Introduction
Gastric cancer (GC) is the fifth most common
malignancy in the world and the third in cancer-related death
(1). One of the reasons behind high
mortality is that patients at the time of diagnosis are usually at
an advanced tumor stage, hence the need for new, more effective
diagnostic and therapeutic approaches is urgent. Also, despite
different therapies (surgery, chemotherapy, targeted therapy) the
prognosis for patients is still poor (2). Among others, the reason for this poor
prognosis may lie in the biological heterogeneity of cancer cells,
comprising their morphology and, more importantly, their
functionality (3). Established tumor
cancer cell lines are a very useful tool for studying cancer cell
biology, heterogeneity or sensitivity to different drugs and
therapies. A comprehensive collection of well-described cell lines
should reflect the diversity of GC and provide adequate models for
its study. Up to date, just a few GC cell lines are available, most
of them being established from Asian patients (4–6), where
this type of cancer is most prevalent.
In the present study we characterize three, new cell
lines established from ascitic fluids of Caucasian patients with
GC. The karyotype (by conventional G-banding) of these cells, their
phenotype [including tumor-associated antigens (TAA) such as c-Met,
Her-2/neu, Tag72, EMA, Epithelial Antigen and EMMPRIN], mRNA
expression profile (Her-2/neu, MAGE-1) and growth in
immunodeficient mice are presented. In addition, the expression and
activity of aldehyde dehydrogenase (ALDH) isoforms relevant to
metastatic potential of cancer cells are documented (7–11).
In conclusion, the presented data widens the current
knowledge on GC cells and provides a liable laboratory model for
anticancer drug testing and tumor proliferation studies.
Materials and methods
Origin of cell lines
Tumor cell lines were established from carcinomatous
ascites of three patients with advanced GC diagnosed at the First
Department of General Gastrointestinal and Oncology Surgery of the
Jagiellonian University Medical College (Krakow, Poland). Patients
provided their informed, written consent in the present study. The
study was approved by the Jagiellonian University Ethical Committee
(KBET/491/B/2003). Ascites were harvested into sterile bottles with
heparin, centrifuged at 110 × g for 5 min. Thereafter both the
cells and the ascitic fluids were collected. The ascitic fluids
were filtered and kept at −80°C until use. The cell pellet was
resuspended (1×106/ml) in DMEM medium with high glucose
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) supplemented with
5% fetal bovine serum (FBS; Biowest, Nuaille, France), 40% of
autologous ascitic fluid and 50 µg/ml gentamycin (Sigma-Aldrich;
Merck KGaA). When the cells started to grow rapidly, the ascitic
fluid, after a period of gradual decrease, was completely withdrawn
from the culture. The cells were incubated at 37°C in 5%
CO2 atmosphere and regularly tested for Mycoplasma
sp. contamination by PCR-ELISA kit (Roche, Mannheim, Germany)
and for endotoxin contamination by the Limulus test (Charles River
Laboratories, Wilmington, MA, USA) according to manufacturer's
instruction.
For analysis of cellular morphology, an inverted
phase-contrast microscope (Olympus, Tokyo, Japan) was used.
Doubling time (growth curves)
Cells (1×105/ml) in medium supplemented
with 5% FBS and 50 µg/ml gentamycin (further referred as complete
medium) were seeded in duplicates into 24-well plates (BD Falcon,
Franklin Lakes, NY, USA). Every 24 h, over a period of 5 days,
cells were harvested and counted. The doubling time was estimated
from the growth curves during the exponential phase of cells'
growth.
Karyotyping analysis
The dividing cells, at the exponential growth phase
(after 18 or 24 h), were exposed to the colcemid solution (0,25
µg/ml culture medium; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) for 30 min. Then, the cells were transferred into
10 ml conical centrifuge tubes and centrifuged at 400 × g for 10
min, after which the supernatant was removed and cells were
suspended in 8–9 ml of prewarmed (37°C) hypotonic solution (20 mM
potassium chloride (KCl) and 10 mM sodium citrate
(Na3C6H5O7; POCH S.A.,
Gliwice, Poland) with simultaneous vortexing. Next, the cells were
incubated at 37°C for 30 min and centrifuged at 400 × g for 10 min.
The supernatant was removed without disrupting the pellets and
cells were suspended in 8–9 ml cold (4°C) fixative solution 3:1
(methanol:glacial acetic acid ratio) again with simultaneous
vortexing. The fixation was repeated 3 times. Finally, cells were
resuspended in 1–2 ml of fixative solution and about 0,3 ml was
dropped on a microscopic slide. The spread slides were dried for at
least overnight at 37°C until staining. The G-banding was used as
the routine cytogenetic technique. The metaphase chromosome
staining was performed with gentle digestion in the trypsin
solution [0,25% in 1X phosphate-buffered saline (PBS), POCH S.A.]
and the Giemsa stain (Sigma-Aldrich; Merck KGaA). The karyotypes
for gastric adenocarcinoma cell lines were analyzed with the
OLYMPUS BX51 microscope and the CytoVision Master 3.0 software
(Olympus) according to the 2013 ISCN international guidelines
(12).
Immunophenotyping
The following fluorescein (FITC)-, allophycocyanin
(APC)-or phycoerythrin (PE)-conjugated mouse anti-human monoclonal
antibodies (mAbs) were used: Anti-CD10, -CD11a, CD11c, -CD18,
-CD33, -CD40, -CDD44std, -CD44v5, -CD44v6, -CD54, -CD61, -CD62P,
-CD86, -CD133, -CD206 (MR), -EGFR, -Her-2/neu, -HLA-DR, HLA class
I, -CCR5, -CCR6, Her-2/neu all from BD Pharmingen (San Diego, CA,
USA); anti-CD29, -CD36, -CD51, -CD58 from Immunotech (Marseille,
France); anti-c-MET, -CCR1, -CCR2, -CCR3, -CCR7, -CXCR1, -CXCR2,
-CXCR4 from R&D (Abington, UK); anti-Tag72, -Mucin1 (EMA,
CD227), -EMMPRIN from Santa Cruz Biotechnology, Inc. (Santa Cruz,
CA, USA) and anti-Epithelial Antigen, -Epithelial Membrane Antigen
(EMA) from DAKO (Heverlee, Belgium). Isotype controls included
appropriate FITC-, APC- or PE-labeled mouse IgG1,
IgG2a or IgG2b. Cells were incubated with
mAbs or isotype controls for 20 min at 4°C, washed, resuspended in
PBS and analyzed by flow cytometry (FACS Canto; BD Biosciences
Immunocytometry Systems, San Jose, CA, USA) using FACS DiVa
software.
Western blotting
Cells were lysed in M-PER lysing buffer (Pierce;
Thermo Fisher Scientific, Inc.) containing protease inhibitor
cocktail (Roche). 20 µg of isolated protein was mixed with NuPAGE
LDS Sample Buffer (4X; Thermo Fisher Scientific, Inc.) and NuPAGE
Sample Reducing Agent (10X; Thermo Fisher Scientific, Inc.).
Samples were heated (70°C, 10 min) and electrophoresed in 14%
polyacrylamide gel containing SDS (Bio-Rad, Hercules, CA, USA).
Next, electrophoresed samples were transferred onto the
polyvinylidene fluoride membrane (Bio-Rad). Then, after blocking
for 1 h at room temperature in Tris buffered saline (TBS) with 0,1%
Tween-20 (Sigma-Aldrich; Merck KGaA) and 1% bovine serum albumin
(BSA; Sigma-Aldrich; Merck KGaA), the membranes were incubated
overnight at 4°C with the following antibodies: Goat anti-human
ALDH1A1 (clone L-15), ALDH1A2 (clone N-20), ALDH1A3 (clone C-13),
ALDH2 (clone N-14) and mouse anti-human ALDH3A1 (clone B-8), rabbit
anti-EMMPRIN (clone N-19), -panCEA (clone: H-300), -MAGE-A1 (clone:
FL-309) and -GAPDH (clone: 14C10) (all Santa Cruz Biotechnology,
Dallas, TX, USA). As a loading control, rabbit anti-human GAPDH
(Cell Signaling Technology, Inc., Danvers, MA, USA) was used. After
incubation, membranes were washed in TBS supplemented with BSA and
Tween-20 and incubated for 1 h at room temperature with either goat
anti-rabbit or goat anti-mouse (dilution 1:4,000) secondary
antibody conjugated with horseradish peroxidase (Santa Cruz
Biotechnology, Inc.). The protein bands were visualized with the
SuperSignal West Pico Chemiluminescence Substrate kit according to
the manufacturer's protocol (Pierce; Thermo Fisher Scientific,
Inc.) and analyzed with KODAK GEL LOGIC 1500 Digital Imaging System
(KODAK, Rochester, NY, USA).
Detection of ALDH activity
The ALDEFLUOR kit (StemCells Technologies, Grenoble,
France) was used for identification of cells with ALDH activity.
Cells were incubated (45 min, 37°C, final concentration
1×106/ml) with the Assay Buffer containing
BODIPY-amino-acetaldehyde (BAAA; final concentration 1 µM)-a
fluorescent substrate for ALDH. Cells able to process the BAAA
substrate to its fluorescent form, BODIPY-aminoacetate (BAA), were
considered as ALDH positive (ALDH+). To confirm
specificity of ALDH depended reaction cells were additionally
incubated with specific ALDH inhibitor, diethylaminobenzaldehyde
(DEAB). Cells incubated with DEAB only served as a negative
control. After treatment, cells were washed and suspended in
ice-cold PBS supplemented with 0.5% of BSA and verapamil (50 µM;
Sigma-Aldrich; Merck KGaA) to block Abcg2 transporters activity and
prevent active efflux of the ALDEFLUOR product from viable
cells.
Determination of Her-2/neu and MAGE-1,
−2 mRNA expression in the cell lines using nested quantitative PCR
(qPCR)
The isolation of total RNA and qPCR for MAGE-1, −2
and β-actin was performed as previously described (13). For detection of HER-2/neu mRNA qPCR
was performed using the following primers:
Sense-5′-CCTCTGACGTCCATCATCTC-3′ and
antisense-5′-ATCTTCTCGTGCCGTCGCTT-3′. The cycle profile for
HER-2/neu PCR run was: Initial denaturation at 95°C for 10 min,
then denaturation at 95°C for 0 sec, annealing at 60°C for 35 sec,
and elongation at 72°C for 35 sec for 35 cycles, followed by final
extension at 72°C for 2 min. The results were normalized with
β-actin data and expressed as CT. To verify amplified
product, melting curve analysis using the LightCycler software was
performed for each sample.
Xenografts in non-obese
diabetic/severe combined immunodeficiency (NOD/SCID) mice
Cells (1×106 of each cell line, viability
over 95%) suspended in 200 µl of saline were injected
subcutaneously (s.c.) into dorsal region of 8-week old NOD/SCID
mice (5 mice per group; Charles River Laboratories, Sulzfeld,
Germany). Every three days, tumor's diameter was measured with a
caliper and its volume (v) was calculated according to the formula:
v=ab2/2, where a is the longest dimension, b is the
perpendicular width. When moribund, the tissues were examined
macroscopically for metastasis in various organs and then processed
for histological examination (14).
The study was approved by the Ist Local Ethical Committee on Animal
Testing (no. 128/2012).
Histological analysis
Subcutaneous GC1401, GC1415 and GC1436 tumors in
NOD/SCID mice and other organs (spleen, liver, lung, lymph nodes)
of tumor-bearing mice were cut out, divided into several portions
and fixed in 10% buffered formalin. Some of them, after routine
processing, were embedded in paraffin. 3 µm thick sections were
stained with H&E according to manufacturer's protocol.
Statistical analysis
The non parametric Kruskal-Wallis test was performed
using GraphPad InStat version 4.0 software (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Growth characteristics
The cells were obtained from carcinomatous ascites
of patients diagnosed with gastric adenocarcinoma (Table I). All patients were evaluated as
non-resectable, with peritoneal spread and no liver or lung
metastases.
 | Table I.Patients' characteristics. |
Table I.
Patients' characteristics.
| Cells | Age | Gender | Stage (TNM) | Histology |
|---|
| GC1401 | 72 | F | IV (T4NXM1) | Adenocarcinoma |
| GC1415 | 73 | M | IV (T4NXM1) | Tubular
adenocarcinoma combined with signet ring cell carcinoma |
| GC1436 | 19 | F | IV (T4NXM1) | Mucinous
adenocarcinoma |
At the initiation of the culture, some of the cells
adhered to the plastic surface, while the others did not. In the
case of GC1401 and GC1415 cells, negligible proliferation of tumor
cells was observed for several (6–8) weeks,
instead, the proliferation of fibroblasts was seen. After this
period of adaptation to the in vitro conditions, both GC1401
and GC1415 cells started to grow rapidly. In contrast, the GC1436
cells were rapidly growing from the beginning. With successive
passages, the number of fibroblasts gradually decreased, to be
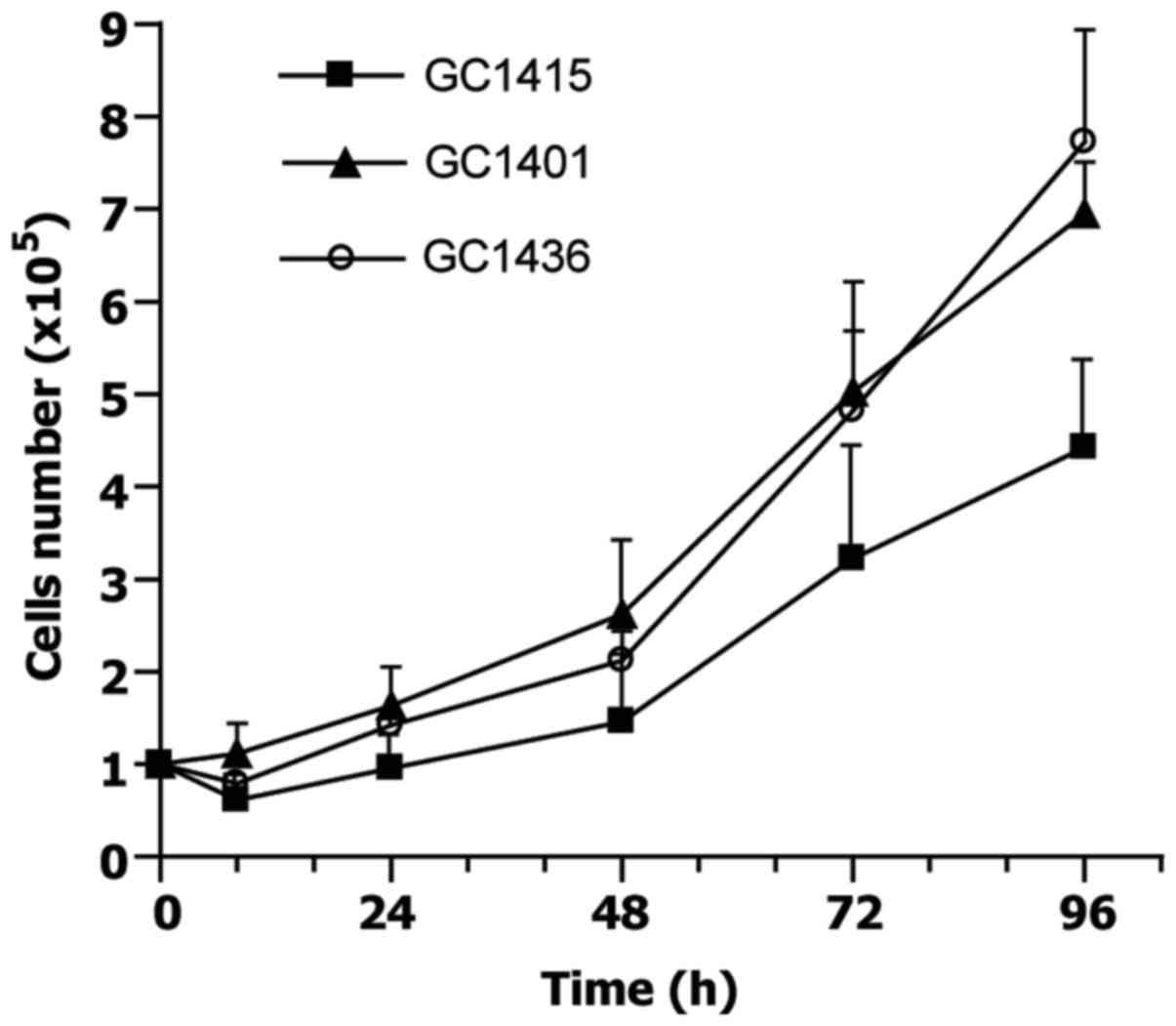
finally replaced by tumor cells. Doubling time, estimated at
exponential phase of growth, for GC1401 and GC1415 cell lines was
about 30 and 34 h, respectively, and about 25 h for GC1436 cells
(Fig. 1). The differences in doubling
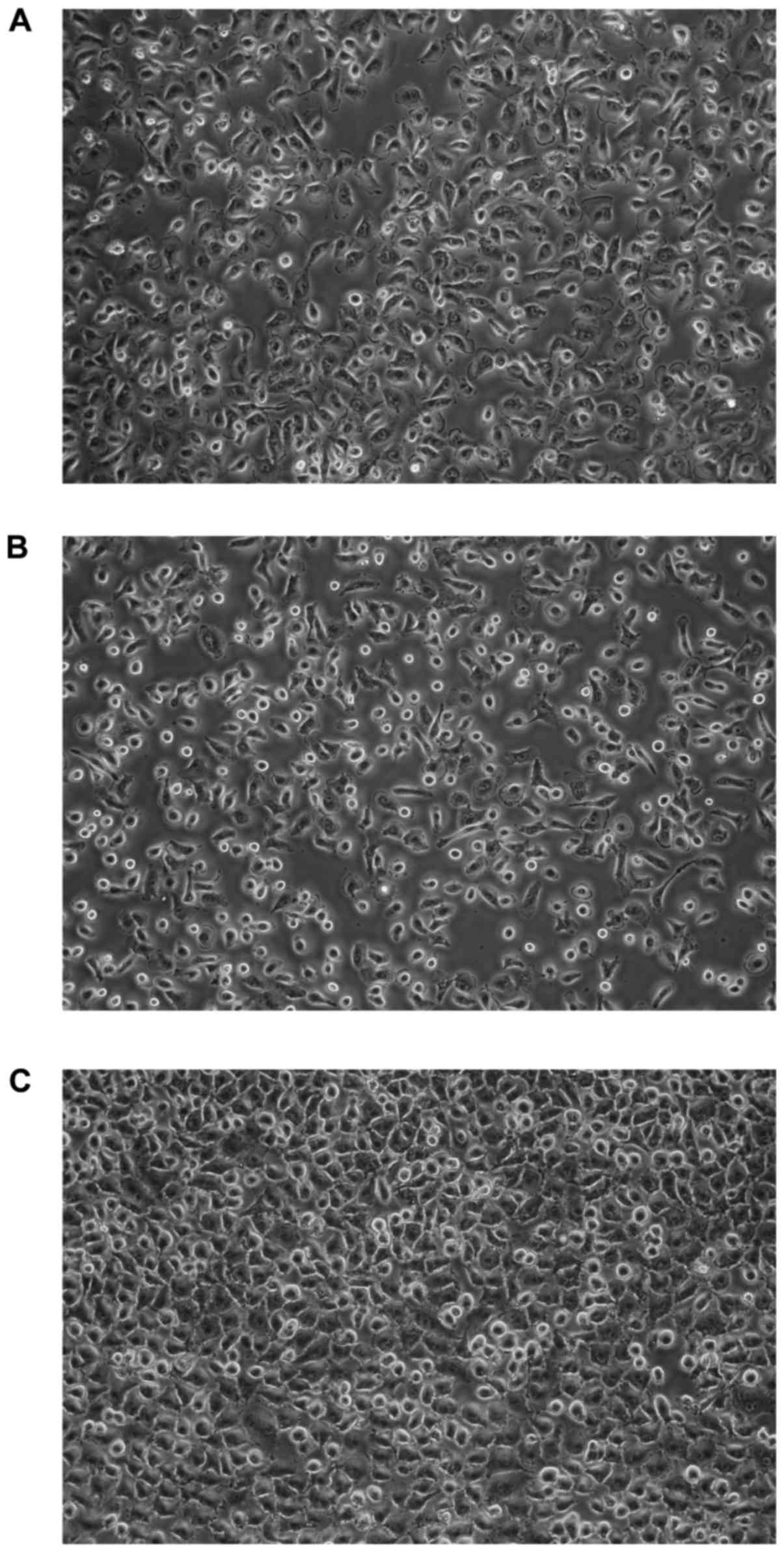
time were not significant. All cells exhibited morphologic features
of epithelial-like cells, creating the sheets of polygonal cells
which attached to the culture flask and formed monolayer at
confluence (Fig. 2).
Karyotyping
Karyotyping analysis showed a great complexity of
all three gastric adenocarcinoma cell lines. The identified variety
involves especially structural chromosomal aberrations.
Additionally, high hyperdiploidy of tumor cell lines were detected;
from 51–56 and 52 chromosomes for GC1415 and GC1401 to 91–106
chromosomes for GC1436. The best cytogenetic characterization was
done for the GC1401 cell line, because of the presence of only two
subclones (Table II). The karyotype
description according to the international guidelines was also
possible for this cell line. The exact result for the GC1436 cell
line was not allowed due to high heterogeneity of the cells and a
low resolution of the karyotype. Additionally, several structural
and numerical chromosomal abnormalities were evident in all cell
lines (Table III).
 | Table II.Karyotypes of gastric adenocarcinoma
cell lines. |
Table II.
Karyotypes of gastric adenocarcinoma
cell lines.
| Cell lines | Karyotype (ISCN
2013) |
|---|
| GC1401 |
52,X,+der(1)t(1;17?)(p13;q11.2?),dup(1)(p13p32),+2,der(6)t(6;17?)(p12;q11.2?),+der(7)t(7;?)(p11.2;?)?add(7)
(q32?),+der(10)t(10;18?)(p11.2;p11.2?),der(12)t(12;14?)(q22;q22?),?dup(14)(q13q24),der(15)t(15;21?)
(p13;q11.2?),der(16)?add(16) (q22 or
q24),der(19)?add(19)(p13.3),rob(21;21)(q10;q10),+2mar[11]/52,X,+der(1)
t(1;17?)(p13;q11.2?),dup(1)(p13p32),+2,der(6)
t(6;17?)(p12;q11.2?),+der(7)t(7;?)(p11.2;?)?add(7)(q32?),+der(10)
t(10;18?)(p11.2;p11.2?),der(12)t(12;14?)(q22;q22?),-13,?dup(14)
(q13q24),der(15)t(15;21?)(p13;q11.2?),der(16)? add(16)(q22 or
q24),der(19)?add(19)(p13.3),rob(21;21)(q10;q10),+3mar[5] |
| GC1415 |
51–56,X,+der(1)t(1;17?)(p13;q11.2?),+2,+der(4)?(q),+5,der(6)t(6;17?)(p12;q11.2?),+7,-8,der(12)?add(12)
(p13),?
dup(14)(q13q24),der(15)t(15;21?)(p13;q11.2?),rob(21;21)(q10;q10),+mar[cp7] |
 | Table III.Common chromosomal aberrations in
gastric adenocarcinoma cell lines. |
Table III.
Common chromosomal aberrations in
gastric adenocarcinoma cell lines.
| Cell lines | GC1401 | GC1415 | GC1436 |
|---|
| Ploidy | 52 chromosomes | 51–56
chromosomes | 91–106
chromosomes |
| Chromosomal
aberrations |
+der(1)t(1;17?)(p13;q11.2?) |
+der(1)t(1;17?)(p13;q11.2?) |
+der(1)t(1;17?)(p13;q11.2?) |
|
| dup(1)(p13p32) |
| dup(1)(p13p32) |
|
| +2 | +2 |
|
|
|
der(6)t(6;17?)(p12;q11.2?) |
der(6)t(6;17?)(p12;q11.2?) |
der(6)t(6;17?)(p12;q11.2?) |
|
|
+der(10)t(10;18?)(p11.2;p11.2?) |
|
der(10)t(10;18?)(p11.2;p11.2?) |
|
|
|
der(12)?add(12)(p13) |
der(12)?add(12)(p13) |
|
|
der(12)t(12;14?)(q22;q22?) |
|
der(12)t(12;14?)(q22;q22?) |
|
|
?dup(14)(q13q24) |
?dup(14)(q13q24) |
?dup(14)(q13q24) |
|
|
der(15)t(15;21?)(p13;q11.2?) |
der(15)t(15;21?)(p13;q11.2?) |
|
|
|
der(19)?add(19)(p13.3) |
|
der(19)?add(19)(p13.3) |
|
|
rob(21;21)(q10;q10) |
rob(21;21)(q10;q10) |
rob(21;21)(q10;q10) |
Expression of surface
determinants
The expression of surface determinants on cells from
in vitro cultures was evaluated using a wide range of mAbs
(Table IV). All three cell lines
showed a similar pattern of surface determinants with similar
levels of expression. All cell lines were HLA-class I positive
(100% of cells) and HLA-DR negative. CD29 and CD51 integrins and
CD58 of the Ig superfamily were expressed on all cells. The
majority of GC1401, GC1415 and GC1436 cells possessed the
expression of CD10, CD40, CD44 and CD61 determinants. There were
differences between the cell lines in the expression of CD44
variants. The lowest level of v5 and v6 was noticed on GC1401 cells
(about 3 and 34%, respectively). GC1415 cells were positive in ~10%
for the v5 and in ~50% positive for the v6. The highest level of
the v5 (app. 40%) and the v6 (app. 60%) positive cells was among
GC1436 cells. Less than 10% of cells were positive for CD33 and
10–20% were positive for CD86. The other determinants tested (i.e.,
CD11a, c, CD18, CD36, CD54, CD62P, CD133, CD206) were not detected
on the cells of all three cell lines.
 | Table IV.Expression of selected surface
markers on gastric adenocarcinoma cell lines. |
Table IV.
Expression of selected surface
markers on gastric adenocarcinoma cell lines.
| Surface marker | GC1401 (% of
cells) | GC1415 (% of
cells) | GC1436 (% of
cells) |
|---|
| CD10 | 50 | 70–80 | 80–90 |
| CD29 | 100 |
97–100 | 100 |
| CD33 | 1 | 8 | 9 |
| CD40 | 91–99 |
85–100 | 65–85 |
| CD44 | 92–97 | 88–93 | 79–83 |
| CD44v5 | 3 | 10 | 41 |
| CD44v6 | 24–44 | 38–70 | 48–80 |
| CD51 | 100 | 95–99 | 96–99 |
| CD58 | 100 | 89–99 |
92–100 |
| CD61 | 90–95 | 87–97 | 97 |
| CD86 | 11–20 | 12–17 | 13–18 |
| HLA-ABC | 100 | 100 | 100 |
| CCR3 | 3 | 10 | 3 |
| CCR6 |
6–15 | 10 | 10 |
| CCR7 | 2 | 1–2 | 1 |
| CXCR1 | 5 | 3 | 3 |
| CXCR4 | 2–3 | 3–4 | 3 |
| c-MET | 98 | 97 | 98 |
| Her-2/neu | 63–98 | 92–98 | 98 |
| Tag72 | 0 | 0 | 2–3 |
| Epithelial
Antigen | 0 | 4–7 | 2–9 |
| Mucin1(CD227) | 2–4 | 4–7 |
5–14 |
| EMMPRIN |
7–17 | 26–66 | 16–24 |
The cells were comparably positive for CCR3
expression which fluctuated between 5 to 25% of positive cells and
CCR6 was present on 6–15% of cells. Very low percent of CCR7
(1–2%), CXCR1 and CXCR4 (up to 5%) and the lack of CCR1, 2, 5, and
CXCR2 expression was observed on cells of all cell lines.
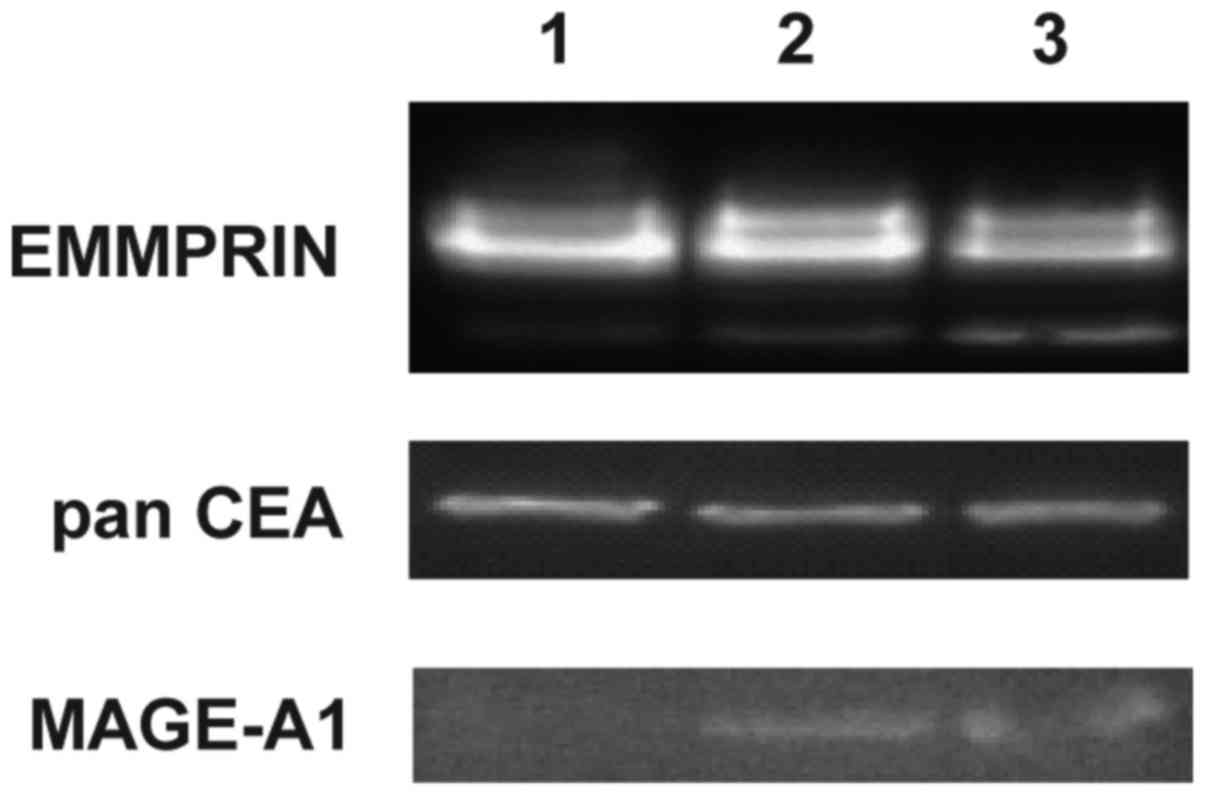
The highest level of EMMPRIN positive cells was
observed in GC1415 cell line (up to 66%), whereas GC1401 and GC1436
cell lines were up to 24% positive. These results corroborate with
western blotting data where EMMPRIN was shown to be present in all
three cell lines (Fig. 3). Mucin1
(EMA) was detected on a small population of GC1401, GC1415 and
GC1436 cells (below 14%). Almost all cells of the three cell lines
were Her-2/neu and c-MET positive. Tag72 was detected on a very low
percent (app. 3%) of GC1436 cells and was not present on GC1401 and
GC1415 cells. Epithelial Antigen was detected on GC1415 and GC1436,
however, the percentage of positive cells was very low (less than
9% of cells). Presence of panCEA was confirmed by western blotting
in all tested cell lines. Expression of MAGE-1 was detected by
western blotting in GC1415 and 1436 only (Fig. 3).
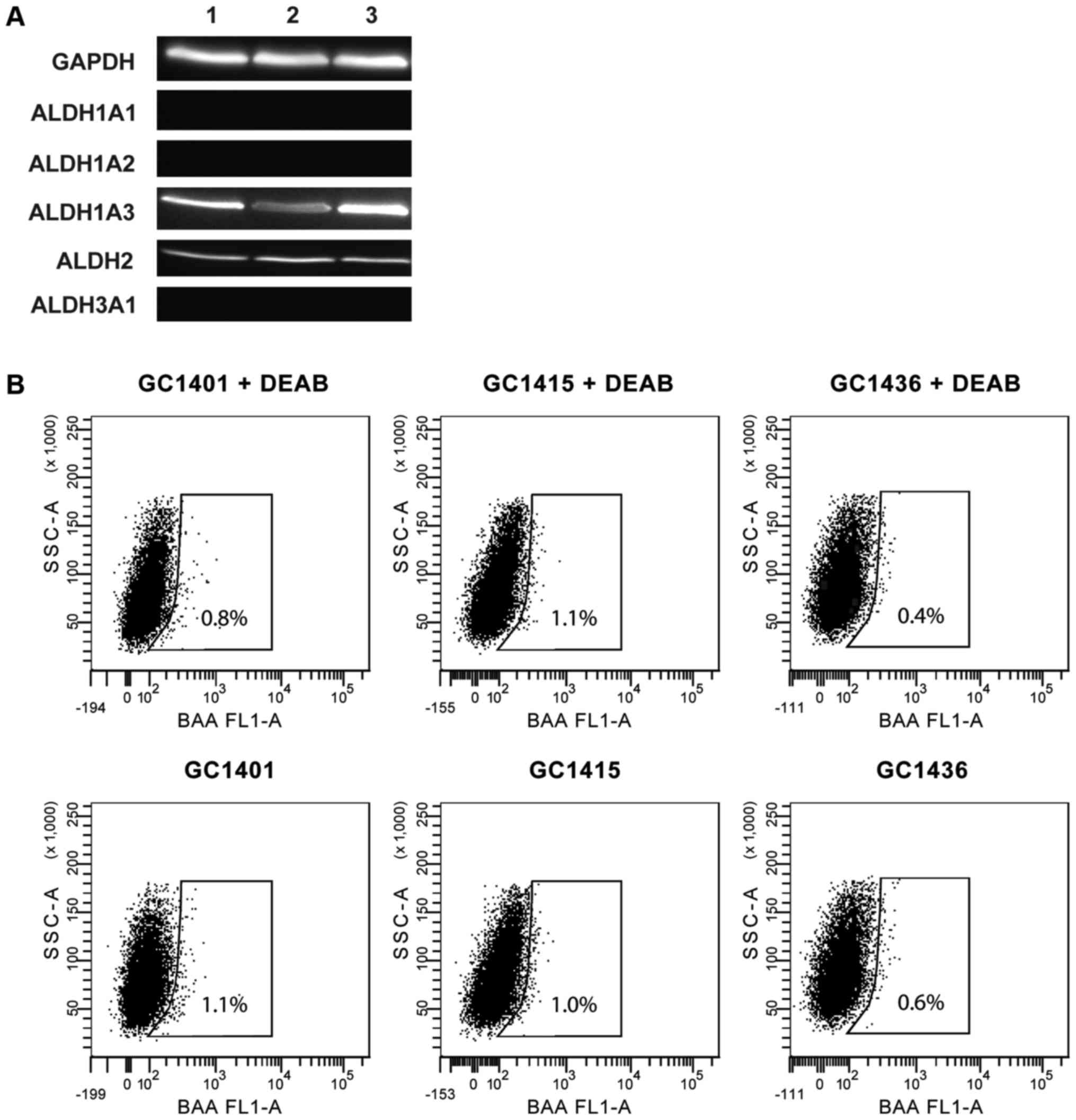
Expression and activity of ALDH
ALDH expression was assessed by western blotting.
All three tested cell lines expressed ALDH1A3 and ALDH2 isoforms,
however, none of them expressed ALDH1A1, ALDH1A2 and ALDH3A1
isoforms (Fig. 4A). The ALDH activity
measured by flow cytometry was very low (Fig. 4B); less than 1% of cells showed such
activity. This observation is consistent with the data that ALDH1A1
is the main enzyme of the ALDH family responsible for the enzymatic
activity as determined by the ALDEFLUOR assay.
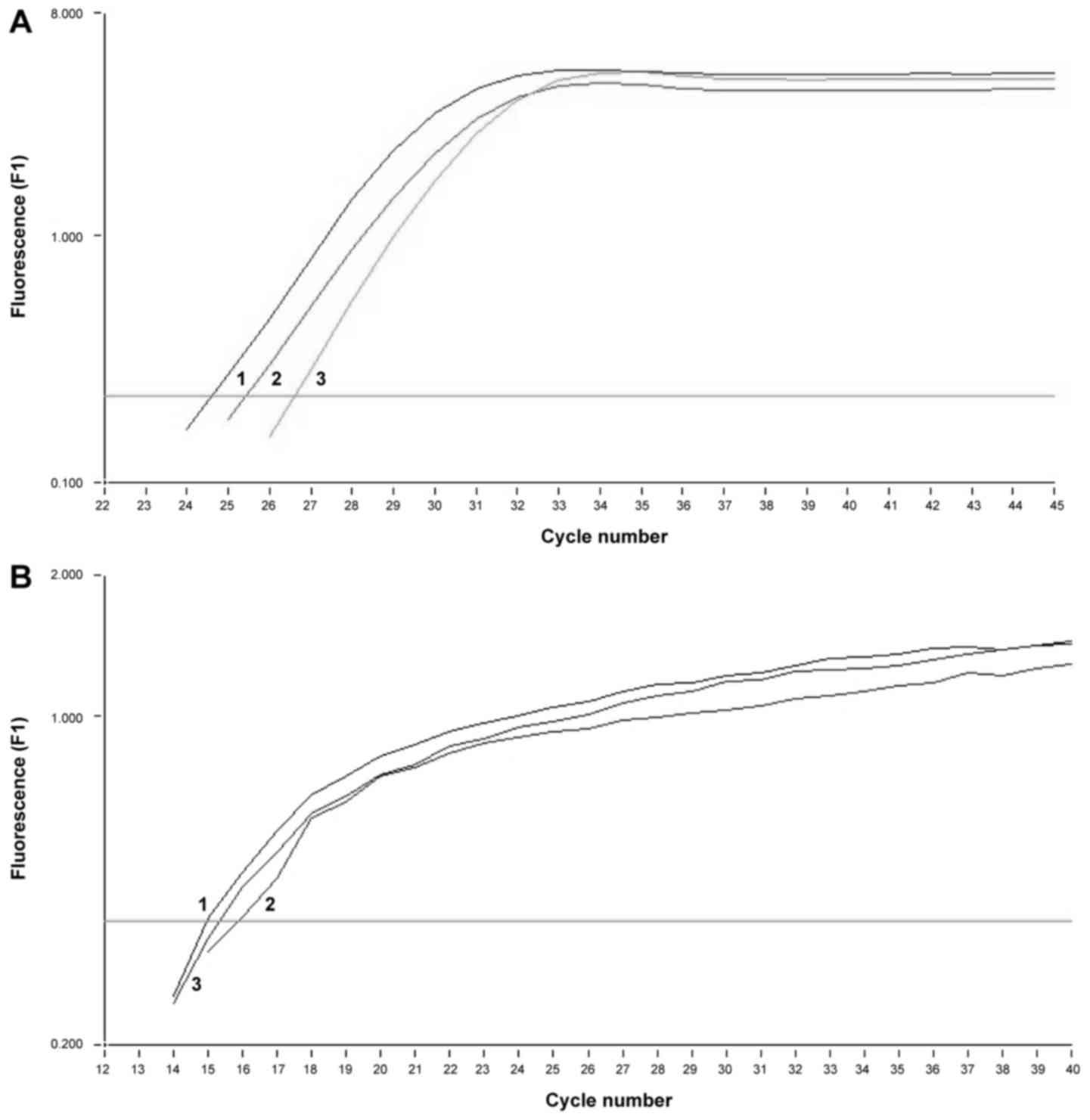
Determination of MAGE-1, −2 and
Her-2/neu mRNA expression by nested qPCR
In all three tumor cell lines similar amounts of
mRNA for MAGE-1, −2 and Her-2/neu were detected. To verify the
amplified product, melting curve analysis was performed for each
sample confirming the presence of MAGE-1 mRNA and Her-2/neu
(respectively Fig. 5).
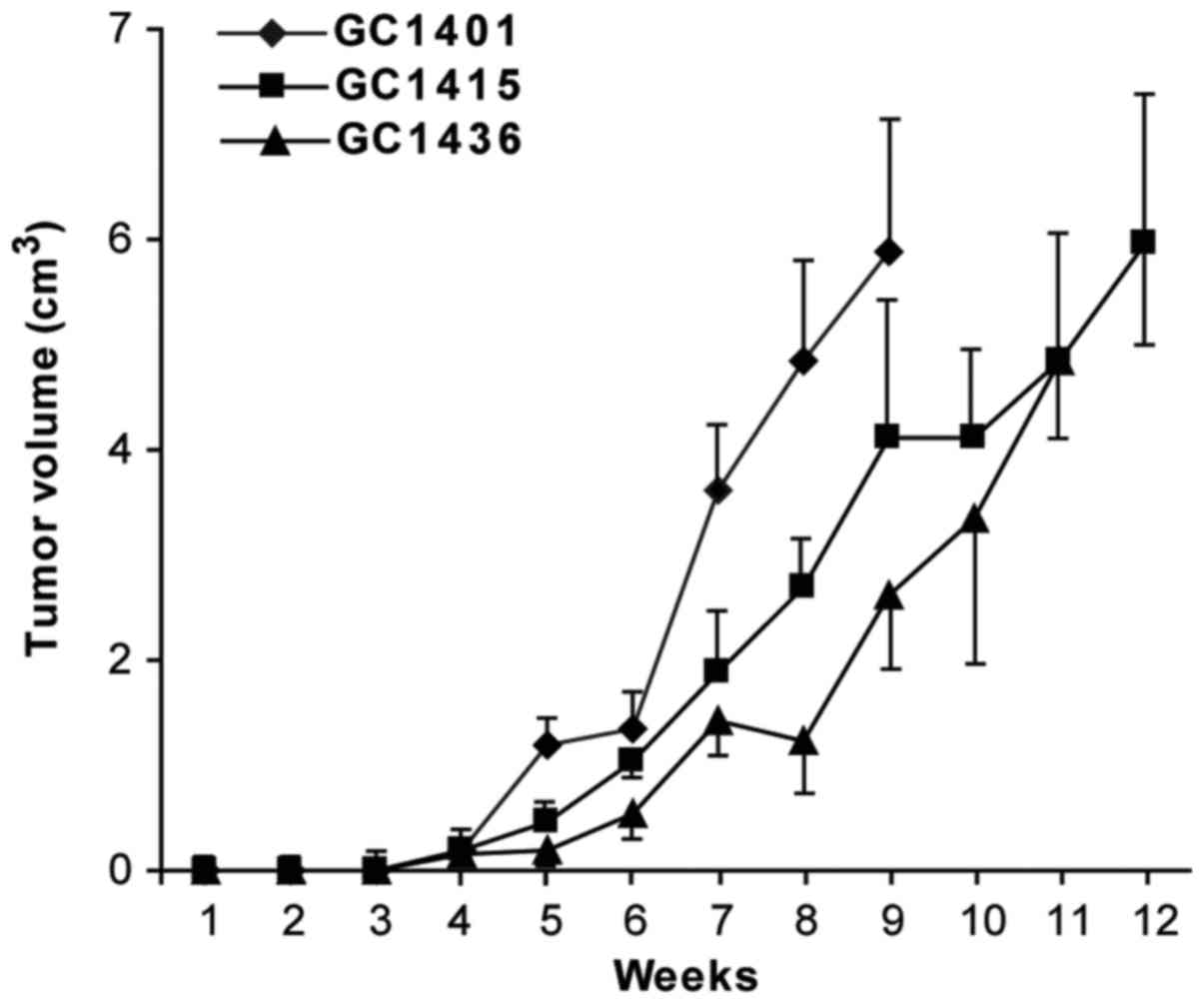
Tumorigenicity and metastasis
evaluation
All three cell lines were tumorigenic in vivo
in NOD/SCID mice. The transplantation of cells from in vitro
culture led usually to the formation of tumors in 13 of 15 mice
(~82–89%). Following s.c. injection of 1×106 tumor
cells, palpable encapsulated tumors were observed within 3–4 weeks
(Fig. 6). The differences in tumor
growth were not statistically significant. 100% mortality was
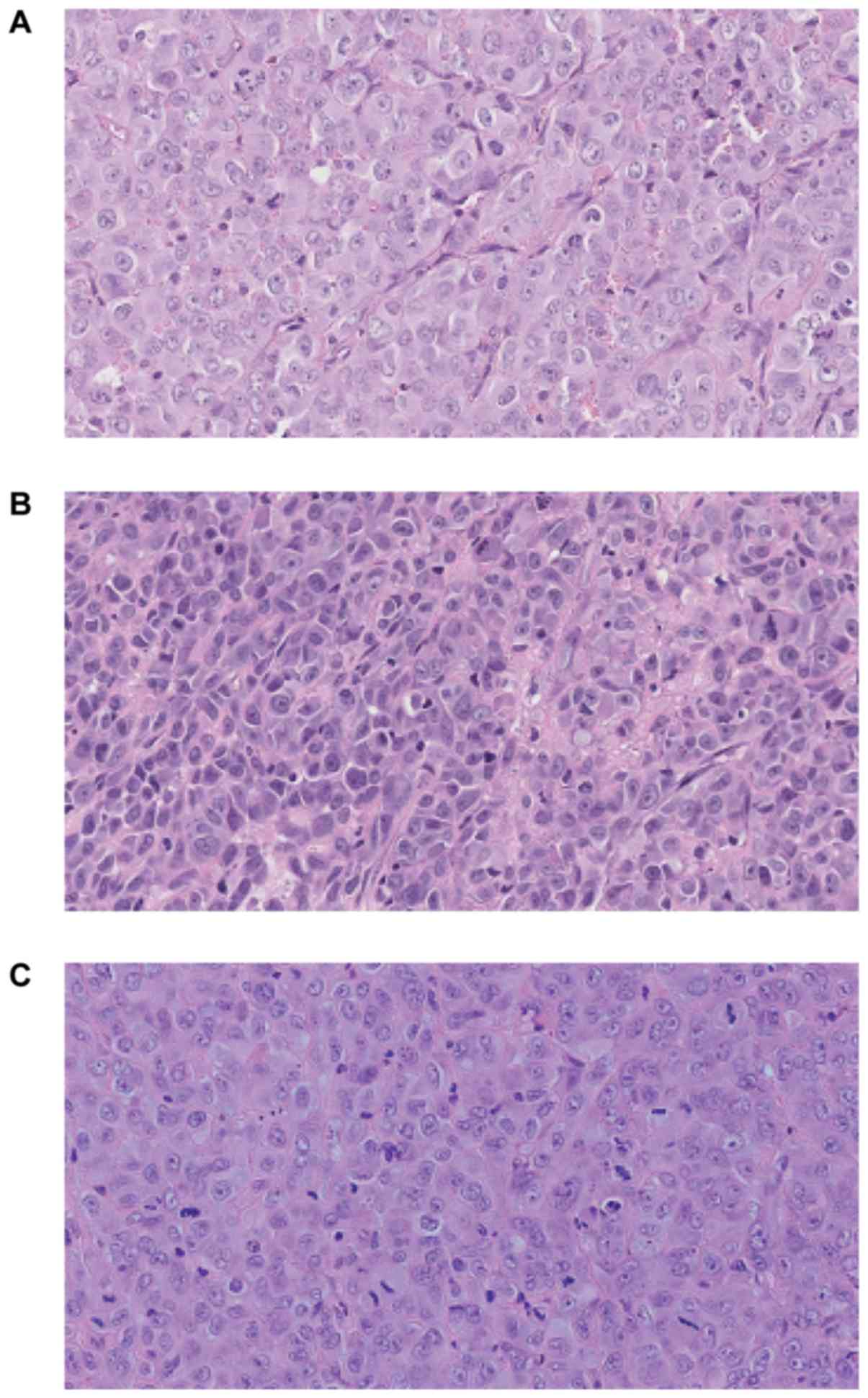
noticed at week 13. Fig. 7 presents
hematoxylin-eosin staining of the tumor sections after 8 weeks of
the tumor growth in vivo. Histologically in all cases
malignant neoplasm of epithelial origin was observed.
No macro- and microscopic metastasis to lungs,
liver, peritoneum, spleen, kidneys or lymph nodes were
observed.
Discussion
There are GC cell lines already established and
characterized, however, due to the heterogeneity of cancer cells
each newly characterized cell line may provide new data useful for
anticancer therapy. The presented manuscript describes the
characterization of three new cell lines established from the
malignant ascites of Caucasian patients diagnosed with gastric
adenocarcinoma. The use of primary tumors as a source of cancer
cells encounters several problems, e.g., the need for mechanical or
enzymatic disruption which often leads to cell damage, which is not
the case, when ascites is being used. Also, the addition of
autologous ascitic fluid improves the culture conditions and cell
survival (especially at the beginning), which is probably due to
the presence of growth-promoting factors (15). Doubling time of established cell lines
fall in the range described for commercially available GC cell
lines e.g., KATO, ATCC®HTB-103, ATCC®CRL-5973
and ATCC®CRL-5973 (21–36 h) (16).
Based on karyotyping analysis, a great cytogenetic
complexity in the investigated cell lines was observed. Numerous
structural and ploidy aberrations were evident in all three cell
lines. This observation is in accordance with high ploidies of
well-defined human stomach-derived cell lines in the American Type
Culture Collection which are mostly hypotetraploid
(ATCC®HTB-103, ATCC®CRL-5973,
ATCC®CRL-5974). To the authors' best knowledge, all of
the identified chromosomal changes are reported for the first time,
and will be subjected to further research. It has been indicated
that 53–94% of advanced gastric adenocarcinomas have an abnormal
chromosomal number (17,18). The chromosomal alterations have been
recorded in gastric adenocarcinomas as gains: 1q, 3q, 7p, 7q, 8q,
9q, 10p, 11q, 13q, 17q, 19q, 20q and losses: 1p, 4p, 4q, 5q, 6q,
9p, 10, 13, 17p, 18q (19–25). The double minute chromosomes were not
found in GC cell lines, but were described in
ATCC®CRL-5971, ATCC®CRL-5973 and
ATCC®CRL-5974 (but not KATO-III). Additionally,
aneuploidy has been detected as a potentially unfavorable
prognostic marker often associated with high proliferative activity
and metastatic potential (17,18).
A complex karyotype is often reported in aggressive
cancers with a tendency to metastasize (26–28). The
structural rearrangements are one of the activation modes of
protooncogenes and may also be a reason for suppressor genes
inactivation, which in turn may induce and drive the neoplastic
process. The classical karyotyping has limitations, primarily with
the resolution of the study and the quality of metaphase
chromosomes. Nevertheless, conventional banding remains the best
technique for the evaluation balanced aberrations.
The phenotype analysis revealed the presence of HLA
class I, CD40 (member of TNFR superfamily) and some adhesion
molecules of the Ig superfamily (CD58), integrins (CD29, CD51,
CD61) and CD44 on almost all cells of all three cell lines. The
level of these markers did not change throughout the culture. CD44
variants 5 and 6 were also present, but their expression has varied
in the course of culture. These markers were already detected in
solid tumors including GC as well as GC cell lines (e.g., KATO-III,
SNU-5, SNU-16) (29,30). Their high expression was observed in
more expanding tumors (31) and was
correlated with decreased patients' survival (32–35).
Among chemokine receptors, CCR3, CCR6, CCR7, CXCR1
and CXCR4 were present at very low levels on the cells of all cell
lines. Chemokine receptors are involved in different activities of
tumor cells such as migration, invasion and adhesion and their high
levels are usually associated with an aggressive character of tumor
cells. The presence of the above receptors was described on
different cancer cells of the gastrointestinal duct, including GC
(36,37), and they were usually upregulated
during metastasis (38–42).
The level of mucin-positive cells was low (3% of
GC1401, 5% of GC1415 and 10% of GC1436). At the same time CD10
expression was relatively high and varied from 50% in GC1401 to 80%
in GC1415 and up to 90% in GC1436 cells. Previously, expression of
CD10 was observed in KATO-III cell line (43). Recently, combined expression of CD10
and MUC was employed to distinguished between gastric and an
intestinal types of GC (44,45). According to this classification, the
expression of CD10 and the absence of MUC-1 (or other proteins from
MUC family) may suggest intestinal type of GC. Moreover, expression
of c-MET and Her-2 may also confirm this histological type
(46). c-MET and Her-2/neu, the
members of receptor tyrosine kinase family, involved in tumor
growth and survival, were present on all cells of each cell line,
as detected at both the protein and mRNA level. Their
overexpression is usually associated with tumor metastasis and poor
prognosis (47,48). Strong expression of EMMPRIN as
described by Zheng et al (49), may contribute to enhanced growth,
invasion and angiogenesis of gastric carcinoma. New,
EMMPRIN-positive cell lines, may help to evaluate the angiogenesis
process.
One of the intriguing questions in this study was
the lack of in vivo metastasis in NOD/SCID mice after
subcutaneous engrafting of cancer cells. It has been already
observed that although human cancer cells proliferate after
injection into nude or SCID mice and form tumors in situ,
their ability to form local or distal metastases is rare (50,51). In
the case of the presented GC cell lines the lack of metastasis may
have arisen from several reasons:
i) Heterotopic human-mice model of subcutaneous
engraftment of human GC in NOD/SCID mice did not reconstruct the
conditions for growth and metastasis of human cancer in human
microenvironment (52).
ii) Presence of a capsule may hamper metastasis
(53).
iii) Low levels or lack of important agents, such as
cytokines/chemokines and/or their receptors (e.g., low expression
of chemokine receptors in the case of presented GC cell lines).
iv) Lack of or very low level of cancer stem cells
(CSC) (presented GC cell lines express CD44 but lack the expression
of CD54 (54) or CD133+
(55) markers characteristic for
CSC).
v) Very low levels of ALDH activity. The expression
of ALDH1A3 and ALDH2 was detected by western blotting assay, but
surprisingly very low amount of cells (app. 1%) exhibiting ALDH
activity, as judged by the ALDEFLUOR assay, were observed. This may
be due to the lack of ALDH1A1 and ALDH1A2 which are potentially
major contributors of ALDH1 activity in different types of cancer
e.g., breast and lung cancer (56,57).
Many studies have shown elevated expression of ALDH
isoforms other than ALDH1A1, but they do not directly prove that
they are the cause of ALDEFLUOR activity in cancers (58–61).
The role of the ALDH1A3 isoform in GC progression
still needs to be elucidated. High levels of this protein were
observed in normal stomach tissue, however, its mRNA overexpression
was detected in patients with GC, and was correlated with worse
patient survival (62). ALDH2 is
constitutively expressed in a variety of tissues (63), however, its role in tumor progression
is limited to genetic polymorphism (rs671) and correlated with
alcohol consumption (64). Our data
may suggest that the low, but noticeable, activity of ALDH may come
from ALDH1A3 or ALDH2 isoforms, which may play a role in
tumorigenesis in a tissue-specific manner.
In summary, cancer metastasis is a complicated,
multi-step process, influenced by many factors. Despite the
presence of some prometastatic determinants (eg. CD29, CD40, CD44,
c-MET, Her-2/neu, composed karyotype) and soluble factors such as
IL-8, VEGF (data not shown), the lack of others (chemokine
receptors, insufficient levels of CSC, activity of ALDH1A1) may
disrupt the progression of metastasis leading to its
inhibition.
The mortality of GC is very high due to its high
heterogeneity even within the same tumor where cell subpopulations
may show a diverse potential to growth and metastasis. In
consequence, conventional therapies are not fully effective, as
subpopulations of cells may differ in response to them. Three novel
cell lines, established and characterized in our laboratory, may
provide models for studies on biological heterogeneity of human GC
cells.
Acknowledgements
This study was supported by the National Science
Centre (grant no. UMO-2012/07B/NZ6/03499).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verdecchia A, Santaquilani M and Sant M:
Survival for cancer patients in Europe. Ann Ist Super Sanita.
45:315–324. 2009.PubMed/NCBI
|
|
3
|
Fidler IJ: Biological heterogeneity of
cancer: Implication to therapy. Hum Vaccines Immunother.
8:1141–1142. 2012. View
Article : Google Scholar
|
|
4
|
Kato M, Shimada Y, Tanaka H, Hosotani R,
Ohshio G, Ishizaki K and Imamura M: Characterization of six cell
lines established from human pancreatic adenocarcinomas. Cancer.
85:832–840. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Park JG, Frucht H, LaRocca RV, Bliss DP
Jr, Kurita Y, Chen TR, Henslee JG, Trepel JB, Jensen RT, Johnson
BE, et al: Characteristics of cell lines established from human
gastric carcinoma. Cancer Res. 50:2773–2780. 1990.PubMed/NCBI
|
|
6
|
Chun YH, Kil JI, Suh YS, Kim SH, Kim H and
Park SH: Characterization of chromosomal aberrations in human
gastric carcinoma cell lines using chromosome painting. Cancer
Genet Cytogenet. 119:18–25. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Croker AK, Goodale D, Chu J, Postenka C,
Hedley BD, Hess DA and Allan AL: High aldehyde dehydrogenase and
expression of cancer stem cell markers selects for breast cancer
cells with enhanced malignant and metastatic ability. J Cell Mol
Med. 13:2236–2252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liao J, Qian F, Tchabo N,
Mhawech-Fauceglia P, Beck A, Qian Z, Wang X, Huss WJ, Lele SB,
Morrison CD and Odunsi K: Ovarian cancer spheroid cells with stem
cell-like properties contribute to tumor generation, metastasis and
chemotherapy resistance through hypoxia-resistant metabolism. PLoS
One. 9:e849412014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mu X, Patel S, Mektepbayeva D, Mahjoub A,
Huard J and Weiss K: Retinal targets ALDH positive cancer stem cell
and alters the phenotype of highly metastatic osteosarcoma cells.
Sarcoma. 2015:7849542015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ajani JA, Wang X, Song S, Suzuki A, Taketa
T, Sudo K, Wadhwa R, Hofstetter WL, Komaki R, Maru DM, et al:
ALDH-1 expression levels predict response or resistance to
preoperative chemoradiation in resectable esophageal cancer
patients. Mol Oncol. 8:142–149. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li XS, Xu Q, Fu XY and Luo WS: ALDH1A1
overexpression is associated with the progression and prognosis in
gastric cancer. BMC Cancer. 14:7052014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schafer LG, McGowan-Jordan J and Schmid M:
ISCN 2013 An International System for Human Cytogenetic
Nomenclature. S. Karger; Basel: 2013
|
|
13
|
Szatanek R, Drabik G, Baran J,
Kolodziejczyk P, Kulig J, Stachura J and Zembala M: Detection of
isolated tumor cells in the blood and bone marrow of patients with
gastric cancer by combined sorting, isolation and determination of
MAGE-1, −2 mRNA expression. Oncol Rep. 19:1055–1060.
2008.PubMed/NCBI
|
|
14
|
Iwanuma Y, Chen FA, Egilmez NK, Takita H
and Bankert RB: Antitumor immune response of human peripheral blood
lymphocytes coengrafted with tumor into severe combined
immunodeficient mice. Cancer Res. 57:2937–2942. 1997.PubMed/NCBI
|
|
15
|
Alama A, Barbieri F, Favre A, Cagnoli M,
Noviello E, Pedullà F, Viale M, Foglia G and Ragni N: Establishment
and characterization of three new cell lines derived from the
ascites of human ovarian carcinomas. Gynecol Oncol. 62:82–88. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Birsoy K, Possemato R, Lorbeer FK,
Bayraktar EC, Thiru P, Yucel B, Wang T, Chen WW, Clish CB and
Sabatini DM: Metabolic determinants of cancer cell sensitivity to
glucose limitation and biguanides. Nature. 508:108–112. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Doak SH: Aneuploidy in upper
gastro-intestinal tract cancers-a potential prognostic marker?
Mutat Res. 651:93–104. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leal MF, Martins do Nascimento JL, da
Silva CE, Vita Lamarão MF, Calcagno DQ, Khayat AS, Assumpção PP,
Cabral IR, de Arruda Cardoso Smith M and Burbano RR: Establishment
and conventional cytogenetic characterization of three gastric
cancer cell lines. Cancer Genet Cytogenet. 195:85–91. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buffart TE, Carvalho B, Mons T, Reis RM,
Moutinho C, Silva P, van Grieken NC, Vieth M, Stolte M, van de
Velde CJ, et al: DNA copy number profiles of gastric cancer
precursor lesions. BMC Genomics. 8:3452007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
David S and Meltzer SJ: Stomach-genetic
and epigenetic alterations of preneoplastic and neoplastic lesions.
Cancer Biomark. 9:493–507. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kimura Y, Noguchi T, Kawahara K, Kashima
K, Daa T and Yokoyama S: Genetic alterations in 102 primary gastric
cancers by comparative genomic hybridization: Gain of 20q and loss
of 18q are associated with tumor progression. Mod Pathol.
17:1328–1337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu CW, Chen GD, Fann CS, Lee AF, Chi CW,
Liu JM, Weier U and Chen JY: Clinical implications of chromosomal
abnormalities in gastric adenocarcinomas. Genes Chromosom Cancer.
35:219–231. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koo SH, Kwon KC, Shin SY, Jeon YM, Park
JW, Kim SH and Noh SM: Genetic alterations of gastric cancer:
Comparative genomic hybridization and fluorescence In situ
hybridization studies. Cancer Genet Cytogenet. 117:97–103. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sakakura C, Hagiwara A, Taniguchi H,
Yamaguchi T, Yamagishi H, Takahashi T, Koyama K, Nakamura Y, Abe T
and Inazawa J: Chromosomal aberrations in human hepatocellular
carcinomas associated with hepatitis C virus infection detected by
comparative genomic hybridization. Br J Cancer. 80:2034–2039. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Stocks S, Pratt N, Sales M, Johnston DA,
Thompson AM, Carey FA and Kernohan NM: Chromosomal imbalances in
gastric and esophageal adenocarcinoma: Specific comparative genomic
hybridization-detected abnormalities segregate with junctional
adenocarcinomas. Genes Chromosomes Cancer. 32:50–58. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Göhring G, Michalova K, Beverloo HB, Betts
D, Harbott J, Haas OA, Kerndrup G, Sainati L, Bergstraesser E,
Hasle H, et al: Complex karyotype newly defined: The strongest
prognostic factor in advanced childhood myelodysplastic syndrome.
Blood. 116:3766–3769. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Orozco JJ and Appelbaum FR: Unfavorable,
complex, and monosomal karyotypes: The most challenging forms of
acute myeloid leukemia. Oncology (Williston Park). 26:706–712.
2012.PubMed/NCBI
|
|
28
|
Höglund M, Frigyesi A, Säll T, Gisselsson
D and Mitelman F: Statistical behavior of complex cancer
karyotypes. Genes Chromosomes Cancer. 42:327–341. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sakakura C, Hagiwara A, Nakanishi M,
Shimomura K, Takagi T, Yasuoka R, Fujita Y, Abe T, Ichikawa Y,
Takahashi S, et al: Differential gene expression profiles of
gastric cancer cells established from primary tumour and malignant
ascites. Brit J Cancer. 87:1153–1161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Washington K, Gottried MR and Telen MJ:
Expression of the cell adhesion molecule CD44 in gastric
adenocarcinomas. Hum Pathol. 25:1043–1049. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yamaguchi K, Ura H, Yasoshima T, Shishido
T, Denno R and Hirata K: Establishment and characterization of a
human gastric carcinoma cell line that is highly metastatic to
lymph nodes. J Exp Clin Cancer Res. 19:113–120. 2000.PubMed/NCBI
|
|
32
|
Mayer B, Lorenz C, Babic R, Jauch KW,
Schildberg FW, Funke I and Johnson JP: Expression of leukocyte cell
adhesion molecules on gastric carcinomas: Possible involvement of
LFA-3 expression in the development of distant metastases. Int J
Cancer. 64:415–423. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wakatsuki K, Yamada Y, Narikiyo M, Ueno M,
Takayama T, Tamaki H, Miki K, Matsumoto S, Enomoto K, Yokotani T
and Nakajima Y: Clinicopathological and prognostic significance of
mucin phenotype in gastric cancer. J Surg Oncol. 98:124–129. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xin Y, Grace A, Gallagher MM, Curran BT,
Leader MB and Kay EW: CD44V6 in gastric carcinoma: A marker of
tumor progression. Appl Immunohistochem Mol Morphol. 9:138–142.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chen J, Zhou J, Lu J, Xiong H, Shi X and
Gong L: Significance of CD44 expression in head and neck cancer: A
systemic review and meta-analysis. BMC Cancer. 14:152014.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ohtani H, Nakayama T and Yoshie O: In situ
expression of the CCL20-CCR6 axis in lymphocyte-rich gastric cancer
and its potential role in the formation of lymphoid stroma. Pathol
Int. 61:645–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arigami T, Natsugoe S, Uenosono Y,
Yanagita S, Arima H, Hirata M, Ishigami S and Aikou T: CCR7 and
CXCR4 expression predicts lymph node status including
micrometastasis in gastric cancer. Int J Oncol. 35:19–24. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jöhrer K, Zelle-Rieser C, Perathoner A,
Moser P, Hager M, Ramoner R, Gander H, Höltl L, Bartsch G, Greil R
and Thurnher M: Up-regulation of functional chemokine receptor CCR3
in human renal cell carcinoma. Clin Cancer Res. 11:2459–2465. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rubie C, Oliveira V, Kempf K, Wagner M,
Tilton B, Rau B, Kruse B, Konig J and Schilling M: Involvement of
chemokine receptor CCR6 in colorectal cancer metastasis. Tumor
Biol. 27:166–174. 2006. View Article : Google Scholar
|
|
40
|
Koizumi K, Hojo S, Akashi T, Yasumoto K
and Saiki I: Chemokine receptors in cancer metastasis and cancer
cell-derived chemokines in host immune response. Cancer Sci.
98:1652–1658. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sugasawa H, Ichikura T, Tsujimoto H,
Kinoshita M, Morita D, Ono S, Chochi K, Tsuda K, Seki S and
Mochizuki H: Prognostic significnce of expression of CCL5/RANTES
receptors in patients with gastric cancer. J Surg Oncol.
97:445–450. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang Y, Du L, Yang X, Qu A, Zhang X, Zhou
C and Wang C: Aberrant CCR4 expression is involved in tumor
invasion of lymph node-negative human gastric cancer. PLoS One.
10:e01200592015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Carl-McGrath S, Lendeckel U, Ebert M,
Wolter AB, Roessner A and Röcken C: The ectopeptidases CD10, CD13,
CD26, and CD143 are upregulated in gastric cancer. Int J Oncol.
25:1223–1232. 2004.PubMed/NCBI
|
|
44
|
Namikawa T and Hanazaki K: Mucin phenotype
of gastric cancer and clinicopathology of gastric-type
differentiated adenocarcinoma. World J Gastroenterol. 16:4634–4639.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Barresi V, Vitarelli E, Grosso M, Tuccari
G and Barresi G: Relationship between immunoexpression of mucin
peptide cores MUC1 and MUC2 and Lauren's histologic subtypes of
gastric carcinomas. Eur J Histochem. 50:301–309. 2006.PubMed/NCBI
|
|
46
|
Yalcin S, Yildiz Y and Sokmensuer C:
Frequency of c-Met, HGF, and HER-2 expression and evaluation of
their association with clinicopathologic and prognostic factors in
gastric cancer. J Clin Oncol. 33 3 Suppl:S882015. View Article : Google Scholar
|
|
47
|
Teng L and Lu J: cMET as a potential
therapeutic target in gastric cancer (Review). Int J Mol Med.
32:1247–1254. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhu GJ, Xu CW, Fang MY, Zhang YP and Li Y:
Detection of Her2/neu expression in gastric cancer: Quantitative
PCR versus immunohistochemistry. Exp Ther Med. 8:1501–1507. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zheng HC, Takahashi H, Murai Y, Zui ZG,
Nomoto K, Miwa S, Tsuneyama K and Takano Y: Upregulated
EMMPRIN/CD147 might contribute to growth and angiogenesis of
gastric carcinoma: A good marker for local invasion and prognosis.
Br J Cancer. 95:1371–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Fidler IJ: Critical factors in the biology
of human cancer metastasis: Twenty-eighth G.H.A. Clowes memorial
award lecture. Cancer Res. 50:6130–6138. 1990.PubMed/NCBI
|
|
51
|
Sharkey FE and Fogh J: Metastasis of human
tumors in athymic nude mice. Int J Cancer. 24:733–738. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zheng MJ, Wang J, Chen YW, Xu L, Xue DD,
Fu W, Zhang YF, Du Q, Zhao Y, Ling LJ, et al: A novel mouse model
of gastric cancer with human gastric microenvironment. Cancer Lett.
325:108–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kyriazis AP, DiPersio L, Michael GJ, Pesce
AJ and Stinnett JD: Growth patterns and metastatic behavior of
human tumors growing in athymic mice. Cancer Res. 38:3186–3190.
1978.PubMed/NCBI
|
|
54
|
Chen T, Yang K, Yu J, Meng W, Yuan D, Bi
F, Liu F, Liu J, Dai B, Chen X, et al: Identification and expansion
of cancer stem cells in tumor tissues and peripheral blood derived
from gastric adenocarcinoma patients. Cell Res. 22:248–258. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wakamatsu Y, Sakamoto N, Oo HZ, Naito Y,
Uraoka N, Anami K, Sentani K, Oue N and Yasui W: Expression of
cancer stem cell markers ALDH1, CD44 and CD133 in primary tumor and
lymph node metastasis of gastric cancer. Pathol Int. 62:112–119.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wu S, Xue W, Huang X, Yu X, Luo M, Huang
Y, Liu Y, Bi Z, Qiu X and Bai S: Distinct prognostic values of
ALDH1 isoenzymes in breast cancer. Tumor Biol. 36:2421–2426. 2015.
View Article : Google Scholar
|
|
57
|
You Q, Guo H and Xu D: Distinct prognostic
values and potential drug targets of ALDH1 isoenzymes in
non-small-cell lung cancer. Drug Des Devel Ther. 9:5087–5097. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Jia J, Parikh H, Xiao W, Hoskins JW,
Pflicke H, Liu X, Collins I, Zhou W, Wang Z, Powell J, et al: An
integrated transcriptome and epigenome analysis identifies a novel
candidate gene for pancreatic cancer. BMC Med Genomics. 6:332013.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kong B, Wu W, Cheng T, Schlitter AM, Qian
C, Bruns P, Jian Z, Jäger C, Regel I, Raulefs S, et al: A subset of
metastatic pancreatic ductal adenocarcinomas depends quantitatively
on oncogenic Kras/Mek/Erk-induced hyperactive mTOR signalling. Gut.
65:647–657. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Saw YT, Yang J, Ng SK, Liu S, Singh S,
Singh M, Welch WR, Tsuda H, Fong WP, Thompson D, et al:
Characterization of aldehyde dehydrogenase isozymes in ovarian
cancer tissues and sphere cultures. BMC Cancer. 12:3292012.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Mao P, Joshi K, Li J, Kim SH, Li P,
Santana-Santos L, Luthra S, Chandran UR, Benos PV, Smith L, et al:
Mesenchymal glioma stem cells are maintained by activated
glycolytic metabolism involving aldehyde dehydrogenase 1A3. Proc
Natl Acad Sci USA. 110:pp. 8644–8649. 2013; View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li K, Guo X, Wang Z, Li X, Bu Y, Bai X,
Zheng L and Huang Y: The prognostic roles of ALDH1 isoenzymes in
gastric cancer. Onco Targets Ther. 9:3405–3414. 2016.PubMed/NCBI
|
|
63
|
Goedde HW and Agarwal DP: Pharmacogenetics
of aldehyde dehydrogenase (ALDH). Pharmacol Ther. 45:345–371. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Hidaka A, Sasazuki S, Matsuo K, Ito H,
Sawada N, Shimazu T, Yamaji T, Iwasaki M, Inoue M and Tsugane S;
JPHC Study Group, : Genetic polymorphisms of ADH1B, ADH1C and
ALDH2, alcohol consumption, and the risk of gastric cancer: The
Japan Public Health Center-based prospective study. Carcinogenesis.
36:223–231. 2015. View Article : Google Scholar : PubMed/NCBI
|