Introduction
Lung cancer, including small-cell lung cancer (SCLC)
and non-SCLC (NSCLC), which is mainly comprised of adenocarcinoma,
squamous cell carcinoma and large cell carcinoma, is the most
common type of malignancy and the leading cause of
cancer-associated mortality worldwide (1). Adenocarcinoma is the most common type of
NSCLC, and epidermal growth factor receptor (EGFR)-tyrosine kinase
inhibitor (TKI) therapy, including erlotinib, gefitinib or
icotinib, is the gold standard of treatment for EGFR-mutant lung
adenocarcinoma (2–4). The incidence of EGFR mutation in NSCLC
in 2005 was higher in East Asian populations when compared with the
incidence in other ethnicities (30 vs. 8%) (5). Patients with lung adenocarcinoma
harboring exon 19 deletions achieved longer progression-free
survival (PFS) and overall survival time (OS) compared with those
with L858R mutations (6). However,
patients ultimately develop acquired resistance, and the most
recently identified mechanism for this is transformation to SCLC
(7). Ahn et al (8) reported six cases of transformation from
lung adenocarcinoma to SCLC. Erlotinib, gefitinib, afatinib and
icotinib were equally as efficient as each other but exhibited
different efficacy-toxicity patterns (4). EGFR-TKI-resistant SCLCs are
differentiated early from the lung adenocarcinoma clones that
harbor completely inactivated retinoblastoma 1 (RB1) and TP53
(9). Molecular mechanisms involved in
the transformation from NSCLC to SCLC include TP53 mutations, RB1
loss, lack of EGFR expression and MYC amplification. The most
studied signaling pathway is the achaete-scute homolog 1 (ASCL1)
which is regulated by four different neurogenic locus notch homolog
(NOTCH) receptors. NOTCH alterations promote ASCL1 and CD56
overexpression (9). These changes
induce cyclin-dependent kinase 5 (CDK5) activity and inactivation
of RB by phosphorylation (9). The
present study reports a case of acquired resistance to icotinib
therapy through transformation to SCLC. The results implicate that
a secondary biopsy is important to clarify the mechanism of TKI
resistance, and NSE may be useful for the early detection of SCLC
transformation in cases that are resistant to EGFR-TKI therapy.
Case report
A 55-year-old man with a history of smoking was
referred to Zhejiang Cancer Hospital (Hangzhou, China) September
22, 2015 due to occur lung metastasis. A right upper lobe lobectomy
was performed June 26, 2009 (48-year-old). The pathological
diagnosis was of lung adenocarcinoma with a mixed acinar and
papillary pattern (June 26, 2009) and the stage of cancer was
pT2aN1M0 (IIB) according to the eighth edition of the
Tumor-Node-Metastasis classification for lung cancer (10). The results of immunohistochemistry
(Primary antibody in Table I;
Secondary antibody: EnVision FLEX/horseradish peroxidase; dilution,
ready-to-use; cat. no., K8000, Dako; Agilent Technologies, Inc.,
Santa Clara, CA, USA) (11–13) markers were as follows: Thyroid
transcription factor 1 (TTF-1)(+), Napsin A(+), synaptophysin
(Syn)(−) and chromogranin A(−). The patient received six cycles of
adjuvant carboplatin/gemcitabine intravenously (1.6 g gemcitabine
on days 1 and 8, and 100 mg carboplatin on days 1, 2 and 3, every
three weeks for one cycle) chemotherapy without complication, but
at 37 months post-surgery, presented with a metachronous solitary
left lower lobe nodule, detected by computed tomography, and
underwent a wedge resection. The tumor was ~0.9×0.8×0.7 cm, without
interlobar lymph node metastasis. The pathological diagnosis using
the aforementioned method was of invasive adenocarcinoma (mainly
papillary accompanied with an acinar pattern; Jan 30, 2013) and was
detected to harbor an EGFR 21 L858R mutation according to
amplification refractory mutation system detection, as previously
described (14). The patient
subsequently received four cycles of pemetrexed (1,000 mg on day 1,
every three weeks for one cycle). After 10 months, the patient
received icotinib treatment (125 mg thrice daily) due to multiple
lung metastasis, detected by computed tomography, and a complete
response was achieved. After another 19 months, multiple lung
metastases appeared again and the right lung hilar lymph node was
determined to be enlarged on surveillance by computed tomography
(Fig. 1A and B). Biopsy by
endobroncheal ultrasonography for the right hilar lymph node
revealed transformation to neuroendocrine carcinoma (Sep 20, 2015)
and the following immunohistochemical results (Primary antibody in
Table I): Cytokeratin (CK)(+),
TTF-1(+), CK7 (weak positive), Ki-67(+, 40%), CD56(+),
carcinoembryonic antigen (CEA)(−), Syn (weak positive), CK5/6(−),
P40(−), chromogranin A(−) and Napsin A(−). The staining analysis
was performed as described previously (11–13). The
post-operative and biopsy specimens were analyzed for 416 genes by
next-generation sequencing, and phosphatidylinositol 3-kinase
catalytic α (PIK3CA) mutation and retinoblastoma (RB) loss were
found (Table II). Neuron-specific
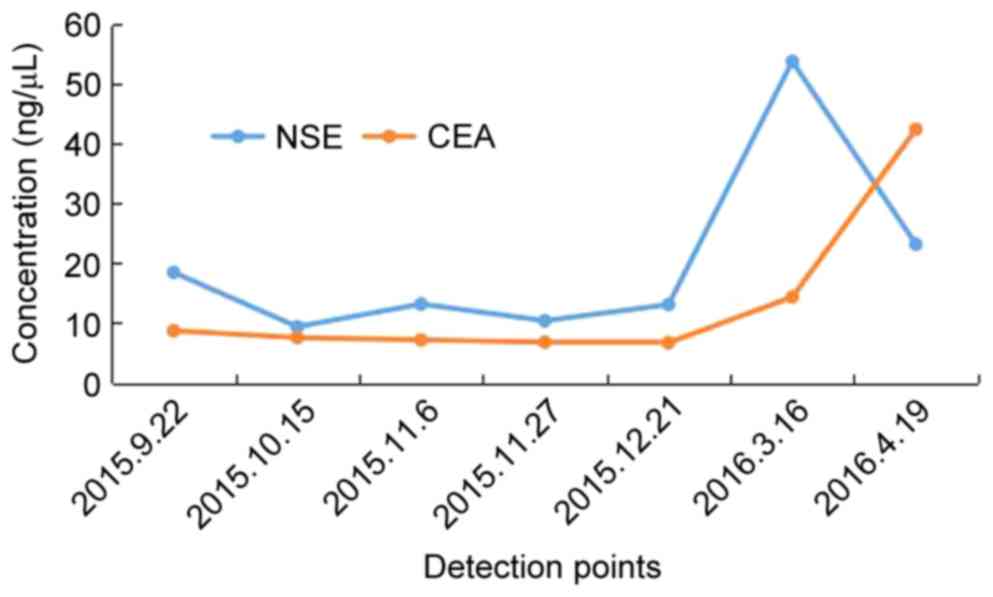
enolase (NSE) level was elevated when transformation to
neuroendocrine carcinoma occurred. A total of five cycles of
intravenous etoposide (180 mg/d1) combined with cisplatin (45 mg
day 1, day 2, day 3, every three weeks for one cycle) were
administered and a partial response was achieved (Fig. 1C and D). Disease progression occurred
accompanied with an elevated NSE level after 6 months (Fig. 1E-H), and bronchoscopy examination
revealed SCLC in the right upper lobe (Mar 16, 2016). The patient
received intravenous chemotherapy consisting of irinotecan (120 mg
on days 1 and 8) combined with carboplatin (600 mg on day 1) every
three weeks for two cycles, and achieved stable disease for two
months (Fig. 1I-L). Subsequently, the
patient was followed up once, 2–3 months later. The patient
succumbed in April 2017 due to deterioration of the condition.
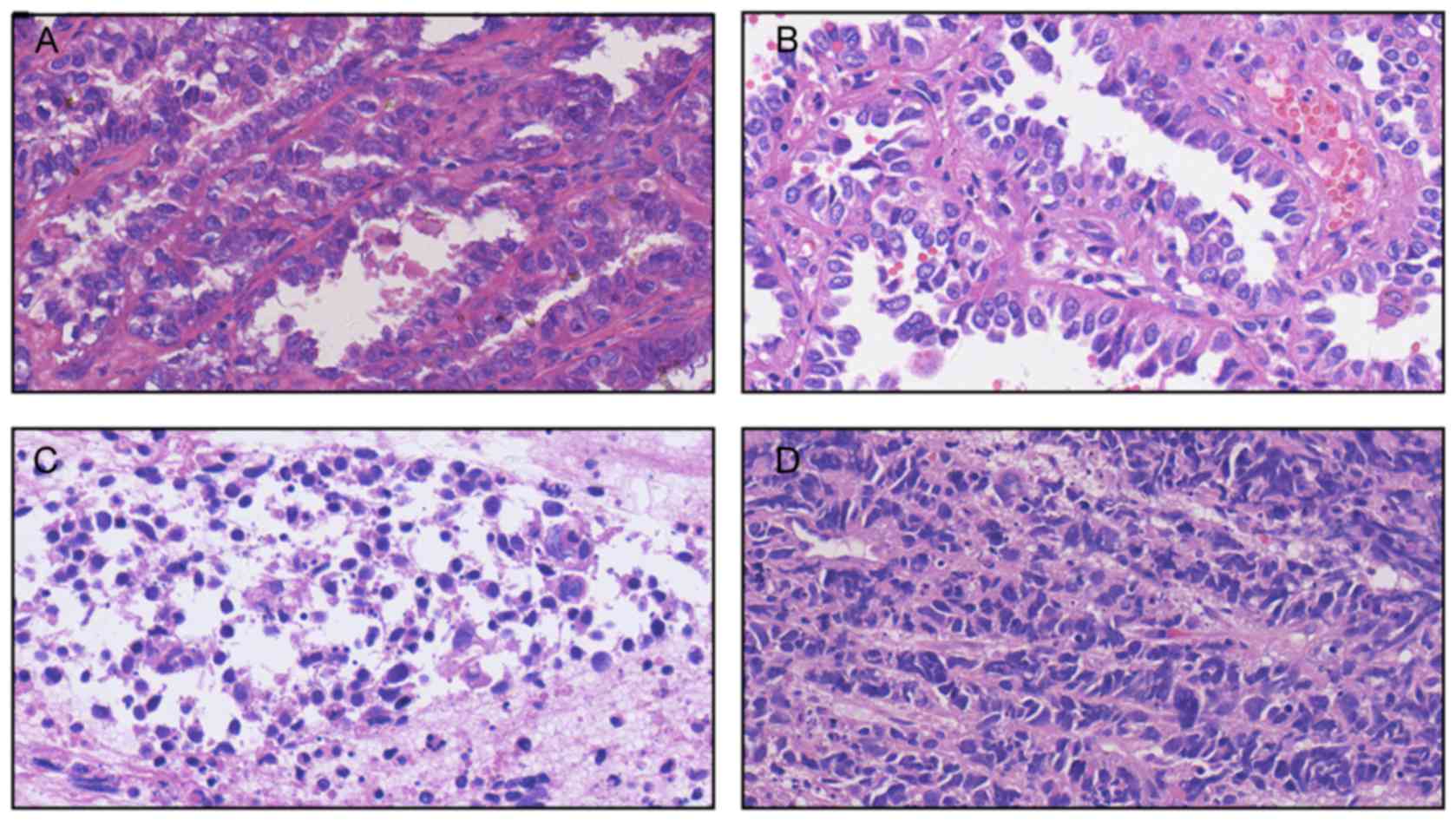
Pathological results of the patient at different times during the
study period is presented in Fig. 2.
The changes in NSE and CEA concentration are presented in Fig. 3. The sequence of anticancer treatments
is presented in Table III. The
patient provided written informed consent for the publication of
the present study.
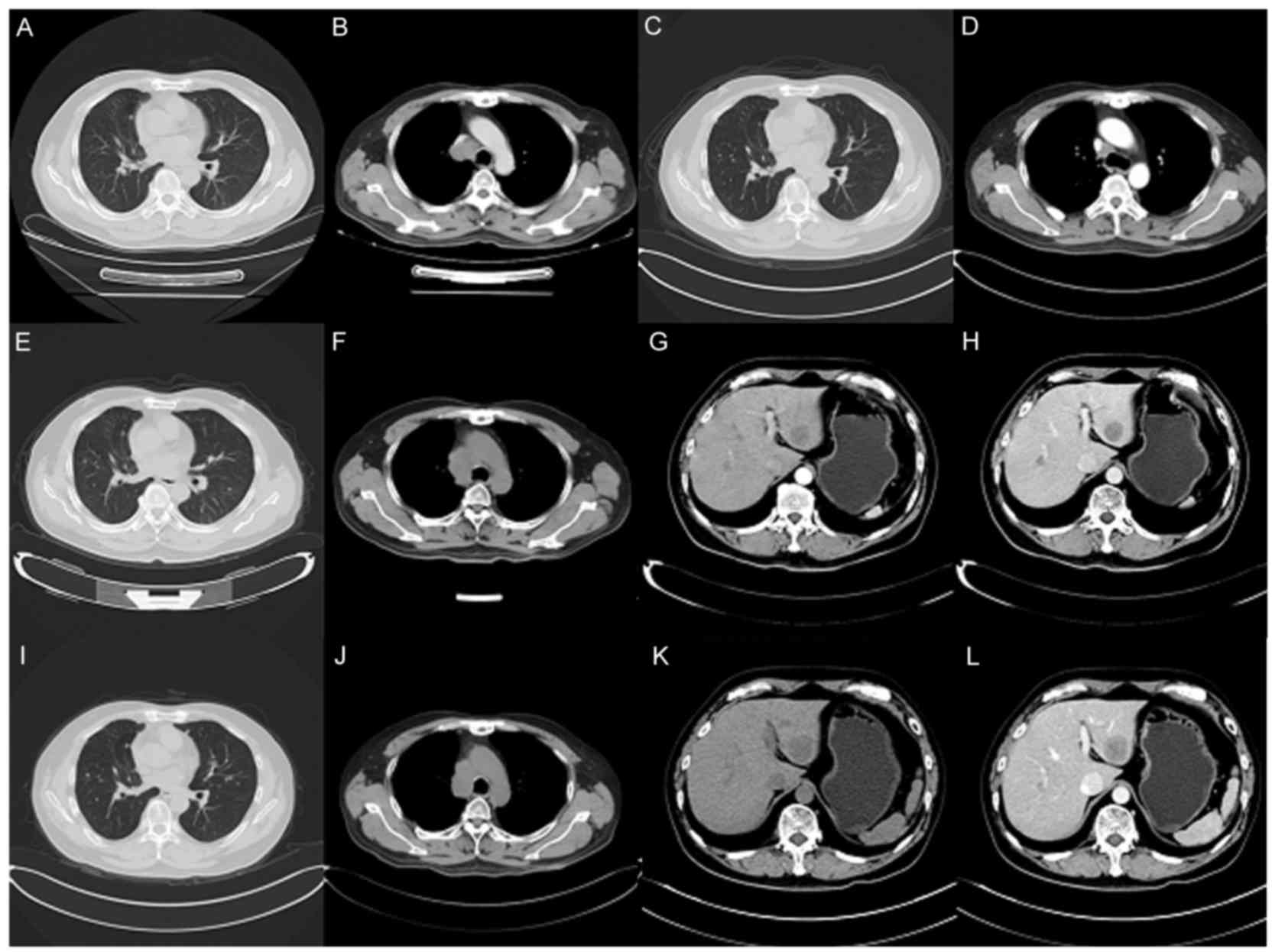 | Figure 1.Computed tomography scans of the
patient. (A and B) Aug 21, 2015: Multiple lung metastases appeared
and the right lung hilar lymph node appeared enlarged, as
visualized in the (A) pulmonary and (H) mediastinal windows. (C and
D) Dec 21, 2015: Regression of lung metastatic neoplasm and notable
shrinking of the right mediastinal lymph node, as visualized in the
(C) pulmonary and (D) mediastinal windows. (E and F) Mar 16, 2016:
Lung metastasis and hilar lymph node was markedly enlarged, as
visualized in the (E) pulmonary and (F) mediastinal windows. (G and
H) Mar 16, 2016: Emerging new liver metastasis as visualized in the
(G) arterial and (H) portal phases. (I and J) May 10, 2016: Lung
metastasis and enlarged mediastinal lymph node, as visualized in
the (I) pulmonary and (J) mediastinal windows. (K and L) May 10,
2016: Liver metastasis as visualized in the (K) arterial and (L)
portal phases. |
 | Table I.Antibody used in the present
study. |
Table I.
Antibody used in the present
study.
| Antibody | Dilution | Catalog number | Supplier |
|---|
| TTF-1 | 1:200 | MAB-0599 | Maxim |
| Napsin A | 1:400 | NCL-L-NapsinA | Leica |
| Syn | RTU | IR660 | DAKO |
| Chromogranin A | 1:200 | MAB-0202 | Maxim |
| CK | RTU | IR 053 | DAKO |
| CK7 | RTU | IR619 | DAKO |
| Ki-67 | RTU | IR626 | DAKO |
| CD56 | RTU | IR628 | DAKO |
| CEA | RTU | IR622 | DAKO |
| CK5/6 | 1:400 | MAB-0276 | Maxim |
| P40 | 1:200 | ACI3066C | BIOCARE |
 | Table II.Genetic alteration for three specimens
obtained from the patient over the study period. |
Table II.
Genetic alteration for three specimens
obtained from the patient over the study period.
| Gene | Specimen from
2009 | Specimen from
2013 | Specimen from
2015 |
|---|
| EGFR (L858R) | 11% | 25% | 78% |
| RB1 (Y567fs) | 6% | 20% | 57% |
| TP53 (S241C) | 20% | 34% | 73% |
| PIK3CA (E545A) | No | No | 3% |
| MYCL
amplification | No | No | 21.6 times |
| RB1 loss | No | Yes | Yes |
| RAC1
amplification | 2.7 times | No | No |
 | Table III.Sequence of anticancer treatments. |
Table III.
Sequence of anticancer treatments.
| Date | Treatment |
|---|
| June 2009 | Right upper
lobectomy |
| December 2009 | Completed adjuvant
gemcitabine-carboplatin chemotherapy |
| January 2013 | Underwent wedge
resection of left lower lobe nodule |
| June 2013 | Completed pemetrexed
chemotherapy |
| August 2013 | Multiple lung
metastasis |
| November 2013 | Commenced icotinib
treatment |
| August 2015 | Multiple lung
metastasis and biopsy by endobroncheal ultrasonography for the
hilar lymph node, which revealed transformation to neuroendocrine
carcinoma |
| December 2015 | Completed
etoposide-cisplatin chemotherapy |
| March 2016 | Multiple lung
metastasis enlarged and hepatic metastasis appeared |
| May 2016 | Complete two cycles
of irinotecan combined with carboplatin |
Discussion
A number of mechanisms of acquired resistance to
EGFR-TKI therapy in EGFR mutant lung adenocarcinoma have been
described, including the EGFR T790M mutation, EGFR amplification,
MET gene amplification, PIK3CA mutation and transformation to SCLC
(7). Transformation of adenocarcinoma
to SCLC in patients with somatic EGFR mutations as the TKI therapy
resistance mechanism has previously been reported (15), and the percentage of transformation
from adenocarcinoma to SCLC was identified in 14% of cases
(7).
Analysis of tumor samples and cell lines derived
from resistant EGFR mutant patients revealed that RB is lost in
100% of such SCLC transformed cases, but rarely in those that
remain NSCLC (16). The gene
detection in the present patient following icotinib resistance also
revealed RB loss and the appearance of a PIK3CA gene mutation. It
is currently indicated that combined-histology tumors and
transformation occur more frequently in lung cancer types with
EGFR-activating mutations compared with EGFR wild-type tumors. The
reason for this may be that the cell of origin of certain
EGFR-mutant adenocarcinomas, type II alveolar cells, also have the
potential to become SCLC (17). Mixed
EGFR-mutant NSCLC/SCLC histology has been reported in a number of
cases, indicating a certain degree of dynamic plasticity between
the two histologies in specific cases, without the selective
pressure of the EGFR TKI (18–20). The
present case was verified as an adenocarcinoma, and not combined
SCLC, through surgical resection and transformation from
adenocarcinoma to SCLC following icotinib resistance. A secondary
biopsy is important in order to evaluate the genetic and
histological changes, and to select an appropriate treatment for
TKI resistance. In the present patient, a secondary biopsy and gene
detection were performed in order to permit the use of a more
rational therapy. It is occasionally difficult to perform a second
biopsy, and so NSE level may be useful for the early detection of
transformation to SCLC in cases that are resistant to EGFR-TKI
therapy. NSE could potentially overcome the limitations of
performing biopsies on single lesions, which may miss the
transformation of another metastatic lesion into SCLC (21–24). It
was concluded that a secondary biopsy was important for the
evaluation of genetic and histological changes and the selection of
an appropriate treatment following TKI resistance, and that NSE may
be useful for the early detection of SCLC transformation.
Acknowledgements
This study was supported by the Zhejiang Provincial
Natural Science Foundation of China (grant no. LY15H290001), the
Public Welfare Technology Application Studies Program of Zhejiang
Province (grant no. 2016C33118), the Zhejiang Province Traditional
Medical Science Fund Project of China (grant no. 2015ZA037) and the
1022 Talent Training Program of Zhejiang Cancer Hospital.
Glossary
Abbreviations
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
NSE
|
neuron-specific enolase
|
|
RB
|
retinoblastoma
|
|
PIK3CA
|
phosphatidylinositol-3-kinase
catalytic α
|
|
SCLC
|
small cell lung cancer
|
|
TKI
|
tyrosine kinase inhibitor
|
|
NSCLC
|
non-small cell lung cancer
|
|
CEA
|
carcinoembryonic antigen
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Brugger W, Triller N, Blasinska-Morawiec
M, Curescu S, Sakalauskas R, Manikhas GM, Mazieres J, Whittom R,
Ward C, Mayne K, et al: Prospective molecular marker analyses of
EGFR and KRAS from a randomized, placebo-controlled study of
erlotinib maintenance therapy in advanced non-small-cell lung
cancer. J Clin Oncol. 29:4113–4120. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liang W, Wu X, Fang W, Zhao Y, Yang Y, Hu
Z, Xue C, Zhang J, Zhang J, Ma Y, et al: Network meta-analysis of
erlotinib, gefitinib, afatinib and icotinib in patients with
advanced non-small-cell lung cancer harboring EGFR mutations. PLoS
One. 9:e852452014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shigematsu H, Lin L, Takahashi T, Nomura
M, Suzuki M, Wistuba II, Fong KM, Lee H, Toyooka S, Shimizu N, et
al: Clinical and biological features associated with epidermal
growth factor receptor gene mutations in lung cancers. J Natl
Cancer Inst. 97:339–346. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng Z, Jin X, Lin B, Su H, Chen H, Fei
S, Zhao L, Deng X, Xie D and Xie C: Efficacy of second-line
tyrosine kinase inhibitors in the treatment of metastatic advanced
non-small-cell lung cancer harboring exon 19 and 21 EGFR mutations.
J Cancer. 8:597–605. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ahn S, Hwang SH, Han J, Choi YL, Lee SH,
Ahn JS, Park K, Ahn MJ and Park WY: Transformation to small cell
lung cancer of pulmonary adenocarcinoma: Clinicopathologic analysis
of six cases. J Pathol Transl Med. 50:258–263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee JK, Lee J, Kim S, Kim S, Youk J, Park
S, An Y, Keam B, Kim DW, Heo DS, et al: Clonal history and genetic
predictors of transformation into small-cell carcinomas from lung
adenocarcinomas. J Clin Oncol. 35:3065–3074. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nicholson AG, Chansky K, Crowley J,
Beyruti R, Kubota K, Turrisi A, Eberhardt WE, van Meerbeeck J and
Rami-Porta R; Staging and Prognostic Factors Committee, Advisory
Boards, and Participating Institutions; Staging and Prognostic
Factors Committee Advisory Boards and Participating Institutions, :
The international association for the study of lung cancer lung
cancer staging project: Proposals for the revision of the clinical
and pathologic staging of small cell lung cancer in the forthcoming
eighth edition of the TNM classification for lung cancer. J Thorac
Oncol. 11:300–311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pelosi G, Fabbri A, Bianchi F, Maisonneuve
P, Rossi G, Barbareschi M, Graziano P, Cavazza A, Rekhtman N,
Pastorino U, et al: ΔNp63 (p40) and thyroid transcription factor-1
immunoreactivity on small biopsies or cellblocks for typing
non-small cell lung cancer: A novel two-hit, sparing-material
approach. J Thorac Oncol. 7:281–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gerdes J, Li L, Schlueter C, Duchrow M,
Wohlenberg C, Gerlach C, Stahmer I, Kloth S, Brandt E and Flad HD:
Immunobiochemical and molecular biologic characterization of the
cell proliferation-associated nuclear antigen that is defined by
monoclonal antibody Ki-67. Am J Pathol. 138:867–873.
1991.PubMed/NCBI
|
|
13
|
Brown AF, Sirohi D, Fukuoka J, Cagle PT,
Policarpio-Nicolas M, Tacha D and Jagirdar J: Tissue-preserving
antibody cocktails to differentiate primary squamous cell
carcinoma, adenocarcinoma, and small cell carcinoma of lung. Arch
Pathol Lab Med. 137:1274–1281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lynch TJ, Bell DW, Sordella R,
Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat
SM, Supko JG, Haluska FG, et al: Activating mutations in the
epidermal growth factor receptor underlying responsiveness of
non-small-cell lung cancer to gefitinib. N Engl J Med.
350:2129–2139. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watanabe S, Sone T, Matsui T, Yamamura K,
Tani M, Okazaki A, Kurokawa K, Tambo Y, Takato H, Ohkura N, et al:
Transformation to small-cell lung cancer following treatment with
EGFR tyrosine kinase inhibitors in a patient with lung
adenocarcinoma. Lung Cancer. 82:370–372. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niederst MJ, Sequist LV, Poirier JT,
Mermel CH, Lockerman EL, Garcia AR, Katayama R, Costa C, Ross KN,
Moran T, et al: RB loss in resistant EGFR mutant lung
adenocarcinomas that transform to small-cell lung cancer. Nat
Commun. 6:63772015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oser MG, Niederst MJ, Sequist LV and
Engelman JA: Transformation from non-small-cell lung cancer to
small-cell lung cancer: Molecular drivers and cells of origin.
Lancet Oncol. 16:e165–e172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Okamoto I, Araki J, Suto R, Shimada M,
Nakagawa K and Fukuoka M: EGFR mutation in gefitinib responsive
small-cell lung cancer. Ann Oncol. 17:1028–1029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tatematsu A, Shimizu J, Murakami Y, Horio
Y, Nakamura S, Hida T, Mitsudomi T and Yatabe Y: Epidermal growth
factor receptor mutations in small cell lung cancer. Clin Cancer
Res. 14:6092–6096. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu HY, Sun WY, Chen B, Zhang YP, Cai JF,
Su D, Wang Z, Zheng YQ and Ma SL: Epidermal growth factor receptor
mutations in small cell lung cancer patients who received surgical
resection in China. Neoplasma. 59:100–104. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen B, Hu B, Li W and Xue J:
Transformation from NSCLC to SCLC: When did it happen? Lancet
Oncol. 16:e3092015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Engelman JA, Oser MG, Niederst MJ and
Sequist LV: Transformation from NSCLC to SCLC: When did it
happen?-Authors' reply. Lancet Oncol. 16:e309–e310. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Norkowski E, Ghigna MR, Lacroix L, Le
Chevalier T, Fadel É, Dartevelle P, Dorfmuller P and Thomas de
Montpréville V: Small-cell carcinoma in the setting of pulmonary
adenocarcinoma: New insights in the era of molecular pathology. J
Thorac Oncol. 8:1265–1271. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y, Li XY, Tang Y, Xu Y, Guo WH, Li
YC, Liu XK, Huang CY, Wang YS and Wei YQ: Rapid increase of serum
neuron specific enolase level and tachyphylaxis of EGFR-tyrosine
kinase inhibitor indicate small cell lung cancer transformation
from EGFR positive lung adenocarcinoma? Lung Cancer. 81:302–305.
2013. View Article : Google Scholar : PubMed/NCBI
|