Introduction
Osteosarcoma, which is derived from primitive
bone-forming mesenchymal cells, is the most common type of
malignant bone tumor, and predominantly occurs in children and
young adults (1). Despite recent
developments in medical technology, including surgery and
chemotherapy, therapy outcomes remain unsatisfactory; this can be
partially attributed to the complex interaction between the tumor
cells and the tumor microenvironment (2–4).
Blood vessels are critical component of the tumor
microenvironment, as they are not only essential for supplying
tumor nutrition, but also for transporting tumor cells to distant
sites (5). A growing body of cancer
research is focusing on angiogenesis. A range of types of drugs
targeting tumor vessels have been developed and applied clinically.
Although such drugs improve the treatment outcome to some extent,
drug resistance is ultimately induced during treatment, and so
therapy outcomes remain unsatisfactory (6,7). Further
endeavor is required to identify more effective targets for
anti-angiogenesis therapy and improve the prognosis for cancer.
Fibroblast activation protein (FAP) was initially
identified as a specific marker for cancer-associated fibroblasts
(8). It was previously reported that
stromal FAP expression was correlated with the progression and
prognosis in a range of tumor types, including colon, breast and
ovarian cancer (9–12). In view of the role of stromal FAP in
cancer progression, its expression in tumor cells has attracted
increasing attention. Mentlein et al (13) reported that FAP was highly expressed
on the surface of bone and soft tissue tumor cells. Yuan et
al (14) reported that the high
expression of FAP in osteosarcoma cells was positively correlated
with an advanced clinical stage, high histological grade, positive
metastasis and poor prognosis. However, the role of FAP in
angiogenesis in osteosarcoma has yet to be characterized.
In the present study, it was identified that FAP
expression in osteosarcoma cells promoted the expression of
vascular endothelial growth factor-A (VEGF-A). Furthermore,
conditioned medium (CM) from osteosarcoma cells with altered FAP
expression could activate AKT, ERK and proliferation in endothelial
cells. Finally, it was demonstrated that the activation of the AKT
and extracellular signal-regulated kinase (ERK) signaling pathways
was associated with the VEGF-A expression induced by FAP.
Materials and methods
Cell lines and cell culture
HOS, U2-OS and MG63 human osteosarcoma cell lines,
and human umbilical vein endothelial cells (HUVECs) were purchased
from American Type Culture Collection (Manassas, VA, USA). HOS,
U2-OS and MG63 cells were cultured in Dulbecco's modified Eagle's
medium (DMEM; Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; Invitrogen;
Thermo Fisher Scientific, Inc.). HUVEC cells were cultured in
endothelial cell medium supplemented with 5% FBS and 1% endothelial
cell growth supplement (all from ScienCell Research Laboratories,
Carlsbad, CA, USA). All cells were cultured in 5% CO2 at
37°C. To study potential molecular mechanism, the AKT inhibitor
LY294002 or ERK inhibitor U0126 were added into osteosarcoma cells
using FAP overexpression for 24 h at 37°C. The final concentrations
of LY294002 and ERK were 50 and 20 µM, respectively.
Transfection
A total of 2×105 cells were plated in
6-well plates. After 24 h, 1.5 µg p-cDNA3.1/FAP-vector and 2 µl
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) were mixed for 20 min at room temperature. Then,
the mixture was added in cells in order to overexpress FAP. A total
of 1.5 µg p-GPU6/FAP-shRNA plasmids (Shanghai GenePharma Co., Ltd.,
Shanghai, China) and 2 µl Lipofectamine® 2000 were mixed
for 20 min at room temperature. Then, the mixture was added in
cells to silence FAP expression. The empty plasmids were used as a
control. After 48 h, RNA and protein were extracted for further
procedures. Subsequent to culture in DMEM without FBS for a further
48 h, the supernatant was collected as CM. The shRNA sequence is
5′-CCCTCAGACAGTTTGCTTATT-3′.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA from cells was extracted with TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was synthesized using the PrimeScript
RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's protocol (25°C for 10 min, 42°C for
60 min, then 70°C for 10 min). The PCR was performed using specific
primers and Universal PCR Master Mix (Thermo Fisher Scientific,
Inc.) with the following thermocycler conditions: 4°C for 5 min,
then 94°C for 30 sec, 57°C for 30 sec and 72°C for 30 sec, followed
by a holding step at 72°C for 5 min. For the FAP PCR, this was
repeated for 40 cycles. For the VEGF-A and GAPDH PCR, this was
repeated for 36 cycles. PCR products were electrophoretically
separated on 1.0% agarose gels. The results were analyzed by
Labwork software, version 4.0 (UVP, Inc., Upland, CA, USA). The
primers used were as follows: FAP forwards,
5′-TTAGTCTGACAAAGAGAAACACTG-3′ and reverse,
5′-ATGAAGACTTGGGTAAAAATCG-3′; VEGF-A forwards,
5′-CGGGCAGGAGGAAGGAGCCT-3′ and reverse, 5′-GTGATGGTGTGGTGGCGGCA-3′;
GAPDH forward, 5′-AGAAGGCTGGGGCTCATTTG-3′ and reverse,
5′-AGGGGCCATCCACAGTCTTC-3′ GAPDH was used as an internal
control.
Western blot analysis
Protein was extracted using radioimmunoprecipitation
assay buffer (Beyotime Institute of Biotechnology, Haimen, China)
containing 1% protease inhibitor. Total protein was measured using
a bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. Then, 300 µg/well
protein was loaded in 5% acrylamide and separated by 10% separating
gel, then transferred to a nitrocellulose membrane. Subsequent to
blocking in TBS with 0.05% Tween-20 (ST825, Beyotime Institute of
Biotechnology) containing 5% non-fat dried milk for 1 h at room
temperature, the membrane was then incubated with primary
antibodies (listed in Table I) at 4°C
overnight, followed by incubation with peroxidase-linked goat
anti-rabbit-IgG (cat. no. ab205718, dilution, 1:4,000; Abcam,
Cambridge, MA, USA) at room temperature for 1 h. Signals were
detected using enhanced chemiluminescence reagents (Pierce; Thermo
Fisher Scientific, Inc.) and measured using Image-Pro software
(version 5.1; Media Cybernetics, Inc., Rockville, MA, USA).
 | Table I.Primary antibodies used for western
blotting in the present study. |
Table I.
Primary antibodies used for western
blotting in the present study.
| Target | Specificity | Dilution | Cat. no. | Company |
|---|
| FAP | Rabbit
anti-human | 1:1,000 | ab53066 | Abcam, Cambridge, MA,
USA |
| VEGF-A | Rabbit
anti-human | 1:1,000 | ab46154 | Abcam |
| Phospho-ERK | Rabbit
anti-human | 1:1,000 | 4370 | Cell Signaling
Technology, Inc., Danvers, MA, USA |
| ERK | Rabbit
anti-human | 1:1,000 | 4695 | Cell Signaling
Technology, Inc. |
| Phospho-AKT | Rabbit
anti-human | 1:1,000 | 4058 | Cell Signaling
Technology, Inc. |
| AKT | Rabbit
anti-human | 1:1,000 | 4691 | Cell Signaling
Technology, Inc. |
| GAPDH | Rabbit
anti-human | 1:4,000 | ab128915 | Abcam |
ELISA
VEGF-A in CM was detected using PGRN ELISA kit (cat.
no. CSB-E11718h; CUSABIO Life Science, Wuhan, China) and VEGF-A
ELISA Kit (CUSABIO Life Science) according to the manufacturer's
instructions. The experiment was repeated at least three times.
HUVEC cell proliferation assay
HUVEC cells were plated in a 96-well plate at 5,000
cells per well, in triplicate. Once the cells had adhered to the
well walls, the medium was replaced with CM from transfected MG63
or U2-OS cells. An MTT assay was performed at 0 and 48 h from the
addition of CM. Cells were incubated with MTT for 4 h, then
formazan was dissolved with 0.1% dimethyl sulfoxide. The absorbance
of the wells was then measured at 490 nm. The proliferation rate
was calculated as OD at 48 h/OD at 0 h.
Statistical analysis
Data was expressed as the mean ± standard deviation.
SPSS software (version 11.0; SPSS, Inc., Chicago, IL, USA) was used
for statistical analysis. Differences between groups were analyzed
using one-way analysis of variance with Dunnett's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
FAP and VEGF-A expression in
osteosarcoma cells
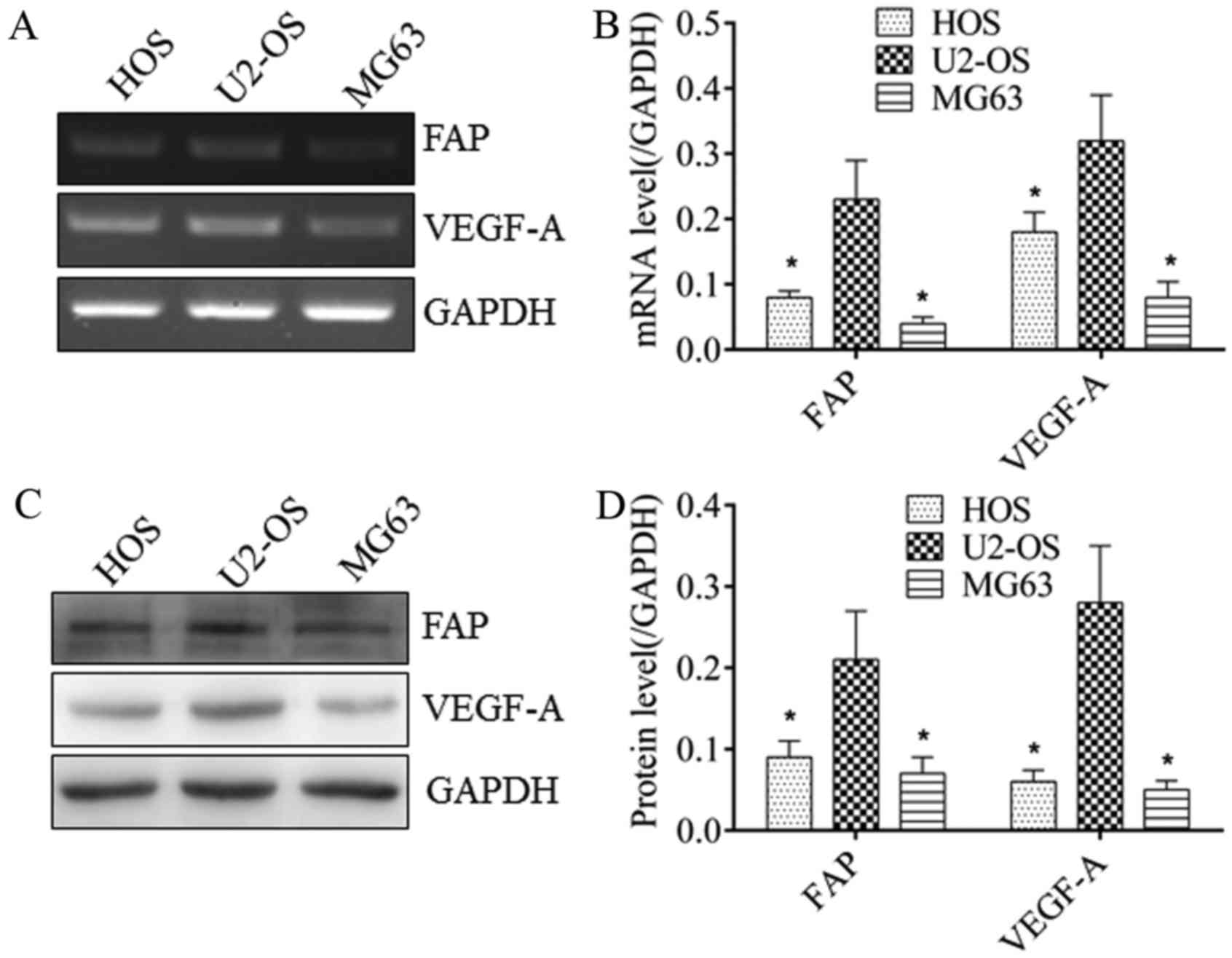
RT-PCR and western blot analysis were performed to
detect the expression of FAP and VEGF-A in osteosarcoma cells. As
demonstrated in Fig. 1, U2-OS cells
exhibited the highest expression of FAP at the mRNA (Fig. 1A and B) and protein (Fig. 1C and D) levels, which was
significantly different from MG63 and HOS cells (P<0.05). MG63
cells exhibited the lowest FAP expression at the mRNA (Fig. 1A and B) and protein (Fig. 1C and D) levels, which were
significantly different from U2-OS and HOS cells (P<0.05). The
VEGF-A expression trend among these three cell lines was consistent
with FAP expression at the mRNA (Fig. 1A
and B) and protein (Fig. 1C and
D) levels. This indicated a potential association between FAP
and VEGF-A expression in osteosarcoma cells.
FAP promotes VEGF-A expression in
osteosarcoma cells
To identify whether an association between FAP and
VEGF-A existed, FAP expression was altered by transfection. As
demonstrated in Fig. 2, when FAP in
MG63 cells was overexpressed, VEGF-A expression was upregulated
significantly at the mRNA (Fig. 2A and
B) and protein (Fig. 2C and D)
levels (P<0.05). Conversely, subsequent to silencing FAP
expression in U2-OS cells, VEGF-A expression was significantly
downregulated at the mRNA (Fig. 2E and
F) and protein (Fig. 2G and H)
levels. In addition, when FAP in MG63 cells was overexpressed, an
increase in the secreted VEGF-A in supernatant was also identified
using ELISA (Fig. 2I). When FAP in
MG63 cells was silenced, a decline in the VEGF-A in supernatant was
also identified with ELISA (Fig. 2J).
This suggested that FAP could promote VEGF-A expression in
osteosarcoma cells, implying its pro-angiogenic properties.
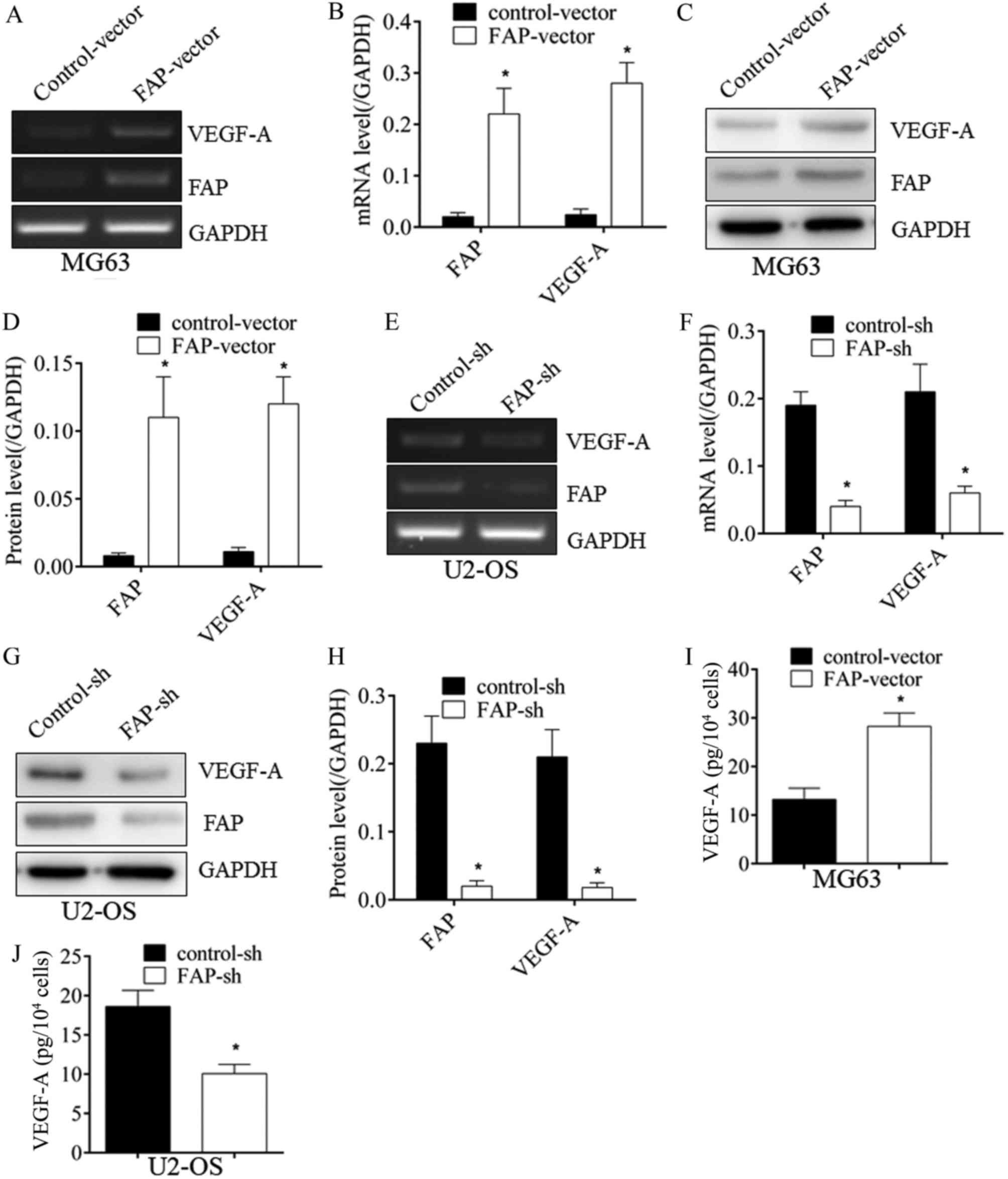 | Figure 2.FAP expression promotes VEGF-A
expression in osteosarcoma cells. (A) Subsequent to the
overexpression of FAP in MG63 cells, VEGF-A mRNA expression was
upregulated, as determined with RT-PCR, which was (B) quantified by
densitometry. (C) In addition, VEGF-A protein expression was
upregulated, which was also (D) quantified. (E) Following the
silencing of FAP expression in U2-OS cells, the expression of
VEGF-A was inhibited at the mRNA level, as determined with RT-PCR,
which was (F) quantified, and at (G) the protein level, which was
(H) quantified. (I) The increase in VEGF-A subsequent to FAP
overexpression and (J) the decrease in VEGF-A subsequent to FAP
silencing were also confirmed by ELISA. *P<0.05 vs. control.
FAP, fibroblast activation protein; VEGF-A, vascular endothelial
growth factor-A; RT-PCR, reverse transcription-polymerase chain
reaction; sh, short hairpin RNA. |
CM from osteosarcoma cells with
alterations in FAP expression affects the angiogenic properties of
HUVEC cells
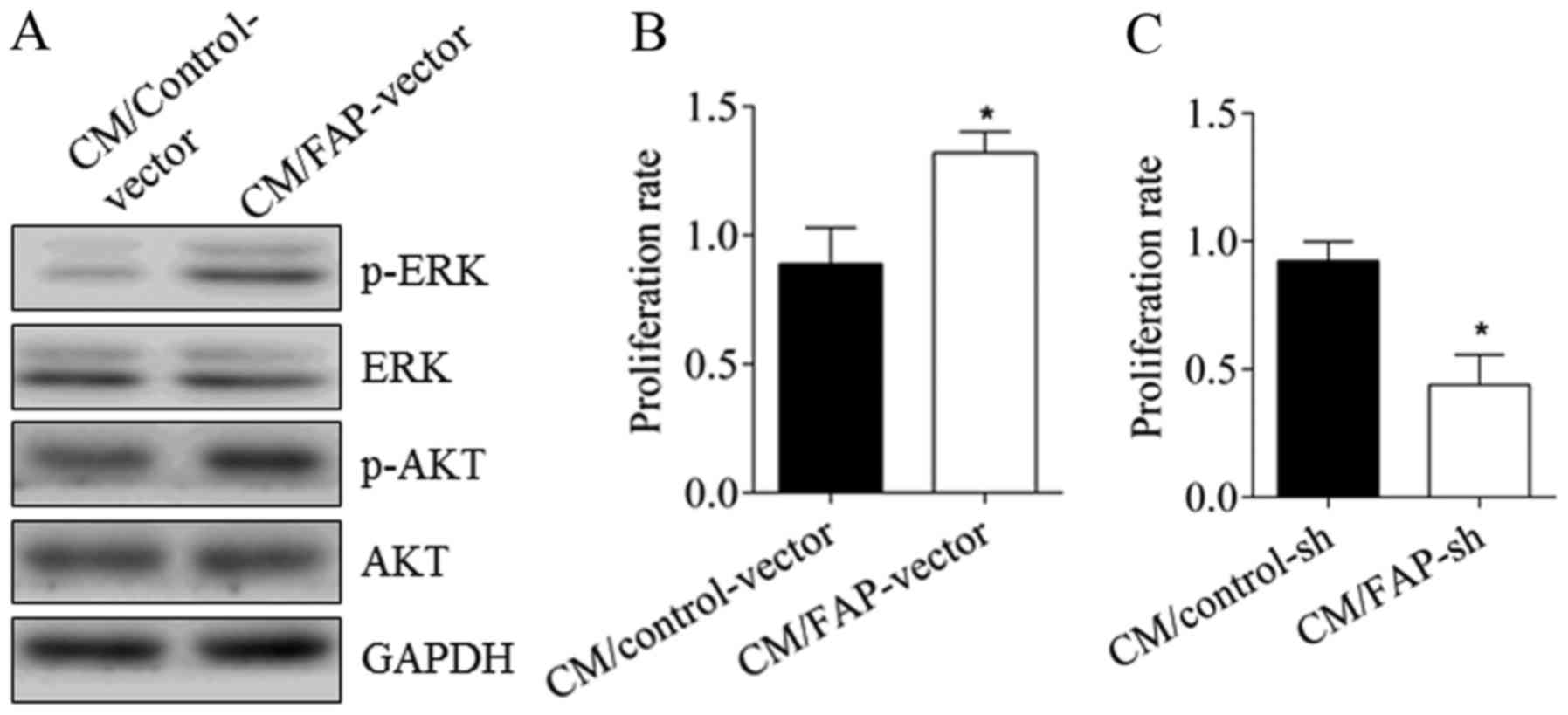
To further confirm the effect of FAP on
angiogenesis, the changes in the angiogenic properties of HUVEC
cells were directly assessed. As demonstrated in Fig. 3, subsequent to treatment with CM from
MG63 cells with over-expressed FAP, compared with the control
group, the phosphorylation of AKT and ERK of HUVEC cells were
activated (Fig. 3A). The
proliferation rate of the HUVECs increased from 0.89±0.13 to
1.32±0.08, representing a significant difference (Fig. 3B; P<0.05). Conversely, subsequent
to treatment with CM from U2-OS cells with silenced FAP for 24 h,
the proliferation rate of HUVEC cells declined from 0.92±0.07 to
0.44±0.07, a significant difference (Fig.
3C; P<0.05). This further demonstrated that FAP expression
promoted angiogenesis.
Phosphoinositide 3-kinase (PI3K)/AKT
and ERK signaling pathways are required for the VEGF-A upregulation
induced by FAP
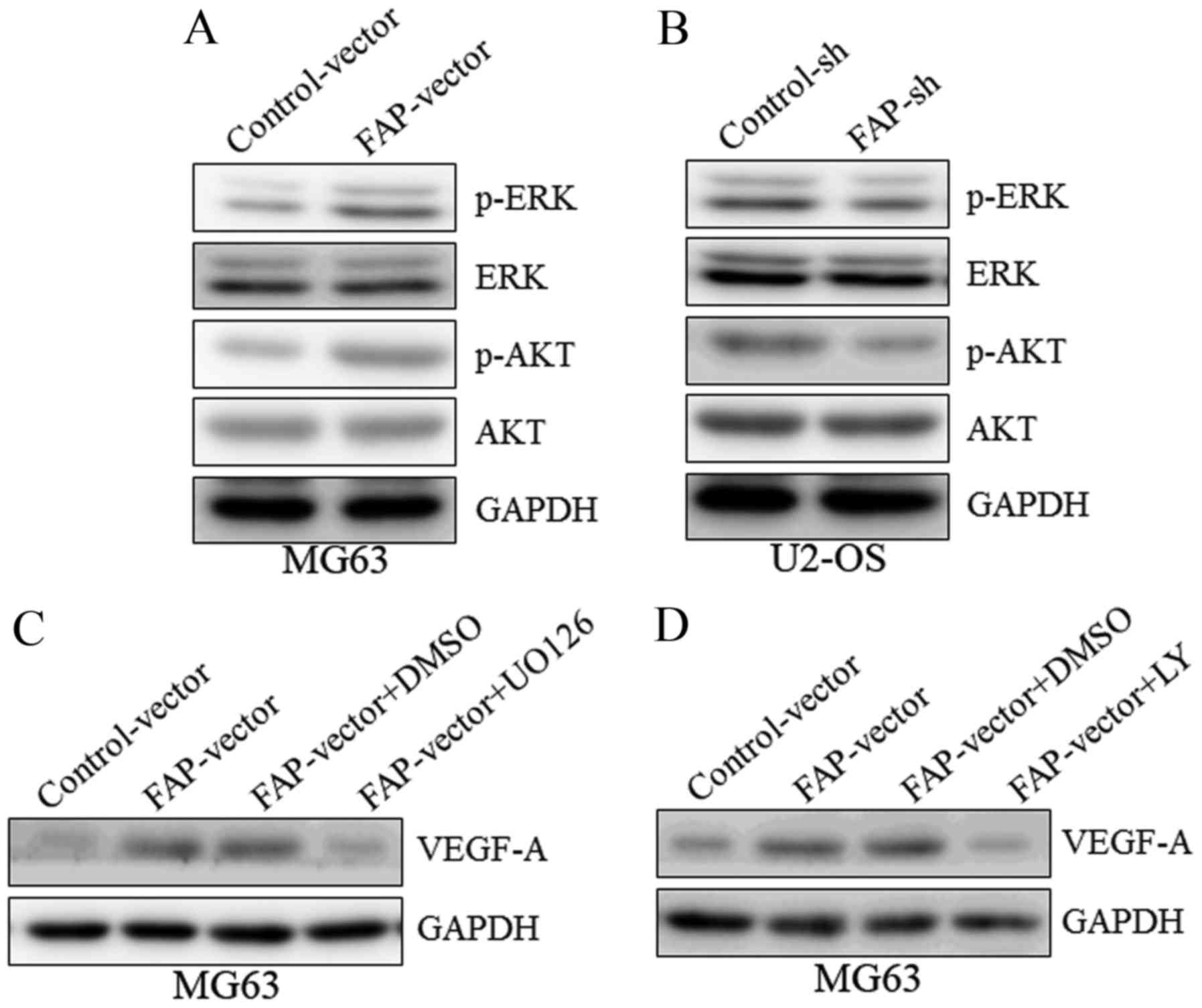
To further investigate the potential molecular
mechanisms of FAP in angiogenesis, AKT and ERK were selected as
candidate targets. A western blot demonstrated that MG63 cells
over-expressing FAP exhibited the activation (phosphorylation) of
AKT and ERK (Fig. 4A), whereas in
U2-OS cells with silenced FAP expression, the phosphorylation of
AKT and ERK were inhibited (Fig. 4B),
implying that AKT and ERK may be downstream targets of FAP.
Subsequent to treatment with an ERK inhibitor (U0126) or AKT
inhibitor (LY294002), the upregulation of VEGF-A expression induced
by FAP overexpression was abrogated (Fig.
4C and D). These results collectively suggest that FAP
regulated VEGF-A expression in osteosarcoma cells via the PI3K/AKT
and ERK signaling pathways.
Discussion
Angiogenesis is a complicated process by which new
vessels form from existing vasculature. This process is controlled
by the balance between proangiogenic factors and antiangiogenic
factors (7). VEGF-A is a crucial
proangiogenic factor that is implicated in the carcinogenesis of
multiple types of tumor (15). It
stimulates signaling pathways in endothelial cells through binding
to vessel endothelial growth factor receptor 2 (VEGFR-2) to enhance
the proliferation, migration and tubule formation abilities of the
cells, thus promoting angiogenesis (16–18).
FAP, initially emerging as a specific marker of
reactive fibroblasts in tumors, is a 170-kDa homodimeric
glycoprotein composed of two 97-kDa subunits (19). It belongs to the serine protease
family and has dipeptidyl peptidase and endopeptidase activity,
allowing the degradation of gelatin and type I collagen (11). The roles of FAP in tumor progression
have been widely studied. Jia et al (20) identified that overexpressing FAP
significantly promoted the growth and motility of MDA-MB-213 human
breast cancer cells in vitro. Shi et al (21) reported that FAP was highly expressed
in carcinoma cells, and was associated with desmoplasia and a worse
prognosis in pancreatic ductal adenocarcinoma. In the present
study, FAP and VEGF-A expression levels were determined in MG63,
U2-OS and HOS cells. It was identified that FAP expression was
consistent with VEGF-A expression in the different osteosarcoma
cell lines. MG63 cells exhibited the lowest expression of FAP and
VEGF-A; U2-OS cells exhibited the highest expression. This may
imply that FAP expression is correlated with VEGF-A expression.
According to the FAP expression level, MG63 and U2-OS cells were
selected for further research. Following the silencing of FAP
expression in U2-OS cells, VEGF-A expression was significantly
downregulated; likewise, over-expressing FAP significantly
upregulated the expression of VEGF-A in MG63 cells. To the best of
our knowledge, this confirmed the effect of FAP on promoting the
expression of pro-angiogenic factors for the first time, further
implying its pro-angiogenic role in osteosarcoma.
Vessel endothelial cells line the inner walls of
blood vessels and serve a critical role in angiogenesis (22). Under the control of angiogenic factors
derived from multiple types of cell, including pericytes and tumor
cells, vessel endothelial cells began to proliferate and migrate,
resulting in angiogenesis (7,23). In the present study, it was identified
that VEGF-A in the supernatant was altered with changes in the FAP
expression of osteosarcoma cells. Subsequent to culture in CM from
MG63 cells overexpressing FAP, the AKT and ERK signal pathways in
HUVEC cells were activated, and the proliferation rate was
increased. Furthermore, CM from U2-OS cells with silenced FAP
expression inhibited the proliferation rate of HUVEC cells. This
demonstrated that the expression of FAP in osteosarcoma cells
could, at least in part, promote angiogenesis through regulating
the expression of VEGF-A. Whether other angiogenic factors may also
be induced by FAP requires further investigation.
The phosphorylation of AKT and ERK has been reported
in multiple cancers. They are members of critical signal pathways
for maintaining the malignancy of tumor cells, and affect a range
of biological behaviors in tumor cells, including proliferation,
migration, differentiation, drug resistance, apoptosis and
phenotype maintenance (24–26). Liu et al (27) reported that microRNA-21 induced
angiogenesis through the activation of AKT and ERK in DU145 human
prostate cancer cells. Wang et al (28) identified that the downregulation of
FAP suppressed cell proliferation and metastasis through the
PI3K/AKT and ERK signaling pathways in oral squamous cell
carcinoma. In the present study, it was identified that the
phosphorylation of AKT and ERK were activated by FAP. The Akt
inhibitor, LY294002, and the ERK inhibitor, U0126, each abrogated
the upregulation of VEGF-A induced by FAP. This is consistent with
previous studies (27,28). However, as there is complexity in the
crosstalk between different signaling pathways, although the
activation of AKT and ERK were confirmed in the present study, the
detailed mechanism of regulation has yet to be fully
elucidated.
In conclusion, FAP and VEGF-A expression levels
corresponded with each other in osteosarcoma cells. The
overexpression of FAP in osteosarcoma cells activated the PI3K/AKT
and ERK signaling pathways, which may promote angiogenesis. This
data may enrich the understanding of the tumor-promoting role of
FAP, and supplies evidence to support the identification of novel
therapy targets against osteosarcoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article
Authors' contributions
CZ designed the present study and wrote the paper;
MW performed the cell experiments and XL performed the cell
experiments and data analysis.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Savage SA, Mirabello L, Wang Z,
Gastier-Foster JM, Gorlick R, Khanna C, Flanagan AM, Tirabosco R,
Andrulis IL, Wunder JS, et al: Genome-wide association study
identifies two susceptibility loci for osteosarcoma. Nat Genet.
45:799–803. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bonuccelli G, Avnet S, Grisendi G, Salerno
M, Granchi D, Dominici M, Kusuzaki K and Baldini N: Role of
mesenchymal stem cells in osteosarcoma and metabolic reprogramming
of tumor cells. Oncotarget. 5:7575–7588. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lu J, Song G, Tang Q, Zou C, Han F, Zhao
Z, Yong B, Yin J, Xu H, Xie X, et al: IRX1 hypomethylation promotes
osteosarcoma metastasis via induction of CXCL14/NF-κB signaling. J
Clin Invest. 125:1839–1856. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Garcia A and Singh H: Bevacizumab and
ovarian cancer. Ther Adv Med Oncol. 5:133–141. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bergers G and Benjamin LE: Tumorigenesis
and the angiogenic switch. Nat Rev Cancer. 3:401–410. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rusckowski M, Wang Y, Blankenberg FG,
Levashova Z, Backer MV and Backer JM: Targeted scVEGF/(177)Lu
radiopharmaceutical inhibits growth of metastases and can be
effectively combined with chemotherapy. EJNMMI Res. 6:42016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zi FM, He JS, Li Y, Wu C, Wu WJ, Yang Y,
Wang LJ, He DH, Yang L, Zhao Y, et al: Fibroblast activation
protein protects bortezomib-induced apoptosis in multiple myeloma
cells through β-catenin signaling pathway. Cancer Biol Ther.
15:1413–1422. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wikberg ML, Edin S, Lundberg IV, Van
Guelpen B, Dahlin AM, Rutegård J, Stenling R, Oberg A and Palmqvist
R: High intratumoral expression of fibroblast activation protein
(FAP) in colon cancer is associated with poorer patient prognosis.
Tumor Biol. 34:1013–1020. 2013. View Article : Google Scholar
|
|
10
|
Hua X, Yu L, Huang X, Liao Z and Xian Q:
Expression and role of fibroblast activation protein-alpha in
microinvasive breast carcinoma. Diagn Pathol. 6:1112011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu R, Li H, Liu L, Yu J and Ren X:
Fibroblast activation protein. Cancer Biol Ther. 13:123–129. 2014.
View Article : Google Scholar
|
|
12
|
Lai D, Ma L and Wang F: Fibroblast
activation protein regulates tumor-associated fibroblasts and
epithelial ovarian cancer cells. Int J Oncol. 41:541–550. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mentlein R, Hattermann K, Hemion C,
Jungbluth AA and Held-Feindt J: Expression and role of the cell
surface protease seprase/fibroblast activation protein-α (FAP-α) in
astroglial tumors. Biol Chem. 392:199–207. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yuan D, Liu B, Liu K, Zhu G, Dai Z and Xie
Y: Overexpression of fibroblast activation protein and its clinical
implications in patients with osteosarcoma. J Surg Oncol.
108:157–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao D, Pan C, Sun J, Gilbert C,
Drews-Elger K, Azzam DJ, Picon-Ruiz M, Kim M, Ullmer W, El-Ashry D,
et al: VEGF drives cancer-initiating stem cells through
VEGFR-2/Stat3 signaling to upregulate Myc and Sox2. Oncogene.
34:3107–3119. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishigami SI, Arii S, Furutani M, Niwano M,
Harada T, Mizumoto M, Mori A, Onodera H and Imamura M: Predictive
value of vascular endothelial growth factor (VEGF) in metastasis
and prognosis of human colorectal cancer. Br J Cancer.
78:1379–1384. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Neufeld G, Cohen T, Gengrinovitch S and
Poltorak Z: Vascular endothelial growth factor (VEGF) and its
receptors. FASEB J. 13:9–22. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cross MJ, Dixelius J, Matsumoto T and
Claesson-Welsh L: VEGF-receptor signal transduction. Trends Biochem
Sci. 28:488–494. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kelly T, Huang Y, Simms AE and Mazur A:
Fibrobl Activation Protein-α: A key modulator of the
microenvironment in multiple pathologies. Int Rev Cell Mol Biol.
297:83–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia J, Martin TA, Ye L and Jiang WG: FAP-α
(Fibroblast activation protein-α) is involved in the control of
human breast cancer cell line growth and motility via the FAK
pathway. BMC Cell Biol. 15:162014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shi M, Yu DH, Chen Y, Zhao CY, Zhang J,
Liu QH, Ni CR and Zhu MH: Expression of fibroblast activation
protein in human pancreatic adenocarcinoma and its
clinicopathological significance. World J Gastroenterol.
18:840–846. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Adeoye OO, Bouthors V, Hubbell MC,
Williams JM and Pearce WJ: VEGF receptors mediate hypoxic
remodeling of adult ovine carotid arteries. J Appl Physiol (1985).
117:777–787. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu Y, Huang A, Li T, Su X, Ding H, Li H,
Qin X, Hou L, Zhao Q, Ge X, et al: MiR-152 reduces human umbilical
vein endothelial cell proliferation and migration by targeting
ADAM17. FEBS Lett. 588:2063–2069. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Steelman LS, Chappell WH, Abrams SL, Kempf
RC, Long J, Laidler P, Mijatovic S, Maksimovic-Ivanic D, Stivala F,
Mazzarino MC, et al: Roles of the Raf/MEK/ERK and
PI3K/PTEN/Akt/mTOR pathways in controlling growth and sensitivity
to therapy-implications for cancer and aging. Aging (Albany NY).
3:192–222. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sasore T and Kennedy B: Deciphering
combinations of PI3K/AKT/mTOR pathway drugs augmenting
anti-angiogenic efficacy in vivo. PLoS One. 9:e1052802014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khan KH, Yap TA, Yan L and Cunningham D:
Targeting the PI3K-AKT-mTOR signaling network in cancer. Chin J
Cancer. 32:253–265. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu LZ, Li C, Chen Q, Jing Y, Carpenter R,
Jiang Y, Kung HF, Lai L and Jiang BH: miR-21 induced angiogenesis
through AKT and ERK Activation and HIF-1α expression. PLoS One.
6:e191392011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang H, Wu Q, Liu Z, Luo X, Fan Y, Liu Y,
Zhang Y, Hua S, Fu Q, Zhao M, et al: Downregulation of FAP
suppresses cell proliferation and metastasis through PTEN/PI3K/AKT
and Ras-ERK signaling in oral squamous cell carcinoma. Cell Death
Dis. 5:e11552014. View Article : Google Scholar : PubMed/NCBI
|