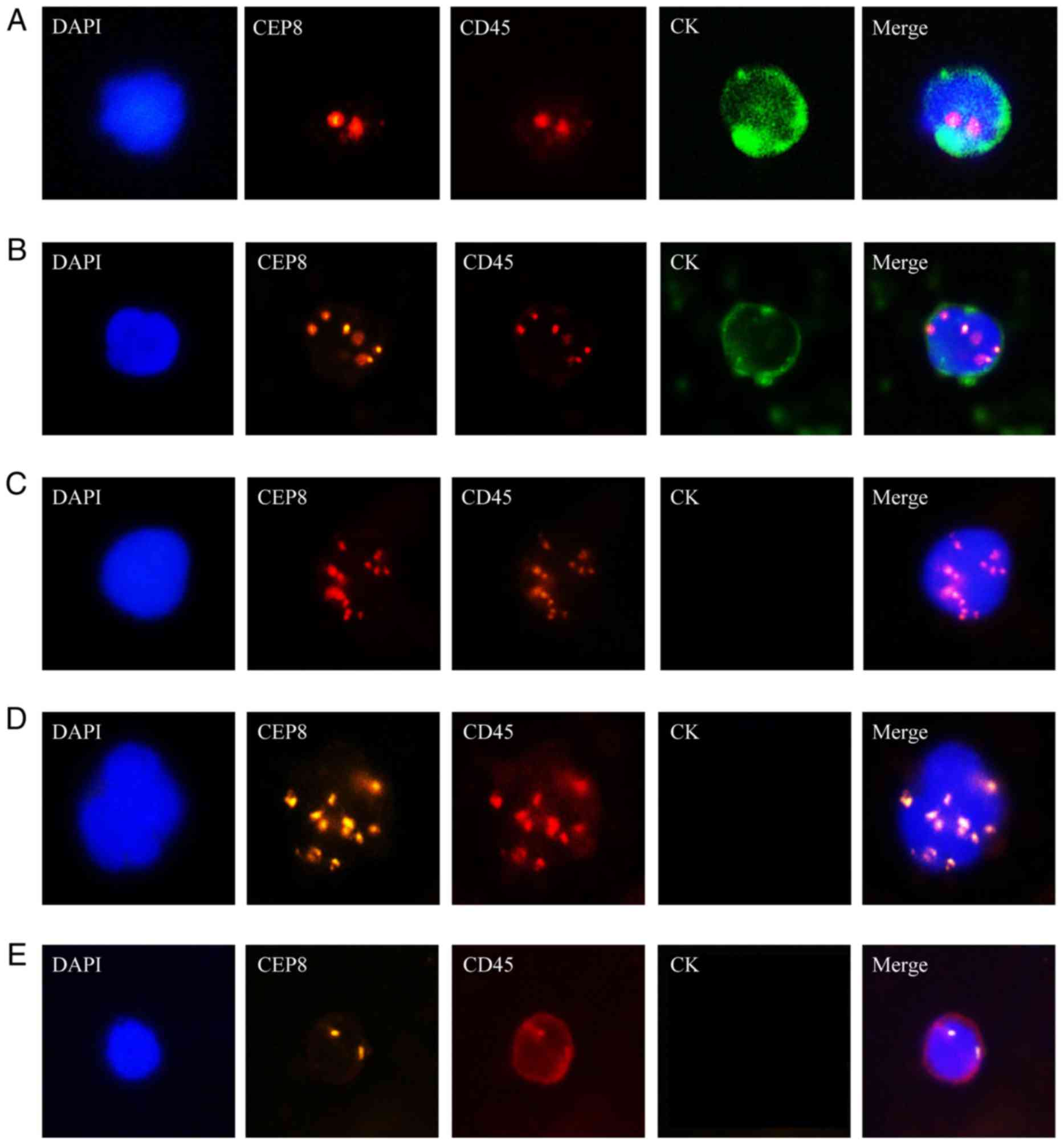
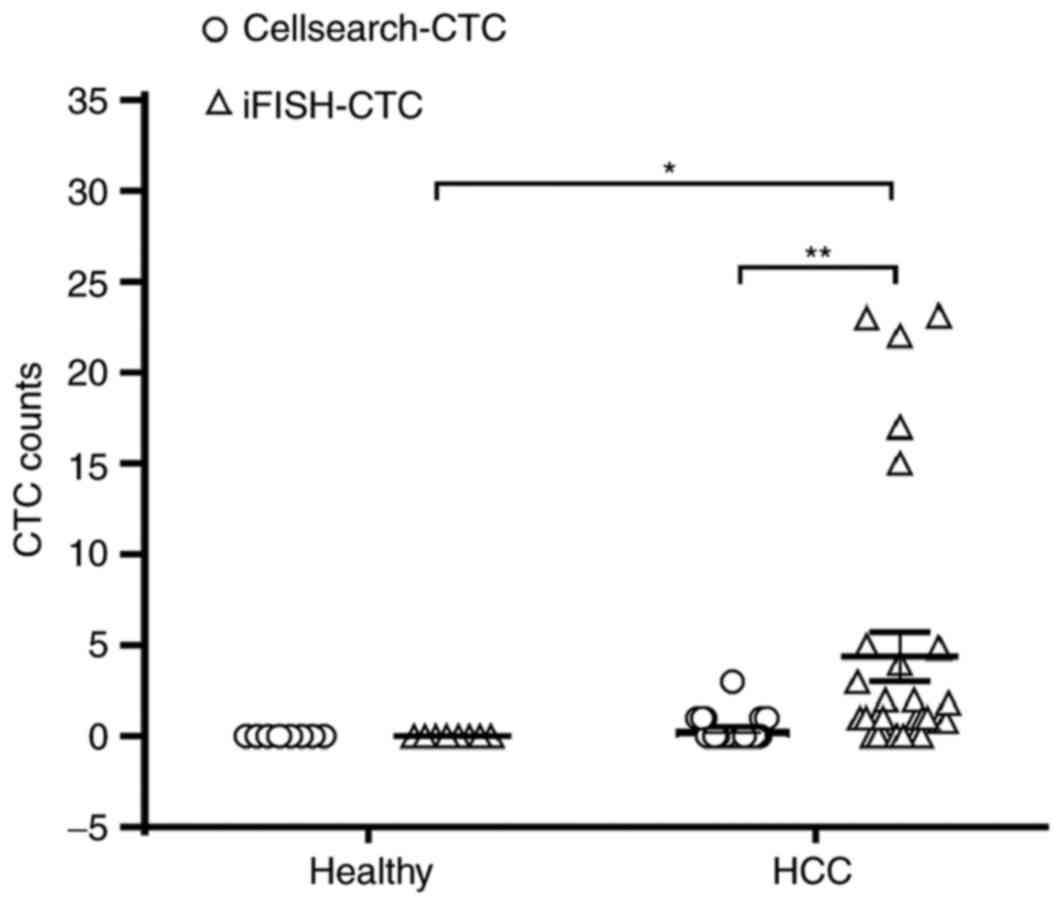
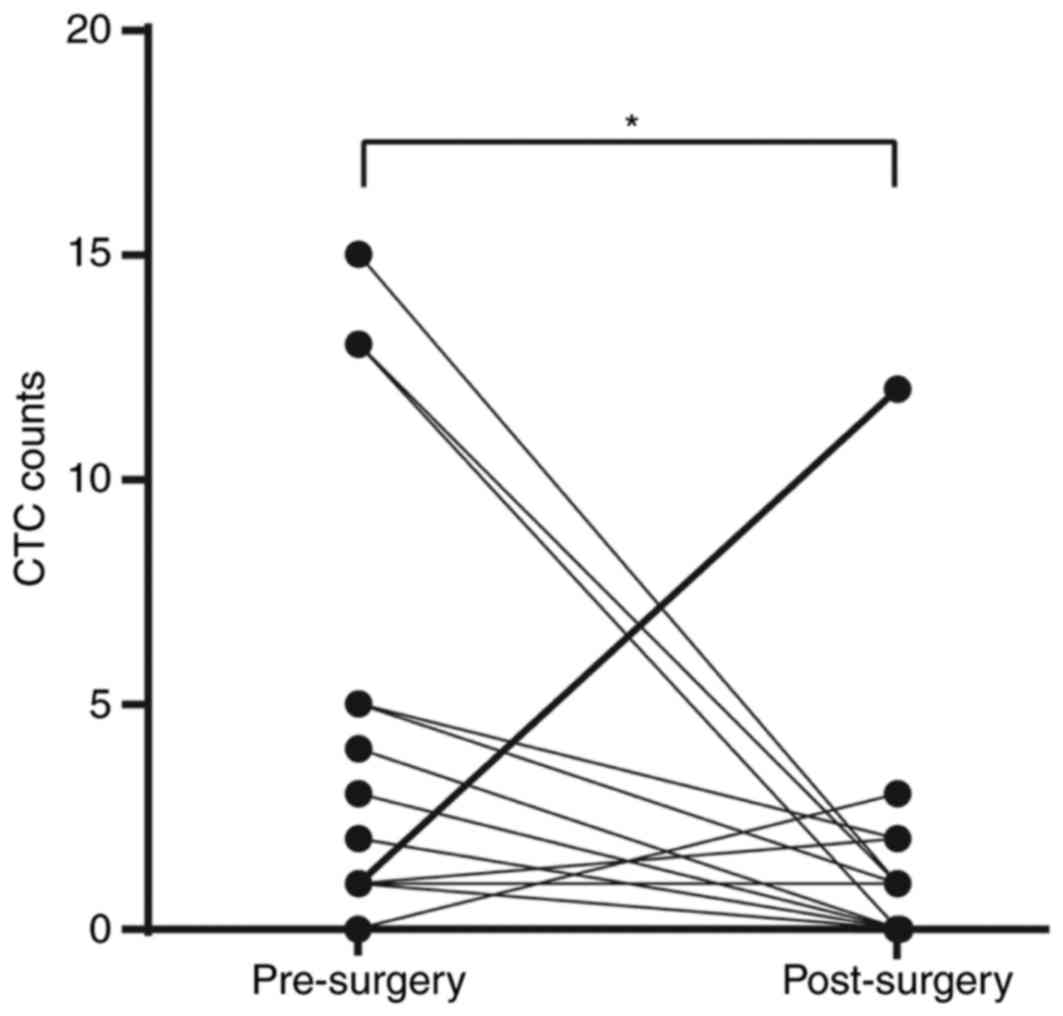
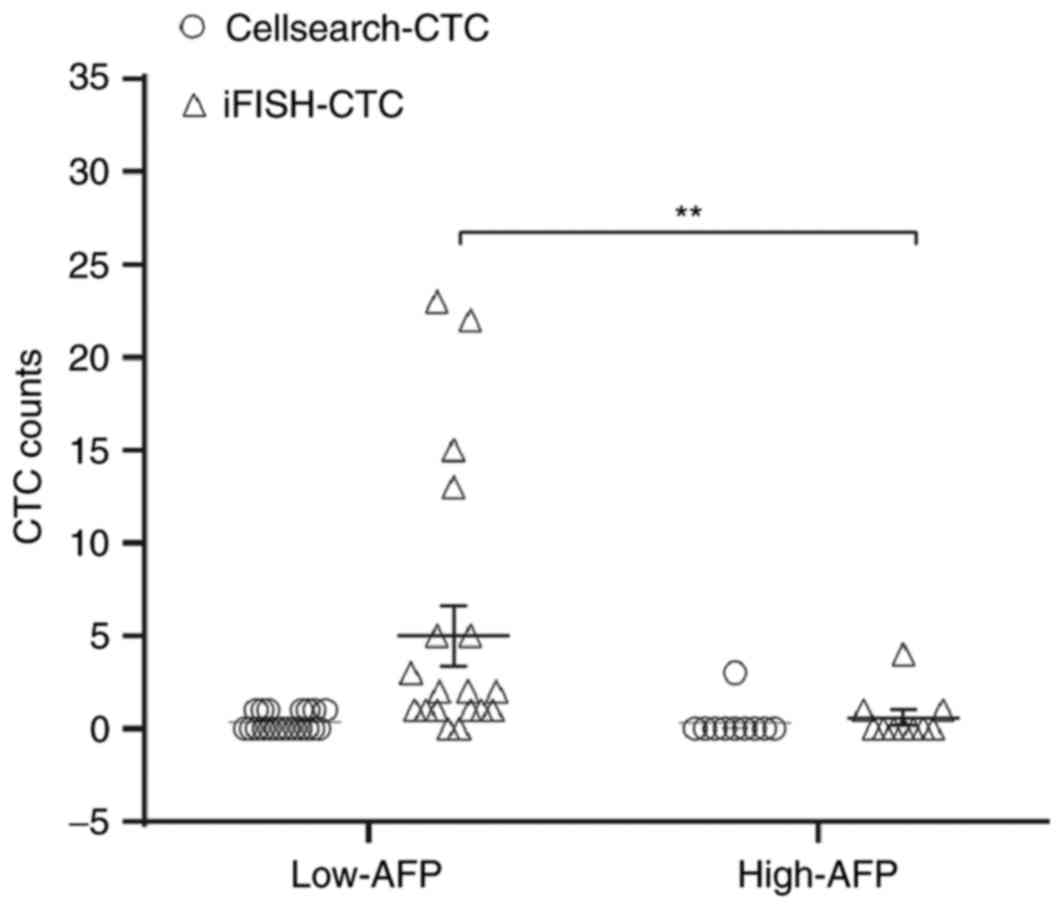
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trojan J, Zangos S and Schnitzbauer AA:
Diagnostics and treatment of hepatocellular carcinoma in 2016:
Standards and developments. Visc Med. 32:116–120. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Welker MW, Bechstein WO, Zeuzem S and
Trojan J: Recurrent hepatocellular carcinoma after liver
transplantation-an emerging clinical challenge. Transpl Int.
26:109–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rich N and Singal AG: Hepatocellular
carcinoma tumour markers: Current role and expectations. Best Pract
Res Clin Gastroenterol. 28:843–853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gorges TM, Tinhofer I, Drosch M, Röse L,
Zollner TM, Krahn T and von Ahsen O: Circulating tumour cells
escape from EpCAM-based detection due to epithelial-to-mesenchymal
transition. BMC Cancer. 12:1782012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schulze K, Gasch C, Staufer K, Nashan B,
Lohse AW, Pantel K, Riethdorf S and Wege H: Presence of
EpCAM-positive circulating tumor cells as biomarker for systemic
disease strongly correlates to survival in patients with
hepatocellular carcinoma. Int J Cancer. 133:2165–2171. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sheng Y, Wang T, Li H, Zhang Z, Chen J, He
C, Li Y, Lv Y, Zhang J, Xu C, et al: Comparison of analytic
performances of Cellsearch and iFISH approach in detecting
circulating tumor cells. Oncotarget. 8:8801–8806. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang J, Wang DD, Yang M, Chen D, Pang L,
Guo S, Cai J, Wery JP, Li L, Li HQ and Lin PP: Comprehensive
characterization of chemotherapeutic efficacy on metastases in the
established gastric neuroendocrine cancer patient derived xenograft
model. Oncotarget. 6:15639–15651. 2015.PubMed/NCBI
|
|
9
|
Ma C, Lv Y, Jiang R, Li J, Wang B and Sun
L: Novel method for the detection and quantification of malignant
cells in the CSF of patients with leptomeningeal metastasis of lung
cancer. Oncol Lett. 11:619–623. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gao Y, Zhu Y, Zhang Z, Zhang C, Huang X
and Yuan Z: Clinical significance of pancreatic circulating tumor
cells using combined negative enrichment and
immunostaining-fluorescence in situ hybridization. J Exp Clin
Cancer Res. 35:662016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lin PP: Integrated EpCAM-independent
subtraction enrichment and iFISH strategies to detect and classify
disseminated and circulating tumors cells. Clin Transl Med.
4:382015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Llovet JM, Bru C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mazzaferro V, Regalia E, Doci R, Andreola
S, Pulvirenti A, Bozzetti F, Montalto F, Ammatuna M, Morabito A and
Gennari L: Liver transplantation for the treatment of small
hepatocellular carcinomas in patients with cirrhosis. N Engl J Med.
334:693–699. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aceto N, Bardia A, Miyamoto DT, Donaldson
MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, et al:
Circulating tumor cell clusters are oligoclonal precursors of
breast cancer metastasis. Cell. 158:1110–1122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Felden J, Schulze K, Krech T, Ewald F,
Nashan B, Pantel K, Lohse AW, Riethdorf S and Wege H: Circulating
tumor cells as liquid biomarker for high HCC recurrence risk after
curative liver resection. Oncotarget. 8:89978–89987.
2017.PubMed/NCBI
|
|
16
|
Guo W, Yang XR, Sun YF, Shen MN, Ma XL, Wu
J, Zhang CY, Zhou Y, Xu Y, Hu B, et al: Clinical significance of
EpCAM mRNA-positive circulating tumor cells in hepatocellular
carcinoma by an optimized negative enrichment and qRT-PCR-based
platform. Clin Cancer Res. 20:4794–4805. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu
SJ, Shi RY, Hu B, Zhou J and Fan J: Circulating stem cell-like
epithelial cell adhesion molecule-positive tumor cells indicate
poor prognosis of hepatocellular carcinoma after curative
resection. Hepatology. 57:1458–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trevisani F, D'intino PE, Morselli-Labate
AM, Mazzella G, Accogli E, Caraceni P, Domenicali M, De Notariis S,
Roda E and Bernardi M: Serum alpha-fetoprotein for diagnosis of
hepatocellular carcinoma in patients with chronic liver disease:
Influence of HBsAg and anti-HCV status. J Hepatol. 34:570–575.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Debruyne EN and Delanghe JR: Diagnosing
and monitoring hepatocellular carcinoma with alpha-fetoprotein: New
aspects and applications. Clin Chim Acta. 395:19–26. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abdul-Hamid S, Fox R and Martin I:
Maternal serum screening for trisomy 21 in women with a false
positive result in last pregnancy. J Obstet Gynaecol. 24:374–376.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
European Association for the Study of The
Liver, ; European Organisation For Research and Treatment of
Cancer, . EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y, Shi ZL, Yang X and Yin ZF:
Targeting of circulating hepatocellular carcinoma cells to prevent
postoperative recurrence and metastasis. World J Gastroenterol.
20:142–147. 2014. View Article : Google Scholar : PubMed/NCBI
|