
malignant transformation (26). The
results of the present study suggested that downregulating miR200a
significantly suppressed p-ERK protein expression in A549 cells.
Liu et al (27) demonstrated
that miR200a expression was associated with the neurotrophic
receptor tyrosine kinase 2/ERK/protein kinase B signaling pathway
in mice exposed to chronic, unpredictable, moderate stress.
To conclude, the present study suggested that
downregulating miR200a significantly suppressed proliferation and
promoted apoptosis in A549 cells in vitro, partly through
the regulation of the EGFR and ERK1/2 signaling pathways, and
thereby may facilitate the development of aggressive tumors. The
results of the present study suggested that
miR200a/EGFR/ERK1/2-based prevention and therapeutics in patients
with NSCLC may prove clinically beneficial.
References
|
1
|
Allendorf DJ, Bordoni RE, Grant SC, Saleh
MN, Reddy VB, Jerome ML, Dixon PM, Miley DK, Singh KP and Robert F:
Phase I/IIa study of sequential chemotherapy regimen of
bendamustine/irinotecan followed by etoposide/carboplatin in
untreated patients with extensive disease small cell lung cancer
(EDSCLC). Cancer Chemother Pharmacol. 76:949–955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Balabko L, Andreev K, Burmann N, Schubert
M, Mathews M, Trufa DI, Reppert S, Rau T, Schicht M, Sirbu H, et
al: Increased expression of the Th17-IL-6R/pSTAT3/BATF/RorγT-axis
in the tumoural region of adenocarcinoma as compared to squamous
cell carcinoma of the lung. Sci Rep. 4:73962014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Köhler J and Schuler M: LUX-Lung 3:
Redundancy, toxicity or a major step forward? Afatinib as
front-line therapy for patients with metastatic EGFR-mutated lung
cancer. Future Oncol. 10:533–540. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang S, Zhang B, Li C, Cui C, Yue D, Shi
B, Zhang Q, Zhang Z, Zhang X and Wang C: Prognostic value of number
of negative lymph node in patients with stage II and IIIa non-small
cell lung cancer. Oncotarget. 8:79387–79396. 2017.PubMed/NCBI
|
|
5
|
Belani CP, Yamamoto N, Bondarenko IM,
Poltoratskiy A, Novello S, Tang J, Bycott P, Niethammer AG,
Ingrosso A, Kim S and Scagliotti GV: Randomized phase II study of
pemetrexed/cisplatin with or without axitinib for non-squamous
non-small-cell lung cancer. BMC Cancer. 14:2902014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shen H, Du G, Liu Z, Bao J, Yu Q, Jia C,
Liang X and Shan L: Assessment and prognostic analysis of EGFR
mutations or/and HER2 overexpression in Uygur's non-small cell lung
cancer. Int J Clin Exp Med. 8:22300–22309. 2015.PubMed/NCBI
|
|
7
|
Li N, Li H, Su F, Li J, Ma X and Gong P:
Relationship between epidermal growth factor receptor (EGFR)
mutation and serum cyclooxygenase-2 Level, and the synergistic
effect of celecoxib and gefitinib on EGFR expression in non-small
cell lung cancer cells. Int J Clin Exp Pathol. 8:9010–9020.
2015.PubMed/NCBI
|
|
8
|
Lococo F, Paci M, Rapicetta C, Rossi T,
Sancisi V, Braglia L, Cavuto S, Bisagni A, Bongarzone I, Noonan DM,
et al: Preliminary evidence on the diagnostic and molecular role of
circulating soluble EGFR in non-small cell lung cancer. Int J Mol
Sci. 16:19612–19630. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Furcht CM, Muñoz Rojas AR, Nihalani D and
Lazzara MJ: Diminished functional role and altered localization of
SHP2 in non-small cell lung cancer cells with EGFR-activating
mutations. Oncogene. 32:2346–2355. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Metzger B, Chambeau L, Begon DY, Faber C,
Kayser J, Berchem G, Pauly M, Boniver J, Delvenne P, Dicato M and
Wenner T: The human epidermal growth factor receptor (EGFR) gene in
European patients with advanced colorectal cancer harbors
infrequent mutations in its tyrosine kinase domain. BMC Med Genet.
12:1442011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang X, Fan J, Li Y, Lin S, Shu P, Ni J,
Qin S and Zhang Z: Polymorphisms in epidermal growth factor
receptor (EGFR) and AKT1 as possible predictors of clinical outcome
in advanced non-small-cell lung cancer patients treated with EGFR
tyrosine kinase inhibitors. Tumour Biol. 37:1061–1069. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real time quantitative PCR and
the 2(-Delta Delta C(T)) methods. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JH, Kim HS and Kim BJ: Prognostic
value of smoking status in non-small-cell lung cancer patients
treated with immune checkpoint inhibitors: A metaanalysis.
Oncotarget. 8:93149–93155. 2017.PubMed/NCBI
|
|
14
|
Cimino D, De Pittà C, Orso F, Zampini M,
Casara S, Penna E, Quaglino E, Forni M, Damasco C, Pinatel E, et
al: miR148b is a major coordinator of breast cancer progression in
a relapse-associated microRNA signature by targeting ITGA5, ROCK1,
PIK3CA, NRAS, and CSF1. FASEB J. 27:1223–1235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ardizzoni A, Manegold C, Debruyne C,
Gaafar R, Buchholz E, Smit EF, Lianes P, ten Velde G, Bosquee L,
Legrand C, et al: European organization for research and treatment
of cancer (EORTC) 08957 phase II study of topotecan in combination
with cisplatin as second-line treatment of refractory and sensitive
small cell lung cancer. Clin Cancer Res. 9:143–150. 2003.PubMed/NCBI
|
|
16
|
Du B, Wang Z, Zhang X, Feng S, Wang G, He
J and Zhang B: MicroRNA-545 suppresses cell proliferation by
targeting cyclin D1 and CDK4 in lung cancer cells. PLoS One.
9:e880222014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li J, Tan Q, Yan M, Liu L, Lin H, Zhao F,
Bao G, Kong H, Ge C, Zhang F, et al: miRNA-200c inhibits invasion
and metastasis of human non-small cell lung cancer by directly
targeting ubiquitin specific peptidase 25. Mol Cancer. 13:1662014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhao X, He W, Li J, Huang S, Wan X, Luo H
and Wu D: MiRNA-125b inhibits proliferation and migration by
targeting SphK1 in bladder cancer. Am J Transl Res. 7:2346–2354.
2015.PubMed/NCBI
|
|
19
|
Liao WT, Li TT, Wang ZG, Wang SY, He MR,
Ye YP, Qi L, Cui YM, Wu P, Jiao HL, et al: microRNA-224 promotes
cell proliferation and tumor growth in human colorectal cancer by
repressing PHLPP1 and PHLPP2. Clin Cancer Res. 19:4662–4672. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang XC, Du LQ, Tian LL, Wu HL, Jiang XY,
Zhang H, Li DG, Wang YY, Wu HY, She Y, et al: Expression and
function of miRNA in postoperative radiotherapy sensitive and
resistant patients of non-small cell lung cancer. Lung Cancer.
72:92–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin K, Cheng J, Yang T, Li Y and Zhu B:
EGFR-TKI down-regulates PD-L1 in EGFR mutant NSCLC through
inhibiting NF-κB. Biochem Biophys Res Commun. 463:95–101. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhen Q, Liu J, Gao L, Liu J, Wang R, Chu
W, Zhang Y, Tan G, Zhao X and Lv B: MicroRNA-200a targets EGFR and
c-Met to inhibit migration, invasion and gefitinib resistance in
non-small cell lung cancer. Cytogenet Genome Res. 146:1–8. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang X, Pesakhov S, Weng A, Kafka M, Gocek
E, Nguyen M, Harrison JS, Danilenko M and Studzinski GP: ERK 5/MAPK
pathway has a major role in 1α,25-(OH)2 vitamin D3-induced terminal
differentiation of myeloid leukemia cells. J Steroid Biochem Mol
Biol. 144:223–227. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Paolo M, Assunta S, Antonio R, Claudia SP,
Anna BM, Clorinda S, Francesca C, Fortunato C and Cesare G:
Selumetinib in advanced non small cell lung cancer (NSCLC)
harbouring KRAS mutation: Endless clinical challenge to KRAS-mutant
NSCLC. Rev Recent Clin Trials. 8:93–100. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito N, Mine N, Kufe DW, Von Hoff DD and
Kawabe T: CBP501 inhibits EGF-dependent cell migration, invasion
and epithelial-to-mesenchymal transition of non-small cell lung
cancer cells by blocking KRas to Calmodulin binding. 8:1–74018.
2017.
|
|
26
|
Wainstein E and Seger R: The dynamic
subcellular localization of ERK: Mechanisms of translocation and
role in various organelles. Curr Opin Cell Biol. 39:15–20. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu BB, Luo L, Liu XL, Geng D, Liu Q and
Yi LT: 7-Chlorokynurenic acid (7-CTKA) produces rapid
antidepressant-like effects: Through regulating hippocampal
microRNA expressions involved in TrkB-ERK/Akt signaling pathways in
mice exposed to chronic unpredictable mild stress.
Psychopharmacology (Berl). 232:541–550. 2015. View Article : Google Scholar : PubMed/NCBI
|