Introduction
Gastric cancer is one of the leading causes of
cancer-associated mortality in the developing world. Accumulating
evidence indicates that dendritic cells (DCs) are important in
tumor immunology, including that of gastric cancer (1). Certain DC-associated inflammatory
factors are useful in predicting the prognosis of gastric cancer
(2). For example, CD83+ DC
cells in primary lesions and regional lymph nodes are inversely
correlated with the prognosis of gastric cancer (3), and peripheral HLA-G-expressing DC-10
cells are elevated in patients with gastric cancer (4). In addition, the infiltration of certain
DC subsets in gastric cancer tissue has been shown to correlate
with 5-year survival rate (5,6). Several clinical trials have used
DC-based anti-gastric cancer therapy strategies (7). Human DC cells can be divided into four
subsets according to the expression of specific markers:
CD303+ plasmacytoid DCs (pDCs), CD1c+
classical myeloid DCs (cDCs/mDCs), CD141+ classical
myeloid DCs (cDCs/mDCs) and inflammatory DCs (8). The improved characterization of
different DC subsets is likely to provide novel avenues for their
tumor therapeutic regulation.
pDCs are a multifunctional subset of bone
marrow-derived immune cells, which produce interferons (IFNs) and
act as antigen-presenting cells (9).
High pDC infiltration has been observed in several types of cancer,
including melanoma, head and neck cancer, breast cancer, ovarian
cancer and prostate cancer, with infiltrated pDCs being involved in
tumor promotion and inhibition, which may depend on their
maturity/gene expression (9,10). In addition, peripheral pDCs have been
reported to show prognostic relevance in certain types of cancer.
For example, patients with late-stage breast cancer had
significantly lower levels of circulating pDCs (11) and patients suffering from prostate
cancer showed a marked reduction in circulating pDCs (12). However, there are few reports
concerning circulating pDCs in gastric cancer.
The aim of the present study was to investigate the
presence and distribution of circulating pDCs and CD1c+
DCs in patients with gastric cancer. The results showed that
patients with gastric cancer had increased numbers of circulating
pDCs and CD1c+ DCs. In addition, there was a trend
toward elevated circulating pDCs with advanced cancer stage and
lymph node metastasis.
Materials and methods
Human subjects
A total of 32 patients with gastric cancer were
recruited from Zhongnan Hospital of Wuhan University (Wuhan,
China). The patients had no other tumors, trauma, infectious
diseases or autoimmune diseases. They had not received
radiotherapy, chemotherapy or immunotherapy. In addition, 35
healthy volunteers were recruited as controls. The age range of
those recruited was 43–78 years, with an average age of 56 years.
Peripheral blood samples were collected from the two groups. All
participants signed informed consent and the study was approved by
the Ethical Committee of Wuhan University (permit no.
2010-10007).
Peripheral DC staining and subset
analysis
The fresh heparinized blood samples were processed
within 2 h following collection according to the protocol of the
human blood dendritic cell enumeration kit (Miltenyi Biotec, Inc.,
Auburn CA, USA). Briefly, the procedure was as follows: An aliquot
(300 µl) of the blood sample was stained with 20 µl anti-BDCA
cocktail and PE-Cy7-conjugated anti-PD-L1 or isotype control.
Following incubation with dead cell detector and red blood cell
lysis solution, the cells were washed and fixed for subsequent flow
cytometric analysis (BD FACSAria™ III flow cytometer; BD
Biosciences, Franklin Lakes, NJ, USA). A total of 105
events in the leukocyte gate were collected.
Absolute enumeration of periphery
leukocytes and DC subsets
The absolute number of leukocytes was determined by
a hemocytometer (XT-1800i; Sysmex Europe, Norderstedt, Germany).
The absolute number of each DC subset per ml of blood was
calculated as follows: percentage of DC subset × number of
leukocytes per ml blood.
Statistical analysis
All values are expressed as the mean ± standard
derivation. Student's t-test was used to compare two groups and a
one-way analysis of variance (ANOVA) followed by Tukey's post-hoc
test was used to compare multiple groups. P<0.05 was considered
to indicate a statistically significant difference. The software
used was Graphpad Prism version 5 (GraphPad Software, Inc., La
Jolla, CA, USA).
Results
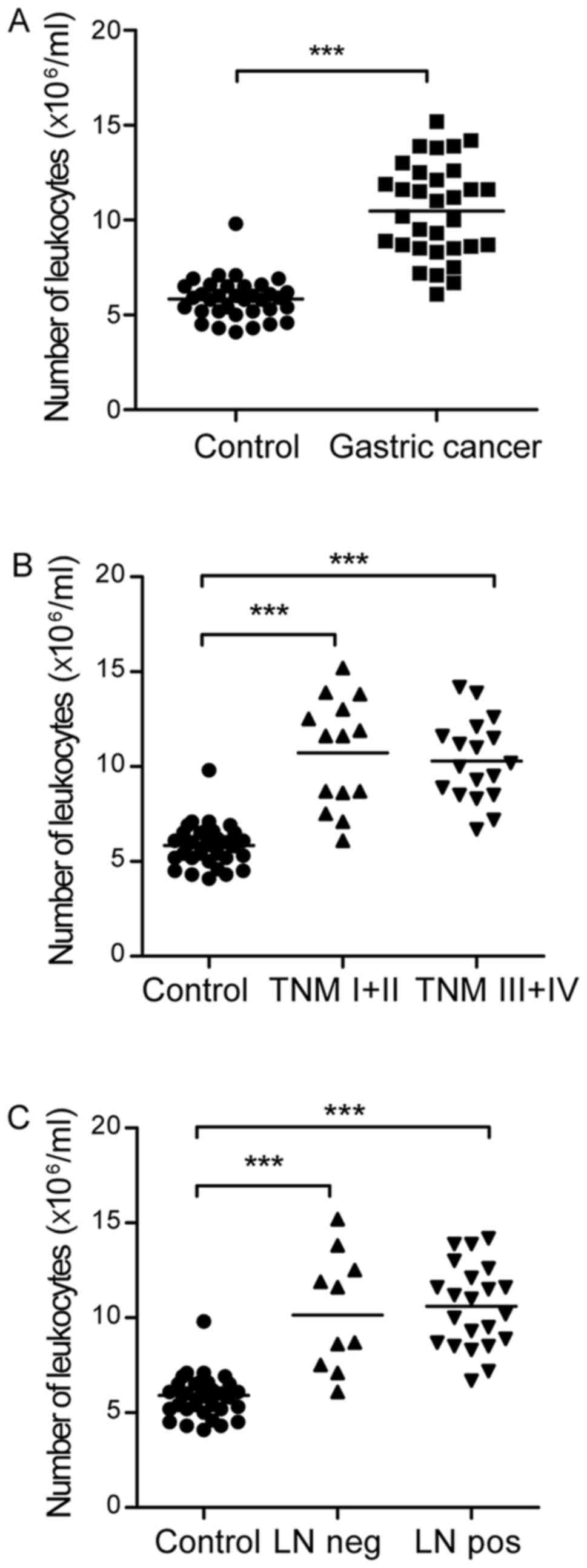
Number of peripheral leukocytes is
increased in patients with gastric cancer
In order to calculate the absolute number of DC
subsets, the number of peripheral leukocytes was first determined.
It was found that there were a significantly increased number of
peripheral leukocytes in the patients with gastric cancer, compared
with that in the healthy controls (10.48±2.46 vs.
5.48±1.08×106/ml blood; Fig.
1A). There was no significant difference in the number of
leukocytic cells between the tumor-node-metastasis (TNM) I+II and
TNM III+IV groups (10.73±2.89 vs. 10.29±2.14×106/ml
blood; Fig. 1B) or the lymph node
negative and lymph node metastasis groups (10.30±3.10 vs.
10.56±2.19×106/ml blood; Fig.
1C) in patients with gastric cancer. The clinical and
pathological characteristics of the patients with gastric cancer
are shown in Table I.
 | Table I.Clinical and pathological
characteristics of patients with gastric cancer. |
Table I.
Clinical and pathological
characteristics of patients with gastric cancer.
| Characteristic | Subcategory | Number |
|---|
| Age (years) | >60 | 17 |
|
| ≤60 | 15 |
| Sex | Male | 24 |
|
| Female | 8 |
| TNM stage | I | 5 |
|
| II | 9 |
|
| III | 9 |
|
| IV | 9 |
| Primary tumor | T1 | 2 |
|
| T2 | 9 |
|
| T3 | 12 |
|
| T4 | 9 |
| Lymph node
metastasis | Negative | 10 |
|
| Positive | 22 |
| Distant
metastasis | Negative | 26 |
|
| Positive | 6 |
| Histology | Adenocarcinoma | 25 |
|
| Signet ring cell
carcinoma | 7 |
Peripheral pDCs and mDC1s are elevated
in patients with gastric cancer
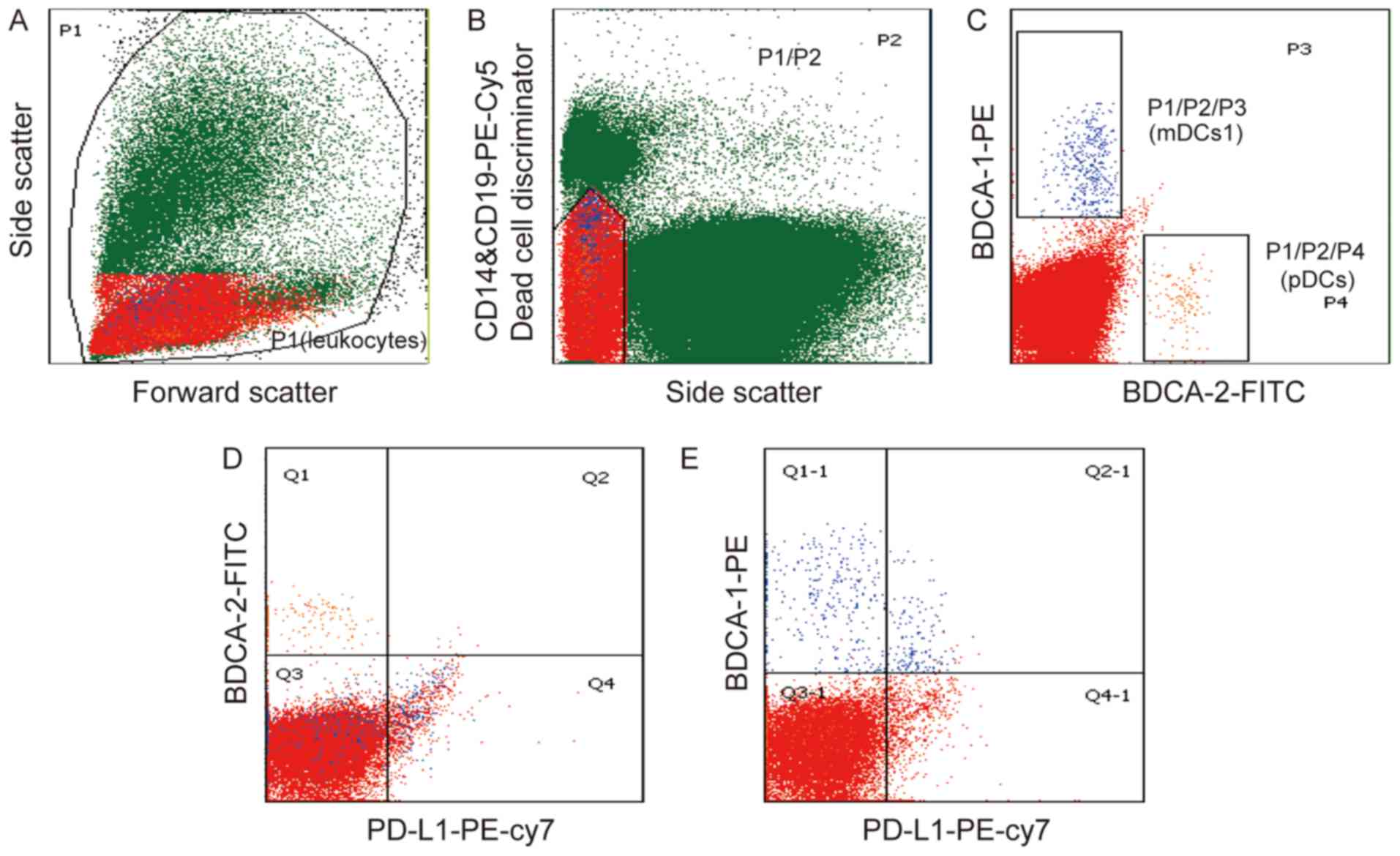
To investigate the role of DCs in gastric cancer,
the present study evaluated two DC subsets using flow cytometric
analysis and the gating strategy, as shown in Fig. 2. The pDCs were identified as
SSClow/−CD14low/−CD19low/−BDCA-2+
(Fig. 2A-C), whereas the mDC1s were
identified as
SSClow/−CD14low/−CD19low/−BDCA-1+
(Fig. 2A-C). The expression of PD-L1
in the pDCs and mDC1s in gastric cancer was also examined. It was
found that the majority of pDCs did not express PD-L1 (Fig. 2D), whereas the mDC1s population showed
partial expression of PD-L1 (Fig.
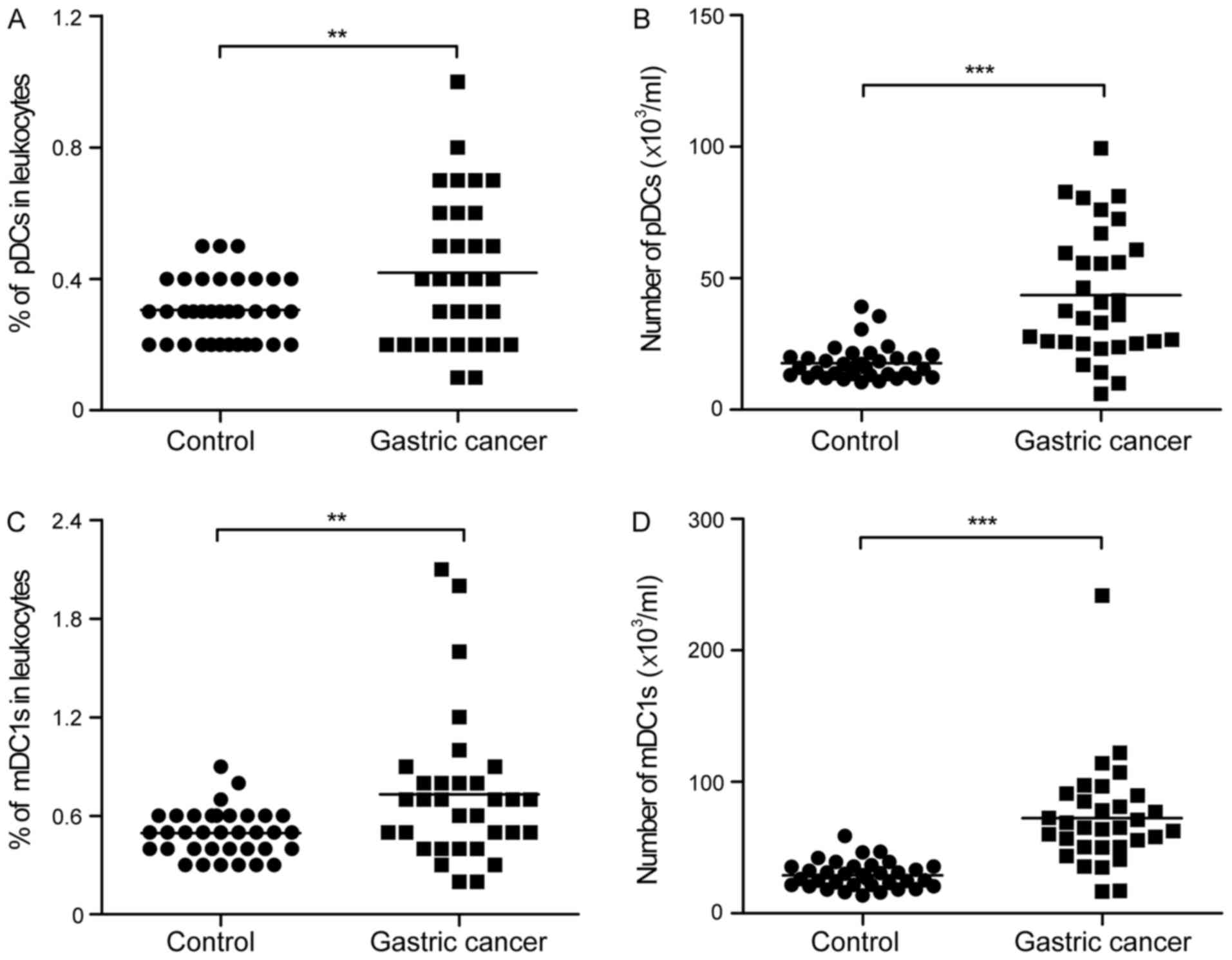
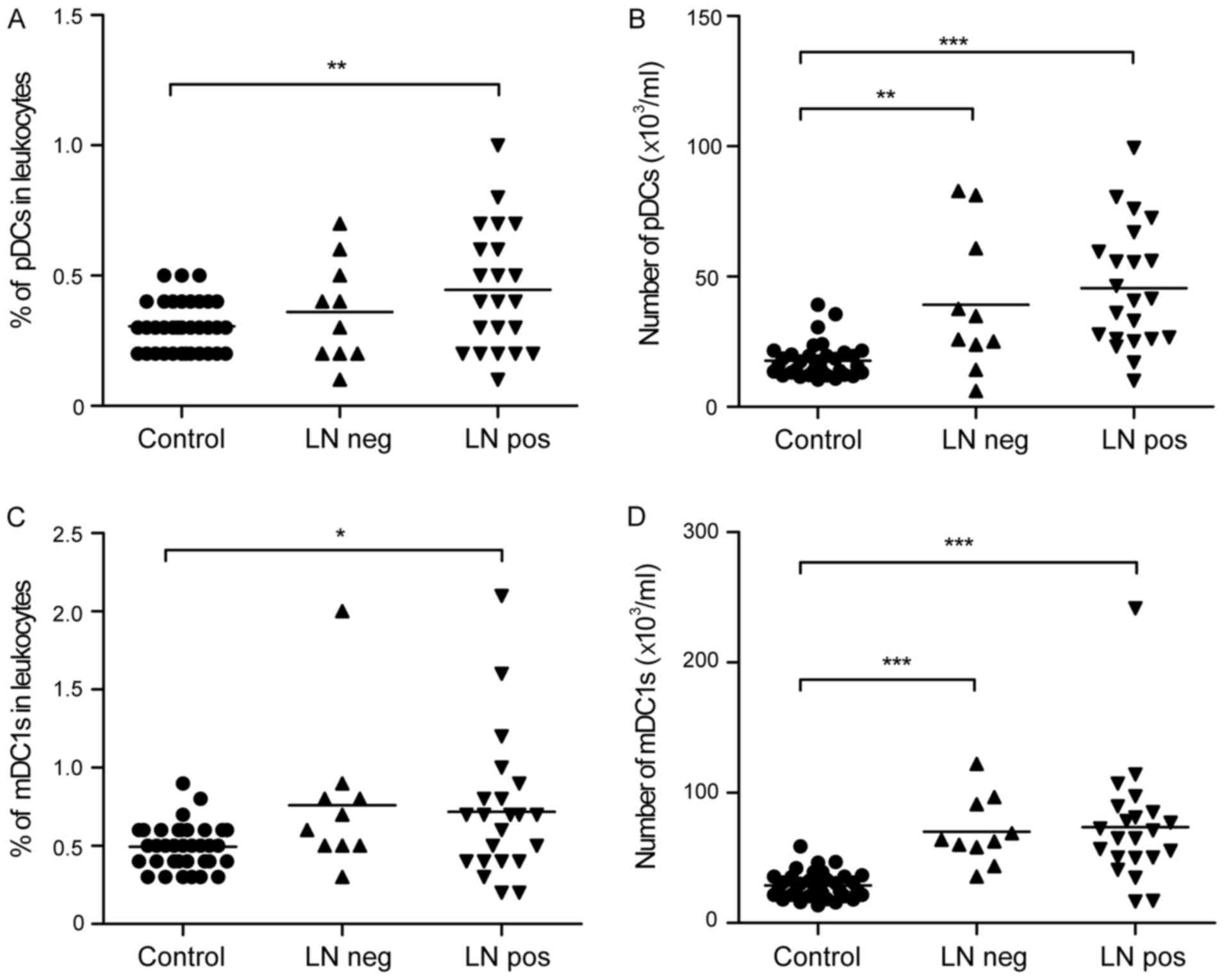
2E). Of note, there was a significant increase in the
percentage and number of pDCs in the peripheral leukocytes from the
patients with gastric cancer, compared with those from the healthy
controls (0.42±0.23 vs. 0.31±0.10%; 43.57±24.25 vs.
17.72±6.64×103/ml; Fig. 3A and
B). Similarly, the percentage and number of mDC1s was
significantly higher in the patients with gastric cancer, compared
with that in the healthy controls (0.73±0.45 vs. 0.49±0.14%;
72.49±39.99 vs. 28.91±10.10×103/ml; Fig. 3C and D).
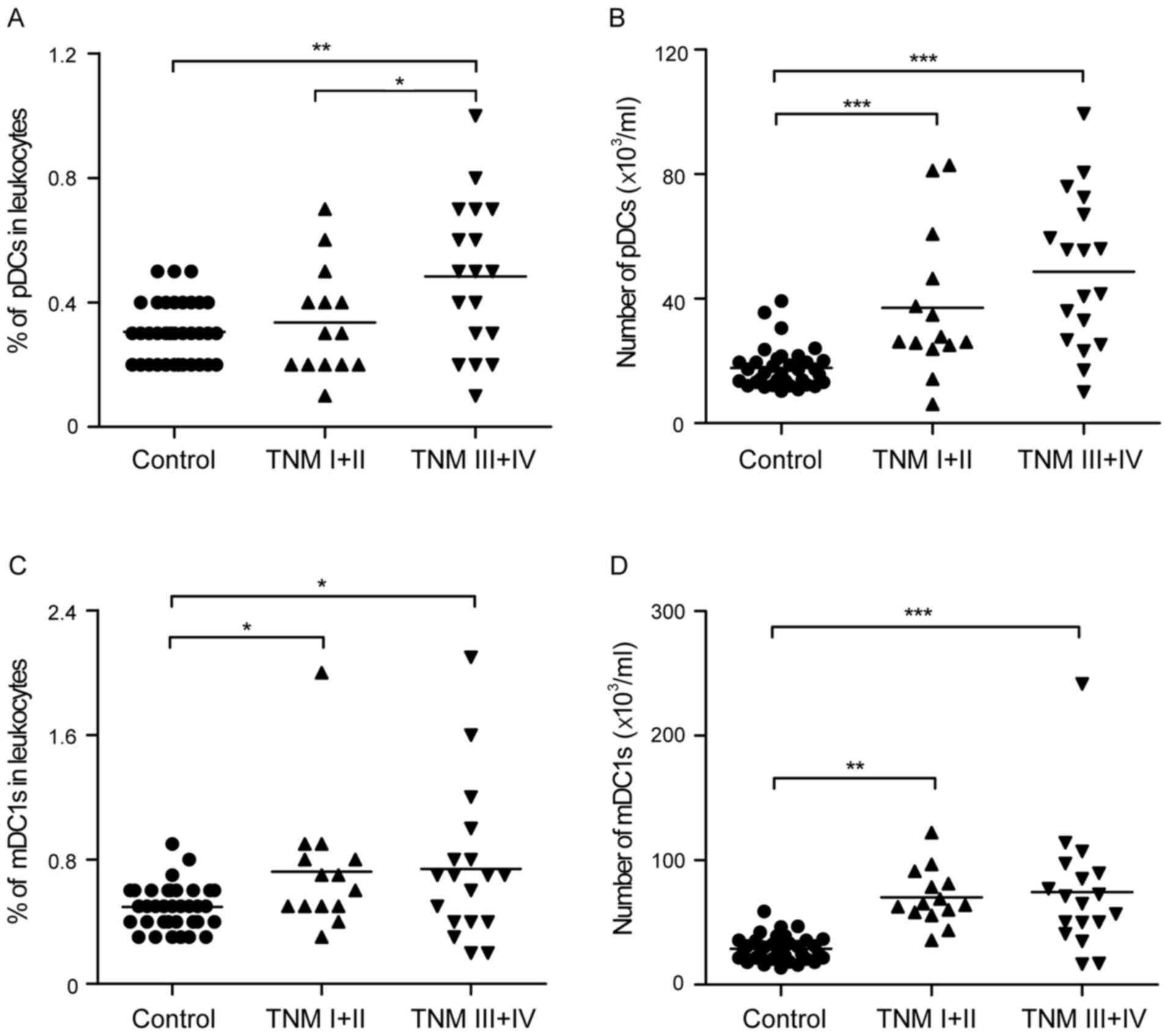
Enrichment of peripheral pDCs in
patients with gastric cancer at advanced stages
The present study further analyzed the peripheral
pDCs and mDC1s in patients with different stages of gastric cancer.
Notably, an increase in peripheral pDCs was found as follows:
Healthy controls <TNM I+II <TNM III+IV groups (0.31±0.10 vs.
0.34±0.17 vs. 0.48±0.24%, respectively; and 17.72±6.64 vs.
37.02±23.13 vs. 48.66±24.51×103/ml, respectively;
Fig. 4A and B). Although certain
trends did not show statistical significance, the percentage and
absolute number of pDCs was significantly higher in the TNM III+IV
group, compared with that in the healthy controls. In addition,
there were significantly elevated peripheral mDC1 cell percentages
(0.72±0.41, vs. 0.74±0.49%; Fig. 4C)
and mDC1 cell numbers (70.16±22.37, vs.
74.29±50.25×103/ml; Fig.
4D) in the TNM I+II and TNM III+IV groups, compared with the
healthy controls, respectively.
Enrichment of peripheral pDCs in
patients with gastric cancer with lymph node metastasis
To further investigate the changes of pDCs and mDC1s
during tumor invasion, the present study analyzed the peripheral
pDCs and mDC1s of patients with different lymph node metastasis
status. It was observed that peripheral pDCs increased as follows:
Healthy controls <lymph node negative group <lymph node
metastasis group in terms of the percentage (0.31±0.10 vs.
0.36±0.20 vs. 0.45±0.24%; Fig. 5A)
and number (17.72±6.64 vs. 39.20±26.86 vs.
45.55±23.36×103/ml; Fig.
5B) of pDCs. Certain trends were not statistically significant,
however, the percentage and absolute number of pDCs were
significantly higher in the lymph node metastasis group, compared
with those in the healthy controls. No significant differences were
found in peripheral mDC1 cell percentages (0.72±0.45 vs.
0.76±0.47%; Fig. 5C) or mDC1 cell
numbers (73.52±45.47 vs. 70.22±25.99×103/ml; Fig. 5D) between the lymph node metastasis
and negative groups.
Discussion
The present study indicated that patients with
gastric cancer had markedly higher numbers of peripheral pDCs and
mDC1s. pDCs were identified as
SSClow/−CD14low/−CD19low/−BDCA-2+.
Huang et al reported pDCs as Lin−
HLA−DR+CD11c−CD123high
(13). Although using different
surface markers to detect pDCs, the results of these two studies
showed a higher proportion of circulating pDCs in patients with
gastric cancer, compared with that in healthy controls. Defining
circulating pDCs as positive prognostic indicators for gastric
cancer is likely to enable easier prediction of disease course
without biopsies and also provide useful information on the control
of cancer by the immune system.
It has been shown that the pDCs infiltrated in the
tumor microenvironment are mainly immature, and appear to be
predominantly immunosuppressive/tolerogenic (14). The increased circulating pDCs in
patients with gastric cancer may also have an important
immunosuppressive role. However, the data obtained in the present
study showed the circulating pDCs in patients with gastric cancer
did not express PD-L1, which is important in the immunosuppression
of gastric cancer (15). Further
investigations involving sorting of the pDCs and analysis of their
inflammatory cytokine profile, including IFNs and interleukin-10,
and function in vitro are likely to provide additional
clues. In addition, it has been reported that properly activated
pDCs can trigger an antitumor response (16,17),
therefore, modifying circulating pDCs may be a potentially useful
gastric cancer therapeutic strategy.
The present study provided evidence that circulating
pDCs were positively correlated with advanced stages and lymph node
metastasis in gastric cancer. Although the increase of pDCs in
advanced stages and the lymph node metastasis of gastric cancer
were not statistically significant, the trends were observed,
compared with those of mDC1s. It has been reported that pDCs may
have a pathological role in metastasis. pDCs have been shown to be
accumulated in positive (with metastasis) sentinel lymph nodes in
melanoma (18). In mouse models of
breast cancer bone metastasis, the depletion of pDCs inhibited
tumor growth and prevented metastasis (19). Therefore, the data in the present
study provide a rationale for investigating pDCs in the metastasis
of gastric cancer.
In conclusion, the present study suggested that
circulating pDCs can be a positive prognostic indicator in patients
with gastric cancer of different stages. The future
characterization of pDCs is likely to shed light on the systemic
understanding of pDC immunity in the development of gastric
cancer.
Acknowledgements
This study was supported by grants awarded to Dr.
Zan Tong. The authors would like to thank Jieyun Wu of Zhongnan
Hospital for collecting blood samples and information from the
patients with gastric cancer and healthy controls.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Veglia F and Gabrilovich DI: Dendritic
cells in cancer: The role revisited. Curr Opin Immunol. 45:43–51.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chang WJ, Du Y, Zhao X, Ma LY and Cao GW:
Inflammation-related factors predicting prognosis of gastric
cancer. World J Gastroenterol. 20:4586–4596. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kashimura S, Saze Z, Terashima M, Soeta N,
Ohtani S, Osuka F, Kogure M and Gotoh M: CD83(+) dendritic cells
and Foxp3(+) regulatory T cells in primary lesions and regional
lymph nodes are inversely correlated with prognosis of gastric
cancer. Gastric Cancer. 15:144–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu DP, Shi WW, Zhang TT, Lv HY, Li JB, Lin
A and Yan WH: Elevation of HLA-G-expressing DC-10 cells in patients
with gastric cancer. Hum Immunol. 77:800–804. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsukayama S, Omura K, Yoshida K, Tanaka Y
and Watanabe G: Prognostic value of CD83-positive mature dendritic
cells and their relation to vascular endothelial growth factor in
advanced human gastric cancer. Oncol Rep. 14:369–375.
2005.PubMed/NCBI
|
|
6
|
Ishigami S, Natsugoe S, Tokuda K, Nakajo
A, Xiangming C, Iwashige H, Aridome K, Hokita S and Aikou T:
Clinical impact of intratumoral natural killer cell and dendritic
cell infiltration in gastric cancer. Cancer Lett. 159:103–108.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Niccolai E, Taddei A, Prisco D and Amedei
A: Gastric cancer and the epoch of immunotherapy approaches. World
J Gastroenterol. 21:5778–5793. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coutant F and Miossec P: Altered dendritic
cell functions in autoimmune diseases: Distinct and overlapping
profiles. Nat Rev Rheumatol. 12:703–715. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Swiecki M and Colonna M: The multifaceted
biology of plasmacytoid dendritic cells. Nat Rev Immunol.
15:471–485. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lombardi VC, Khaiboullina SF and Rizvanov
AA: Plasmacytoid dendritic cells, a role in neoplastic prevention
and progression. Eur J Clin Invest. 45 Suppl 1:S1–S8. 2015.
View Article : Google Scholar
|
|
11
|
Kini Bailur J, Gueckel B and Pawelec G:
Prognostic impact of high levels of circulating plasmacytoid
dendritic cells in breast cancer. J Transl Med. 14:1512016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sciarra A, Lichtner M, Autran GA,
Mastroianni C, Rossi R, Mengoni F, Cristini C, Gentilucci A, Vullo
V and Di Silverio F: Characterization of circulating blood
dendritic cell subsets DC123+ (lymphoid) and
DC11C+ (myeloid) in prostate adenocarcinoma patients.
Prostate. 67:1–7. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang XM, Liu XS, Lin XK, Yu H, Sun JY,
Liu XK, Chen C, Jin HL, Zhang GE, Shi XX, et al: Role of
plasmacytoid dendritic cells and inducible costimulator-positive
regulatory T cells in the immunosuppression microenvironment of
gastric cancer. Cancer Sci. 105:150–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Demoulin S, Herfs M, Delvenne P and Hubert
P: Tumor microenvironment converts plasmacytoid dendritic cells
into immunosuppressive/tolerogenic cells: Insight into the
molecular mechanisms. J Leukoc Biol. 93:343–352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tamura T, Ohira M, Tanaka H, Muguruma K,
Toyokawa T, Kubo N, Sakurai K, Amano R, Kimura K, Shibutani M, et
al: Programmed death-1 Ligand-1 (PDL1) expression is associated
with the prognosis of patients with stage II/III gastric cancer.
Anticancer Res. 35:5369–5376. 2015.PubMed/NCBI
|
|
16
|
Kalb ML, Glaser A, Stary G, Koszik F and
Stingl G: TRAIL(+) human plasmacytoid dendritic cells kill tumor
cells in vitro: Mechanisms of imiquimod- and IFN-α-mediated
antitumor reactivity. J Immunol. 188:1583–1591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tel J, Smits EL, Anguille S, Joshi RN,
Figdor CG and de Vries IJ: Human plasmacytoid dendritic cells are
equipped with antigen-presenting and tumoricidal capacities. Blood.
120:3936–3944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gerlini G, Urso C, Mariotti G, Di Gennaro
P, Palli D, Brandani P, Salvadori A, Pimpinelli N, Reali UM and
Borgognoni L: Plasmacytoid dendritic cells represent a major
dendritic cell subset in sentinel lymph nodes of melanoma patients
and accumulate in metastatic nodes. Clin Immunol. 125:184–193.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sawant A, Hensel JA, Chanda D, Harris BA,
Siegal GP, Maheshwari A and Ponnazhagan S: Depletion of
plasmacytoid dendritic cells inhibits tumor growth and prevents
bone metastasis of breast cancer cells. J Immunol. 189:4258–4265.
2012. View Article : Google Scholar : PubMed/NCBI
|