Introduction
Primary ovarian cancer is the leading cause of
gynecological cancer-associated mortality cause of death in
patients with and most cases are diagnosed at an advanced stage
with lymph node metastasis (1–3). Overall
5-year survival rates of patients with advanced ovarian cancer
undergoing comprehensive surgical procedures remain low, owing to
the spread of ovarian cancer cells throughout the peritoneal cavity
(4). The majority of women are
diagnosed with stage III or IV cancer (5,6). Vascular
endothelial growth factor (VEGF) is a potent mediator of
angiogenesis that induces the formation of novel blood vessels, and
the growth and metastasis of cancers (7,8). The
growth and spread of tumors depends on the formation of an adequate
vascular support and amoeboid mode of tumor cell invasion (9). Furthermore, several studies have
indicated that VEGF serves a role in promoting endothelial cell
proliferation and migration, and induces a direct effect on cell
proliferation and invasiveness (3,10,11). Previous studies have indicated that
suppressing the expression or activity VEGF in ovarian cancer cells
may represent a potential anticancer therapy (12–14).
MicroRNAs (miRNAs/miRs) are 22-nucleotide-long
non-coding RNAs that regulate the expression of target mRNAs and
are involved in cellular processes, including proliferation,
differentiation, apoptosis and development (15–17).
miR-126 is upregulated in the endothelium and originates from a
common precursor structure located within the epidermal growth
factor-like protein 7 gene (18,19).
Several online databases are available to identify potential miRNA
targets. Analysis preformed using the PicTar algorithm (http://www.pictar.org/) (20), miRBase (www.mirbase.org) (21)
and TargetScan target prediction (version 6.2; www.targetscan.org) (22) tools revealed that VEGF was the
putative target of miR-126 that may serve a role in angiogenesis
owing to regulation of VEGF signaling (23,24).
Alteration of expression of miR-126 was also reported in various
breast, lung and prostate cancer cells (24–26).
However, the cellular mechanisms underlying the effect of miR-126
on ovarian cancer cells remains to be elucidated.
The present study aimed to characterize the role of
miR-126, and the signaling pathway that it may be involved in, in
the pathogenesis of ovarian cancer cells. Using SKOV3 ovarian
cancer cells with up or downregulated miR-126, the present study
attempted to identify the role of miR-126 in ovarian cancer
cells.
Materials and methods
Cell culture
The ovarian cancer SKOV3 cell line (American Type
Culture Collection, Manassas, VA, USA) was used in the present
study. Cells were maintained and propagated in vitro by
serial passage in Dulbecco's modified Eagle's medium (DMEM; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with
10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 IU/ml penicillin and 100 µg/ml streptomycin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in a humidified
environment in 5% CO2 at 37°C.
Plasmid construction, lentivirus
package and cell infection
The lentiviral vector pGLV3/H1/GFP+Puro (pGLV3;
Shanghai GenePharma Co., Ltd., Shanghai, China) was used to
construct the pGLV3-miR-126 plasmid. miR-126 mimics, miR-126
inhibitor and negative control (NC) oligonucleotides were
synthesized by Shanghai GenePharma Co., Ltd. The following
sequences were used: miR-126, 5′-TCGTACCGTGAGTAATAATGCG-3′;
hsa-miR-126 inhibitor, 5′-CGCATTATTACTCACGGTACGA-3′; and miR-NC,
5′-TTCTCCGAACGTGTCACGT-3′. miR-126 shDNA double chain template
sequence was synthesized artificially and cloned into pGLV3-miRNA
lentivirus plasmid. The miR-126 mimic sequence was chemosynthesized
by Shanghai GenePhama Co., Ltd. using the following primers:
hsa-miR-126-BamHI forward,
5′-GATCCGTCGTACCGTGAGTAATAATGCGTTCAAGAGACGCATTATTACTCACGGTACGACTTTTTTG-3′
and hsa-miR-126-EcoRI reverse,
5′-AATTCAAAAAAGTCGTACCGTGAGTAATAATGCGTCTCTTGAACGCATTATTACTCACGGTACGACG-3′.
The miRNA-126 inhibitor sequence was synthesized using the
following primers: hsa-miR-126-BamHI forward,
5′-GATCCGAGCATGGCACTCATTATTACGCTTCAAGAGAGCGTAATAATGAGTGCCATGCTCTTTTTTG-3′
and hsa-miR-126-EcoRI reverse,
5′-AATTCAAAAAAGAGCATGGCACATGCTCG-3′. The NC was pGLV3-shDNA-NC and
the following sequence was used: NC-BamHI forward,
5′-GATCCGTCGTACCGTGAGTAATAATGCGTTCAAGAGACGCATTATTACTCACGGTACGACTTTTTTG-3′
and shNC-EcoRI reverse,
5′-AATTCAAAAAAGTCGTACCGTGAGTAATAATGCGTCTCTTGAACGCATTATTACTCACGGTACGACG-3′.
All the sequences of resulting vectors were verified with sequence
analysis by Shanghai GenePhama Co., Ltd.
The 293T cell line (Type Culture Collection of the
Chinese Academy of Sciences, Shanghai, China) was maintained in
DMEM, 10% FBS, 4.0 mM L-glutamine, 100 U/ml penicillin and 100
µg/ml streptomycin (Sigma-Aldrich; Merck KGaA) at 37°C and 95%
CO2 in order to produce lentivirus packing plasmids. At
1 day prior to transfection, 5×106 cells were seeded
into a 15-cm dish. pGLV3-miR-126 or pGLV3 vector and packing
plasmids, including pGag/Pol, pRev and pVSV-G were co-transfected
using RNAi-mate transfection reagent (both from Shanghai GenePharma
Co., Ltd.), according to the manufacturer's protocol. A total of 72
h following transfection, the supernatant was collected and
centrifuged at 8,500 × g at 4°C for 4 min, and passed through a
0.45-µm syringe filter. Subsequently, the sample was centrifuged
again at 48,400 × g at 4°C for 2 h. The viral titer of miR-126
mimic, miR-126 inhibitor and the negative control was measured
according to the expression level of green fluorescent protein
(GFP) according to the manufacturer's protocol (Shanghai GenePhama
Co. Ltd.). Packaged lentiviruses were named LV3-has-miR-126,
LV3-has-miR-126 inhibitor and LV3-NC. All sequences of resulting
vectors were verified with sequence analysis by Shanghai GenePhama
Co., Ltd.
SKOV3 cells were infected with LV3-has-miR-126,
LV3-has-miR-126 inhibitor and LV3-NC as LV3-has-miR-126 mimic
group, LV3-has-miR-126 inhibitor group and NC group respectively,
while uninfected SKOV3 cells were the untreated SKOV3 cells group.
The SKOV3 cells of the first three groups were infected at a
multiplicity of infection (MOI) of 15 in the presence of 5 µg/ml
polybrene (Shanghai GenePharma Co., Ltd.). Efficiency of infection
was ~90% as assessed by GFP and fluorescent microscopy. Cells were
used for subsequent experimentation 72 h after transfection.
Cell cycle assay
Cells from all treatment groups were harvested at 48
h following transfection, fixed in 70% ice-cold ethanol overnight
at 0°C and washed with 1X Buffer A (centrifugation at 4,200 × g for
5 min at 0°C). Subsequently, 5 µl propidium iodide (Nanjing KeyGen
Biotech Co., Ltd., Nanjing, China) was added into 0.5 ml of cell
suspension (5×105 cells) for 15 min at room temperature.
Experiments were performed in triplicate for each sample and
analyses were performed using a flow cytometer (FACSCanto II; BD
Biosciences, Franklin Lakes, NJ, USA) in accordance with the
manufacturer's protocol. Multicycle software for Windows (Phoenix
Flow Systems, San Diego, CA, USA) was used to determine the
percentage of cells in G1, G2 and S
stages.
Cell invasion assay
A total of 48 h following transfection, the invasive
ability of cells was assayed using a Transwell invasion assay (8-mm
pore size). Transwell inserts were put into the 24-well plates. A
total of 0.1 ml Matrigel (50 mg/ml; BD Biosciences) was added onto
the plate surface and incubated for 4–5 h. SKOV3 cells were freshly
trypsinized (0.25% trypsin) at 37°C for 3 min and washed with PBS,
then suspended in DMEM with 0.1% BSA (Gibco; Thermo Fisher
Scientific, Inc.). Subsequently, 0.1 ml cell suspension (1×105
cells) was added to the upper chamber of each insert coated with
Matrigel. A total of 0.5 ml DMEM containing 20% FBS was added into
the lower chamber and cells were allowed to invade for 24 h at 37°C
in a 5% CO2 humidified incubator. Following the
incubation, cells on the membrane were removed using a cotton swab
and 0.05% crystal violet stain was used to identify migratory cells
at 20°C for 30 min The number of migratory cells on the lower
surface of the membrane was counted under Nikon Labophot 2 light
microscope (Nikon Corp., Tokyo, Japan; 10 fields of vision) at a
magnification of ×200.
Immunofluorescence staining and
western blot analysis
Analysis preformed using the PicTar algorithm
(http://www.pictar.org/) (20), miRBase (www.mirbase.org) (21)
and TargetScan target prediction (version 6.2; www.targetscan.org) (22) tools revealed that VEGF was the
putative target of miR-126. Therefore, the expression of VEGF was
explored in all groups. A total of 48 h after transfection, cells
were fixed in 4% paraformaldehyde for 30 min at 20°C, washed three
times with PBS, and incubated for 5 min at −20°C in 95% ethanol
(v/v in PBS). Cells were subsequently washed three times with PBS,
blocked for 1 h at 20°C in 5% normal goat serum (Wuhan Boster
Biological Technology, Ltd., Wuhan, China) in PBS with 0.1% Triton
X-100 and incubated overnight with anti-VEGF antibodies (cat no.
BA0407; 1:200; Wuhan Boster Biological Technology, Ltd.) at 4°C.
Cells were subsequently washed three times with PBS and incubated
for 40 min at 37°C with the corresponding secondary antibody (goat
anti-rabbit immunoglobulin (Ig)G (H+L)-tetramethylrhodamine (TRITC;
cat no. BA1090; 1:100; Wuhan Boster Biological Technology, Ltd.).
Cells were washed with PBS and mounted onto the glass slides.
Immunostained SKOV3 cultures were examined under a Zeiss laser
scanning confocal microscope at a magnification of ×200 (LSM 510
META; Carl Zeiss AG, Oberkochen, Germany) for detection of the
TRITC fluorophore by semi-quantitative confocal laser scanning
analysis-Image-Pro Plus 6.0 (Media Cybernetics, Inc., Rockville, MD
USA).
For western blot analysis, proteins were extracted
from SKOV3 cells. Proteins were solubilized in
Radioimmunoprecipitation assay buffer (Sangon Biotech Co., Ltd.,
Shanghai, China) and proteins quantified using the BCA Protein
Assay kit (Beyotime Institute of Biotechnology, Haimen, China) were
separated at 30 µg per lane on 10% SDS-PAGE and electro-transferred
onto polyvinylidene difluoridemembrane. The membrane was blocked in
5% skimmed milk powder prepared in Tris-buffered saline with 0.1%
Tween-20 (TBS-T; Sangon Biotech Co., Ltd.) for 30 min at 20°C. For
VEGF detection, membranes were incubated at 4°C overnight with
anti-VEGF antibodies (cat no. BA0407; 1:500; Wuhan Boster
Biological Technology, Ltd.) and anti-β-actin antibodies (cat no.
BM0627; 1:200; Wuhan Boster Biological Technology, Ltd.). Membranes
were washed 3 times for 10 min in TBS-T and incubated with a
1:5,000 dilution of horseradish peroxidase-conjugated goat
anti-rabbit IgG (cat no. BA1054; Wuhan Boster Biological
Technology, Ltd.) for 2 h. Membranes were washed 6 times for 20 min
with TBS-T prior to development with a standard enhanced
chemiluminescence kit (ECL kit; Nanjing KeyGen Biotech Co., Ltd.,
Nanjing, China). Densitometry analysis of the bands was performed
using Quantity One software (version 4.6; Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Statistical analysis
Data are presented as the mean ± standard deviation
(n=5). Statistical analysis was performed using SPSS software
(version 14.0; IBM Corp., Armonk, NY, USA). The significance of any
differences was evaluated using one-way analysis of variance
followed by a Tukey post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
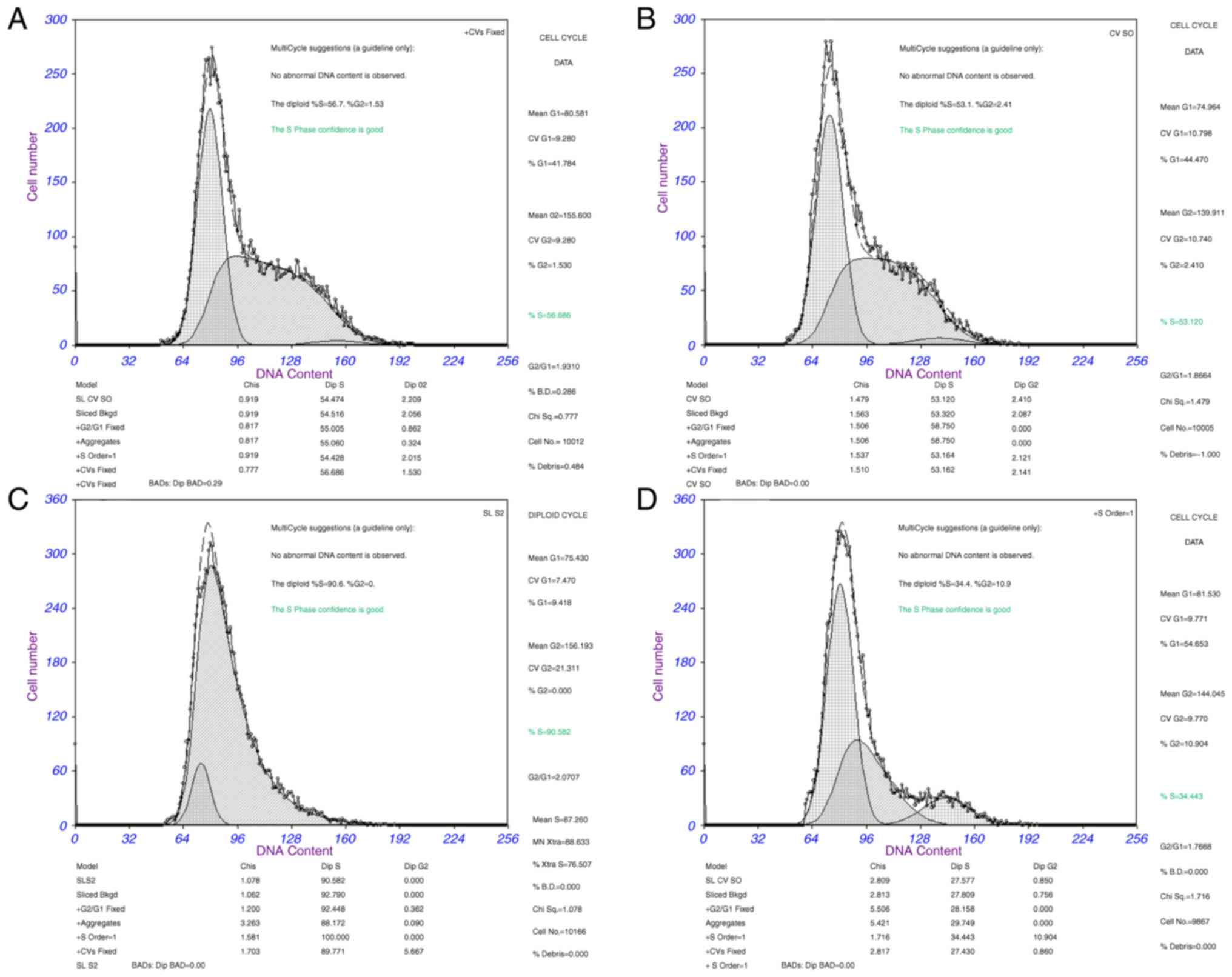
Cell cycle assay
Cell cycle analysis was used to determine whether
the effect of miR-126 on proliferation of SKOV3 cells was due to
cell cycle alterations. The results demonstrated that the
percentage of cells in G1 phase increased to 54.65%,
whereas the percentage of cells in S phase decreased to 34.44% in
the miR-126 mimic group compared with the NC group (G1
phase, 44.47% and S phase, 53.12% in the NC group). The percentage
of cells in G2 phase was 2.41% in the NC group and
10.90% in the miR-126 mimic group (P<0.05). These results
indicate that the overexpression of miR-126 in SKOV3 cells induced
G1 cell cycle arrest. Cell cycle analysis was also
conducted in the miR-126 inhibitor group. Following transduction
with the LV3-miR-126 inhibitor for 48 h, flow cytometry analysis
revealed that SKOV3 cells were promoted from the G1
phase into the S phase. The percentage of cells in G1
phase was from 44.47% in the NC group and 9.42% in the miR-126
inhibitor group, while the percentage of cells in S phase was
53.12% in the NC group and 90.58% in the miR-126 inhibitor group
(P<0.05). There were no significant alterations in cell cycle
ratios between the untreated group and the NC group (P>0.05;
Fig. 1). The results demonstrated
that miR-126 mimic arrested SKOV3 cells at the G1 phase
while the miR-126 inhibitor induced cell cycle progression.
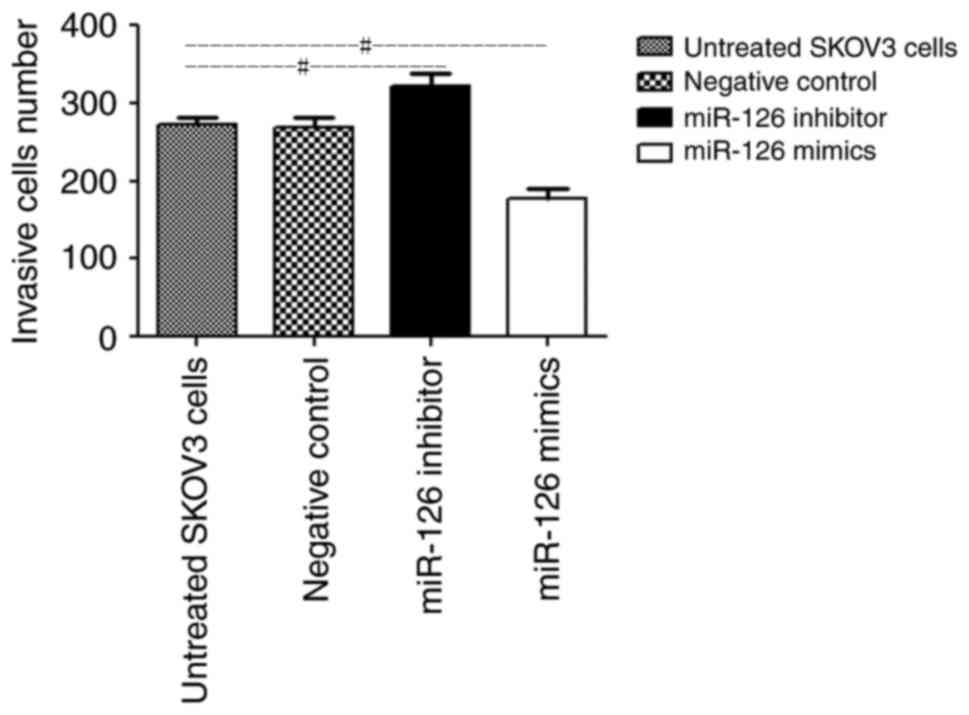
Cell invasion assay
The number of SKOV3 cells in the miR-126 mimic group
that invaded through the Matrigel-coated membranes was
significantly lower than that in the untreated group (164.6±25.8
vs. 253.1±14.9 cells, respectively; P<0.05; Fig. 2). Furthermore, the depletion of
miR-126 significantly promoted SKOV3 cell invasion through
Matrigel-coated membranes, compared with the untreated group
(290.9±13.2 cells vs. 253.1±14.9 cells, respectively; P<0.05;
Fig. 2). There were no significant
differences between the number of invaded NC cells and that of the
untreated cells (256.5±15.2 vs. 253.1±14.9 cells respectively;
P>0.05; Fig. 2). These results
indicated that downregulation of miR-126 may promote cell invasion,
whereas upregulation of miR-126 inhibits the invasion of cells
through Matrigel-coated membranes.
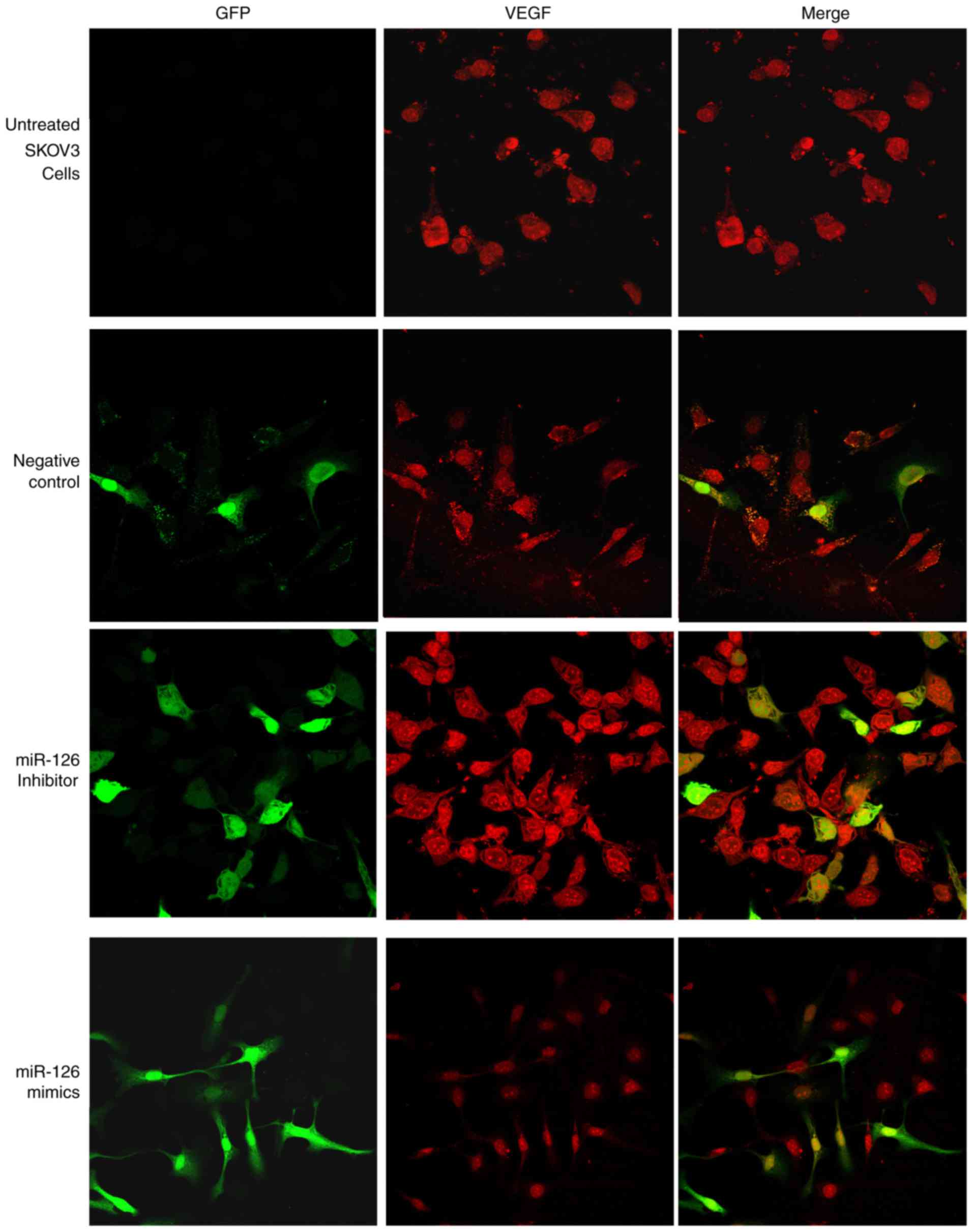
Immunofluorescence staining and
western blot analysis
Immunofluorescence double staining and
semi-quantitative confocal laser scanning analysis revealed that
miRNA vectors and VEGF were expressed in the four treatment groups.
Green fluorescence, indicating vector-transfected cells, was
detected in all cell nuclei and only certain cytoplasmic areas. The
red fluorescence detected in the cytoplasm of SKOV3 cells indicated
VEGF expression. The results of immunofluorescence staining
demonstrated that transfection with miR-126 mimics mediated the
inhibition of VEGF expression. The mean immunofluorescence
intensity of VEGF in the miR-126 inhibitor group was significantly
increased compared with that in the control group (P<0.05;
Fig. 3). Furthermore, the expression
level of VEGF decreased as a result of overexpression of miR-126 in
LV3-has-miR-126-transfected SKOV3 cells, compared with untreated
and negative control cells (Fig. 3).
These results further indicated that miR-126 has an inhibitory role
on VEGF expression in SKOV3 cells.
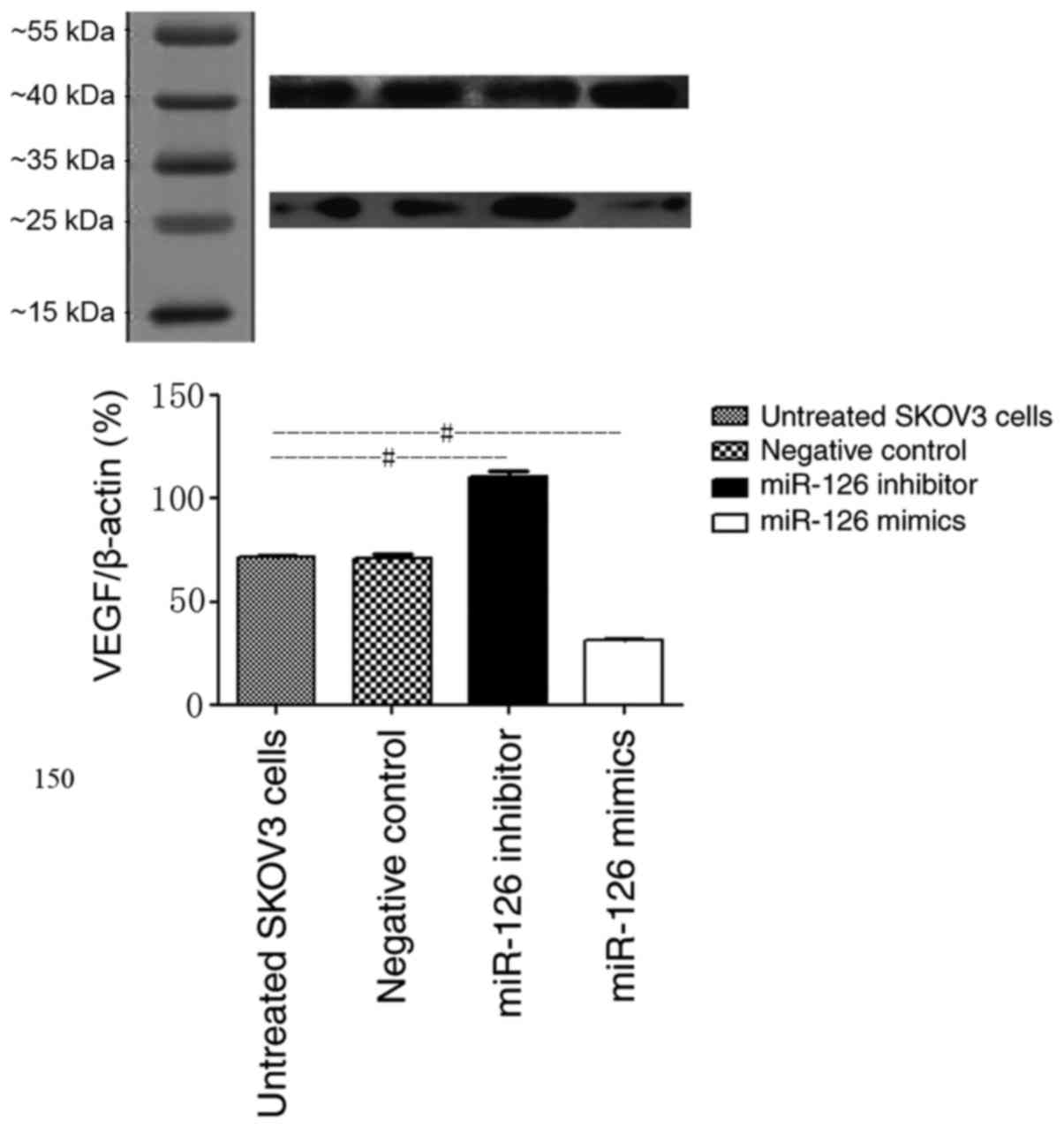
VEGF protein was detected in all treatment groups as
a single band at ~28 kDa. Densitometry analysis of the VEGF bands
revealed a significant increase in expression levels (110.4±6.8%)
in the LV3-has-miR-126 inhibitor group and a significant decrease
(31.2±2.2%) in the LV3-has-miR-126 mimic group, compared with the
untreated group (71.4±2.8%; both P<0.05; Fig. 4). There was no significant difference
between the NC control group and the untreated group (70.9±3.8 vs.
71.4±2.8%, respectively; P>0.05; Fig.
4). The above results indicate that VEGF was upregulated in
SKOV3 cells transfected with LV3-has-miR-126 inhibitor, whereas
VEGF expression was inhibited following LV3-has-miR-126 mimic
transfection.
Discussion
miR-126 has been reported to be downregulated in
human breast, lung, stomach, cervix and pancreas cancer (24,27–30).
Furthermore, patients with non-small cell lung cancer and primary
breast tumors exhibiting low miR-126 expression displayed poor
survival compared with patients with high miR-126 levels (25). These studies indicated the presence of
an association between the expression of miR-126 and
metastasis-free survival. The present study demonstrated that
ovarian cancer cells transfected with miR-126 exhibited a
significantly increased frequency of cells in G1phase
and a lower frequency of cells in S phase. The percentage of cells
in G1 phase in the miR-126 mimic group was markedly
higher than that in the NC group. These results demonstrated that
miR-126 could induce ovarian cancer cell cycle arrest, whereas
inhibition miR-126 promoted cell cycle progression and induced
ovarian tumor cell proliferation.
Previous studies demonstrated that VEGF serves a
role in the modulation of cellular functions, including
angiogenesis, tumor cell proliferation and invasiveness (31–33). VEGF
increases vascular permeability and leads to malignant ascites in
later stages of ovarian cancer, indicating disease progression and
treatment failure (31,34). Therefore, anti-VEGF treatment could
aid in improving patient survival (35). Clinical trials have demonstrated that
certain anti-VEGF agents, including bevacizumab, can increase the
risk of treatment-associated death caused by pulmonary hemorrhage,
hypertension, proteinuria or bleeding, owing to disruption of
normal vasculature (36,37). These side effects limit the
therapeutic usage of anti-VEGF agents. The targeting of endogenous
miRNAs provides an alternative approach to anti-VEGF treatment
(38). Online accessible algorithms,
including PicTar, miRBase and TargetScan, demonstrated that one of
the potential targets of miR-126 is VEGF (18,19). The
present study demonstrated that miR-126 was a target of VEGF and
the upregulation of miR-126 markedly inhibited VEGF expression. The
present study aimed to elucidate the interaction between miR-126
and VEGF in ovarian cancer cells. The results demonstrated that
SKOV3 cells infected with LV-miR-126 can efficiently reduce the
expression of VEGF. Furthermore, SKOV3 cells infected with
LV-miR-126 inhibitor exhibited overexpression of VEGF and the cell
proliferation as 90.58% cells of S phase vs. 53.12% in the NC
group.
Invasion through the basement membrane is one of the
features of aggressive tumors (39);
therefore, the present study further investigated the association
between the expression of miR-126 and ovarian cell invasion. Cells
transfected with LV-has-miR-126 inhibitor exhibited increased
invasiveness compared with the untreated group. The results also
demonstrated that increasing the expression of miR-126 in ovarian
cancer cells could reduce the invasiveness. We hypothesize that low
expression of miR-126 promotes VEGF expression and cell invasion,
leading to the development of ovarian cancer.
In conclusion, to the best of our knowledge, the
present study is the first to present data indicating that miR-126
is a tumor suppressor that can induce ovarian cancer cell cycle
arrest and suppress invasion, at least in part, by targeting VEGF
expression. miR-126 may be used as a potential marker for clinical
prognosis and treatment of ovarian cancer in the future.
Acknowledgements
The present study was supported by the Natural
Science Foundation of Zhejiang Province (grant no. LY13H160018) and
the National Natural Science Foundation of China (grant no.
81371881). The authors would like to thank Dr Faisal Rehman
(Zhejiang University, Zhejiang, China) for critical proofreading of
the manuscript and language correction.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bertone-Johnson ER: Epidemiology of
ovarian cancer: A status report. Lancet. 365:101–102. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Smolle E, Taucher V and Haybaeck J:
Malignant ascites in ovarian cancer and the role of targeted
therapeutics. Anticancer Res. 34:1553–1561. 2014.PubMed/NCBI
|
|
3
|
Sun L, Li L, Li Z, Hong S, Yang Q, Qu X
and Kong B: Alterations in the serum proteome profile during the
development of ovarian cancer. Int J Oncol. 45:2495–2501. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Matsuda A and Katanoda K: Five-year
relative survival rate of ovarian cancer in the USA, Europe and
Japan. Jpn J Clin Oncol. 44:1962014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ovarian cancer, five-year stage-specific
relative survival rates (2004–2008). J Natl Cancer Inst.
103:12872011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mury D, Woelber L, Jung S, Eulenburg C,
Choschzick M, Witzel I, Schwarz J, Jaenicke F and Mahner S:
Prognostic and predictive relevance of CA-125 at primary surgery of
ovarian cancer. J Cancer Res Clin Oncol. 137:1131–1137. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Patterson DM, Gao D, Trahan DN, Johnson
BA, Ludwig A, Barbieri E, Chen Z, Diaz-Miron J, Vassilev L, Shohet
JM and Kim ES: Effect of MDM2 and vascular endothelial growth
factor inhibition on tumor angiogenesis and metastasis in
neuroblastoma. Angiogenesis. 14:255–266. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bieri M, Oroszlan M, Farkas A, Ligeti N,
Bieri J and Mohacsi P: Anti-HLA I antibodies induce VEGF production
by endothelial cells, which increases proliferation and
paracellular permeability. Int J Biochem Cell Biol. 41:2422–2430.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qiao L, Liang N, Zhang J, Xie J, Liu F, Xu
D, Yu X and Tian Y: Advanced research on vasculogenic mimicry in
cancer. J Cell Mol Med. 19:315–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Awazu Y, Mizutani A, Nagase Y, Tsuchiya S,
Nakamura K, Kakoi Y, Kitahara O, Takeuchi T, Yamasaki S, Miyamoto
N, et al: Anti-angiogenic and anti-tumor effects of TAK-593, a
potent and selective inhibitor of vascular endothelial growth
factor and platelet-derived growth factor receptor tyrosine kinase.
Cancer Sci. 104:486–494. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu P, Liu W, Wang L, Yang M and Du J: High
circulating VEGF level predicts poor overall survival in lung
cancer. J Cancer Res Clin Oncol. 139:1157–1167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rinck-Junior JA, Oliveira C, Lourenço GJ,
Sagarra RA, Derchain SF, Segalla JG and Lima CS: Vascular
endothelial growth factor (VEGF) polymorphism and increased risk of
epithelial ovarian cancer. J Cancer Res Clin Oncol. 141:69–73.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim KK, Singh AP, Singh RK, Demartino A,
Brard L, Vorsa N, Lange TS and Moore RG: Anti-angiogenic activity
of cranberry proanthocyanidins and cytotoxic properties in ovarian
cancer cells. Int J Oncol. 40:227–235. 2012.PubMed/NCBI
|
|
14
|
He Z, Li B, Rankin GO, Rojanasakul Y and
Chen YC: Selecting bioactive phenolic compounds as potential agents
to inhibit proliferation and VEGF expression in human ovarian
cancer cells. Oncol Lett. 9:1444–1450. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nikitina EG, Urazova LN and Stegny VN:
MicroRNAs and human cancer. Exp Oncol. 34:2–8. 2012.PubMed/NCBI
|
|
16
|
Zheng X, Dong J, Gong T, Zhang Z, Wang Y,
Li Y, Shang Y, Li K, Ren G, Feng B, et al: MicroRNA library-based
functional screening identified miR-137 as a suppresser of gastric
cancer cell proliferation. J Cancer Res Clin Oncol. 141:785–795.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo P, Fei J, Zhou J and Zhang W:
microRNA-126 suppresses PAK4 expression in ovarian cancer SKOV3
cells. Oncol Lett. 9:2225–2229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kuhnert F, Mancuso MR, Hampton J,
Stankunas K, Asano T, Chen CZ and Kuo CJ: Attribution of vascular
phenotypes of the murine Egfl7 locus to the microRNA miR-126.
Development. 135:3989–3993. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Yang P, Sun T, Li D, Xu X, Rui Y,
Li C, Chong M, Ibrahim T, Mercatali L, et al: miR-126 and miR-126*
repress recruitment of mesenchymal stem cells and inflammatory
monocytes to inhibit breast cancer metastasis. Nat Cell Biol.
15:284–294. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Krek A, Grün D, Poy MN, Wolf R, Rosenberg
L, Epstein EJ, MacMenamin P, da Piedade I, Gunsalus KC, Stoffel M,
et al: Combinatorial microRNA target predictions. Nat Genet.
37:495–500. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozomara A and Griffiths-Jones S: miRBase:
Annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42:D68–D73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lewis BP, Burge CB and Bartel DP:
Conserved seed pairing, often flanked by adenosines, indicates that
thousands of human genes are microRNA targets. Cell. 120:15–20.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sasahira T, Kurihara M, Bhawal UK, Ueda N,
Shimomoto T, Yamamoto K, Kirita T and Kuniyasu H: Downregulation of
miR-126 induces angiogenesis and lymphangiogenesis by activation of
VEGF-A in oral cancer. Br J Cancer. 107:700–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu N, Zhang D, Xie H, Zhou Z, Chen H, Hu
T, Bai Y, Shen Y, Yuan W, Jing Q and Qin Y: Endothelial-specific
intron-derived miR-126 is down-regulated in human breast cancer and
targets both VEGFA and PIK3R2. Mol Cell Biochem. 351:157–164. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jusufović E, Rijavec M, Keser D, Korošec
P, Sodja E, Iljazović E, Radojević Z and Košnik M: Let-7b and
miR-126 are down-regulated in tumor tissue and correlate with
microvessel density and survival outcomes in non-small-cell lung
cancer. PLoS One. 7:e455772012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Musiyenko A, Bitko V and Barik S: Ectopic
expression of miR-126*, an intronic product of the vascular
endothelial EGF-like 7 gene, regulates prostein translation and
invasiveness of prostate cancer LNCaP cells. J Mol Med (Berl).
86:313–322. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Miko E, Margitai Z, Czimmerer Z, Várkonyi
I, Dezso B, Lányi A, Bacsó Z and Scholtz B: miR-126 inhibits
proliferation of small cell lung cancer cells by targeting SLC7A5.
FEBS Lett. 585:1191–1196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Feng R, Chen X, Yu Y, Su L, Yu B, Li J,
Cai Q, Yan M, Liu B and Zhu Z: miR-126 functions as a tumour
suppressor in human gastric cancer. Cancer Lett. 298:50–63. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu L, Wang YL and Wang JF: Differential
expression of miR-21, miR-126, miR-143, miR-373 in normal cervical
tissue, cervical cancer tissue and Hela cell. Sichuan Da Xue Xue
Bao Yi Xue Ban. 43:536–539. 2012.(In Chinese). PubMed/NCBI
|
|
30
|
Frampton AE, Krell J, Jacob J, Stebbing J,
Castellano L and Jiao LR: Loss of miR-126 is crucial to pancreatic
cancer progression. Expert Rev Anticancer Ther. 12:881–884. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Herr D, Sallmann A, Bekes I, Konrad R,
Holzheu I, Kreienberg R and Wulff C: VEGF induces ascites in
ovarian cancer patients via increasing peritoneal permeability by
downregulation of Claudin 5. Gynecol Oncol. 127:210–216. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kaneko S, Ishibashi M and Kaneko M:
Vascular endothelial growth factor expression is closely related to
irinotecan-mediated inhibition of tumor growth and angiogenesis in
neuroblastoma xenografts. Cancer Sci. 99:1209–1217. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang L, Liu X, Wang H and Wang S:
Correlation of the expression of vascular endothelial growth factor
and its receptors with microvessel density in ovarian cancer. Oncol
Lett. 6:175–180. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng D, Liang B and Li Y: Serum vascular
endothelial growth factor (VEGF-C) as a diagnostic and prognostic
marker in patients with ovarian cancer. PLoS One. 8:e553092013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Teng LS, Jin KT, He KF, Zhang J, Wang HH
and Cao J: Clinical applications of VEGF-trap (aflibercept) in
cancer treatment. J Chin Med Assoc. 73:449–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Elice F and Rodeghiero F: Side effects of
anti-angiogenic drugs. Thromb Res. Suppl 1:S50–S53. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
des Guetz G, Uzzan B, Chouahnia K and
Morere JF: Cardiovascular toxicity of anti-angiogenic drugs. Target
Oncol. 6:197–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang F, Ren X and Zhang X: Role of
microRNA-150 in solid tumors. Oncol Lett. 10:11–16. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Srinivasan D and Plattner R: Activation of
Abl tyrosine kinases promotes invasion of aggressive breast cancer
cells. Cancer Res. 66:5648–5655. 2006. View Article : Google Scholar : PubMed/NCBI
|