Introduction
Colorectal carcinoma (CRC) is the third most common
type of cancer worldwide, which accounts for 10% of all tumors
(1,2).
In previous years, the incidence of CRC has increased yearly, which
can be attributed to changes in lifestyle, diet and deteriorating
environmental factors. Despite the advances made in the prevention
and treatment of CRC, the 5-year survival rate of patients is 35%
in most regions of the world (3–5). Recent
investigations have revealed that the incidence, progression,
invasion and metastases of CRC result from the interaction of
multiple genes and factors (6–8). Even
after undergoing curative surgery, a substantial proportion of
patients with CRCs, experience tumor recurrence (2,9). Despite
the current advancements in molecular biology, few of these
techniques have been introduced into clinical practice for
treatment. The aim of the present study was to identify a potential
biomarker that may predict the prognoses of patients with CRC.
The G protein-coupled receptor kinases (GRKs) are a
versatile family of kinases, which serve a critical role in G
protein-coupled receptor homologous desensitization. GRKs promote
the receptor-arrestin interaction and the uncoupling of the
receptor from its G protein, by phosphorylating specific serine and
threonine residues in the cytoplasmic domains of the activated
receptor (10,11). Despite the critical role of GRKs in
homologous desensitization, aberrant GRK activity has been
identified in cases of opiate addiction, heart failure, and tumor
progression and metastasis (12–14).
Therefore, GRKs are hypothesized to be valuable therapeutic
targets. GRK6, a member of the GRK family, is present in many human
tissues, and has been implicated in multiple disease pathways
(15,16). Additionally, present studies indicate
that GRK6 may be implicated in the metastasis of several
carcinomas, including hepatic and lung cancer, medulloblastoma and
multiple myelomas (17–19). However, the prognostic value and the
clinicopathological significance of GRK6 in CRC have not been
previously reported.
The purpose of the present study was to investigate
GRK6 expression and evaluate the clinicopathological features in
patients with CRC and to investigate the potential association of
GRK6 in the origin and progression of CRC.
Materials and methods
The reporting of the present study was in accordance
with the REMARK guidelines (20).
Tissue specimens and
clinicopathological data
A total of 83 patients with CRC were enrolled in the
present study at the Affiliated Hospital of Nantong University
(Nantong, China) from March 2009 to February 2010. A total of 39
females and 44 males were included in the present study, ranging
from 25–65 years, with a mean age of 57 years. Specimens of tumor
tissues and adjacent paracancerous histological normal tissues
(PCHNTs) were collected during surgery. The PCHNT specimens were
extracted >3 cm from the tumor margin and assessed
microscopically for the presence of normal cells and the absence of
dysplastic cells. Following surgical removal, the fresh specimens
were divided into two equal halves. One half was fixed in formalin,
embedded in paraffin, and used for immunohistochemical staining.
The other half was preserved at −80°C after freezing in liquid
nitrogen for further analysis by western blotting and RT-qPCR.
Clinicopathological data were obtained, and the
tumor-node-metastasis (TNM) stages and lymph node status were
determined. The stages and grades of tumors were determined
according to the criteria listed in the fifth edition of TNM
classification of the International Union Against Cancer (21). None of the patients with CRC had
received preoperative radiotherapy or chemotherapy prior to
surgery. Following surgery, all patients received standard
treatments according to the National Comprehensive Cancer Network
guidelines (22). The present study
was approved by the Ethics Committee of the Affiliated Hospital of
Nantong University. All experiments were performed in accordance
with the approved guidelines of the Affiliated Hospital of Nantong
University and the 1964 Helsinki Declaration and its later
amendments. Written informed consent was obtained from all
participants prior to undergoing surgery.
Immunohistochemistry analysis
The paraffin-embedded tissue sections (~2 mm), were
dewaxed in xylene, rehydrated in a series of ethanol (70% for 30
min, 80% for 60 min, 90% for 60 min, 95% for 60 min, 95% for 60
min, 100% for 60 min and 100% for 60 min) and immersed in 3%
hydrogen peroxide at room temperature for 5 min to suppress the
endogenous peroxidase activity. The tissues sections were heated at
100°C for 10 min in 0.01 mol/l sodium citrate buffer (pH 6.0) to
facilitate antigen retrieval. After three washes with
phosphate-buffered saline (PBS) for 5 min each, the sections were
incubated at room temperature for 2 h with a polyclonal rabbit
anti-human GRK6 primary antibody (cat. no. sc-566; 1:200; Abcam,
Cambridge, UK) diluted in PBS. The anti-GRK6 monoclonal antibody
was used to determine the expression of the GRK6 protein. The
sections were washed three times in PBS for 5 min each, and
incubated with a HRP-conjugated goat anti-rabbit secondary
immunoglobulin G (cat. no. SA00004-6; 1:200; ProteinTech Group,
Inc., Chicago, IL, USA) for 1 h at room temperature. After three
additional washes with PBS, the antigen-antibody complexes were
developed with diaminobenzidine (DAB, 20 µl + PBS, 1,000 µl + 3%
H2O2, 5 µl) staining at room temperature for
10 min.
A total of three observers determined the
immunohistochemical staining scores (ISS), which is based on
staining frequency and intensity. Staining frequency was scored as
follows: No staining was scored as 0; 1–25% of stained cells was
scored as 1; 25–50%, as 2; 51–75%, as 3; and >75%, as 4.
Staining intensity was rated on a scale of 0–3 as follows: 0,
negative; 1, weak; 2, moderate; and 3, strong. The raw data were
converted into ISS by multiplying the corresponding frequency
scores and the staining intensity scores. Therefore, the ISS could
range from 0 to 12. An ISS of 9–12 was designated as strong
immunoreactivity (+++); 5–8, as moderate (++); 1–4, as weak (+);
and 0, as negative (−) immunoreactivity. The sections with staining
in the cell cytoplasm of >25% tumor cells were deemed as
positive for GRK6. Staining was scored independently by three
individuals. Based on the results of immunohistochemical staining
for GRK6 expression, patients with CRC were divided into two
groups, one with low GRK6 expression (− to +) and another with high
GRK6 expression (++ to +++).
Western blot analysis
Total protein was extracted using a lysis buffer
(Thermo Fisher Scientific, Inc., Waltham, MA, USA) that contains
protease inhibitors. A total of 30 µg of protein were separated by
10% SDS-PAGE gel electrophoresis and transferred to polyvinylidene
fluoride (PVDF) membranes. Non-specific binding was blocked by
incubating the membranes with 5% non-fat milk in Tris-buffered
saline containing 0.1% Tween-20 (TBST) for 2 h at room temperature.
Following incubation with the polyclonal rabbit anti-human GRK6
antibody (dilution 1:1,000; cat. no. sc-566; Abcam), or rabbit
anti-GAPDH antibody (dilution 1:2,000; cat. no. SAB2701826;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), overnight at 4°C,
the membranes were washed three times with TBST for 5 min. The
membranes were then incubated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (dilution
1:1,000; Sigma-Aldrich; Merck KGaA) at room temperature for 2 h.
The signals were detected using enhanced chemiluminescence (GE
Healthcare, Chicago, IL, USA) followed by film development.
In addition, 19 other pairs of samples of cancerous
tissues and matched non-cancerous fresh frozen tissues were
collected for reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) analysis. Similarly, western blot analysis was
performed on 19 other pairs of cancerous tissues and matched
non-cancerous fresh frozen tissues, by the aforementioned
method.
RT-qPCR
GRK6 mRNA expression was analyzed by RT-qPCR
(23). Total RNA was extracted using
TRIzol® reagent (Gibco; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions from the CRC tissues.
Reverse transcription was performed at 42°C for 60 min followed by
70°C for 5 min using the Transcriptor First Strand cDNA Synthesis
kit (Roche Diagnostics GmbH, Mannheim, Germany). RT-qPCR was
performed using FastStart Universal SYBR-Green Master (Rox; Roche
Diagnostics GmbH). According to the protocol, 2 µl cDNA was added
to a 20 µl final reaction volume. The RT-qPCR analysis was
performed in 96-well plates in a thermocycler (ABI Prism 7500;
Applied Biosystems; Thermo Fisher Scientific, Inc.). The cycling
parameters used were as follows: Hot start at 95°C for 10 min, 40
cycles of amplification, quantification at 95°C for 15 sec, and
57°C for 1 min, during which time fluorescence was measured, at
72°C for 30 sec. Continuous fluorescence acquisition was performed
at 65–97°C in order to perform melting curve analysis. These
cycling parameters generated a single amplification for the primer
set used, according to the presence of a single melt peak. GAPDH
was selected as the internal reference. All RT-qPCR reactions were
repeated 3 times for each gene, and each sample was set up in
triplicate. Primer sequences were designed based on published human
gene sequences. The primer sequences were as follows: GRK6 forward
primer, 5′-AAAACACCTTCAGGCAATACCG-3′; and reverse primer,
5′-AGGCCAAGCTCACTACAAACCTA-3′. GAPDH forward primer,
5′-CATGAGAAGTATGACAACAGCCT-3′; and GAPDH reverse primer,
5′-AGTCCTTCCACGATACCAAAGT-3′. The 2−ΔΔCq method was used
to calculate relative changes in gene expression (24).
Statistical analysis
SPSS statistical software (version 17.0; SPSS Inc.,
Chicago, IL, USA) was used for data analyses. The GRK6 mRNA levels
in CRC and PCHNT specimens were compared using the paired Student's
t-test. Student's t-test for independent samples was performed to
compare the means of two groups. The χ2 test was used to
determine the significance of GRK6 expression and the
clinicopathological variables of CRC. Kaplan-Meier analysis was
used to compute survival rates, and statistical significance was
assessed by using the log-rank test. A univariate analysis with the
Cox regression model was used to determine the identified
prognostic factors, and multivariate analysis with the Cox
regression model was used to investigate the combined effects.
Spearman's rank correlation analysis was used to analysis the
association between GRK6 expression and TNM stage in colorectal
carcinoma. The results are presented as mean ± standard deviation
of a minimum of three independent experiments. P<0.05 was
considered to indicate a statistically significant difference
(25). Furthermore, the survival
rates of patients based on GRK6 positivity were analyzed by
separating the patients into two groups based on the stage of
disease: I–II and III–IV.
Results
Upregulation of GRK6 expression in
primary colorectal tumors
The results of the immunohistochemical analysis
demonstrated that GRK6 expression was significantly increased in
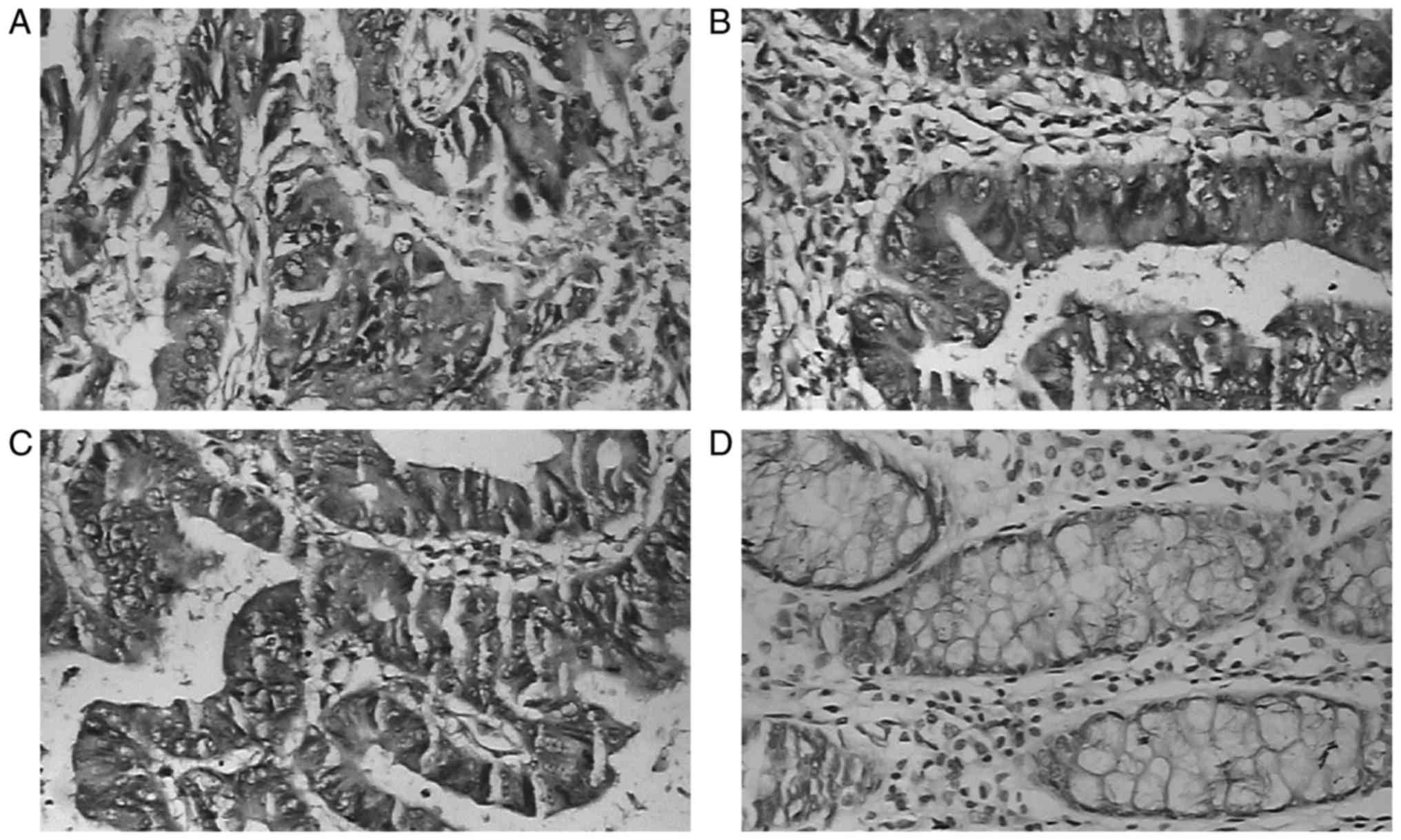
patients with CRC compared with normal CRC tissues (Fig. 1). GRK6 expression was observed in 70
of 83 patients with CRC (84.3%); 33 patients demonstrated strong
expression (33/70×100=47.1%; Fig.
1A), and 37 demonstrated weak expression (37/70×100=52.9%;
Fig. 1B). In the majority of the
PCHNT and non-cancerous specimens, negative or weakly positive
immunostaining of GRK6 in the cytoplasmic region was observed
(Fig. 1C and D). GRK6 expression was
significantly higher in carcinoma tissues compared with PCHNT and
non-cancerous tissues (P<0.05; Table
I). However, there were no statistically significant
differences in GRK6 expression between normal colorectal tissues
and PCHNT specimens (P=0.289).
 | Table I.GRK6 expression in CRC, PCHNT and
normal colorectal tissues. |
Table I.
GRK6 expression in CRC, PCHNT and
normal colorectal tissues.
|
|
| GRK6 expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | n | − | + | ++ | +++ | Positive rate
(%) |
|---|
| CRC | 83 | 13 | 37 | 22 | 11 | 84.3 |
| PCHNT | 83 | 73 | 7 | 3 | 0 | 12.0 |
| Normal tissue | 19 | 17 | 2 | 0 | 0 | 10.5 |
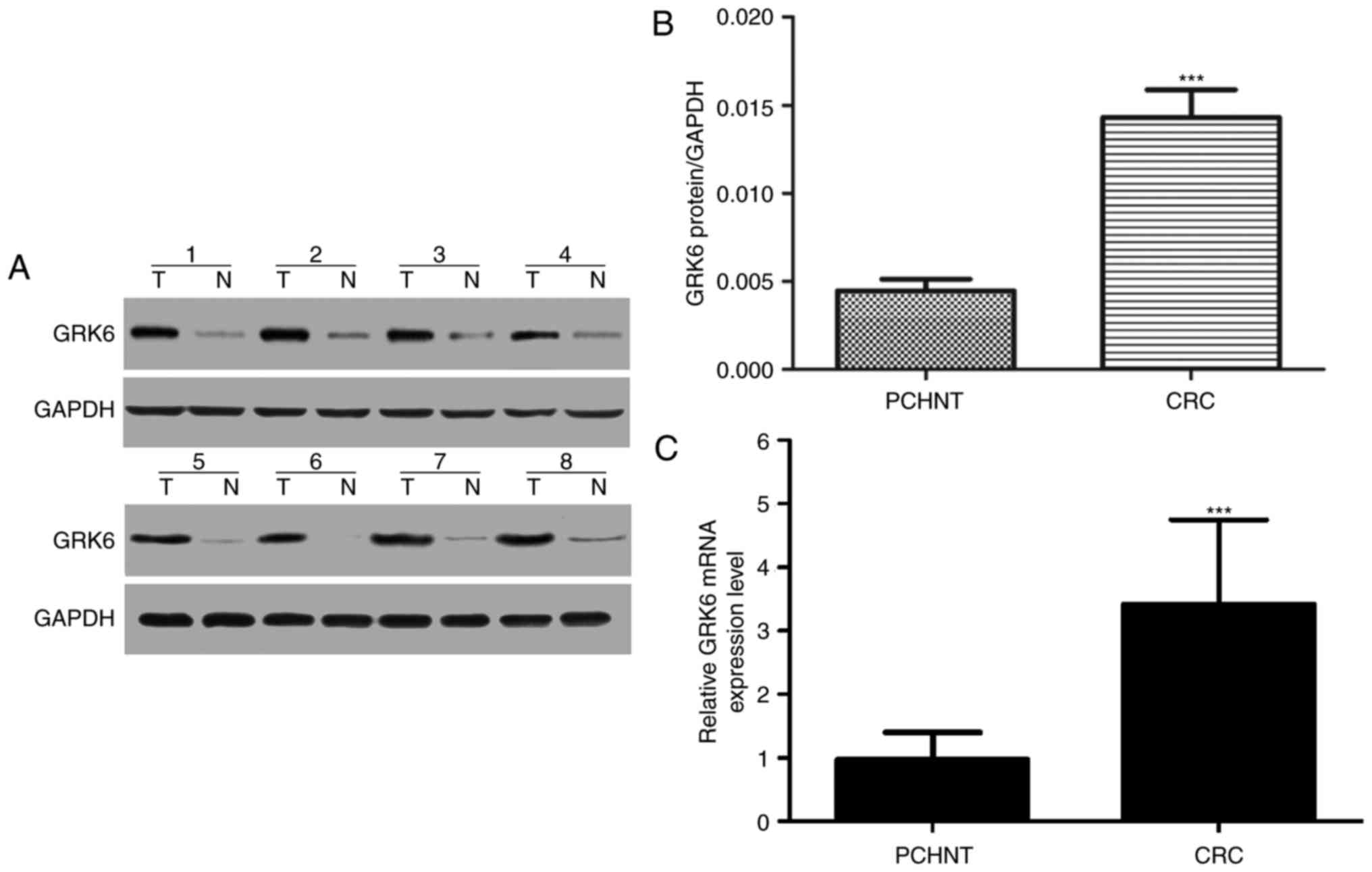
The expression of GRK6 protein was examined by
western blotting in 19 pairs of randomly selected specimens of CRC
tissues and their matched normal colorectal tissues. The
representative results of western blotting in 8 cases are presented
in Fig. 2A. The expression levels of
GRK6 protein were markedly increased in tumor tissues compared with
adjacent non-cancerous tissues (Fig.
2B). Therefore, GRK6 may be a potential candidate for the
regulation of CRC initiation and progression.
The aforementioned results were further confirmed by
RT-qPCR analysis. GRK6 mRNA expression was examined in 19 CRC
tissues and corresponding PCHNT specimens, which were randomly
selected. GRK6 expression was significantly upregulated in samples
of CRC tissues compared with PCHNT specimens (P<0.05; Fig. 2C).
Association between GRK6 expression
and clinicopathological factors in patients with CRC
The association between GRK6 expression and
clinicopathological factors in patients with CRC is summarized in
Table II. The overexpression of GRK6
was significantly associated with histological differentiation,
lymph node metastasis, venous invasion, depth of invasion, distant
metastasis, and TNM stages (P=0.001, P=0.045, P=0.009, P=0.026,
P<0.0001, P=0.020, respectively) in patients with CRC. However,
no significant associations were observed between GRK6 expression
and age, sex, tumor size and growth patterns in patients with CRC.
In addition, no significant association was observed between the
levels of preoperative carcinoembryonic antigen (CEA) (P=0.705),
CA19-9 (P=0.443) and GRK6 expression. The results demonstrated that
GRK6 expression in CRC specimens was positively associated with TNM
stage, which indicated that advanced TNM stages corresponded to
increased GRK6 expression in CRC (rs=0.467, P<0.01;
Table III).
 | Table II.Associations between GRK6 protein
expression and clinicopathological parameters in colorectal cancer
tissues. |
Table II.
Associations between GRK6 protein
expression and clinicopathological parameters in colorectal cancer
tissues.
|
|
| GRK6
expression |
|
|---|
|
|
|
|
|
|---|
| Clinical
parameters | Total | High | Low-moderate | Absent | P-value |
|---|
| Sex |
|
|
|
| 0.688 |
|
Male | 44 | 7 | 31 | 6 |
|
|
Female | 39 | 4 | 28 | 7 |
|
| Age (years) |
|
|
|
| 0.399 |
|
≥50 | 58 | 5 | 44 | 9 |
|
|
≤50 | 25 | 6 | 15 | 4 |
|
| Tumor size
(cm) |
|
|
|
| 0.133 |
|
>3 | 36 | 7 | 26 | 3 |
|
| ≤3 | 47 | 4 | 33 | 10 |
|
| Growth pattern |
|
|
|
| 0.714 |
|
Expanding type | 31 | 4 | 22 | 5 |
|
|
Infiltration type | 12 | 3 | 8 | 1 |
|
|
Ulcerative type | 40 | 4 | 29 | 7 |
|
|
Differentiation |
|
|
|
| 0.001a |
|
Well/moderate | 55 | 2 | 42 | 11 |
|
|
Poor | 28 | 9 | 17 | 2 |
|
| Lymph node
invasion |
|
|
|
| 0.045a |
|
Negative | 48 | 3 | 35 | 10 |
|
|
Positive | 35 | 8 | 24 | 3 |
|
| Venous
invasion |
|
|
|
| 0.009a |
|
Negative | 68 | 6 | 53 | 9 |
|
|
Positive | 15 | 5 | 6 | 4 |
|
| Depth of
invasion |
|
|
|
| 0.026a |
|
T1/T2 | 37 | 1 | 28 | 8 |
|
|
T3/T4 | 46 | 10 | 31 | 5 |
|
| Distant
metastasis |
|
|
|
|
<0.0001a |
| M0 | 70 | 2 | 56 | 12 |
|
| M1 | 13 | 9 | 3 | 1 |
|
| TNM stage |
|
|
|
| 0.020a |
|
I/II | 53 | 3 | 42 | 8 |
|
|
III/IV | 30 | 8 | 17 | 5 |
|
| CEA level (ng
ml−1) |
|
|
|
| 0.705 |
|
>5 | 44 | 5 | 33 | 6 |
|
| ≤5 | 39 | 6 | 26 | 7 |
|
| CA19-9 level (U
ml−1) |
|
|
|
| 0.443 |
|
>37 | 23 | 4 | 14 | 5 |
|
|
≤37 | 60 | 7 | 45 | 8 |
|
 | Table III.Association between GRK6 expression
and TNM stage in colorectal carcinoma. |
Table III.
Association between GRK6 expression
and TNM stage in colorectal carcinoma.
|
| GRK6
expression |
|
|
|
|---|
|
|
|
|
|
|
|---|
| TNM stage | − | + | ++ | +++ | Total | rs | P-value |
|---|
| I | 5 | 8 | 3 | 0 | 16 |
|
|
| II | 3 | 20 | 11 | 3 | 37 |
|
|
| III | 5 | 7 | 7 | 3 | 22 |
|
|
| IV | 0 | 2 | 1 | 5 | 8 | 0.459 |
<0.01a |
| Total | 13 | 37 | 22 | 11 | 83 |
|
|
Association between GRK6 expression
and survival rates in patients with CRC
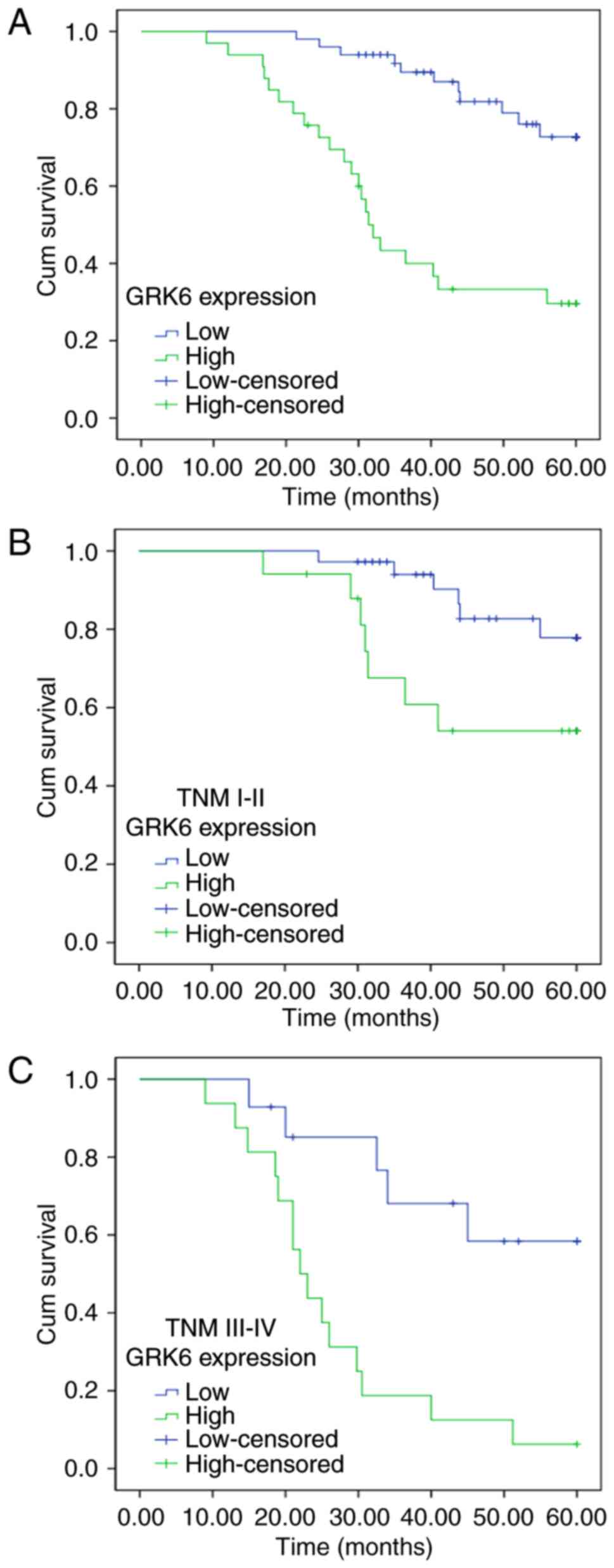
The survival statuses of 83 patients with CRC were
evaluated by Kaplan-Meier survival curves and the log-rank test at
the end of clinical follow-up. Based on the results of
immunohistochemical staining for GRK6 expression, patients with CRC
were divided into two groups, namely, the patient group with low
GRK6 expression (− to +) and the patient group with high GRK6
expression (++ to +++). There were 50 patients in the group with
low levels of GRK6 expression, of which 11 succumbed to disease.
The 5-year overall survival rate was 78%. There were 33 patients
with high levels of GRK6 expression, of which 22 succumbed to
disease. The 5-year overall survival rate was 33.3%. Therefore, the
overall survival of patients with CRC and low GRK6 expression was
significantly longer compared with patients with CRC with high GRK6
expression (Fig. 3A).
Furthermore, the survival rates of patients based on
GRK6 positivity were analyzed by separating the patients into two
groups based on the stage of disease: I–II and III–IV. In patients
with stage I–II disease, there were 36 patients with low GRK6
expression (− to +), of which 6 succumbed to disease, and there
were 17 patients with high GRK6 expression (++ to +++), of which 7
succumbed to disease. In patients with stage III–IV disease there
were 14 patients with low GRK6 expression, of which 5 succumbed to
disease. There were 16 patients in the high GRK6 expression group,
of which 15 succumbed to disease. The overall survival of the group
with high GRK6 expression was significantly shorter compared with
the group with low GRK6 expression, irrespective of the differences
in the stage of disease (Fig. 3B and
C).
Prognostic value of GRK6 expression in
patients with CRC
To evaluate the independent prognostic value of GRK6
expression on overall survival in patients with CRC, multivariate
analysis was performed to evaluate the prognostic factors in the
present study cohort using a Cox proportional hazard model
(Table IV). In these patients, TNM
stages, lymph node metastases, histological differentiation, tumor
invasion, GRK6 expression, and the levels of CEA and CA19-9 were
associated with poor prognosis (P<0.001, P=0.023, P<0.001,
P=0.021, P=0.003, P=0.001, P=0.034, respectively).
 | Table IV.Cox proportional hazards model
analysis of prognostic factors. |
Table IV.
Cox proportional hazards model
analysis of prognostic factors.
|
|
Characteristics |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Factors | Unfavorable | Favorable | Hazard ratio | 95% CI | P-value |
|---|
| TNM stage | III/IV | I/II | 3.195 | 1.691–6.250 |
<0.001a |
| Lymph node | Present | None | 2.317 | 1.210–4.976 | 0.023a |
| Tumor invasion | T3/T4 | T1/T2 | 2.660 | 1.413–5.199 | 0.021a |
| Histological
differentiation | Poor | Well/moderate | 2.530 | 1.752–7.136 |
<0.001a |
| GRK6
expression | Positive | Negative/weak | 2.020 | 1.052–3.889 | 0.003a |
| CEA level (µg
l−1) | ≥5 | <5 | 2.114 | 1.786–3.136 | 0.001a |
| CA19-9 level (µg
l−1) | ≥37 | <37 | 2.977 | 1.236–3.929 | 0.034a |
Discussion
CRC is one of the leading causes of
cancer-associated mortalities worldwide. Despite numerous advances
in diagnostic methods, combination chemotherapy and radiation
therapy, the prognosis and quality of life for patients with CRC
remains poor (26). Due to the
frequent failures of conventional treatment strategies, many
molecular biomarkers have been characterized for the development of
novel anticancer therapies, including targeted drugs (7,27). To
date, prognostic treatment strategies largely depend on clinical
staging and histopathological criteria. However, the current
staging classifications do not accurately predict patient outcomes
(8). Therefore, it is critical to
investigate biological markers that could appropriately determine
the risk of poor prognoses in patients.
GRKs are a versatile family of kinases, which
contribute to important functions in the desensitization of G
protein-coupled receptor homologous. GRKs promote the
receptor-arrestin interaction and the uncoupling of the receptor
from its G protein by phosphorylating specific serine and threonine
residues in the cytoplasmic domains of the activated receptor
(10,11). GRK6 is the most recently identified
member of the family of GRKs (10).
Recent studies demonstrated that the overexpression of GRK6 exerts
an important role in the process of transduction of pain signals
(11,28–31).
Previous studies also revealed that endogenous GRK6 molecules
contribute to the desensitization of M3 muscarinic acetylcholine in
the human SH-SY5Y neuroblastoma cell line (32). Furthermore, the ‘silencing’ of GRK6 in
myeloma cells reduced the levels of myeloid cell leukemia 1 and
suppressed the phosphorylation of signal transducer and activator
of transcription 3 (STST3), thereby causing a tumor-inhibitory
effect (16). Additionally, Chen
et al (33) demonstrated that
GRK6 serves critical roles in cell adhesion and migration of three
cancer cell lines (pC3, MB231 and HeLa cells) (33). Additionally, GRK6 provides a scaffold
for signaling molecules to regulate cell adhesion and organization
of cytoskeleton by interacting with G protein-coupled receptor
kinase interacting ArfGAP 1 and indirectly trans-activating
epidermal growth factor receptor, which in turn affects the
migration and invasion of cancer cells (14). GRK6 may affect the migration and
invasion of cancer cells through secondary messengers, including
cAMP and calmodulin (34). Based on
these studies, it may be inferred that GRK6 contributes an
important role in angiogenesis, tumor progression, metastasis and
cell proliferation. Therefore, its was proposed that GRK6
expression in CRC tissues may predict the prognoses of patients
with this disease.
Immunohistochemical staining was used to detect GRK6
protein expression in CRC tissues. Notably, GRK6 expression in CRC
tissues was higher compared with normal colorectal tissues. This
observation was further confirmed by RT-qPCR and western blot
analysis. The association between GRK6 expression and
clinicopathological features in CRC was further examined, including
the prognosis of patients. GRK6 expression was positively
associated with histological differentiation, venous invasion,
depth of invasion, lymph node metastasis, distant metastasis, and
TNM staging in CRC. Therefore, high GRK6 expression may be a
potential diagnostic biomarker for specific subtypes of CRC. In
survival analysis as assessed by Kaplan-Meier method, the overall
5-year survival of patients with negative or weakly positive GRK6
expression was significantly longer compared with patients with
strongly positive GRK6 expression. Additionally, it was observed
that the survival time of patients with stage I–II CRC were
significantly longer compared with the survival times of patients
with stage III–IV CRC (P<0.05). Furthermore, Spearman's rank
correlation analysis indicated that the more advanced TNM stages
corresponded to higher GRK6 expression levels in CRC. Previous
studies have revealed that GRK6 expression contributes to the
progression of several types of cancer (11,16,18,28–31).
Despite these diverse studies, the precise functions
and the mechanistic actions of GRK6 have not been defined
completely. In univariate survival analysis, GRK6 expression was
significantly associated with prognosis, and this prognostic value
was retained in multivariate survival analysis. This indicates that
high GRK6 expression may predict poor prognosis in patients with
CRC. Furthermore, the overexpression of GRK6 was correlated with
poor differentiation, venous invasion, deep tissue invasion,
distant metastasis, lymph node metastasis and high TNM grades
(P<0.05). This also indicates that GRK6 contributes an important
role in the invasion, progression and metastasis of CRC. The
results from the present study reveal that increased GRK6
expression may help to identify patients with CRC, who have poor
prognosis. However, the precise mechanism of action of GRK6 in CRC
and other tumors remains unclear. It is worthwhile to explore
further functional investigations of the effect of GRK6 expression,
and provide further evidence, which yields molecular targets for
diagnosing and treating CRC and other types of tumors.
In conclusion, the present study has provided new
insights into the role of GRK6 expression in the development of
CRC. The results of the present study support the interpretation
that GRK6 is a tumor-promoting factor in CRC, and therefore it may
serve as an independent biomarker for poor survival in patients
with CRC. Therefore, high GRK6 expression may identify high-risk
patients and be a potential novel therapeutic target in CRC.
Acknowledgements
Not applicable
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LM and RT had the concept for and designed the
study. RT and QL acquired, analysed and interpreted the data. XG
analysed and interpreted the data. RT, QL and XG drafted the
manuscript. RT and QL critically revised the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Affiliated Hospital of Nantong University
(Nantong, China). All experiments were performed in accordance with
the approved guidelines of the Affiliated Hospital of Nantong
University and the 1964 Helsinki Declaration and its later
amendments.
Consent for publication
Written informed consent was obtained from all
participants prior to undergoing surgery.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cassidy J, Saltz L, Twelves C, Van Cutsem
E, Hoff P, Kang Y, Saini JP, Gilberg F and Cunningham D: Efficacy
of capecitabine versus 5-fluorouracil in colorectal and gastric
cancers: A meta-analysis of individual data from 6171 patients. Ann
Oncol. 22:2604–2609. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Zeng H and Zhang S: The
updated incidences and mortalities of major cancers in China, 2011.
Chin J Cancer. 34:502–507. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sugarbaker PH: Second-look surgery for
colorectal cancer: Revised selection factors and new treatment
options for greater success. Int J Surg Oncol.
2011:9150782011.PubMed/NCBI
|
|
4
|
Liu QZ, Gao XH, Chang WJ, Gong HF, Fu CG,
Zhang W and Cao GW: Expression of ITGB1 predicts prognosis in
colorectal cancer: A large prospective study based on tissue
microarray. Int J Clin Exp Pathol. 8:12802–12810. 2015.PubMed/NCBI
|
|
5
|
Uhry Z, Belot A, Colonna M, Bossard N,
Rogel A, Iwaz J, Mitton N, Grosclaude P and Remontet L: National
cancer incidence is estimated using the incidence/mortality ratio
in countries with local incidence data: Is this estimation correct?
Cancer Epidemiol. 37:270–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Alsop K, Mead L, Smith LD, Royce SG,
Tesoriero AA, Young JP, Haydon A, Grubb G, Giles GG, Jenkins MA, et
al: Low somatic K-ras mutation frequency in colorectal cancer
diagnosed under the age of 45 years. Eur J Cancer. 42:1357–1361.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Brink M, de Goeij AF, Weijenberg MP,
Roemen GM, Lentjes MH, Pachen MM, Smits KM, de Bruïne AP, Goldbohm
RA and van den Brandt PA: K-ras oncogene mutations in sporadic
colorectal cancer in the netherlands cohort study. Carcinogenesis.
24:703–710. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jimi S, Yasui T, Hotokezaka M, Shimada K,
Shinagawa Y, Shiozaki H, Tsutsumi N and Takeda S: Clinical features
and prognostic factors of bone metastases from colorectal cancer.
Surg Today. 43:751–756. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lan YT, Chang SC, Yang SH, Lin CC, Wang
HS, Jiang JK, Chen WS, Lin TC, Chiou SH and Lin JK: Comparison of
clinicopathological characteristics and prognosis between early and
late recurrence after curative surgery for colorectal cancer. Am J
Surg. 207:922–930. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vroon A, Heijnen CJ and Kavelaars A: GRKs
and arrestins: Regulators of migration and inflammation. J Leukoc
Biol. 80:1214–1221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raghuwanshi SK, Smith N, Rivers EJ, Thomas
AJ, Sutton N, Hu Y, Mukhopadhyay S, Chen XL, Leung T and Richardson
RM: G protein-coupled receptor kinase 6 deficiency promotes
angiogenesis, tumor progression and metastasis. J Immunol.
190:5329–5336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dorn GW II: GRK mythology: G-protein
receptor kinases in cardiovascular disease. J Mol Med. 87:455–463.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Metaye T, Gibelin H, Perdrisot R and
Kraimps JL: Pathophysiological roles of G-protein-coupled receptor
kinases. Cell Signal. 17:917–928. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Premont RT, Claing A, Vitale N, Freeman
JL, Pitcher JA, Patton WA, Moss J, Vaughan M and Lefkowitz RJ:
Beta2-Adrenergic receptor regulation by GIT1, a G protein-coupled
receptor kinase-associated ADP ribosylation factor
GTPase-activating protein. Proc Natl Acad Sci USA. 95:pp.
14082–14087. 1998; View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmed MR, Berthet A, Bychkov E, Porras G,
Li Q, Bioulac BH, Carl YT, Bloch B, Kook S, Aubert I, et al:
Lentiviral overexpression of GRK6 alleviates L-dopa-induced
dyskinesia in experimental Parkinson's disease. Sci Transl Med.
2:28ra282010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tiedemann RE, Zhu YX, Schmidt J, Yin H,
Shi CX, Que Q, Basu G, Azorsa D, Perkins LM, Braggio E, et al:
Kinome-wide RNAi studies in human multiple myeloma identify
vulnerable kinase targets, including a lymphoid-restricted kinase,
GRK6. Blood. 115:1594–1604. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yao S, Zhong L, Liu J, Feng J, Bian T,
Zhang Q, Chen J, Lv X, Chen J and Liu Y: Prognostic value of
decreased GRK6 expression in lung adenocarcinoma. J Cancer Res Clin
Oncol. 142:2541–2549. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li YP: GRK6 expression in patients with
hepatocellular carcinoma. Asian Pac J Trop Med. 6:220–223. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan L, Zhang H, Liu J, Rubin JB, Cho YJ,
Shu HK, Schniederjan M and MacDonald TJ: Growth factor
receptor-Src-mediated suppression of GRK6 dysregulates CXCR4
signaling and promotes medulloblastoma migration. Mol Cancer.
12:182013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Altman DG, McShane LM, Sauerbrei W and
Taube SE: Reporting recommendations for tumor marker prognostic
studies (REMARK): Explanation and elaboration. PLoS Med.
9:e10012162012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sobin LH and Fleming ID: TNM
classification of malignant tumors, fifth edition (1997). Union
internationale contre le cancer and the american joint committee on
cancer. Cancer. 80:1803–1804. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gupta S, Provenzale D, Regenbogen SE,
Hampel H, Slavin TP Jr, Hall MJ, Llor X, Chung DC, Ahnen DJ, Bray
T, et al: NCCN guidelines insights: Genetic/Familial high-risk
assessment: Colorectal, 2008. J Natl Compr Canc Netw. 15:9–20.
2007.
|
|
23
|
Li Q, Zhi X, Zhou J, Tao R, Zhang J, Chen
P, Røe OD, Sun L and Ma L: Circulating tumor cells as a prognostic
and predictive marker in gastrointestinal stromal tumors: A
prospective study. Oncotarget. 7:36645–36654. 2016.PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi R, Wang L, Wang T, Xu J, Wang F and Xu
M: NEDD9 overexpression correlates with the progression and
prognosis in gastric carcinoma. Med Oncol. 31:8522014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tian Y, Xu T, Huang J, Zhang L, Xu S,
Xiong B, Wang Y and Tang H: Tissue metabonomic phenotyping for
diagnosis and prognosis of human colorectal cancer. Sci Rep.
6:207902016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ikeda K, Kojima M, Saito N, Sakuyama N,
Koushi K, Watanabe T, Sugihara K, Akimoto T, Ito M and Ochiai A:
Current status of the histopathological assessment, diagnosis and
reporting of colorectal neuroendocrine tumors: A web survey from
the japanese society for cancer of colon and rectum. Pathol Int.
66:94–101. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shitara K, Mizota A, Yatabe Y, Kondo C,
Nomura M, Yokota T, Takahari D, Ura T and Muro K: Lapatinib plus
trastuzumab for a patient with heavily pre-treated gastric cancer
that progressed after trastuzumab. Jan J Clin Oncol. 41:663–665.
2011. View Article : Google Scholar
|
|
29
|
Chua TC and Merrett ND: Clinicopathologic
factors associated with HER2-positive gastric cancer and its impact
on survival outcomes-a systematic review. Int J Cancer.
130:2845–2856. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Benovic JL and Gomez J: Molecular cloning
and expression of GRK6. A new member of the G protein-coupled
receptor kinase family. J Biol Chem. 268:19521–19527.
1993.PubMed/NCBI
|
|
31
|
Zhou Y, Li RJ, Li M, Liu X, Zhu HY, Ju Z,
Miao X and Xu GY: Overexpression of GRK6 attenuates neuropathic
pain via suppression of CXCR2 in rat dorsal root ganglion. Mol
Pain. 12:17448069166463812016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Willets JM, Challiss RA and Nahorski SR:
Endogenous G protein-coupled receptor kinase 6 Regulates M3
muscarinic acetylcholine receptor phosphorylation and
desensitization in human SH-SY5Y neuroblastoma cells. J Biol Chem.
277:15523–15529. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Lu B, Yang Q, Fearns C, Yates JR
III and Lee JD: Combined integrin phosphoproteomic analyses and
small interfering RNA-based functional screening identify key
regulators for cancer cell adhesion and migration. Cancer Res.
69:3713–3720. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ribas C, Penela P, Murga C, Salcedo A,
García-Hoz C, Jurado-Pueyo M, Aymerich I and Mayor F Jr: The G
protein-coupled receptor kinase (GRK) interactome: Role of GRKs in
GPCR regulation and signaling. Biochim Biophys Acta. 1768:913–922.
2007. View Article : Google Scholar : PubMed/NCBI
|