Introduction
According to 2012 report of the World Health
Organization (1), cancer is the
leading cause of morbidity and mortality worldwide. Lung cancer is
the most common type of cancer and results in the highest
cancer-associated mortality rate worldwide, as well as in Vietnam
(1). Traditional cancer treatments,
including surgery, chemotherapeutic agents and ionizing radiation,
have been used to eliminate tumors in patients. However, the rising
resistance to drugs or radiation leads to metastasis of cancer
cells and reduces the survival rate of patients with cancer.
In the past few years, cancer immunotherapy has been
considered to be a novel promising method for cancer treatment,
particularly when combined with other traditional methods (2,3).
Immunotherapy increases the strength of immune responses against
tumors by stimulating the activities of specific components of the
immune system. Based on the function of immune cells in targeting
cancer, the autologous immune enhancement therapy (AIET) involves
multiplying autologous immune cells ex vivo and injecting
them into the body in order to destroy the cancer cells (2–4). Several
studies have demonstrated that the higher number and higher rate of
activity of infiltrating natural killer (NK) cells and cytotoxic T
lymphocytes (CTLs) to the tumor are closely correlated with
positive prognosis, tumor size decrease and longer survival of
patients with cancer (5,6).
NK cells, first identified in 1975 as a unique
lymphocyte subset, have the morphology of large granular
lymphocytes, and are capable of recognizing and killing
abnormalities that are missing or not expressing the ‘self’ markers
of major histocompatibility complex class I. These cells are
characterized by the expression of CD56 and the lack of CD3
expression (termed CD56+CD3− lymphocytes),
which can also be distinguished according to the level of CD56
expression as CD56bright and CD56dim subsets
(7). NK cells directly kill target
tumor cells through the apoptosis mechanism by releasing
cytoplasmic granules containing perforin and granzymes, or by
expressing death receptor ligands on their cell surface (8). In addition, NK cells secrets various
effective molecules, including interferon (IFN)-γ, and function in
coordination with other immune cells, such as dendritic cells and T
lymphocyte, to exert antitumor functions in various manners
(9,10). In cancer patients, the NK cell number
in the peripheral blood and tumor infiltrate, as well as the
cytokine production and expression of activating receptors, are
decreased; by contrast, the inhibitory receptors are overexpressed
(10).
CTLs, also known as CD8+ or killer T
cells, are characterized by the expression of CD3 and CD8
(CD3+CD8+). These cells are a critical
component of adaptive immunity to destroy infected or malignant
cells. CTLs secrete cytokines including primarily tumor necrosis
factor (TNF)-α and IFN-γ, which have antitumor and anti-viral
microbial effects. Another major function of CTLs is the production
and release of cytotoxic granules, which are also found in NK cells
and contain two families of proteins, namely perforin and
granzymes. Furthermore, CTLs also cause the destruction of infected
cells via the Fas/FasL interaction (11–15).
The AIET method mainly uses a dual combination of NK
cells and CTLs, as they have a definite advantage in targeting
abnormal expressing MHC class I and MHC antigen expressing cancer
cells. In addition, NK cells and CTLs preferentially kill cancer
stem cells, which is an added benefit to their use, since cancer
stem cells are resistant to the majority of therapies and serve a
major role in cancer recurrence (16–18).
Considering this evidence, it is suggested that AIET would be an
effective treatment method for cancer patients by destroying
circulating tumor cells, thereby preventing metastasis and cancer
recurrence. For AIET, obtaining a sufficient number of functional
immune cells is critical in clinical protocols. Therefore, the
number and purity of expanded immune cells is considered as a key
factor. Several researchers have attempted the use of various
methods to achieve large-scale NK cell and CTL ex vivo
expansion (19–23), and have applied these methods in
clinical trials with positive results reported in India, Japan and
China (18,24–26).
The aim of the present study is to evaluate the
effectiveness of BINKIT® for the expansion of NK cells
and CTLs collected from the peripheral blood of Vietnamese patients
with lung cancer for the application of AIET. The BINKIT medium was
successfully developed by the Biotherapy Institute of Japan (Tokyo,
Japan). The use of this medium for immune cell expansion and
activation in AIET in clinical applications has been previously
examined (4,25,26). To
the best of our knowledge, the present study is the first to
identify the use of BINKIT for AIET in lung cancer.
Materials and methods
Patients
A total of 7 patients with lung cancer with an
Eastern Cooperative Oncology Group/Performance Status (ECOG/PG) of
≥3, who were admitted to the Department of Oncology of the Vinmec
International Hospital (Hanoi, Vietnam) between April 2016 and
August 2016, were enrolled into the present study. The exclusion
criteria included severe infection, autoimmune disease, use of
anti-rejection drugs or T cell lymphomas. Patients provided a
written informed consent, and the study was approved by the Ethics
Committee of the Vinmec International Hospital. This study was
conducted with the permission of the Vietnam Ministry of Health
(document no. 2517/BYT-KCB; Hanoi, Vietnam). In total, 7 peripheral
blood samples were obtained and marked as PT1 to PT7, corresponding
to the 7 patients.
Isolation and large-scale expansion of
NK cells and CTLs from peripheral blood
Peripheral blood mononuclear cells (PBMNCs) were
obtained from the peripheral blood sample (70 ml) of each cancer
patient by density gradient centrifugation using Ficoll-Paque media
(GE Healthcare Life Sciences, Uppsala, Sweden) following the
manufacturer's instructions. Subsequently, PBMNCs were cultured
using BINKIT (Biotherapy Institute of Japan, Japan). Briefly, the
PBMNCs were divided into two equal parts for NK cell and CTLs
expansion, and seeded at a density of 1×106 cells/ml in
the cell initial medium. For NK cell expansion, the medium
contained 0.01 KE/ml OK-432 (Chugai Pharmaceutical Co., Ltd.,
Tokyo, Japan) and 700 IU/ml recombinant human interleukin-2
(rhIL-2; Chiron Corp, Emeryville, CA, USA) supplemented with 5%
heat-inactivated autologous plasma, and the cells were cultured in
an anti-CD16 monoclonal antibody (Beckman Coulter, Inc., Brea, CA,
USA)-immobilized culture flask. For CTL expansion, PBMNCs were
culture in cell initial medium containing 700 IU/ml rhIL-2 in an
anti-CD3 monoclonal antibody-immobilized flask. The cells were
incubated at 37°C in an atmosphere with 5% CO2 for 3
days. After 3 days, the culture medium was changed and subcultured
every 2–3 days in cell subculture medium (provided in the kit)
containing 350 IU/ml rhIL-2 supplemented with 5% heat-inactivated
autologous plasma to maintain a concentration of
0.8–1.0×106 cells/ml, without discarding the old medium.
When the number of cell increased logarithmically, the cultured
cells were transferred into culture bags (Nipro, Osaka, Japan)
until day 21 of culture.
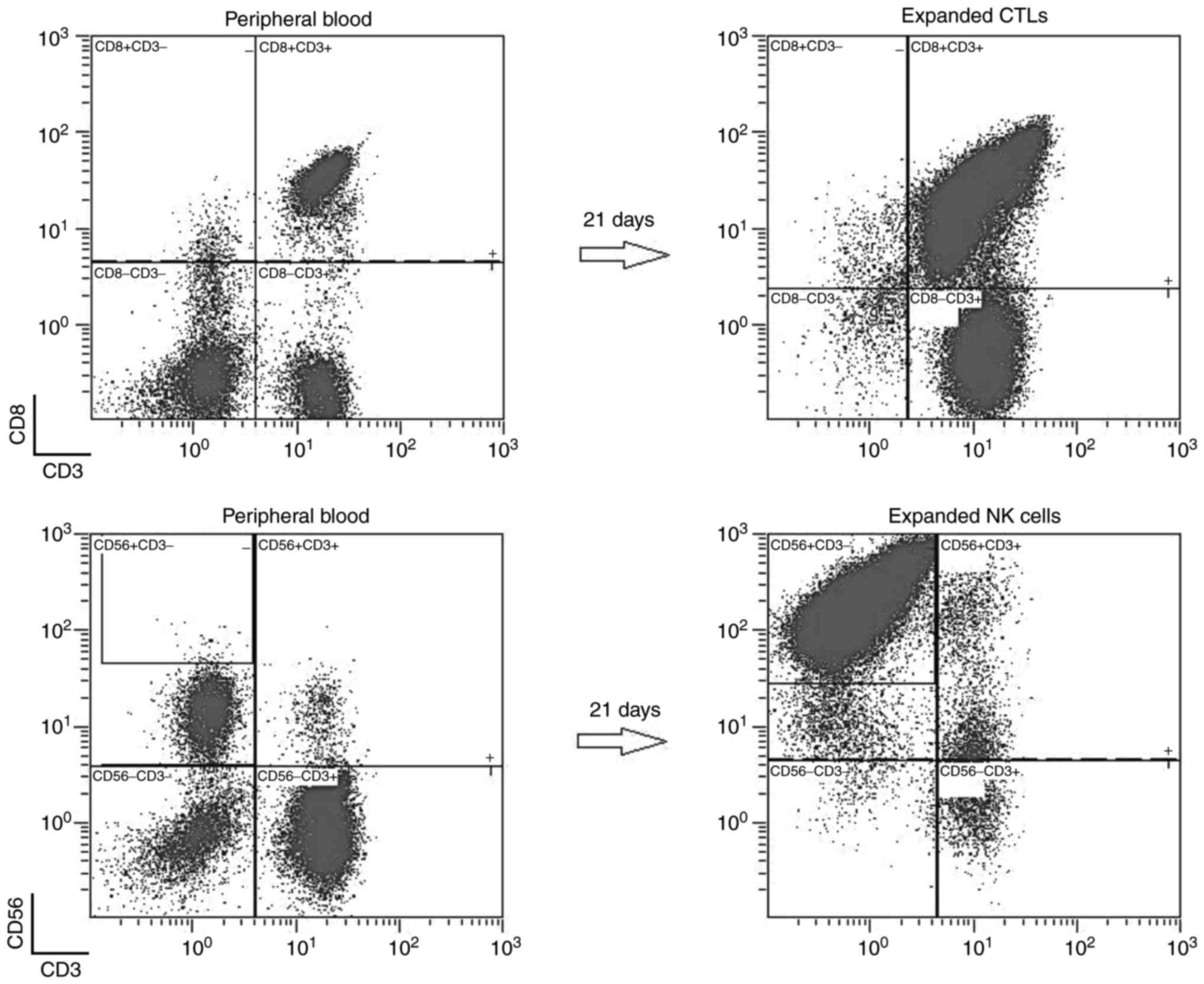
Phenotypic analysis
The phenotype of expanded cells and PBMNCs at
baseline (day 0) and at the end of the culture (day 21) was
analyzed by flow cytometry. Monoclonal antibodies specific for CD3,
CD8, CD56 and CD4 that were conjugated with Pacific Blue,
fluorescein isothiocyanate, R-phycoerythrin and
Allophycocyanin-Alexa Flour 750, respectively (Beckman Coulter,
Inc.), and the corresponding isotype were used for the
characterization of cell population. Cells were analyzed by Navios
flow cytometer (Beckman Coulter, Inc.), and data were acquired by
Navios software (version 3.2) according to the manufacturer's
instructions.
Quality control testing
Quality control testing was examined by assessing
samples obtained during the culture period and at the final
product. For sterility examination, the BacT/ALERT Plus
microbiological detection system (bioMérieux, Marcy-l'Étoile,
France) was used, while a MycoAlert Mycoplasma Detection kit (Lonza
Group, Ltd., Basel, Switzerland) was applied for mycoplasma
contamination testing. The viability of expanded cells was measured
by trypan blue exclusion assay and tested for endotoxin by a
kinetic colorimetric LAL assay using the Endosafe-PTS portable test
system (Charles River Laboratories, Inc., Wilmington, MA, USA).
Safety evaluation
The safety evaluation was conducted using the
ECOG/PS scale (27). The evaluations
were conducted at the time patients were enrolled in the research
(from April to August 2016), and then 18 months later.
Statistical analysis
Statistical analyses were performed with STATA
software (version 12.0; StataCorp LLC, College Station, TX, USA) to
determine the Prob>F, R2 coefficients of correlation
and P-value. A value of P<0.05 was considered to indicate a
difference that was statistically significant.
Results
Patient characteristics
Between December 2015 and June 2016, a total of 7
patients (6 females and 1 male) were enrolled into the present
study. Table I lists the patient
clinical characteristics. The mean age of the participants was
55.5±17.4 years, with an age range of 30–84 years. All patients
included in the present study had an ECOG/PS value of ≥3 (Table I), suggesting that they were only
capable of limited self-care, confined to bed or chair for >50%
of their waking hours (27).
 | Table I.Clinicopathological data of the lung
cancer patients enrolled in the present study. |
Table I.
Clinicopathological data of the lung
cancer patients enrolled in the present study.
| Case | Age (years) | Sex | Tumor
sizea | Lymph
nodeb | Disease
stagec | Prior
treatmentd | ECOG/PS at blood
collection | ECOG/PS at last
evaluatione | Estimated survival
(months) | Survival
(time)f |
|---|
| PT1 | 84 | F | T4 | N3 | M1m (bone,
lung) | Chemotherapy,
targeted Taxol | 4 | 3 (3) | 6 | + (18) |
| PT2 | 64 | F | T3 | N3 | M0 |
Chemo/radiotherapy | 3 | 2 (3) | 6 | + (18) |
| PT3 | 30 | F | T2 | N0 | M1m (lung,
bone) | Targeted Taxol | 3 | 1 (11) | 6 | + (18) |
| PT4 | 53 | F | T4 | N2 | M1m (lung, brain,
bone) | Chemotherapy,
targeted Taxol, checkpoint inhibitor | 3 | 2 (6) | 6 | + (18) |
| PT5 | 56 | F | T1 | N0 | M0 | Surgery | 3 | 2 (6) | 6 | + (15) |
| PT6 | 62 | M | T3 | N2 | M1m (brain,
bone) |
Chemo/radiotherapy | 3 | 2 (8) | 6 | + (16) |
| PT7 | 40 | F | T2 | N2 | M1m (bone) | Targeted Taxol | 3 | 1 (6) | 6 | − (17) |
Immune cell expansion
Subsequent to seeding, the PBMNC number was counted
every 2 days starting on day 3 of the culture using trypan blue
staining. It was observed that the number of cells decreased after
3 days of culture at the stimulation step. The total cell number at
day 3 was ~68.4% as compared with that on day 0. Subsequently, the
cell number began to growth at day 7 and reached logarithm growth
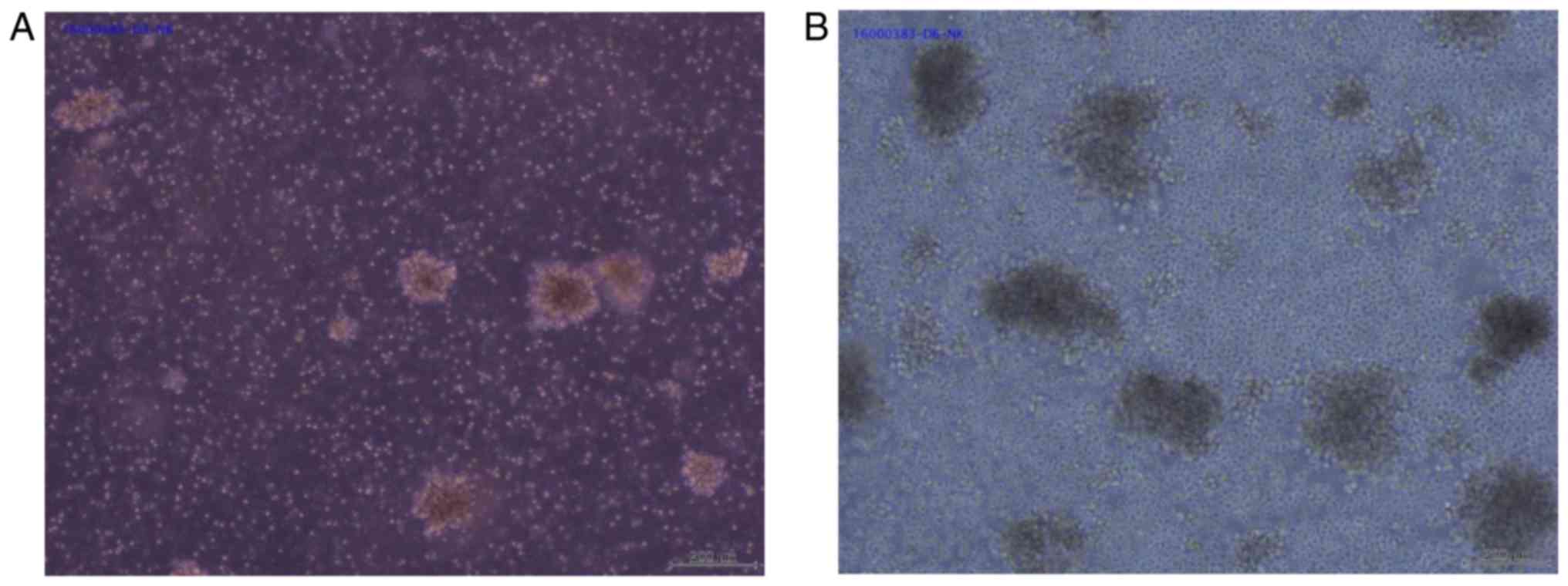
on continuous days until the day 20. In this period, cells grew as
large clumps, which is a characteristic of proliferating immune
cells (Fig. 1). In particular, the
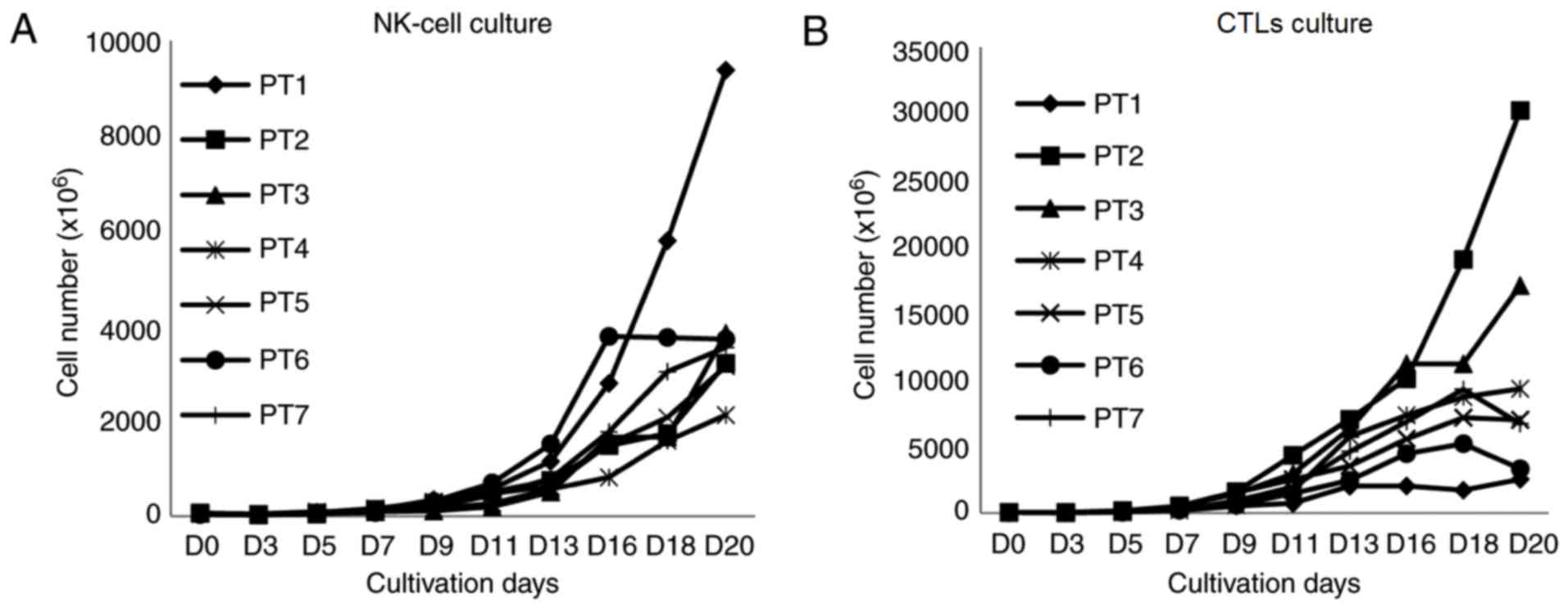
growth stopped in two samples in the CTL culture on day 18 and the
cell number decreased subsequently (Fig.
2).
From 70 ml of peripheral blood, PBMNCs were
collected with the Ficoll-Paque method and divided into two equal
parts for NK cell and CTL expansion. The average number of cells at
seeding for each culture condition was 53.4±13.5×106.
After 21 days of culture, the average total number of cells in the
NK culture was 4,137.2×106
(2,123.0–9,373.2×106 cells), which increased by
79.8-fold (range, 34.4- to 142.7-fold), while the cell viability
was 94.7% (91–97.3%). In the CTL culture, the average total number
of cells was 10,896.1×106
(2,544.0–30,240.0×106 cells), which increased by
192.5-fold (range, 38.7- to 448.0-fold), with a cell viability of
95.7% (92.8–98%; Table II).
 | Table II.Number of cell population pre- and
post-expansion. |
Table II.
Number of cell population pre- and
post-expansion.
| Case | PBMNC
count/collection for each culture (×106) | Final cell count
for NK cell (×106) | Total cell fold
expansion in NK (×106) | Final cell count
for CTLs culture (×106) | Total cell fold
expansion in αβT (×106) |
|---|
| PT1 | 65.7 | 9,373.2 | 142.7 | 2,544 | 38.7 |
| PT2 | 67.5 | 3,210 | 47.6 | 30,240 | 448.0 |
| PT3 | 59.0 | 3,840 | 65.1 | 17,088 | 289.6 |
| PT4 | 61.8 | 2,123 | 34.4 | 9,345 | 151.3 |
| PT5 | 47.8 | 3,152 | 65.9 | 7,008 | 146.6 |
| PT6 | 33.2 | 3,720 | 112.0 | 3,328 | 100.2 |
| PT7 | 38.9 | 3,542 | 91.1 | 6,720 | 172.8 |
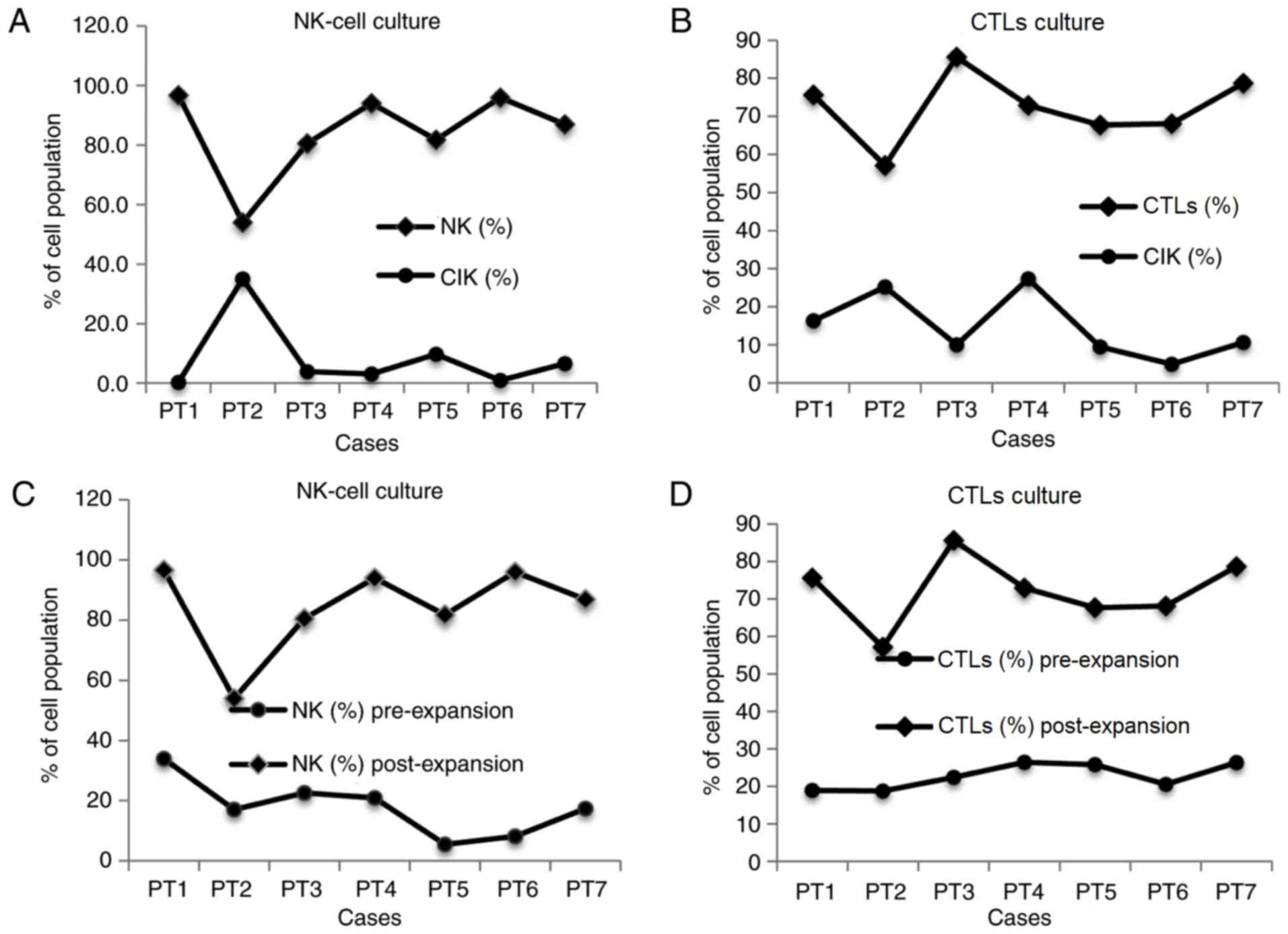
Characteristics of immune cell
population
The targeted immune cells increased sharply in the
selected culture medium (Fig. 3). The
average absolute number of NK cells in the NK culture at day 21 was
3,587.4×106, demonstrating an increase of 637.5-fold
(range, 167.1- to 1,613-fold) and accounting for 84.3±14.9%
(54–96.7%) of the total cell culture compared with the value of
17.9±9.5% (5.5–33.9%) at seeding. Similarly, the absolute number of
CTLs in the CTL culture was 7,561.4×106, demonstrating
an increase of 742.3-fold (range, 191.6- to 1,545.1-fold) and
accounting for 72.2±9.1% (57.1–85.6%) of the total cell culture
compared with the percentage of 22.7±3.5% (18.7–26.4%) at seeding
(Tables III and IV).
 | Table III.Results of immunophenotyping analysis
(average) pre- and post-expansion. |
Table III.
Results of immunophenotyping analysis
(average) pre- and post-expansion.
|
| PBMNCs (%) | CTL culture
(%) | NK cell culture
(%) |
|---|
|
|
|
|
|
|---|
| Case |
CD3+ |
CD3+CD4+ |
CD3+CD8+ |
CD3−CD56+ |
CD3+ |
CD3+CD4+ |
CD3+CD8+ |
CD3−CD56+ |
CD3+ |
CD3+CD4+ |
CD3+CD8+ |
CD3−CD56+ |
|---|
| PT1 | 48.9 | 29.2 | 18.9 | 33.9 | 84.8 | 11 | 75.6 | 11.9 | 1.1 | 0.6 | 0.6 | 96.7 |
| PT2 | 62.5 | 34.5 | 18.7 | 17 | 95.4 | 5.9 | 57.1 | 3.4 | 44.8 | 1.8 | 3.8 | 54 |
| PT3 | 48.5 | 25.5 | 22.4 | 22.6 | 96.1 | 12.2 | 85.6 | 2.6 | 17.5 | 0.5 | 16.4 | 80.5 |
| PT4 | 62.2 | 33.7 | 26.4 | 20.9 | 96.5 | 38 | 72.9 | 2.2 | 5.7 | 2.7 | 2.2 | 94 |
| PT5 | 69.4 | 40.8 | 25.8 | 5.5 | 97.9 | 30.6 | 67.7 | 2.1 | 21.8 | 6.1 | 6.5 | 81.8 |
| PT6 | 51.7 | 29.5 | 20.5 | 8.1 | 96.4 | 29.3 | 68.1 | 5 | 2 | 0.4 | 6 | 96 |
| PT7 | 58 | 30.8 | 26.3 | 17.4 | 88.5 | 11 | 78.7 | 9 | 12.2 | 0.8 | 10.4 | 86.9 |
 | Table IV.Absolute cell number (average) pre-
and post-expansion. |
Table IV.
Absolute cell number (average) pre-
and post-expansion.
|
| CTL culture
(CD3+CD8+), ×106 | NK-cell culture
(CD3−CD56+), ×106 |
|---|
|
|
|
|
|---|
| Case | Pre-expansion | Post-expansion | Fold increase | Pre-expansion | Post-expansion | Fold increase |
|---|
| PT1 | 10.0 | 1,923.3 | 191.6 | 17.71 | 9,063.9 | 511.7 |
| PT2 | 11.4 | 17,267.0 | 1,513.3 | 10.37 | 1,733.4 | 167.1 |
| PT3 | 9.5 | 14,627.3 | 1,545.0 | 9.55 | 3,091.2 | 323.6 |
| PT4 | 13.7 | 6,812.5 | 498.9 | 10.82 | 1,995.6 | 184.4 |
| PT5 | 10.6 | 4,744.4 | 446.7 | 2.26 | 2,578.3 | 1,138.7 |
| PT6 | 5.6 | 2,266.4 | 404.5 | 2.21 | 3,571.2 | 1,613.0 |
| PT7 | 8.9 | 5,288.6 | 596.0 | 5.87 | 3,078.0 | 524.3 |
In the NK cell culture, a strong correlation was
recognized between the number of NK and cytokine-induced killer
(CIK) cells, with a coefficient of determination (R2) of
0.9. The number of CIK cells decreased when NK cells were dominant
in the population and vice versa (coefficient = −1.17; P<0.001;
Fig. 4A), whereas other
CD3+ cells did not display this correlation (P>0.05).
By contrast, in the CTL culture, there was no correlation between
CTLs and CIK cells (P>0.05; Fig.
4B). In the two culture conditions, the initial number of
immune cells at seeding was not correlated with the number of
expanded immune cells subsequent to culturing (Fig. 4C and D). Notably, the percentage of
CD3−CD56bright increased strongly in the NK
cell culture, accounting for 82.1% of cells (52.7–92.8%; n=7) in
average. The absolute number of this cell type was
3,094.3×106 (913.5–8,438.5×106), which
increased by 408.9-fold (range, 121.4- to 958-fold; Table V). All cultures were negative for
mycoplasma and bacteria/fungi, while the endotoxin level was
<0.5 EU/ml.
 | Table V.Percentage of active NK cells
(CD3−CD56+ bright) pre- and
post-expansion. |
Table V.
Percentage of active NK cells
(CD3−CD56+ bright) pre- and
post-expansion.
|
| Pre-expansion | Post-expansion |
|---|
|
|
|
|
|---|
| Cases | Percentage (%) | Absolute number
(×106) | Percentage (%) | Absolute number
(×106) |
|---|
| PT1 | 0.5 | 0.09 | 93.1 | 8,438.5 |
| PT2 | 0.1 | 0.01 | 52.7 | 913.5 |
| PT3 | 0.2 | 0.02 | 78.7 | 2,432.8 |
| PT4 | 0.3 | 0.03 | 91.8 | 1,831.6 |
| PT5 | 0.2 | 0.00 | 77.8 | 2,005.9 |
| PT6 | 0.1 | 0.00 | 95.8 | 3,421.2 |
| PT7 | 0.7 | 0.04 | 85.0 | 2,616.3 |
Safety evaluation
The 7 patients presented an ECOG/PS score of 3 (6
patients) and 4 (1 patient) at the day of blood collection. This
score decreased at the last evaluation, with 1 patient
demonstrating a score of 3, 3 patients with a score of 2 and 2
patients with a score of 1. All patients had been estimated to live
only 6 months upon joining the present study. At the last follow-up
(18 months), 6 patients are alive, whereas 1 patient succumbed
after 17 months (Table I).
Discussion
In the present study, the efficacy of ex vivo
expansion of NK cells and CTLs from the peripheral blood of
Vietnamese cancer patients using BINKIT was evaluated. The two
culture conditions investigated (NK cell and CTL cultures)
demonstrated no clear correlation between the rate of initial
immune cells in the peripheral blood and the corresponding number
following ex vivo expansion (P>0.05).
A relatively pure NK
(CD56+CD3−) cell population of 84.3±14.9% was
generated, which was initially 17.9±9.5% in the whole blood without
any prior NK purification method. In 3 out of 7 cases (42.8%), the
proportion of NK following culture reached >94%. The mean NK
cell expansion was 637.5-fold (range, 167.1- to 1,613-fold) after
21 days of culture. Furthermore, in the specific medium for NK cell
expansion, the percentage of other lymphocyte, including
CD3+CD4+, CD3+CD8+ and
CD3+CD56+ cells, was minimal (mean value,
8.63, 1.46 and 3.74%, respectively). These data indicated that NK
cells were selectively expanded and became the major
population.
Recently, CIK cells have been investigated in the
treatment of cancer as a potential immune cell therapy (2,3). Notably,
the present study identified a close association between the
quantity of CIK cells and the number of NK cells in the NK cell
culture (P<0.05). However, in the CTL culture, no such
correlation between the CIK and CTL cell numbers was observed.
There were several methods to achieve large-scale
expansion of NK cells ex vivo in order to develop NK cell
therapeutics (10,22). PBMNCs have been used as a general
source of NK cells for expansion; however, these are composed of
numerous immune cell types. The most important target of various
studies is identifying a method for specifically culturing NK cells
in PBMNCs. Carlens et al (20)
observed that, after 21 days of culture from total PBMNCs with
IL-12 and OKT3, NK cells were stimulated up to 700-fold with a 55%
purity. Another study by Fujisaki et al (21) in 2009 revealed that, upon
K562-mb15-41BBL cell stimulation, NK cells increased by 277-fold
after 21 days of culture. Torrelli et al (22) used irradiated autologous feeder cells
along with IL-2 and IL-15 for NK cell expansion following
CD3+ T cell depletion and obtained an NK cell increase
by 15.7-fold after 14 days of culture. Terunuma and his co-workers
(4,25,26)
demonstrated that ex vivo culture of NK cells from
peripheral blood using BINKIT is a safe culture method, and that NK
cells expanded by several hundred folds after 2 weeks under GMP
conditions. Compared with these previously published studies, the
present study results obtained the highest purity of the NK cell
population and a high number of NK cells, which was suitable for
clinical application.
Ex vivo expansion of the CTL population in
the present study had a slightly lower purity as compared with that
in NK cells, with a mean proportion of CTLs
(CD8+CD3+) of 72.2±9.1% of the total number
of cells and a highest reported value of 85.6%. In the group of
patients included in the study, the mean fold expansion of immune
cells from PBMNCs was 742.3-fold for CTL cells, while the absolute
mean number of CTLs was 7,561.4×106.
Several CTL expansion methods have been used in
order to develop a relevant time of culture and number of CTLs
suitable for clinical application. An earlier method required a
45–60-day expansion protocol for CTLs (28). In order to improve this culture time,
Dudley et al (29) presented
the rapid expansion protocol using anti-CD3 antibody with high-dose
IL-2 and allogeneic feeder cells to generate T cells. After 14 days
of cultivation, the cell number increased by 1,320-fold (29). In order to avoid using a high dose of
IL-2 and exogenous feeder cells, Porter et al (30) co-stimulated ex vivo T cells
with CD3 and CD28 antibodies and obtained an expansion number of a
113-fold increase. However, this method induced both
CD3+CD4+ and CD3+CD8+ T
cells. Another study also used CD3 and CD28 antibodies to stimulate
T cells together with the removal of CD25 with the aim of
increasing the antigen-specific CD8+ T cells, and
successfully increased the specific T cells by 335-fold (31). Recently, Smith et al (32) investigated the method of using a
xeno-free serum replacement as an alternative to human serum and
fetal bovine serum in culturing, and obtained an >300-fold
increase in the number of T cells following costimulation with CD3
and CD28 antibodies.
In comparison with alternative methods to expand NK
cells and CTLs in clinical practice, the present study data
suggested that large-scale expansion of these cells directly from
PBMNCs using BINKIT without prior purification and feeder cells is
a safe and effective method for immune cell therapy in cancer
treatment.
Notably, it was observed that CD56 was highly
expressed (namely CD56bright) in the expanded NK cell
population. In whole blood, only <1% of CD56bright
cells were identified; however, following culturing, the NK
population contained mainly CD56bright cells (82.1%).
The CD56bright NK cells are considered the efficient
cytokine producers, as their major secretory molecules are IFN-γ,
TNF-α, granulocyte macrophage colony-stimulating factor and IL-10,
all of which serve important roles in destroying cancerous cells
(33). In the study by Chan et
al in 2007 (34),
CD56bright NK cells have longer telomeres as compared
with CD56dim cells, and can be differentiated into
CD56dim in vivo without cell proliferation.
Notably, a previous study demonstrated that CD56dim
cells generated from CD56bright cells had increased
cytotoxicity (33). Therefore, it is
suggested that NK expanded cells have a higher cytotoxicity and are
more efficient in killing cancer cells.
Prior to infusion of cultured cells, an endotoxin,
mycoplasma and sterility detection tests were conducted to confirm
the safety of the intravenous injection. All products in the
present study were negative for mycoplasma and bacteria, while the
concentration of endotoxin was <0.5 EU/ml, which is considered
to be a safe value for intravenous injection. Following
administration of ex vivo expanded cells in all patients, no
adverse reactions were observed. Furthermore, improvement in the
quality of life was observed in almost all patients. These
observations prove the safety of the intravenous administration of
the ex vivo-expanded NK and CTL cells to cancer patients in
the present study. The safety subsequent to receiving the culture
cells was also examined, and patients were followed up. Almost all
patients demonstrated improved life quality, with the majority of
patients presenting a score of 1 or 2 in the ECOG/PS scale.
Furthermore, 6 patients were alive at the last follow-up, and only
1 patient succumbed at 17 months after the treatment. These results
suggest that AIET conducted in the present study was safe.
In conclusion, the present data demonstrated that
BINKIT is an appropriate and efficient method for ex vivo
expansion of NK cells and CTLs from the PBMNCs of lung cancer
patients, and evidently improved their quality of life. The
advantages of the BINKIT protocol is the high level of purity and
the safety for injection of the expanded NK cell and CTL culture
after 3 weeks. To determine the activity of expanded immune cells
and the effects of AIET in cancer treatment, further investigation
must be conducted and clinical data collected. Given the positive
results of this first study using AIET in cancer patients in
Vietnam, it is strongly suggested that this method is a promising
tool for immune cell-based cancer immunotherapy for cancer
patients.
Acknowledgements
The authors would like to thank Dr. Hiroshi Terunuma
and Mr. Tsubasa Takane (Biotherapy Institute of Japan, Tokyo,
Japan) for advices in cell culture.
References
|
1
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC CancerBase No. 11. IARC; Lyon, France: 2013
|
|
2
|
Mellman I, Coukos G and Dranoff G: Cancer
immunotherapy comes of age. Nature. 480:480–489. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dudley ME and Rosenberg SA:
Adoptive-cell-transfer therapy for the treatment of patients with
cancer. Nat Rev Cancer. 3:666–675. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Terunuma H: Autologous immune enhancement
therapy for cancer-our experience since 2004. J Stem Cells Regen
Med. 8:205–206. 2012.PubMed/NCBI
|
|
5
|
Kalinski P, Mailliard RB, Giermasz A, Zeh
HJ, Basse P, Bartlett DL, Kirkwood JM, Lotze MT and Herberman RB:
Natural killer-dendritic cell cross-talk in cancer immunotherapy.
Expert Opin Biol Ther. 5:1303–1315. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalos M and June CH: Adoptive T cell
transfer for cancer immunotherapy in the era of synthetic biology.
Immunity. 39:49–60. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cooper MA, Fehniger TA and Caligiuri MA:
The biology of human natural killer-cell subsets. Trends Immunol.
22:633–640. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Screpanti V, Wallin RP, Ljunggren HG and
Grandien A: A central role for death receptor-mediated apoptosis in
the rejection of tumors by NK cells. J Immunol. 167:2068–2073.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bouwer AL, Saunderson SC, Caldwell FJ,
Damani TT, Pelham SJ, Dunn AC, Jack RW, Stoitzner P and McLellan
AD: NK cells are required for dendritic cell-based immunotherapy at
the time of tumor challenge. J Immunol. 192:2514–2521. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sutlu T and Alici E: Natural killer
cell-based immunotherapy in cancer: Current insights and future
prospects. J Intern Med. 266:154–181. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin Z, Schwartzkopff J, Pradera F,
Kammertoens T, Seliger B, Pircher H and Blankenstein T: A critical
requirement of interferon gamma-mediated angiostasis for tumor
rejection by CD8+ T cells. Cancer Res. 63:4095–4100.
2003.PubMed/NCBI
|
|
12
|
Maher J and Davies ET: Targeting cytotoxic
T lymphocytes for cancer immunotherapy. Br J Cancer. 91:817–821.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weigelin B, Krause M and Friedl P:
Cytotoxic T lymphocyte migration and effector function in the tumor
microenvironment. Immunol Lett. 138:19–21. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Anikeeva N, Somersalo K, Sims TN, Thomas
VK, Dustin ML and Sykulev Y: Distinct role of lymphocyte
function-associated antigen-1 in mediating effective cytolytic
activity by cytotoxic T lymphocytes. Proc Natl Acad Sci USA.
102:pp. 6437–6442. 2005; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ratner A and Clark WR: Role of TNF-alpha
in CD8+ cytotoxic T lymphocyte-mediated lysis. J
Immunol. 150:4303–4314. 1993.PubMed/NCBI
|
|
16
|
Castriconi R, Daga A, Dondero A, Zona G,
Poliani PL, Melotti A, Griffero F, Marubbi D, Spaziante R, Bellora
F, et al: NK cells recognize and kill human glioblastoma cells with
stem cell-like properties. J Immunol. 182:3530–3539. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weng D, Song B, Durfee J, Sugiyama V, Wu
Z, Koido S, Calderwood SK and Gong J: Induction of cytotoxic T
lymphocytes against ovarian cancer-initiating cells. Int J Cancer.
129:1990–2001. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ahmed N, Salsman VS, Kew Y, Shaffer D,
Powell S, Zhang YJ, Grossman RG, Heslop HE and Gottschalk S:
HER2-specific T cells target primary glioblastoma stem cells and
induce regression of autologous experimental tumors. Clin Cancer
Res. 16:474–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cho D and Campana D: Expansion and
activation of natural killer cells for cancer immunotherapy. Korean
J Lab Med. 29:89–96. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Carlens S, Gilljam M, Chambers BJ, Aschan
J, Guven H, Ljunggren HG, Christensson B and Dilber MS: A new
method for in vitro expansion of cytotoxic human
CD3-CD56+ natural killer cells. Hum Immunol.
62:1092–1098. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fujisaki H, Kakuda H, Shimasaki N, Imai C,
Ma J, Lockey T, Eldridge P, Leung WH and Campana D: Expansion of
highly cytotoxic human natural killer cells for cancer cell
therapy. Cancer Res. 69:4010–4017. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Torelli GF, Rozera C, Santodonato L,
Peragine N, D'agostino G, Montefiore E, Napolitano MR, Monque DM,
Carlei D, Mariglia P, et al: A good manufacturing practice method
to ex vivo expand natural killer cells for clinical use. Blood
Transfus. 13:464–471. 2015.PubMed/NCBI
|
|
23
|
Koehl U, Brehm C, Huenecke S, Zimmermann
SY, Kloess S, Bremm M, Ullrich E, Soerensen J, Quaiser A, Erben S,
et al: Clinical grade purification and expansion of NK cell
products for an optimized manufacturing protocol. Front Oncol.
3:1182013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Subramani B, Ratnavelu K, Pullai CR,
Krishnan K, Sugadan SD, Deng X and Hiroshi T: Autologous immune
enhancement therapy: A case report of a stage IV colonic cancer.
Oncol Lett. 5:1611–1614. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dedeepiya V, Terunuma H, Manjunath S,
Senthilkumar R, Thamaraikannan P, Srinivasan T, HelenReena C,
Preethy S and Abraham S: Autologous immune enhancement therapy for
cancer using NK cells and CTLs without feeder layers; our six year
experience in India. J Stem Cells Regen Med. 7:952011.PubMed/NCBI
|
|
26
|
Terunuma H, Deng X, Nishino N and Watanabe
K: NK cell-based autologous immune enhancement therapy (AIET) for
cancer. J Stem Cells Regen Med. 9:9–13. 2013.PubMed/NCBI
|
|
27
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Topalian SL, Muul LM, Solomon D and
Rosenberg SA: Expansion of human tumor infiltrating lymphocytes for
use in immunotherapy trials. J Immunol Methods. 102:127–141. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dudley ME, Wunderlich JR, Shelton TE, Even
J and Rosenberg SA: Generation of tumor-infiltrating lymphocyte
cultures for use in adoptive transfer therapy for melanoma
patients. J Immunother. 26:332–342. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Porter DL, Levine BL, Bunin N, Stadtmauer
EA, Luger SM, Goldstein S, Loren A, Phillips J, Nasta S, Perl A, et
al: A phase 1 trial of donor lymphocyte infusions expanded and
activated ex vivo via CD3/CD28 costimulation. Blood. 107:1325–1331.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rasmussen AM, Borelli G, Hoel HJ, Lislerud
K, Gaudernack G, Kvalheim G and Aarvak T: Ex vivo expansion
protocol for human tumor specific T cells for adoptive T cell
therapy. J Immunol Methods. 355:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Smith C, Økern G, Rehan S, Beagley L, Lee
SK, Aarvak T, Schjetne KW and Khanna R: Ex vivo expansion of human
T cells for adoptive immunotherapy using the novel Xeno-free CTS
immune cell serum replacement. Clin Transl Immunol. 4:e312015.
View Article : Google Scholar
|
|
33
|
Michel T, Poli A, Cuapio A, Briquemont B,
Iserentant G, Ollert M and Zimmer J: Human CD56bright NK Cells: An
Update. J Immunol. 196:2923–2931. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chan A, Hong DL, Atzberger A, Kollnberger
S, Filer AD, Buckley CD, McMichael A, Enver T and Bowness P:
CD56bright human NK cells differentiate into CD56dim
cells: Role of contact with peripheral fibroblasts. J Immunol.
179:89–94. 2007. View Article : Google Scholar : PubMed/NCBI
|