Introduction
Breast cancer is a major public health concern, and
remains the most prevalent cancer type and a major cause of
cancer-associated mortality in women worldwide (1). Invasive ductal carcinomas represent −75%
of all breast cancer cases (2). In
the United States, the breast cancer incidence is 120.7/100,000
women with the breast cancer mortality rate being 24/100,000 women;
the lifetime risk of breast cancer is 12.2% (3). Although, the breast cancer incidence in
China is significantly lower compared with that in the United
States (4), previous models suggest
that breast cancer may soon reach epidemic proportions (5). Over the past three decades, numerous
patients have succumbed to cancer metastasis and recurrence despite
comprehensive advances achieved in the treatment of breast cancer.
A number of risk factors have been identified to be associated with
the initiation and progress of breast cancer (6), including a shift to a western diet and
increased stress (7). However, the
exact mechanisms underlying this malignancy and the potential
biomarkers for prognosis prediction remain to be elucidated.
Phosphorylated RAC-α serine/threonine-protein kinase
(pAkt), the activated form of Akt, also known as protein kinase B,
is a major downstream effector of the phosphatidylinositide
3-kinase (PI3K) pathway. It serves an essential role in the
development, progression and metastatic spread of breast cancer
(8,9).
Extensive preclinical evidence has indicated that PI3K/Akt pathway
inhibition may sensitize breast cancer cells to doxorubicin
(10,11). Furthermore, previous studies have
indicated that the expression of pAkt is associated with the
prognosis in breast cancer (8–11),
although the results have been controversial (8,12–15). The expression of pAkt was revealed to
be negatively correlated with the prognosis of breast cancer
(12–14), which contrasted with the results
demonstrated by another study (15).
The variance in results may be due to the different ethnicities and
pathological types of patients included in each study.
Autophagy is the process of self-digestion in which
lysosomal degradation is used to maintain cellular viability during
periods of metabolic stress, including starvation; however, its
role in the growth of cancer remains controversial (16,17).
Beclin 1 is an essential component for inducing autophagy in a
variety of cancer types (18). It was
demonstrated that the levels of Beclin 1 in breast cancer cells
were significantly lower compared with those in normal breast
epithelial cells (18); however, the
expression pattern of Beclin 1 in breast cancer tissue and its
association with the prognosis of patients has not been extensively
studied.
In the present study, a total of 90 Chinese female
patients with invasive ductal breast cancer were enrolled between
June 1999 and August 2002 at Shanghai First People's Hospital
(Shanghai, China). The expression patterns of pAkt and Beclin 1 in
invasive ductal breast cancer tissues were measured. In addition,
their associations with the prognosis of patients following surgery
were evaluated using Cox regression analysis.
Materials and methods
Patients and tissue specimens
A total of 90 Chinese female patients with invasive
ductal breast cancer were enrolled in the present study between
June 1999 and August 2002 at Shanghai First People's Hospital. At
the time of surgery, the mean age of patients was 55.68±12.18 years
(range, 27–82 years). A complete diagnostic evaluation consisting
of chest X-rays, mammography, ultrasounds of the liver and a
whole-body bone scan prior to surgery was performed for each
patient to exclude the presence of distant metastasis. No patients
had received radiotherapy or neoadjuvant therapy prior to surgery.
The cancer tissue specimens and normal paracancerous tissues were
collected in liquid nitrogen during surgery. All samples were
confirmed by pathological examination. Pathological information was
obtained for the following: Histological tumor type, primary tumor
size, estrogen receptor (ER) status, progesterone receptor (PR)
status, axillary lymph node status and clinical stage. The clinical
stage was classified according to the American Joint Committee on
Cancer tumor node metastasis staging system (19). The present study was approved by the
Ethical Committee and Institutional Review Board of Shanghai First
People's Hospital. Written informed consent was obtained from all
patients for the use of their samples in the present study.
Immunohistochemistry
Formalin-fixed paraffin-embedded tissue sections
were deparaffinized in xylene, rehydrated through graded ethanol
and then boiled at 99°C for 10 min in citrate buffer (10 mM, pH
6.0) for antigen retrieval. Endogenous peroxidase activity was
suppressed by incubation with 3% hydrogen peroxide for 10 min at
room temperature (RT). Slides were then blocked with 5% bovine
serum albumin at RT for 60 min (Wuhan Boster Biological Technology,
Co., Ltd., Wuhan, China), incubated with diluted primary antibodies
against pAkt (1:500; cat. no. ab38449) and Beclin 1 (1:500; cat.
no. ab62557) (both from Abcam, Cambridge, MA, USA) for 1 h at 37°C,
and then incubated with the secondary antibody (horseradish
peroxidase-conjugated anti-rabbit IgG; cat. no. ab205718; Abcam)
for 20 min at 37°C. Slides were visualized with diaminobenzidine
and counterstained with hematoxylin at RT for 2–3 min for light
microscopic examination at 200-fold magnification, (CX31; Olympus
Corporation, Tokyo, Japan). The intensity of staining was scored
from 1 to 3 (1, no staining or light brown staining; 2, weak to
moderate brown staining; and 3, moderate to strong brown staining).
The percentage of positive cells was calculated from 10 random
fields. The intensity of staining and the percentage of positive
staining were used to evaluate protein expression. Cases with ≥10%
positive cells and scores ≥2 were considered positive.
Follow-up
Patients were followed up until June 30, 2016.
Patients were given a physical examination every 3 months for the
first 2 years after surgery and were subsequently examined every 6
months. Disease-free survival (DFS) was calculated as the duration
between the date of surgery and the date of first evidence of local
recurrence, distant metastasis, or diagnosis of a second primary
tumor or cancer-associated mortality. Overall survival (OS) was
calculated as the time between the date of surgery and the date of
mortality from any cause.
Statistical analysis
SPSS software (version 19.0; IBM Corp., Armonk, NY,
USA) was used for all statistical analysis. The Chi-square test or
Fisher's exact test was applied for univariate analysis to
determine the association between the expression of pAkt and Beclin
1 with clinicopathological parameters. Survival curves were
calculated using the Kaplan-Meier estimator method and comparisons
were performed using the log-rank tests. The univariate Cox
regression was used to identify potential factors associated with
the prognosis, then factors with P<0.1 in the univariate
analysis were included in the multivariate Cox regression analysis
to determine the independent prognostic factors and explore their
effects. All P-values presented are two-tailed. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
A total of 90 female patients with invasive ductal
carcinoma of the breast were enrolled in the present study. Among
the 90 breast cancer tissues, 36 (40%) were identified with lymph
node metastasis, and 54 (60%) without lymph node metastasis. For
tumor size, there were 33 patients with tumor size <2 cm, 49
patients with tumor size ≥2 and <5 cm, and 8 patients with tumor
size ≥5 cm. A total of 55 (61.1%) patients were identified as
ER-positive, 48 (53.3%) as PR-positive, and 19 (21.1%) as human
epidermal growth factor receptor 2 (HER2)-positive. In addition,
there were 20 patients with triple-negative breast cancer. There
were 23 (25.6%) patients with clinical stage I, 53 (58.8%) with
clinical stage II, and 14 (15.6%) with clinical stage III. The
clinicopathological characteristics of the patients are presented
in Table I.
 | Table I.Summary of clinicopathological
characteristics of 90 patients with breast cancer. |
Table I.
Summary of clinicopathological
characteristics of 90 patients with breast cancer.
|
Characteristics | Value |
|---|
| Mean age
(years) |
|
| Age range, years
(n/%) | 55.68±12.18 |
|
<50 | 31/34.4 |
|
≥50 | 59/65.6 |
| Lymph node
metastasis (n/%) |
|
|
Positive | 36/40 |
|
Negative | 54/60 |
| Tumor size, cm
(n/%) |
|
|
<2 | 33/36.7 |
| ≥2 and
<5 | 49/54.4 |
| ≥5 | 8/8.9 |
| ER status
(n/%) |
|
|
Positive | 55/61.1 |
|
Negative | 35/38.9 |
| PR status
(n/%) |
|
|
Positive | 48/53.3 |
|
Negative | 42/46.7 |
| HER2 status
(n/%) |
|
|
Positive | 19/21.1 |
|
Negative | 71/78.9 |
| TNBC (n/%) |
|
|
Yes | 20/22.2 |
| No | 70/77.8 |
| Clinical stage
(n/%) |
|
| I | 23/25.6 |
| II | 53/58.8 |
|
III | 14/15.6 |
Expression pattern of pAkt and Beclin
1, and their association with clinicopathological parameters
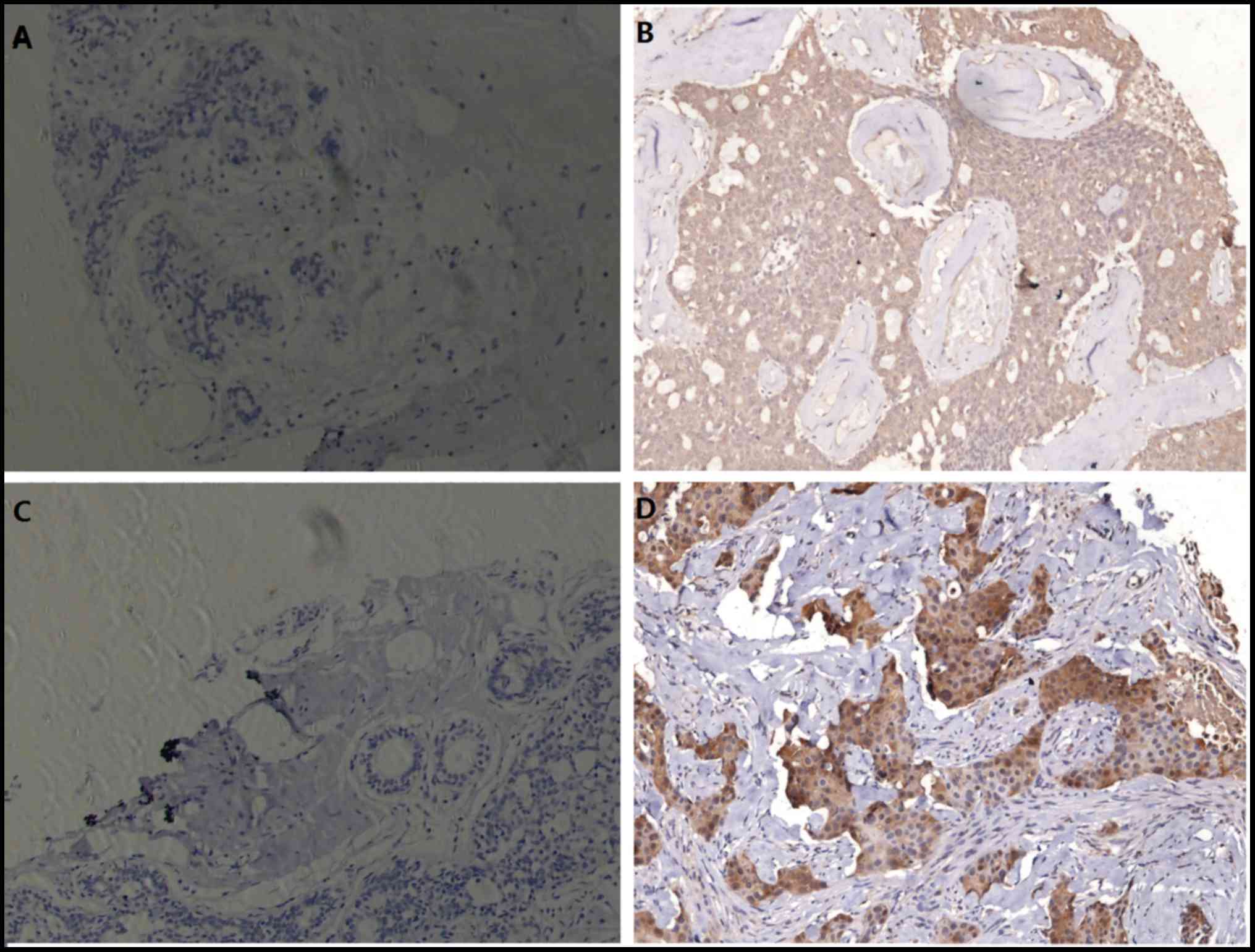
In 90 invasive ductal breast cancer samples, pAkt
expression was detected in 17 (18.9%) samples and Beclin 1 in 33
(36.7%) samples, both of which were not detected in normal
paracancerous samples (Fig. 1). The
positive reaction for pAkt and Beclin 1 were primarily localized to
the cytoplasm with slight positive staining on the membrane.
The association between protein expression and
clinicopathological parameters was analyzed. As presented in
Tables II and III, no significant associations were
identified between the expression levels of pAkt and Beclin 1, and
any clinicopathological parameters.
 | Table II.Association between pAkt expression
and clinicopathologic parameters. |
Table II.
Association between pAkt expression
and clinicopathologic parameters.
|
| pAkt |
|
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Negative | Positive | Total | P-value |
|---|
| Total case | 73 | 17 | 82 |
|
| Age, years |
|
|
|
|
|
<50 | 28 | 3 | 31 | 0.156 |
|
≥50 | 45 | 14 | 59 |
|
| Tumor size, cm |
|
|
|
|
|
<2 | 28 | 5 | 33 | 0.752 |
| ≥2 and
<5 | 39 | 10 | 49 |
|
| ≥5 | 6 | 2 | 8 |
|
| Lymph node
status |
|
|
|
|
|
Negative | 45 | 9 | 54 | 0.586 |
|
Positive | 28 | 8 | 36 |
|
| Clinical stage |
|
|
|
|
| I | 18 | 5 | 23 | 0.854 |
| II | 43 | 10 | 53 |
|
|
III | 12 | 2 | 14 |
|
| ER |
|
|
|
|
|
Negative | 26 | 9 | 35 | 0.269 |
|
Positive | 47 | 8 | 55 |
|
| PR |
|
|
|
|
|
Negative | 31 | 11 | 42 | 0.113 |
|
Positive | 42 | 6 | 48 |
|
| HER2 |
|
|
|
|
|
Negative | 59 | 12 | 71 | 0.342 |
|
Positive | 14 | 5 | 19 |
|
| TNBC |
|
|
| 0.518 |
|
Yes | 15 | 5 | 20 |
|
| No | 58 | 12 | 70 |
|
 | Table III.Association between Beclin 1
expression and clinicopathologic parameters. |
Table III.
Association between Beclin 1
expression and clinicopathologic parameters.
|
| Beclin 1 |
|
|
|---|
|
|
|
|
|
|---|
|
Characteristics | Negative | Positive | Total | P-value |
|---|
| Total case | 57 | 33 | 90 |
|
| Age, years |
|
|
|
|
|
<50 | 22 | 9 | 31 | 0.359 |
|
≥50 | 35 | 24 | 59 |
|
| Tumor size, cm |
|
|
|
|
|
<2 | 21 | 12 | 33 | 0.998 |
| ≥2 and
<5 | 31 | 18 | 49 |
|
| ≥5 | 5 | 3 | 8 |
|
| Lymph node
status |
|
|
|
|
|
Negative | 32 | 22 | 54 | 0.377 |
|
Positive | 25 | 11 | 36 |
|
| Clinical stage |
|
|
|
|
| I | 16 | 7 | 23 | 0.518 |
| II | 31 | 22 | 53 |
|
|
III | 10 | 4 | 14 |
|
| ER |
|
|
|
|
|
Negative | 20 | 5 | 35 | 0.295 |
|
Positive | 37 | 18 | 55 |
|
| PR |
|
|
|
|
|
Negative | 25 | 17 | 42 | 0.517 |
|
Positive | 32 | 16 | 48 |
|
| HER2 |
|
|
|
|
|
Negative | 45 | 26 | 71 | 0.986 |
|
Positive | 12 | 7 | 19 |
|
| TNBC |
|
|
| 0.068 |
|
Yes | 9 | 11 | 20 |
|
| No | 48 | 22 | 70 |
|
Risk factors associated with patient
survival
All 90 patients were followed up from 5 months to
13.5 years (mean period, 107.8 months). A total of 10 mortalities
(6 patients with lymph node metastasis and 4 patients without lymph
node metastasis) and 4 recurrences (2 patients with lymph node
metastasis and 2 patients without lymph node metastasis) were
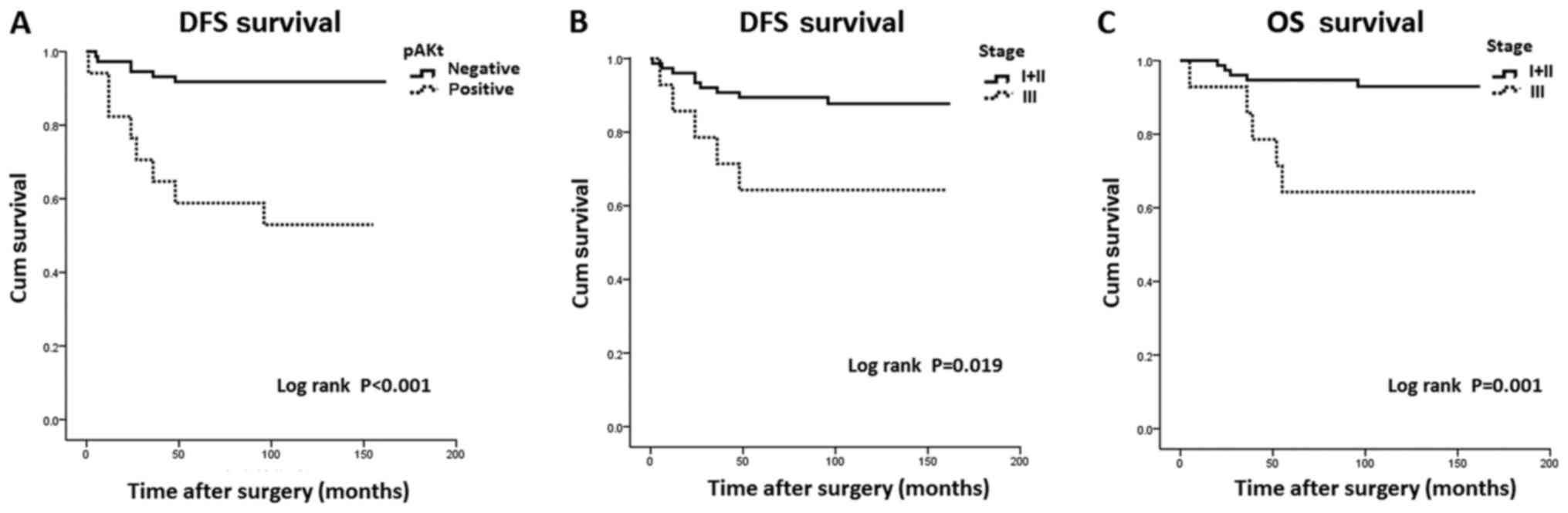
reported during this period. Survival analysis revealed that
patients with pAkt expression exhibited significantly shorter DFS
times compared with those negative for pAkt (97.12 vs. 150.64
years; P<0.001; Fig. 2A).
Furthermore, a trend was observed between pAkt expression and
shorter OS times, but it did not reach statistical significance
(128.63 years vs. 151.32 years; P=0.071). In addition, patient and
shorter clinical stage I–II had significantly longer DFS (146.15
years) and OS (153.70 years) times compared with those in clinical
stage III (DFS, 111.79 years; and OS, 116.21 years) (P=0.019 and
P=0.001, respectively; Fig. 2B and
C).
To determine the risk factors associated with the
survival, univariate Cox regression analysis was performed for
several potential factors, including age, tumor size, lymph node
status, clinical stage, ER status, PR status and HER2 status,
expression of pAkt, and Beclin 1. The results revealed that pAkt
expression was negatively associated with DFS [hazard ratio (HR),
0.148; 95% confidence interval (CI), 0.051–0.427; P<0.001] and
OS times (HR, 0.331; 95% CI, 0.093–1.173; P=0.087). Furthermore,
clinical stage III was negatively associated with both DFS
[HR=0.293, 95% CI=0.098–0.874, P=0.028] and OS [HR=0.165, 95%
CI=0.048–0.571, P=0.004]. Other factors were not associated with
survival (Table IV). Then,
multivariate Cox regression analysis was conducted, and the results
indicated that pAkt expression was independently associated with
DFS (HR, 0.208; 95% CI, 0.067–0.646; P=0.007) and OS times (HR,
0.246; 95% CI, 0.067–0.911; P=0.036). Additionally, clinical stage
III was an independent risk factor associated with DFS (HR, 0.208;
95% CI, 0.067–0.646; P=0.007) and OS times (HR, 0.134; 95% CI,
0.037–0.482; P=0.002) (Table V).
 | Table IV.Univariate Cox regression analysis of
survival of 90 patients with breast cancer. |
Table IV.
Univariate Cox regression analysis of
survival of 90 patients with breast cancer.
|
| Univariate Cox
regression analysis |
|---|
|
|
|
|---|
|
| DFS | OS |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Beclin 1 (positive
vs. negative) | 0.537
(0.187–1.540) | 0.248 | 0.509
(0.146–1.771) | 0.289 |
| pAkt (positive vs.
negative) | 0.148
(0.051–0.427) | 0.000 | 0.331
(0.093–1.173) | 0.087 |
| Age (≥50 vs. <50
years) | 0.731
(0.229–2.334) | 0.597 | 0.768
(0.198–2.972) | 0.702 |
| Clinical stage
(I+II vs. III) | 0.293
(0.098–0.874) | 0.028 | 0.165
(0.048–0.571) | 0.004 |
| Tumor size (≥5 vs.
<5 cm) | 0.530
(0.119–2.369) | 0.406 | 0.222
(0.071–1.571) | 0.165 |
| ER (positive vs.
negative) | 1.205
(0.418–3.473) | 0.730 | 1.655
(0.479–5.717) | 0.426 |
| PR (positive vs.
negative) | 1.543
(0.535–4.447) | 0.422 | 2.696
(0.697–10.429) | 0.151 |
| HER2 (positive vs.
negative) | 0.444
(0.149–1.328) | 0.146 | 0.593
(0.153–2.294) | 0.449 |
| Lymph node status
(N1-N3 vs. N0) | 0.462
(0.160–1.333) | 0.153 | 0.434
(0.122–1.538) | 0.196 |
 | Table V.Multivariate Cox regression analysis
of survival of 90 patients with breast cancer. |
Table V.
Multivariate Cox regression analysis
of survival of 90 patients with breast cancer.
|
| Multivariate Cox
regression analysis |
|---|
|
|
|
|---|
|
| DFS | OS |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| pAkt (positive vs.
negative) | 0.120
(0.040–0.355) | <0.001 | 0.246
(0.067–0.911) | 0.036 |
| Clinical stage
(I+II vs. III) | 0.208
(0.067–0.646) | 0.007 | 0.134
(0.037–0.482) | 0.002 |
Discussion
Multiple factors have been identified to be
associated with breast cancer carcinogenesis, progression,
metastasis and recurrence; however, the precise underlying
molecular mechanisms remain poorly understood. Therefore, sensitive
and specific prognostic indicators for breast cancer are required
in clinical practice.
Overexpression of the PI3K/Akt signaling pathway
proteins has been identified in several cancer types (20), including breast cancer. Thus, the
present study also detected the activation of the PI3K/Akt pathway
in breast cancer tissues, which was not present in normal
paracancerous tissues. pAkt was detected in 17 (18.9%) patient
samples by immunohistochemical analyses, which was lower compared
with that reported in previous study demonstrating that the
PI3K/Akt pathway is activated in half of these tumors (21,22). The
difference in results obtained may have been due to the different
pathological types or clinical stages studies. Additionally, no
significant association between pAkt expression and any of the
clinicopathological parameter examined was observed in the present
study. Notably, the clinical value of pAkt has been noted in
previous studies on certain cancer types, including ovarian cancer,
while, others, including the present study did not (23). The reason in differences may be due to
the small cohort size in the current study.
The prognostic value of pAkt in invasive ductal
breast cancer was detected next using survival analysis. The
results of the present study revealed that patients with pAkt
expression exhibited significantly shorter DFS times compared with
those negative for pAkt. Cox regression analysis indicated pAkt
expression was an independent risk factor associated with shorter
DFS and OS times, indicating pAkt expression was associated with a
poor prognosis, and may be used as a potential prognostic biomarker
in invasive ductal breast cancer. However, these results oppose
those demonstrated by a previous study by Badve et al
(15), which demonstrated that
nuclear localization of pAkt was associated with a more improved
outcome in patients with breast cancer. Previously, different
expression patterns of nuclear and cytoplasmic pAkt were observed,
and it was speculated that pAkt has different actions depending on
its subcellular localization (24).
Only nuclear localized pAkt has been demonstrated to predict a more
improved prognosis (24). In the
present study, pAkt expression was detected primarily in the
cytoplasm, which may explain why pAkt expression was able to
predict a poor prognosis in patients with breast cancer. In
addition, other reasons may be the due to the different genetic
background and pathological types in the present study versus other
studies. To the best of our knowledge, this is the first study to
identify cytoplasmic pAkt expression as a potential biomarker to
predict poor prognosis in Chinese women with invasive ductal breast
cancer. Considering this, the detection of pAkt expression in
breast cancer tissue may provide additional information on the
prognosis, which may favor early detection of the development of
recurrence and distant metastasis, and allow for successful salvage
treatments for these patients in a timely manner.
Autophagy is a physiological process in eukaryotic
cells, and is essential to maintaining the stability of the
internal environment and adapting to external environment changes.
It has been revealed that the expression changes of
autophagy-associated proteins may contribute to the development or
progression of cancer (25). Beclin 1
is well-known as a reliable autophagy-related protein in various
cancer types, and is involved in the signaling pathway that
activates autophagy and in the initial step of autophagosome
formation, leading to tumor development, metastasis and recurrence
(26). In the current study, Beclin 1
expression was detected in 33 (36.7%) breast cancer samples, but
not detected in any normal samples, indicating the upregulation of
autophagy in breast cancer. While other studies have demonstrated
that Beclin 1 expression was frequently decreased in breast cancer
compared with that in normal cells, decreased Beclin 1 was also
observed in other cancer types, including hepatocellular carcinoma
and lung cancer (26–28).
An in vivo study has demonstrated that Beclin
1 knockdown provides an oncogenic stimulus, causing malignant
transformation and spontaneous tumors (29). However, other studies have reported
that autophagy is upregulated in tumors, including
gastrointestinal, pancreatic and gallbladder cancer (30–32). In
addition, the results in the present study revealed that Beclin 1
expression was not significantly associated with any
clinicopathological parameter, including age, tumor size, clinical
stage, lymph node metastasis, ER, PR and HER2 status in patients
with breast cancer. Furthermore, no significant differences in DFS
and OS rates were noted between the Beclin 1-positive and -negative
groups. Cox regression analysis identified no significant
association between Beclin 1 expression and clinical prognosis.
Similar results have been observed in other cancer types, including
cervical and lung cancer (33).
Conversely, other studies have revealed that Beclin 1 exhibits
significant negative associations with clinical stage, lymph node
metastasis and the prognosis of cervical cancer (34), as well as pancreatic cancer (31), gastric carcinoma (35), esophageal squamous cell carcinoma
(36), and hepatocellular carcinoma
(37).
These controversial findings may be explained by the
biphasic function of autophagy during carcinogenesis. Autophagy is
implicated in different functions in diverse tumors and different
phases of tumor development. For instance, autophagy may suppress
tumorigenesis in the early phase of tumor development; however,
autophagy may be a key tumor cell survival mechanism in response to
microenvironmental stress in the late phase of tumor development.
Certain studies have revealed that autophagy enhances the survival
of tumor cells, in vitro and in vivo, under the
condition of metabolic stress (38,39),
while, other studies indicated that autophagy inhibits tumor cells,
and autophagy defects were associated with increasing
carcinogenesis (40,41). Previous studies demonstrated that
autophagy represents a key survival mechanism in which tumor cells
respond to microenvironmental stress during cancer development and
promote tumor cell survival (42).
Based on the results of the present study, we propose that
autophagy is upregulated in invasive ductal breast cancer.
Although, the small number of cancer tissue samples included in the
present may limit the interpretation of these results. Therefore,
large sample studies should be conducted to confirm the role of
autophagy in invasive ductal breast cancer.
Additionally, the present study demonstrated that
clinical stage III was an independent risk factor associated with
shorter DFS and OS times, consistent with previous findings
(43). There were certain limitations
in the present study. First, the immunohistochemistry method on
tissue samples is a semi-quantitative method, thus not highly
informative. However, the primary aim of this study was to identify
whether pAkt and Beclin 1 expression in breast cancer tissue were
potential prognostic biomarkers for breast cancer. Furthermore, the
expression of these genes in tissue sample may be more objective
and useful compared with the detection in tumor cells. Second, the
number of cases, particularly the mortality cases in the study was
limited. In the future studies, a larger patient cohort is required
to further evaluate and validate these promising findings.
To conclude, the results of the present study
indicated that pAkt expression was independently associated with a
poor outcome in patients with invasive ductal breast cancer,
suggesting the use of pAkt as a potential prognostic biomarker. The
detection of pAkt expression in breast cancer tissue may provide
useful information on the prognosis, which may favor early
detection of the development of recurrence and distant metastasis,
and allow for successful salvage treatments for these patients in a
timely manner.
Acknowledgements
The present study was partially supported by the
Youth Foundation of Shanghai Municipal Health Bureau (grant no.
2012y144).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li CI, Anderson BO, Daling JR and Moe RE:
Trends in incidence rates of invasive lobular and ductal breast
carcinoma. JAMA. 289:1421–1424. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kohler BA, Ward E, McCarthy BJ, Schymura
MJ, Ries LA, Eheman C, Jemal A, Anderson RN, Ajani UA and Edwards
BK: Annual report to the nation on the status of cancer, 1975–2007,
featuring tumors of the brain and other nervous system. J Nat
Cancer Inst. 103:714–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang YC, Wei LJ, Liu JT, Li SX and Wang
QS: Comparison of cancer incidence between China and the USA.
Cancer Biol Med. 9:128–132. 2012.PubMed/NCBI
|
|
5
|
Linos E, Spanos D, Rosner BA, Linos K,
Hesketh T, Qu JD, Gao YT, Zheng W and Colditz GA: Effects of
reproductive and demographic changes on breast cancer incidence in
China: A modeling analysis. J Natl Cancer Inst. 100:1352–1360.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McPherson K, Steel CM and Dixon JM: ABC of
breast diseases. Breast cancer-epidemiology, risk factors, and
genetics. BJM. 321:624–628. 2000. View Article : Google Scholar
|
|
7
|
Chia KS, Reilly M, Tan CS, Lee J, Pawitan
Y, Adami HO, Hall P and Mow B: Profound changes in breast cancer
incidence may reflect changes into a Westernized lifestyle: A
comparative population-based study in Singapore and Sweden. Int J
Cancer. 113:302–306. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pérez-Tenorio G and Stål O; Southeast
Sweden Breast Cancer Group, : Activation of AKT/PKB in breast
cancer predicts a worse outcome among endocrine treated patients.
Br J Cancer. 86:540–545. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Roudier E, Mistafa O and Stenius U:
Statins induce mammalian target of rapamycin mTOR)-mediated
inhibition of Akt signaling and sensitize p53-deficient cells to
cytostatic drugs. Mol Cancer Ther. 5:2706–2715. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mondesire WH, Jian W, Zhang H, Ensor J,
Hung MC, Mills GB and Meric-Bernstam F: Targeting mammalian target
of rapamycin synergistically enhances chemotherapy-induced
cytotoxicity in breast cancer cells. Clin Cancer Res. 10:7031–7042.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vestey SB, Sen C, Calder CJ, Perks CM,
Pignatelli M and Winters ZE: Activated Akt expression in breast
cancer: Correlation with p53, Hdm2 and patient outcome. Eur J
Cancer. 41:1017–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tokunaga E, Kimura Y, Oki E, Ueda N,
Futatsugi M, Mashino K, Yamamoto M, Ikebe M, Kakeji Y, Baba H and
Maehara Y: Akt is frequently activated in HER2/neu-positive breast
cancers and associated with poor prognosis among hormone-treated
patients. Int J Cancer. 118:284–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Benesch C, Schneider C, Voelker HU, Kapp
M, Caffier H, Krockenberger M, Dietl J, Kammerer U and Schmidt M:
The clinicopathological andprognostic relevance of pyruvate kinase
M2 and pAkt expression in breast cancer. Anticancer Res.
30:1689–1694. 2010.PubMed/NCBI
|
|
15
|
Badve S, Collins NR, Bhat-Nakshatri P,
Turbin D, Leung S, Thorat M, Dunn SE, Geistlinger TR, Carroll JS,
Brown M, et al: Subcellular localization ofactivated AKT in
estrogen receptor- and progesterone receptor-expressing
breastcancers: Potential clinical implications. Am J Pathol.
176:2139–2149. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Levine B and Kroemer G: Autophagy in the
pathogenesis of disease. Cell. 132:27–42. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mizushima N, Levine B, Cuervo AM and
Klionsky DJ: Autophagy fights disease through cellular
self-digestion. Nature. 451:1069–1075. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishimura K, Semba S, Aoyagi K, Sasaki H
and Yokozaki H: Mesenchymal stem cells provide an advantageous
tumor microenvironment for the restoration of cancer stem cells.
Pathobiology. 79:290–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Singletary SE, Allred C, Ashley P, Bassett
LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, et
al: Staging system for breast cancer: Revisions for the 6th adition
of the AJCC cancer staging manual. Surg Clin North Am. 83:803–819.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Markman B, Atzori F, Pérez-García J,
Tabernero J and Baselga J: Status of PI3K inhibition and biomarker
development in cancer therapeutics. Ann Oncol. 21:683–691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Altomare DA, Wang HQ, Skele KL, De Rienzo
A, Klein-Szanto AJ, Godwin AK and Testa JR: AKT and mTOR
phosphorylation is frequently detectedin ovarian cancer and can be
targeted to disrupt ovarian tumor cell growth. Oncogene.
23:5853–5857. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Verhaak RG, Tamayo P, Yang JY, Hubbard D,
Zhang H, Creighton CJ, Fereday S, Lawrence M, Carter SL, Mermel CH,
et al: Prognostically relevant gene signatures of high-grade
serouso varian carcinoma. J Clin Invest. 123:517–525.
2013.PubMed/NCBI
|
|
23
|
Woenckhaus J, Steger K, Sturm K, Münstedt
K, Franke FE and Fenic I: Prognostic value of PIK3 CA and
phosphorylated AKT expression in ovarian cancer. Virchows Arch.
450:387–395. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Azim HA, Kassem L, Treilleux I, Wang Q, El
Enein MA, Anis SE and Bachelot T: Analysis of PI3K/mTOR pathway
biomarkers and their prognostic value in women with hormone
receptor-positive, HER2-negative early breast cancer. Transl Oncol.
9:114–123. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen J, Xi B, Zhao Y, Yu Y, Zhang J and
Wang C: High-mobility group protein B1 (HMGB1) is a novel biomarker
for human ovarian cancer. Gynecol Oncol. 126:109–117. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jiang ZF, Shao LJ, Wang WM, Yan XB and Liu
RY: Decreased expression of Beclin-1 and LC3 in human lung cancer.
Mol Biol Rep. 39:259–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zarzynska JM: The importance of autophagy
regulation in breast cancer development and treatment. Biomed Res
Int. 2014:7103452014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding ZB, Shi YH, Zhou J, Qiu SJ, Xu Y, Dai
Z, Shi GM, Wang XY, Ke AW, Wu B and Fan J: Association of autophagy
defect with a malignant phenotype and poor prognosis of
hepatocellular carcinoma. Cancer Res. 68:9167–9175. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dalby KN, Tekedereli I, Lopez-Berestein G
and Ozpolat B: Targeting the prodeath and prosurvival functions of
autophagy as novel therapeutic strategies in cancer. Autophagy.
6:322–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yoshioka A, Miyata H, Doki Y, Yamasaki M,
Sohma I, Gotoh K, Takiguchi S, Fujiwara Y, Uchiyama Y and Monden M:
LC3, an autophagosome marker, is highly expressed in
gastrointestinal cancers. Int J Oncol. 33:461–468. 2008.PubMed/NCBI
|
|
31
|
Fujii S, Mitsunaga S, Yamazaki M, Hasebe
T, Ishii G, Kojima M, Kinoshita T, Ueno T, Esumi H and Ochiai A:
Autophagy is activated in pancreatic cancer cells and correlates
with poor patient outcome. Cancer Sci. 99:1813–1819.
2008.PubMed/NCBI
|
|
32
|
Park JY, Kim HS, Cho H, Kim NC, Chae KH,
Park WS and Kim YW: Clinicopathologic correlation of
autophagy-related Beclin-1 expression in gallbladder cancer.
Hepatogastroenterology. 61:1494–1500. 2014.PubMed/NCBI
|
|
33
|
Jiang ZF, Shao LJ, Wang WM, Yan XB and Liu
RY: Decreased expression of Beclin-1 and LC3 in human lung cancer.
Mol Biol Rep. 39:259–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng HY, Zhang YN, Wu QL, Sun XM, Sun JR
and Huang X: Expression of beclin 1, an autophagy-related protein,
in human cervical carcinoma and its clinical significance. Eur J
Gynaecol Oncol. 33:15–20. 2012.PubMed/NCBI
|
|
35
|
Chen YB, Hou JH, Feng XY, Chen S, Zhou ZW,
Zhang XS and Cai MY: Decreased expression of Beclin 1 correlates
with a metastatic phenotypic feature and adverse prognosis of
gastric carcinomas. J Surg Oncol. 105:542–547. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Y, Lu Y, Lu C and Zhang L: Beclin-1
expression is a predictor of clinical outcome in patients with
esophageal squamous cell carcinoma and correlated to
hypoxia-inducible factor HIF)-1alpha expression. Pathol Oncol Res.
15:487–493. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Osman NA, Abd El-Rehim DM and Kamal IM:
Defective Beclin-1 and elevated hypoxia-inducible factor (HIF)-1α
expression are closely linked to tumorigenesis, differentiation,
and progression of hepatocellular carcinoma. Tumour Biol.
36:4293–4299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mathew R, Karantza-Wadsworth V and White
E: Role of autophagy in cancer. Nat Rev Cancer. 7:961–967. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Degenhardt K, Mathew R, Beaudoin B, Bray
K, Anderson D, Chen G, Mukherjee C, Shi Y, Gélinas C, Fan Y, et al:
Autophagy promotes tumor cell survival and restricts necrosis,
inflammation, and tumorigenesis. Cancer Cell. 10:51–64. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lorin S, Hamaï A, Mehrpour M and Codogno
P: Autophagy regulation and its role in cancer. Semin Cancer Biol.
23:361–379. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choi AM, Ryter SW and Levine B: Autophagy
in human health and disease. N Engl J Med. 368:651–662. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liang XH, Jackson S, Seaman M, Brown K,
Kempkes B, Hibshoosh H and Levine B: Induction of autophagy and
inhibition of tumorigenesis by beclin 1. Nature. 402:672–676. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Zhang Y, Xiu HQ, et al: Clinical
features of TNM staging of triple negative breast cancer and risk
factors affecting its prognosis. Chin J Breast Dis. 6:30–35.
2012.
|