Introduction
Colorectal cancer (CRC) is the third most commonly
diagnosed cancer in men and the second most common in women
worldwide, with an estimated 1.4 million cases and 693,900 deaths
occurring in 2012 (1). In several
Asian countries, the incidence of CRC is increasing, which may be
related to an increased prevalence of risk factors for CRC,
including unhealthy diet, obesity, and smoking (2,3). Although
early CRC diagnosis and treatment strategies have improved in
recent decades, most patients still present with advanced disease
at diagnosis, and their prognoses remain poor (4). The main reason is that CRC is usually
diagnosed at late stages, often with lymph node or distant
metastases. In the past decade, the survival of metastatic CRC
patients has significantly improved due to the development of new
combinations of chemotherapy drugs, including 5-fluorouracil,
irinotecan, and oxaliplatin. In particular, the introduction of new
targeted therapies such as monoclonal antibodies (cetuximab and
panitumumab) against epidermal growth factor receptor (EGFR) or
monoclonal antibodies (bevacizumab, ramucirumab, and aflibercept)
against vascular endothelial growth factor has made a large impact.
The addition of targeted therapies to chemotherapy regimens results
in increased toxicity and treatment costs (5) and therefore requires the identification
of markers to identify patients who are likely to respond to them.
The chimeric IgG1 monoclonal antibody cetuximab is effective for
patients with wild-type RAS (6,7) and
left-side CRC patients (8,9). However, the response rate (RR) is 40–60%
for CRC patients with wild-type RAS. Therefore, approximately 50%
of patients with wild-type RAS are still resistant to cetuximab.
Identifying a novel predictor for improving therapy would be
significant. In the present study, we explored new predictive
markers correlated with cetuximab efficacy in metastatic CRC
(mCRC).
SGLT1, an active glucose transporter that maintains
enough glucose for cell survival, was downregulated by EGFR
knockdown in cancer cells (10).
Cetuximab, which targets EGFR, may also affect SGLT1 function and
glucose metabolism. Our previous study found that SGLT1 expression
(P<0.010) was higher in colon cancer tissues than in normal
tissues and was related to clinical stage (P=0.030) (11). We found that in an in vitro
colon cancer cell line, cetuximab treatment downregulated
hexokinase 2 (HK2), the key enzyme for aerobic glycolysis. Based on
these results, we considered it significant to study the connection
between aerobic glycolysis and cetuximab efficacy in colon
cancer.
Aerobic glycolysis is a hallmark of cancer cells,
which exhibit lactate production even in the presence of ample
oxygen, a phenomenon known as the Warburg effect (12). An important advantage of the increased
glycolysis of tumor cells is the production of energy without the
consumption of oxygen and glycolytic intermediates, such as amino
acids, nucleotides, phospholipids and triglycerides, which are used
as macromolecules to synthesize structural elements for new cells.
Three key enzymes, namely, HK2, pyruvate kinase muscle isozyme M2
(PKM2) and lactate dehydrogenase A (LDHA), are critical glycolysis
regulators. Although the expression of HK2, PKM2 and LDHA has been
individually reported to be correlated with cancer cell growth,
their predictive role in patients with mCRC remains unclear. In the
present study, we evaluated the clinical value of these glycolytic
enzymes in predicting the effect of cetuximab on mCRC. HK2, PKM2
and LDHA expression in CRC and normal tissue was compared. The
correlation of their expression with clinical pathological
features, the progression-free survival (PFS) of first-line
palliative therapy and survival was evaluated to explore their
predictive and prognostic values for CRC treated with
cetuximab.
Patients and methods
Patient eligibility
Patients with mCRC who had ever been administered
cetuximab combined with first-line or later chemotherapy at our
institution between January 1, 2005, and October 1, 2015, were
identified. Although Cetuximab was first introduced into China in
2006, some people had enough money to buy cetuximab in 2005 in Hong
Kong. That is why patients in the present study accepted cetuximab
during 2005–2015. At first, 235 patients were enrolled, but only 68
patients with paraffin embedding tissue were available for
immunohistochemical testing. All of the eligible patients in the
present study satisfied the following requirements: i) intact
medical data, ii) explicit pathological diagnosis of colon cancer,
iii) at least two experts confirming metastasis based on clinical
and imaging methods, iv) at least two cycles of palliative
chemotherapy with or without first-line cetuximab and v) sufficient
cancer specimens in the archives of the Tissue Bank to test HK2,
PKM2 and LDHA expression. Furthermore, we collected negative margin
specimens from most patients as normal tissue. Written informed
consent was received from the sample donors, and approval was
granted by the Institute Research Medical Ethics Committee of Sun
Yat-sen University (Guangzhou, China). The basic clinical
characteristics for all of the patients are reported in Table I.
 | Table I.Correlation between HK2, PKM2 and LDHA
expression and clinicopathological characteristics in CRC
patients. |
Table I.
Correlation between HK2, PKM2 and LDHA
expression and clinicopathological characteristics in CRC
patients.
|
|
| HK2 expression | PKM2 expression | LDHA expression |
|---|
|
|
|
|
|
|
|---|
| Variable | N=68 (%) | Low | High | P | Low | High | P | Low | High | P-value |
|---|
| Sex |
|
|
| 0.663 |
|
| 0.973 |
|
| 0.891 |
| Male | 44 (64.7) | 10 | 28 |
| 11 | 28 |
| 15 | 21 |
|
|
Female | 24 (35.3) | 4 | 15 |
| 5 | 13 |
| 10 | 13 |
|
| Age, years |
|
|
| 0.807 |
|
| 0.829 |
|
| 0.549 |
|
<65 | 55 (80.9) | 11 | 37 |
| 13 | 36 |
| 19 | 28 |
|
| ≥65 | 13 (19.1) | 3 | 6 |
| 3 | 5 |
| 6 | 6 |
|
| Family history of
cancer |
|
|
| 0.816 |
|
| 0.236 |
|
| 0.819 |
| No | 55 (80.9) | 11 | 35 |
| 15 | 31 |
| 20 | 28 |
|
| Yes | 13 (19.1) | 3 | 8 |
| 1 | 10 |
| 5 | 6 |
|
| Tumor location |
|
|
| 0.895 |
|
| 0.685 |
|
| 0.935 |
|
Left-side | 41 (60.3) | 7 | 28 |
| 10 | 27 |
| 18 | 19 |
|
|
Right-side | 14 (20.6) | 2 | 9 |
| 4 | 6 |
| 6 | 6 |
|
| NA | 13 (19.1) |
|
|
|
|
|
|
|
|
|
| Pathological
differentiation |
|
|
| 0.298 |
|
| 0.955 |
|
| 0.623 |
|
Low | 17 (25.0) | 5 | 8 |
| 4 | 10 |
| 6 | 10 |
|
| Median
+ high | 44 (64.7) | 7 | 30 |
| 10 | 26 |
| 17 | 21 |
|
| NA | 7 (10.3) |
|
|
|
|
|
|
|
|
|
| T stage |
|
|
| 0.780 |
|
| 0.747 |
|
| 0.07 |
| T1 + T2
+ T3 | 41 (60.3) | 9 | 26 |
| 11 | 25 |
| 11 | 23 |
|
| T4 | 22 (32.4) | 4 | 14 |
| 4 | 14 |
| 12 | 9 |
|
| NA | 5 (7.4) |
|
|
|
|
|
|
|
|
|
| N stage |
|
|
| 0.15 |
|
| 0.839 |
|
| 0.903 |
| N0 | 22 (32.4) | 3 | 18 |
| 4 | 16 |
| 7 | 10 |
|
| N1 +
N2 | 36 (52.9) | 9 | 19 |
| 8 | 22 |
| 13 | 20 |
|
| NA | 10 (14.7) |
|
|
|
|
|
|
|
|
|
|
Synchronous/metachronous metastasis |
|
|
| 0.091 |
|
| 0.586 |
|
| 0.569 |
| I + II
+ III | 23 (33.8) | 8 | 13 |
| 7 | 14 |
| 7 | 12 |
|
| IV | 43 (63.2) | 6 | 28 |
| 9 | 25 |
| 17 | 21 |
|
| NA | 2 (2.9) |
|
|
|
|
|
|
|
|
|
Immunohistochemistry (IHC)
Standard immunohistochemical procedures were
performed to determine the location and levels of HK2, PKM2 and
LDHA expression in CRCs. Briefly, formalin-fixed paraffin-embedded
(FFPE) tissue blocks were cut into 4 µm sections for IHC and
hematoxylin and eosin staining. Polyclonal rabbit anti-HK2
antibody, polyclonal rabbit anti-PKM2 antibody or polyclonal rabbit
anti-LDHA antibody (all Cell Signaling Technology, Danvers, MA,
USA) diluted 1:150 was used in the present study. Tissue sections
were de-waxed in xylene, rehydrated through a graded ethanol
series, and incubated in retrieval buffer solution for antigen
recovery. Next, the samples were incubated with hydrogen peroxide
for 10 min to block intrinsic peroxidases, washed in phosphate
buffered saline (PBS), and blocked with normal serum for 10 min.
The samples were incubated with a primary antibody for 40 min at
37°C followed by EnVision ™ Detection systems (DAKO; Agilent
Technologies, Inc., Glostrup, Denmark) for 30 min at 37°C. The
signal was visualized with diaminobenzidine (DAB). Negative
controls were obtained by substituting non-immune rabbit serum for
the primary antibodies.
Evaluation of immunostaining
results
Protein expression was evaluated by two individuals
using an Olympus CX31 microscope (Olympus, Center Valley, PA). The
mean percentage of positive tumor cells was determined from five
areas at magnification, ×400 and assigned a 0–100% value. The
intensity of immunostaining was scored as follows: negative, 0;
weak, 1; moderate, 2; and intense, 3. For the percentage of
positive tumor cells, the score was classified as 0–4 (10%, 11–25%,
26–50%, 51–75%, >75%). Thus, the percentage of positive tumor
cells and the staining intensity were multiplied to produce a total
score for each sample. Then, the total HK2 and PKM2 scores were
categorized as low expression (≤3) and high expression (>3)
(13). The total scores for LDHA were
categorized as low expression (<6) and high expression (≥6).
Data analysis
IBM SPSS 20 software was used to perform statistical
analyses. Associations between discrete variables were assessed
using the chi-square test or Fisher's exact test as appropriate.
The statistical test used to analyze data derived from such small
cohorts is Chi square test for continuity correction (14). The Kaplan-Meier method was used to
estimate tumor progression in mCRC, and the log-rank test was used
to determine the statistical significance. The groups were compared
with respect to survival using Cox regression analysis. Hazard
ratios were determined using univariate and multivariate Cox
regression analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Basic patient characteristics
A total of 68 patients consisting of 42 men (61.8%)
and 26 women (38.2%) were included in the present study. The age of
the patients ranged from 23 to 77 years, with a median age of 54.5
years. Overall survival (OS) was analyzed in 68 patients (98.5%),
whereas PFS was assessed in 63 patients (92.6%). The median PFS for
first-line chemotherapy was 7.4 months (0.5–32.9), and the median
OS was 29.9 months (2.2–137). The objective response rate (ORR) and
disease control rate (DCR) were 47.8 and 71.6% based on data from
67 patients. The basic clinical and pathological characteristics of
all the cases and their correlations with HK2, PKM2 and LDHA are
shown in Table I. Both N1 and N2
indicated lymph node metastasis. Besides, the total cases was
small, separating N1 and N2 will make the subgroup number smaller.
Therefore we combined N1 and N2 as a group.
HK2 is significantly upregulated in
CRC tissues
According to the quantitative scoring method
described before, HK2, PKM2 and LDHA were divided into high
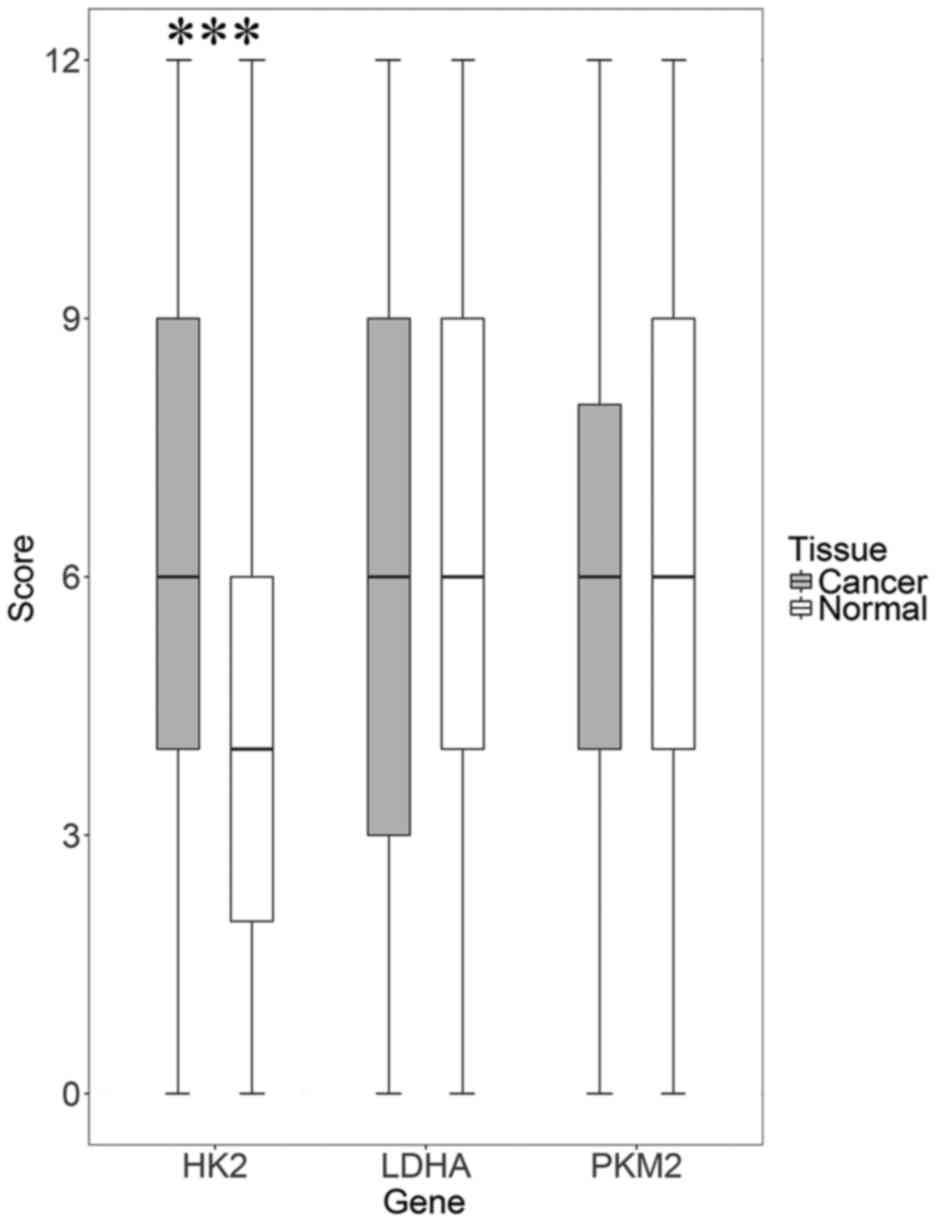
expression and low expression. As shown in Fig. 1, HK2 expression was significantly
upregulated in tumor specimens compared to normal specimens (75.4%
vs. 40%, P<0.001). However, PKM2 and LDHA expression did not
differ between CRC and normal tissue (71.9% vs. 61.2%, P=0.243 and
57.6% vs. 40%, P=0.067). The overall average in expression has no
difference, but there is still some people have strong staining in
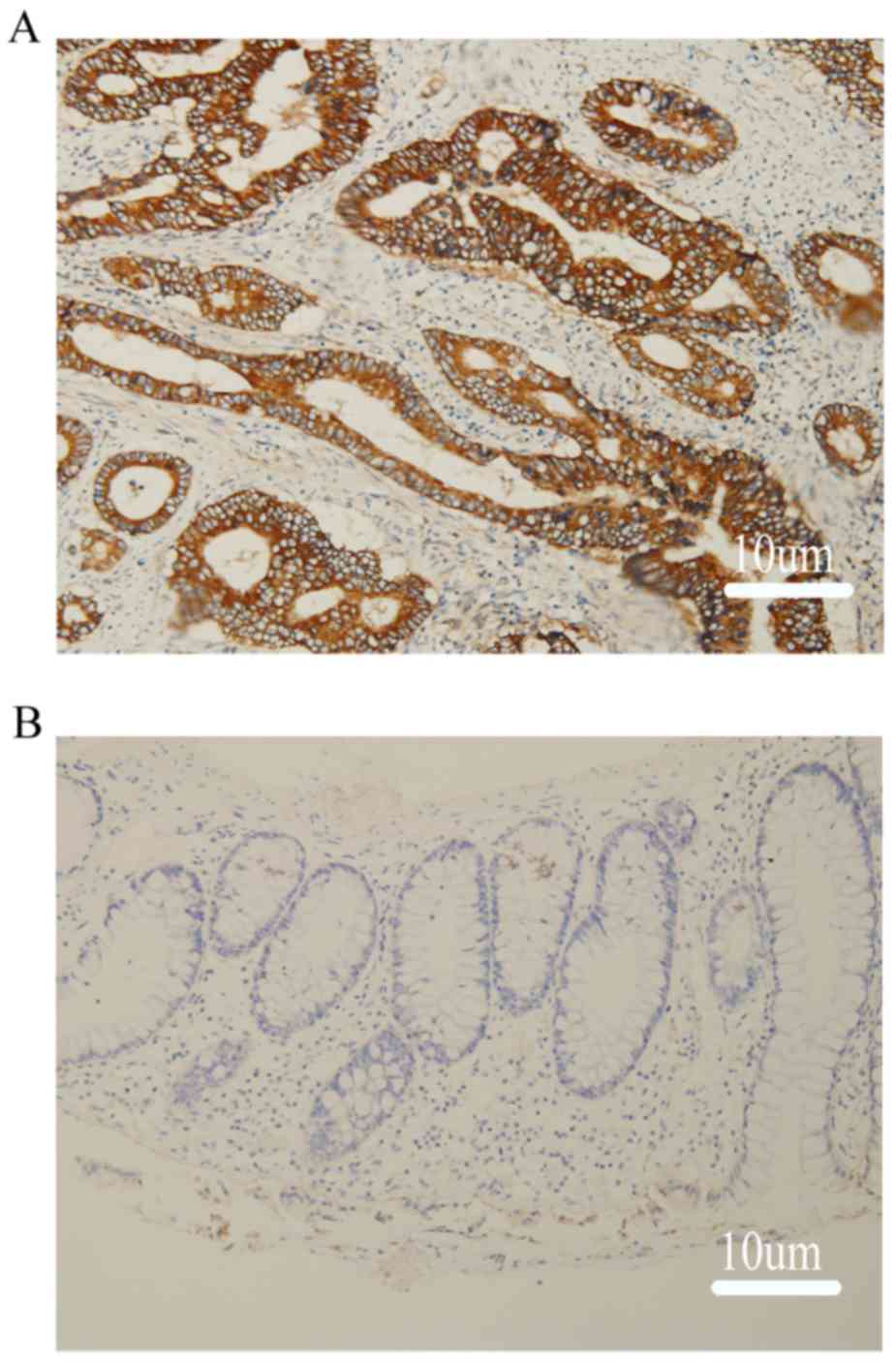
cancer tissue and low staining in normal tissue. We performed
immunohistochemical staining for HK2, LDHA and PKM2 in CRC samples
and in negative margins as a normal tissue control (Fig. 2).
Correlation between HK2, PKM2 and LDHA
expression and clinicopathological parameters in CRC patients
The correlation between HK2 expression and the
corresponding clinicopathological parameters of CRC patients were
calculated using the Pearson chi-square test. The results are shown
in Table I. HK2, PKM2 and LDHA
expression was not significantly correlated with sex, age, family
history of cancer, tumor location, pathological differentiation, T
stage, N stage or synchronous/metachronous metastasis.
Predictive value of HK2, PKM2 and LDHA
expression in CRC patients treated with cetuximab
We studied the correlation of HK2, PKM2 and LDHA in
cancer tissue with PFS in two groups: one group of 33 patients
treated with cetuximab plus first-line chemotherapy and another
group of 35 cases administered chemotherapy alone. As shown in
Table II, when a combination of
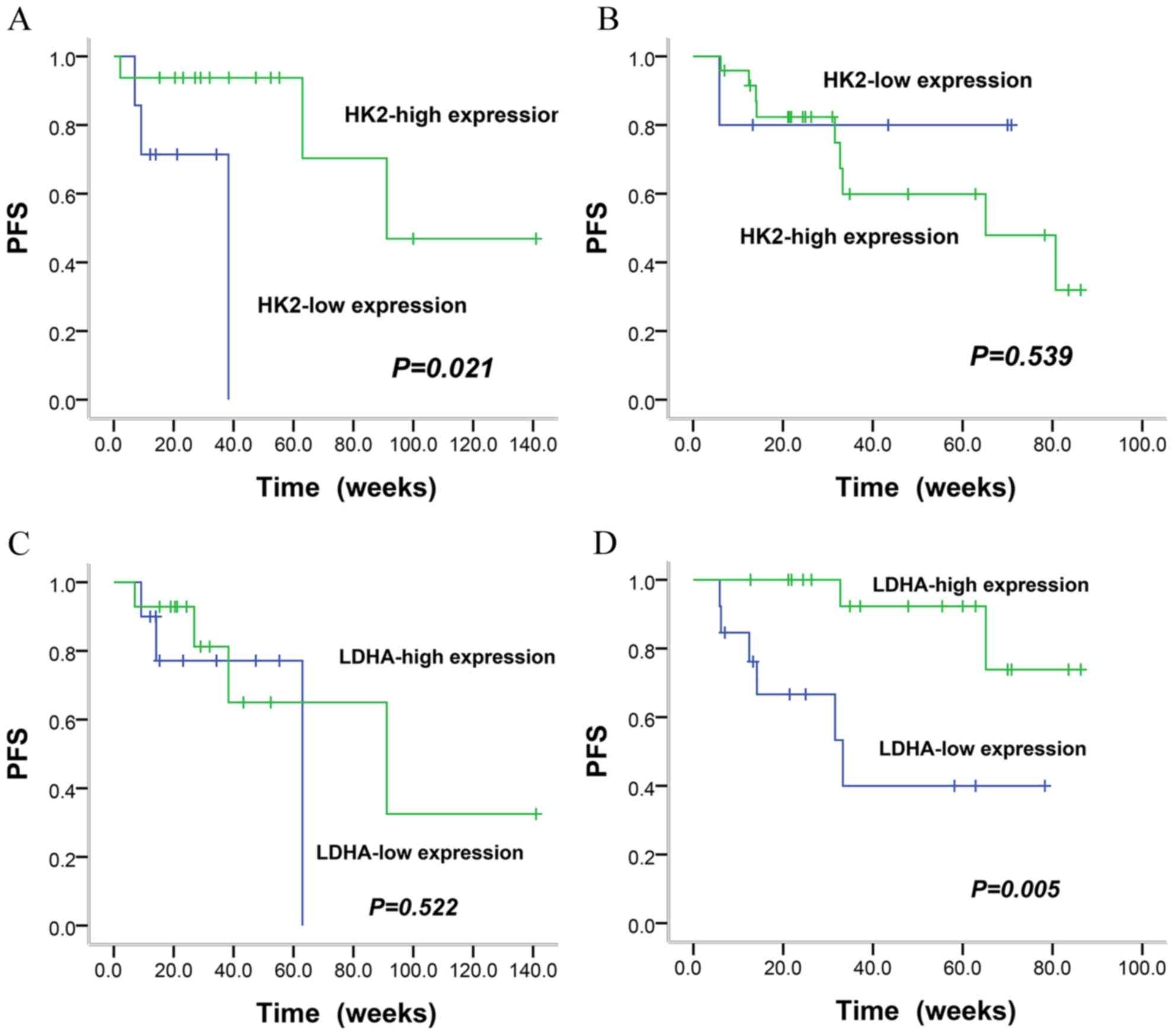
cetuximab and chemotherapy was administered, patients with high HK2
expression had longer first-line PFS than those patients with low
HK2 expression (23.9 months vs. 6.9 months; P=0.021) (Fig. 3A). However, the PFS of patients
receiving chemotherapy alone did not differ between the groups with
high HK2 expression and low HK2 expression (13.4 months vs. 13.5
months, P=0.539) (Fig. 3B). In
contrast to HK2, LDHA expression was associated with first-line PFS
in patients treated with chemotherapy alone, with a PFS of 18.3 and
10.1 months for high and low expression, respectively (P=0.005)
(Fig. 3D), whereas this relationship
was not observed in cases treated with first-line cetuximab plus
chemotherapy (19.9 months vs. 12 months, P=0.522) (Fig. 3C). Furthermore, high LDHA expression
correlated with high ORR (72.2% vs. 15.4%, P=0.006) but not DCR
(88.9% vs. 53.8%, P=0.074) in patients who received chemotherapy.
Neither DCR nor ORR was related to HK2 expression regardless of
first-line therapy with or without cetuximab. Additionally, PKM2
expression had no effect on PFS, DCR or ORR for first-line therapy
with or without cetuximab.
 | Table II.Relationship of HK2, PKM2 and LDHA
with the short-efficacy of cetuximab plus chemotherapy vs.
chemotherapy alone in the first line of mCRC patients. |
Table II.
Relationship of HK2, PKM2 and LDHA
with the short-efficacy of cetuximab plus chemotherapy vs.
chemotherapy alone in the first line of mCRC patients.
|
| Factors | DCR | ORR | PFS |
|---|
|
|
|
|
|
|
|---|
|
Subgroup/factors | Expression | N (%) | N (%) | P | N (%) | P | Months | P-value |
|---|
| Chemotherapy plus
cetuximab |
| 33 |
|
|
|
|
|
|
| HK2 | High | 19 (57.6) | 16 (84.2) | 0.102 | 10 (52.6) | 0.686 | 23.9 | 0.021 |
|
| Low | 9 (27.3) | 5 (55.6) |
| 4 (44.4) |
| 6.9 |
|
|
| NA | 5 (15.1) |
|
|
|
|
|
|
| PKM2 | High | 19 (57.6) | 14 (73.7) | 0.740 | 10 (52.6) | 0.658 | 22.1 | 0.84 |
|
| Low | 7 (21.2) | 4 (57.1) |
| 3 (42.9) |
| 12.8 |
|
|
| NA | 7 (21.2) |
|
|
|
|
|
|
| LDHA | High | 16 (48.5) | 10 (62.5) | 0.962 | 5 (41.7) | 0.930 | 19.9 | 0.522 |
|
| Low | 12 (36.4) | 9 (75.0) |
| 6 (40.0) |
| 12 |
|
|
| NA | 5 (15.1) |
|
|
|
|
|
|
| Chemotherapy
alone |
| 35 |
|
|
|
|
|
|
| HK2 | High | 24 (68.6) | 15 (62.5) | 0.817 | 10 (41.7) | 0.285 | 13.4 | 0.539 |
|
| Low | 5 (14.3) | 4 (80.0) |
| 4 (80.0) |
| 13.5 |
|
|
| NA | 6 (17.1) |
|
|
|
|
|
|
| PKM2 | High | 22 (62.9) | 16 (72.7) | 0.613 | 11 (50.0) | 0.779 | 15.1 | 0.331 |
|
| Low | 9 (25.7) | 5 (55.6) |
| 4 (44.4) |
| 12.8 |
|
|
| NA | 4 (11.4) |
|
|
|
|
|
|
| LDHA | High | 18 (51.5) | 16 (88.9) | 0.074 | 13 (72.2) | 0.006 | 18.3 | 0.005 |
|
| Low | 13 (37.1) | 7 (53.8) |
| 2 (15.4) |
| 10.1 |
|
|
| NA | 4 (11.4) |
|
|
|
|
|
|
Prognostic value of HK2, PKM2 and LDHA
in mCRC patients for cetuximab and chemotherapy treatment
For all patients, HK2, PKM2 and LDHA had no impact
on OS (P=0.462, P=0.418 and P=0.243) by the Kaplan-Meier method. By
univariate Cox regression analysis, LDHA expression (P=0.016) and
tumor site (P=0.035) were risk factors for first-line PFS, and
pathological differentiation (P<0.001) and
synchronous/metachronous metastasis (P=0.043) were risk factors
affecting OS. Multivariate Cox regression analysis revealed that
LDHA expression (P=0.005), pathological differentiation (P=0.019)
and synchronous/metachronous metastasis (P=0.014) were independent
prognostic factors for PFS, and pathological differentiation was a
prognostic factor for OS (P=0.002). The detailed data are shown in
Table III.
 | Table III.Univariate and multivariate analysis
of predictive and prognostic factors for survival in patients with
CRC. |
Table III.
Univariate and multivariate analysis
of predictive and prognostic factors for survival in patients with
CRC.
|
| PFS |
|---|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
|
| HRa (95%
CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P | HR (95% CI) | P-value |
|---|
| HK2 expression | 0.639
(0.200–2.044) | 0.450 |
|
| 0.751
(0.350–1.613) | 0.463 |
|
|
| PKM2
expression | 0.594
(0.215–1.639) | 0.314 |
|
| 1.384
(0.628–3.052) | 0.420 |
|
|
| LDHA
expression | 0.254
(0.083–0.774) | 0.016 | 0.061
(0.009–0.429) | 0.005 | 1.475
(0.765–2.844) | 0.246 |
|
|
| Sex | 1.470
(0.578–3.742) | 0.419 |
|
| 0.858
(0.446–1.650) | 0.647 |
|
|
| Age | 1.026
(0.294–3.579) | 0.968 |
|
| 1.268
(0.602–2.669) | 0.532 |
|
|
| Family history of
caner | 1.095
(0.383–3.133) | 0.866 |
|
| 1.223
(0.581–2.573) | 0.597 |
|
|
| Tumor location | 3.087
(1.081–8.817) | 0.035 |
|
| 1.223
(0.581–2.573) | 0.597 |
|
|
| Pathological
differentiation | 1.893
(0.636–5.640) | 0.252 | 9.902
(1.464–66.977) | 0.019 | 4.021
(1.973–8.196) | 0.000 | 4.255
(1.695–10.682) | 0.002 |
| T stage | 2.391
(0.836–6.838) | 0.104 |
|
| 1.293
(0.675–2.476) | 0.438 |
|
|
| N stage | 2.877
(0.916–9.031) | 0.070 |
|
| 1.477
(0.773–2.823) | 0.237 |
|
|
|
Synchronous/metachronous metastasis | 0.551
(0.217–1.399) | 0.210 | 0.120
(0.022–0.646) | 0.014 | 1.994
(1.023–3.887) | 0.043 |
|
|
Discussion
Although many new predictive markers have been found
in other cancers in the era of precision medicine, targeted therapy
for mCRC has shown little progress. Therefore, understanding how to
assess known target molecules is important for treating mCRC. We
evaluated the role of the key glycolytic enzymes HK2, PKM2 and LDHA
for predicting cetuximab efficacy in mCRC. We found that HK2
expression was higher in CRC tissue. HK2 predicts cetuximab
efficacy as a first-line therapy, and LDHA is predictive for
chemotherapy. PKM2 did not predict cetuximab efficacy. For patients
who received cetuximab as first- or later-line treatment, LDHA
expression, pathological differentiation and
synchronous/metachronous metastasis were independent prognostic
factors for PFS, and pathological differentiation was an
independent prognostic factor for OS. HK2, PKM2 and LDHA did not
impact OS.
Some studies have reported that HK2 expression was
higher in tumor tissue than adjacent normal tissue in many
malignant tumor types, such as gastric cancers (15), renal cell carcinomas (16), and hepatocellular carcinomas (17). The present study also found that HK2
was overexpressed in CRC samples compared to normal tissue (75.4%
vs. 40%, P<0.001), which is consistent with a malignant tumor
depending on glycolysis to survive. Patra et al (18) confirmed that HK2 is required for tumor
initiation and maintenance in mouse cancer models. Tumor cells are
dependent on HK2 for proliferation. In the present study, we found
that high HK2 expression was correlated with better PFS when
treated by cetuximab plus chemotherapy compared with low expression
(23.9 months vs. 6.9 monthsl; P=0.021), suggesting that cetuximab
plus chemotherapy will have greater efficacy in patients with high
HK2 expression. HK2 expression has association with PFS but no with
ORR and DCR, which may result from bias of small samples.
Simultaneously, for patients treated with chemotherapy alone, HK2
had no effect on PFS. Therefore, we concluded that HK2 may be a new
marker for predicting cetuximab efficacy.
In the present study, we observed an interesting
phenomenon in which high LDHA expression was correlated with ORR
and PFS in patients treated with chemotherapy alone compared to
those treated with chemotherapy plus cetuximab. In patients treated
with chemotherapy alone, high LDHA expression indicated a better
ORR (72.2% vs. 15.4%, P=0.006) and PFS (18.3 months vs. 10.1
months, P=0.005). However, in patients treated with chemotherapy
plus cetuximab, LDHA expression had no effect on ORR or PFS. We
conclude that LDHA is a useful predictor for the efficacy of
chemotherapy alone. Both HK2 and LDHA are key glycolysis enzymes,
but they predict the efficacy of chemotherapy or cetuximab in
different ways, which is an interesting phenomenon to explore. As
shown in Table II, the PFS in
patients receiving chemotherapy alone was >10 months in each
subgroup, which was much better than the data shown in CRYSTAL and
OPUS study (19,20). In the present study, the percent of
left-sided colon cancer was higher than that of right-sided colon
cancer in the subgroup receiving chemotherapy alone (81.2% vs.
18.8%). In this subgroup, there is higher rate of male and lower T
stage patients, with a rate of (male 71.4% vs. female 28.6%) and T
stage (T1 + T2 + T3 stage 81.2% vs. T4 stage 18.8%). Maybe this can
explain the PFS in patients receiving chemotherapy alone is >10
months.
In our study, we concluded that the three key
glycolysis enzymes HK2, PKM2, and LDHA had no effect on patient OS.
However, whether that meant that HK2, PKM2 and LDHA were not
prognostic factors for mCRC treated with cetuximab remained
unknown. Many studies have reported that high expression of HK2 was
associated with poor survival outcomes in some tumor types, such as
breast cancer (21), gastric cancer
(15), non-small-cell lung cancer
(22), non-Hodgkin lymphoma (23), nasopharyngeal carcinoma (13), hepatocellular carcinoma (17), cervical squamous cell carcinoma
(24), and pancreatic cancer
(25), among others. A meta-analysis
revealed that HK2 overexpression was significantly associated with
a worse OS and PFS in solid tumors (26). Another study reported that high HK2
expression was associated with reduced recurrence-free survival
(RFS) in CRC patients (27). Our
results regarding HK2 association with PFS or OS in CRC treated
with cetuximab plus chemotherapy are not completely consistent with
studies in other fields, and this discrepancy may be explained by
several reasons. The first is the small number of samples in our
study. Second, we studied different cancers than those in other
reports. Third, we chose only mCRC cases, and our patients received
similar but not identical regimens compared to those of other CRC
studies. A more rigorously designed trial is needed to confirm our
primary results. Additionally, our study shows that pathological
differentiation is an independent prognostic factor for PFS and OS
in CRC patients.
In the present study, we not only confirmed the
selective upregulation of HK2 in CRC cells compared with the
adjacent margin but also verified that HK2 predicts cetuximab
efficacy and that LDHA predicts chemotherapy efficacy. These
findings may aid physicians' decision to administer chemotherapy or
cetuximab plus chemotherapy for a patient in the clinic. This is a
small sample study and has some limits. The results in the present
study gives us a hint: HK2 maybe a good biomarker for the treatment
of cetuximab, but the final conclusion needs more study to
confirm.
Our study had several shortcomings: First, it was
retrospective, the patients received different therapies after
first-line treatment, and the number of cases was small. Second,
our study focus on HK2 expression with cetuximab efficacies, so we
do not detect KRAS exon 3, 4 and NRAS beyond KRAS 2. However, we
identified a new predictor, HK2, for cetuximab treatment efficacy
in mCRC and the association between HK2 and cetuximab was firstly
reported up to our known, so the present study was significant in
spite of small simple. On the other hand we confirmed the
correlation of LDHA and chemotherapy efficacy.
Acknowledgements
The present study was supported by Guangdong
Provincial Natural Science Foundation (grant no. 2017A030313685)
and Guangdong Provincial Traditional Chinese Medicine Bureau of
Scientific Research Projects (grant no. KY040217, 2017).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Edwards BK, Ward E, Kohler BA, Eheman C,
Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I,
Seeff LC, et al: Annual report to the nation on the status of
cancer, 1975–2006, featuring colorectal cancer trends and impact of
interventions (risk factors, screening and treatment) to reduce
future rates. Cancer. 116:544–573. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yee YK, Tan VP, Chan P, Hung IF, Pang R
and Wong BC: Epidemiology of colorectal cancer in Asia. J
Gastroenterol Hepatol. 24:1810–1816. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levin B, Lieberman DA, McFarland B, Smith
RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR,
et al: Screening and surveillance for the early detection of
colorectal cancer and adenomatous polyps, 2008: A joint guideline
from the American Cancer Society, the US Multi-Society Task Force
on Colorectal Cancer and the American College of Radiology. CA
Cancer J Clin. 58:130–160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schrag D: The price tag on
progress-chemotherapy for colorectal cancer. N Engl J Med.
351:317–319. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lievre A, Bachet JB, Le Corre D, Boige V,
Landi B, Emile JF, Côté JF, Tomasic G, Penna C, Ducreux M, et al:
KRAS mutation status is predictive of response to cetuximab therapy
in colorectal cancer. Cancer Res. 66:3992–3995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller
C, Kahl C, Seipelt G, et al: FOLFIRI plus cetuximab versus FOLFIRI
plus bevacizumab as first-line treatment for patients with
metastatic colorectal cancer (FIRE-3): A randomised, open-label,
phase 3 trial. Lancet Oncol. 15:1065–1075. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alan P and Venook DN: Impact of primary
(1º) tumor location on overall survival (OS) and progression-free
survival (PFS) in patients (pts) with metastatic colorectal cancer
(mCRC): Analysis of CALGB/SWOG 80405 (Alliance) [Abstract]. 2016
ASCO Annual Meeting. 34:pp. 35042016;
|
|
9
|
Alan P and Venook DN: Impact of primary
(1º) tumor location on Overall Survival (OS) and Progression Free
Survival (PFS) in patients (pts) with metastatic colorectal cancer
(mCRC): Analysis of All RAS wt patients on CALGB/SWOG 80405
(Alliance) [Abstract]. ESMO Congress. 2016; [Abstract]. ESMO
Congress, 2016.
|
|
10
|
Weihua Z, Tsan R, Huang WC, Wu Q, Chiu CH,
Fidler IJ and Hung MC: Survival of cancer cells is maintained by
EGFR independent of its kinase activity. Cancer Cell. 13:385–393.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guo GF, Cai YC, Zhang B, Xu RH, Qiu HJ,
Xia LP, Jiang WQ, Hu PL, Chen XX, Zhou FF and Wang F:
Overexpression of SGLT1 and EGFR in colorectal cancer showing a
correlation with the prognosis. Med Oncol. 28 Suppl 1:S197–S203.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang MX, Hua YJ, Wang HY, Zhou L, Mai HQ,
Guo X, Zhao C, Huang WL, Hong MH and Chen MY: Long-term prognostic
implications and therapeutic target role of hexokinase II in
patients with nasopharyngeal carcinoma. Oncotarget. 7:21287–21297.
2016.PubMed/NCBI
|
|
14
|
Grizzle E.: J: Continuity Correction in
the χ 2 -Test for 2×2 Tables. 1967.
|
|
15
|
Sun X, Sun Z, Zhu Z, Guan H, Zhang J,
Zhang Y, Xu H and Sun M: Clinicopathological significance and
prognostic value of lactate dehydrogenase A expression in gastric
cancer patients. PLoS One. 9:e910682014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Girgis H, Masui O, White NM, Scorilas A,
Rotondo F, Seivwright A, Gabril M, Filter ER, Girgis AH, Bjarnason
GA, et al: Lactate dehydrogenase A is a potential prognostic marker
in clear cell renal cell carcinoma. Mol Cancer. 13:1012014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang ZF, Feng XS, Chen H, Duan ZJ, Wang
LX, Yang D, Liu PX, Zhang QP, Jin YL, Sun ZG and Liu H: Prognostic
significance of synergistic hexokinase-2 and beta2-adrenergic
receptor expression in human hepatocelluar carcinoma after curative
resection. BMC Gastroenterol. 16:572016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Patra KC, Wang Q, Bhaskar PT, Miller L,
Wang Z, Wheaton W, Chandel N, Laakso M, Muller WJ, Allen EL, et al:
Hexokinase 2 is required for tumor initiation and maintenance and
its systemic deletion is therapeutic in mouse models of cancer.
Cancer Cell. 24:213–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bokemeyer C, Bondarenko I, Makhson A,
Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G,
Stroh C, et al: Fluorouracil, leucovorin, and oxaliplatin with and
without cetuximab in the first-line treatment of metastatic
colorectal cancer. J Clin Oncol. 27:663–671. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Van Cutsem E, Köhne CH, Hitre E, Zaluski
J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G
and Roh JK: Cetuximab and chemotherapy as initial treatment for
metastatic colorectal cancer. N Engl J Med. 360:1408–1417. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang X, Li X and Xie X, Ye F, Chen B,
Song C, Tang H and Xie X: High expressions of LDHA and AMPK as
prognostic biomarkers for breast cancer. Breast. 30:39–46. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koukourakis MI, Giatromanolaki A, Sivridis
E, Bougioukas G, Didilis V, Gatter KC and Harris AL; Tumour and
Angiogenesis Research Group, : Lactate dehydrogenase-5 (LDH-5)
overexpression in non-small-cell lung cancer tissues is linked to
tumour hypoxia, angiogenic factor production and poor prognosis. Br
J Cancer. 89:877–885. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu R, Jiang M, Chen Z, Xu X, Hu H, Zhao X,
Gao X and Guo L: Lactate dehydrogenase 5 expression in Non-Hodgkin
lymphoma is associated with the induced hypoxia regulated protein
and poor prognosis. PLoS One. 8:e748532013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Huang X, Liu M, Sun H, Wang F, Xie X, Chen
X, Su J, He Y, Dai Y, Wu H and Shen L: HK2 is a radiation resistant
and independent negative prognostic factor for patients with
locally advanced cervical squamous cell carcinoma. Int J Clin Exp
Pathol. 8:4054–4063. 2015.PubMed/NCBI
|
|
25
|
Ogawa H, Nagano H, Konno M, Eguchi H,
Koseki J, Kawamoto K, Nishida N, Colvin H, Tomokuni A, Tomimaru Y,
et al: The combination of the expression of hexokinase 2 and
pyruvate kinase M2 is a prognostic marker in patients with
pancreatic cancer. Mol Clin Oncol. 3:563–571. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Wu K, Shi L, Xiang F, Tao K and
Wang G: Prognostic significance of the metabolic marker
hexokinase-2 in various solid tumors: A meta-analysis. PLoS One.
11:e01662302016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hamabe A, Yamamoto H, Konno M, Uemura M,
Nishimura J, Hata T, Takemasa I, Mizushima T, Nishida N, Kawamoto
K, et al: Combined evaluation of hexokinase 2 and phosphorylated
pyruvate dehydrogenase-E1α in invasive front lesions of colorectal
tumors predicts cancer metabolism and patient prognosis. Cancer
Sci. 105:1100–1108. 2014. View Article : Google Scholar : PubMed/NCBI
|