Introduction
Colorectal cancer (CRC) is becoming one of the most
common malignancies globally (1).
Each year, almost 1.4 million new CRC cases occur and 700,000
individuals succumb to mortality from CRC globally (1–3). Despite
advancements in surgery, radiation therapy, chemotherapy and
targeted therapy, the survival rate of patients remains low
(4,5).
Although extensive studies have demonstrated that CRC is a
multi-factorial disease and is associated with physical, chemical,
biological and genetic factors (6),
the pathophysiology of CRC remains to be fully elucidated.
The phosphatidylinositol 3-kinase (PI3K) signaling
pathway serves crucial functions in normal cellular processes,
including cell proliferation, growth, apoptosis and motility
(7). PI3K, protein kinase B (Akt) and
mammalian target of rapamycin (mTOR) represent key nodes in the
PI3K pathway. Class IA PI3K is a heterodimeric lipid kinase
composed of a catalytic subunit,
phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α
(p110α) and a regulatory subunit (p85) (8). Akt is the downstream effector of PI3K
and regulates the effects of PI3K on tumor growth and progression
(9,10). The phosphorylation at serine 473 in
its C-terminus fully activates Akt, and the activity of Akt may be
evaluated using antibodies against phosphorylated (p)-Akt-S473
(11,12). An evolutionarily conserved
serine/threonine kinase known as mTOR is the downstream target of
Akt signaling. Akt may directly phosphorylate serine 2448 in mTOR
(9,13). Akt serves important functions in cell
proliferation. Cyclin D1 is a key cell cycle regulatory protein and
its expression levels are important in the G1/S phase transition
(7,14). The activation of Akt may phosphorylate
and inhibit glycogen synthesis kinase-3 β, which results in the
stabilization of cyclin D1 (14).
Activated Akt may control the assembly of cyclin D1-cyclin
dependent kinase (CDK)4 complexes by modulating cyclin D1 levels
and contributing to the regulation of cell cycle progression
(15). Akt may also affect cell
survival by the indirect regulation of the nuclear factor-κβ
(NF-κβ) signaling pathway (7).
However, the elevated expression of RELA proto-oncogene, NF-κβ
subunit (p65), a subunit of activated NF-κβ, may increase the
phosphorylation and expression of Akt in the NIH3T3 cell line and
primary endothelial cells (16). The
Ras oncoprotein is one of the main regulators of the NF-κβ
signaling pathway, and in its active conformation, Ras may
phosphorylate the Raf/mitogen-activated protein kinase kinase
(MEK)/extracellular signal-regulated kinase (ERK) and the
PI3K/Akt/mTOR signaling pathways to regulate normal cell growth and
malignant transformation (17).
Studies have demonstrated that the PI3K/Akt/mTOR and the
Raf/MEK/ERK cascades are associated, with multiple points of
convergence, cross-talk and feedback loops (10,17).
ERK1/2 may mediate the phosphorylation of the essential scaffolding
protein of mTOR complex 1 (C1) to promote mTOR signaling (18). The associated factors and key
components of the P13K pathway include p110α, Akt, mTOR, cyclinD1,
p65, Ras and ERK1/2; the aforementioned studies suggest that these
factors may constitute a biochemical network to regulate cellular
functions.
Numerous studies have demonstrated that the altered
expression and/or activation of the aforementioned factors
contribute to the tumorigenesis and progression of various types of
human cancer, including CRC (7,10,13,14,17,19–31).
However, systemic research on the expression and function of all
these factors in CRC is limited.
In the present study, the expression of the
associated factors and key components of the PI3K pathway in
patients with CRC, including p110α, p-Akt Ser473, p-mTOR Ser2448,
cyclin D1, CDK4, p65, Ras and ERK1/2, were explored. Their clinical
significance in CRC was additionally analyzed.
Materials and methods
Patients and controls
Between January 2013 and December 2014, a total of
65 CRC and 15 colonic adenoma tissue samples were collected from
the Department of Pathology of the Jinan Central Hospital
Affiliated to Shandong University (Shandong, China) for a
retrospective study. All tissues were confirmed by postoperative
pathology. The group of colonic adenoma cases comprised 7 females
and 8 males with an age range of 34–82 years (mean age, 64.2). The
group of patients with CRC consisted of 34 males and 31 females,
and the mean age was 66 years (range, 32–88 years). A total of 36
of the tumors were moderately differentiated and 29 cases were
poorly differentiated according to pathology reports. According to
the tumor-node-metastasis (TNM) staging system (32), there were 3 cases of stage I, 25 cases
of stage II, 24 cases of stage III and 13 cases of stage IV. Lymph
node metastasis occurred in 33 CRC cases. The present study was
approved by the Institutional Review Board of Jinan Central
Hospital Affiliated to Shandong University, and written informed
consent was obtained from all patients or the patient's family.
Immunohistochemistry (IHC)
IHC was performed to detect the expression of p110α,
p-Akt Ser473, p-mTOR Ser2448, cyclin D1, CDK4, p65, Ras and ERK1/2
in CRC and colonic adenoma tissues. All tissue samples were 10%
formalin-fixed at room temperature for 1 h, paraffin-embedded, cut
into 4-µm sections and then placed on slides pretreated with
3-aminopropyltriethoxysilane. Following deparaffinization and
rehydration (xylene for 10 min; 100, 95, 75 and 50% EtOH for 2 min
each; PBS for 5 min), the sections were microwaved for heat-induced
epitope retrieval at 100°C for 3 min in a citrate buffer solution
(10 mM sodium citrate and 0.05% Tween 20; pH 6.0). The sections
were then washed using PBS and the endogenous peroxidase activity
was inhibited with 3% hydrogen peroxide for 10 min. The specimens
were then blocked with PBS containing 5% normal goat serum (cat
no., ab7481; Abcam, Shanghai, China) at 37°C for 30 min.
Subsequently, the sections were incubated with antibodies overnight
at 4°C. The primary antibodies used included a rabbit anti-p110α
antibody (cat no. 4249; 1:200), a rabbit anti-p-Akt Ser473 antibody
(cat no. 4060; 1:200), a rabbit anti-p-mTOR Ser2448 antibody (cat
no. 2976; 1:200), a rabbit anti-cyclin D1 antibody (cat no. 2978;
1:200), a rabbit anti-CDK4 antibody (cat no. 12790; 1:200), a
rabbit anti-NF-κB p65 antibody (cat no. 8242; 1:200), a rabbit
anti-Erk1/2 antibody (cat no. 4695; 1:200; all Cell Signaling
Technology, Inc., Danvers, MA, USA) and a rabbit anti-Ras (H,K,N)
antibody (cat no. LS-C176193; 1:200; LifeSpan BioSciences, Inc.,
Seattle, WA, USA). Following rinsing with PBS, the slides were
incubated at room temperature for 30 min with a secondary
anti-rabbit antibody conjugated with horseradish peroxidase (1:200;
cat no., sc-2357; Santa Cruz Biotechnology, Inc., Dallas, TX, USA).
The slides were then exposed to 3,3′-diaminobenzidine for
visualization, and hematoxylin at room temperature for 5 min, for
nuclear counterstaining.
Evaluation of immunostaining
parameters
Tissue sections were evaluated at a ×200
magnification using light microscopy by two pathologists in a
blinded manner. Slides with debatable evaluations were re-evaluated
until a consensus was reached. First, proportion scores were
assigned to represent the estimated proportion of positive tumor
cells (0, <5%; 1, 6–25%; 2, 26–50%; 3, 51–75%; 4, ≥76%).
Secondly, intensity scores were assigned to represent the average
intensity of the positive tumor cells (0, no color; 1, pale yellow;
2, tan; 3, brown). The proportion and intensity scores were then
multiplied to obtain total scores. A total of 5 typical regions
were assessed for each sample and the average scores were obtained.
For further statistical analysis, all specimens were divided into
four groups: Negative expression (−), 0–1; weak positive expression
(+), 2–4; positive expression (++), 5–8; or strong positive
expression (+++) >9. Scores with a range from 1–2 were also
added to the positive expression group.
Statistical analysis
SPSS 17.0 (SPSS, Inc., Chicago, IL, USA) software
package was used for statistical analyses. The χ2 test
was performed to analyze the differences in various target proteins
between CRC tissues and colonic adenoma tissues, in addition to
associations between the expression levels of the various proteins
and the clinical parameters. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of p110α, p-Akt Ser473,
cyclin D1, CDK4, p-mTOR Ser2448, p65, Ras and ERK1/2 in CRC tissues
and colonic adenoma tissues
IHC staining results revealed that, of the 65 CRC
cases, 52 cases (80.00%) were p110α-positive, 48 cases (73.85%)
were p-Akt Ser473-positive, 45 cases (69.23%) were cyclin
D1-positive, 41 cases (63.08%) were CDK4-positive, 51 cases
(78.46%) were p-mTOR Ser2448-positive, 43 cases (66.15%) were
p65-positive, 40 cases (61.54%) were Ras-positive, and 46 cases
(70.77%) were ERK1/2-positive. However, positive staining of p110α,
p-Akt Ser473, cyclin D1, CDK4, p-mTOR Ser2448, p65, Ras and ERK1/2
was only observed in 3 cases (20.00%), 5 cases (33.33%), 2 cases
(13.33%), 3 cases (20.00%), 4 cases (26.67%), 5 cases (33.33%), 4
cases (26.67%) and 4 cases (26.67%) of 15 colon adenoma cases,
respectively. The expression of target proteins in CRC tissues were
all significantly increased compared with in colon adenoma tissues
(Table I; P<0.05). The
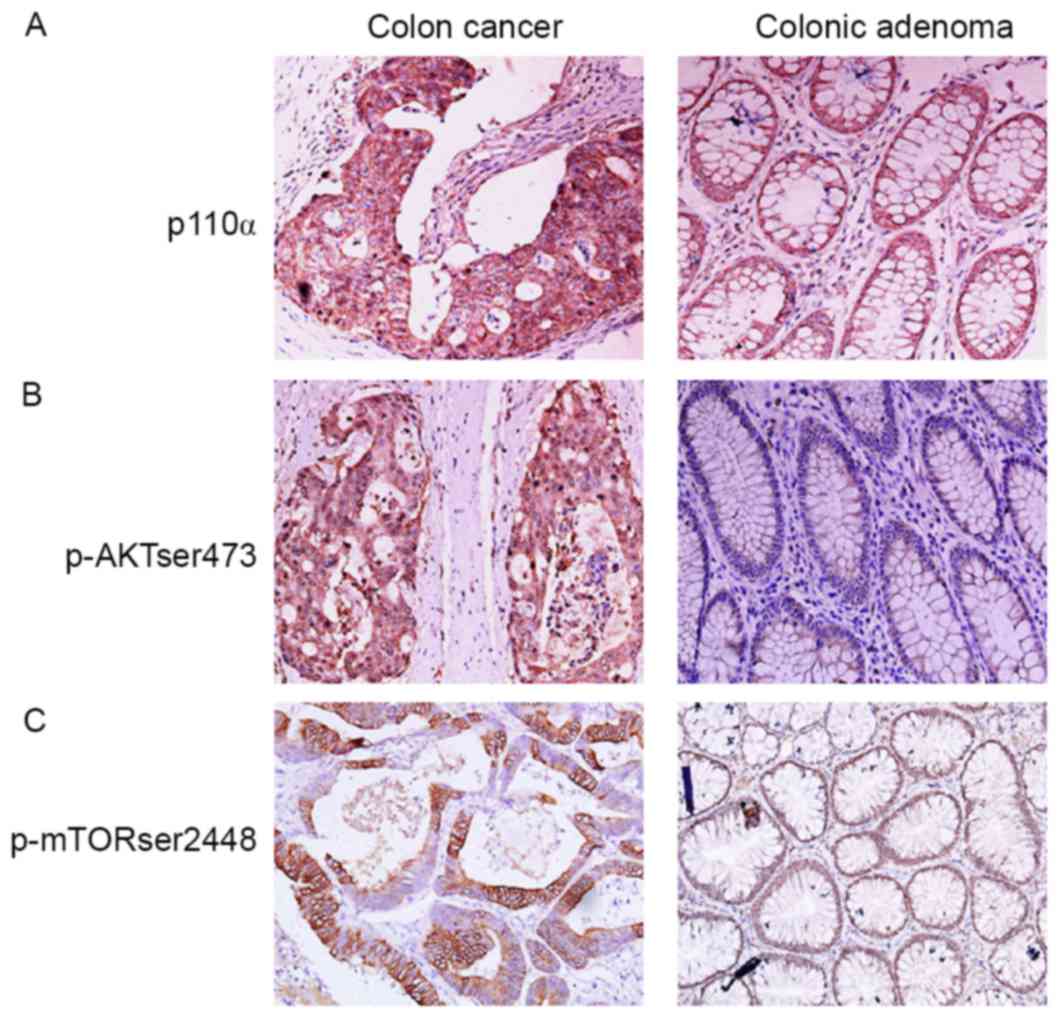
localization of p110α and p-Akt Ser473 were primarily in the
cytoplasm of CRC cells (Fig. 1A and
B). The location of p-mTOR Ser2448 was mainly in the
perinuclear and cytoplasmic regions of CRC cells, and in the nuclei
of colonic adenoma cells (Fig. 1C).
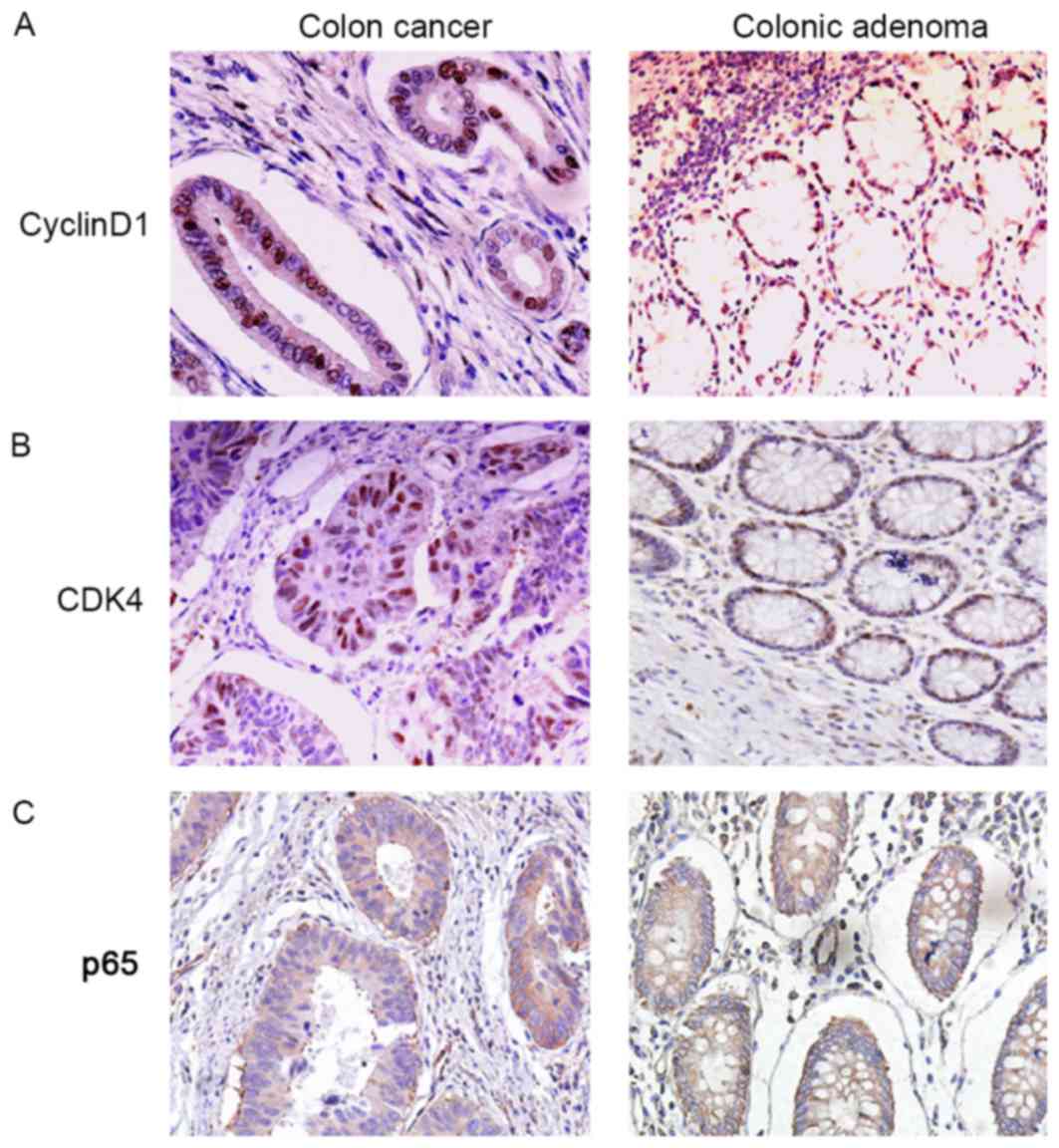
Cyclin D1 and CDK4 presented in the nuclei of CRC cells (Fig. 2A and B). The localization of p65 was
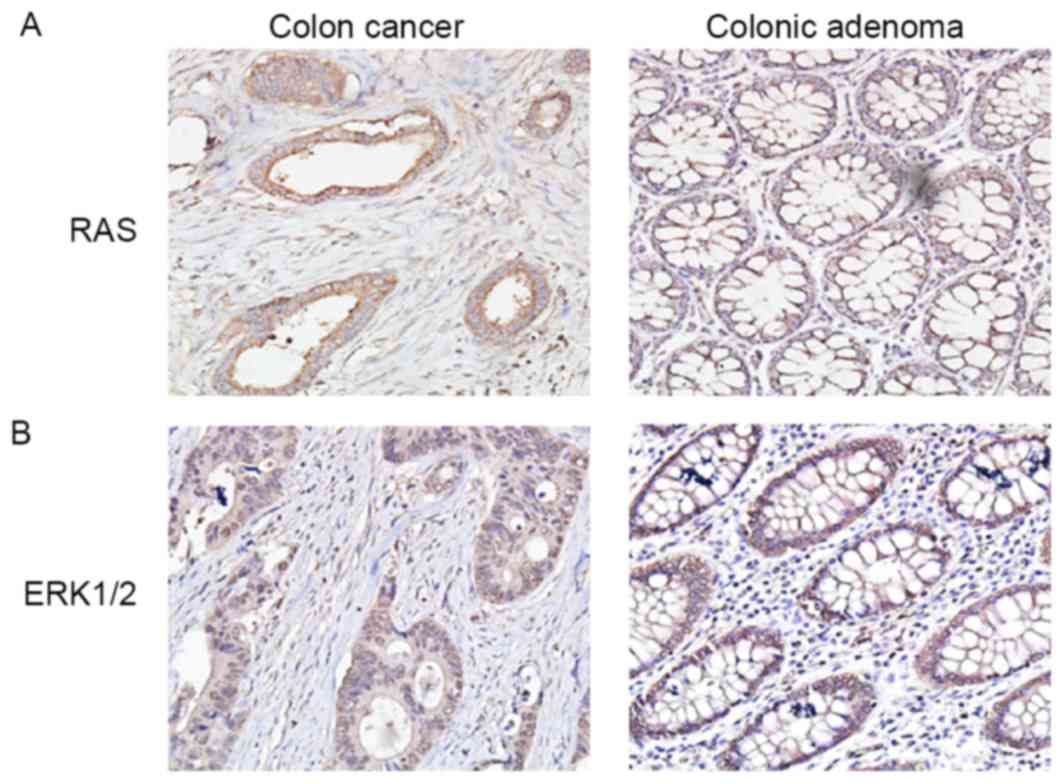
mainly in the nuclei and cytoplasm of CRC cells (Fig. 2C). Ras and ERK1/2 were also observed
in the cytoplasm of CRC cells (Fig. 3A
and B).
 | Table I.Expression of different targets in
colon cancer and colonic adenoma tissues. |
Table I.
Expression of different targets in
colon cancer and colonic adenoma tissues.
| Groups | − | + | ++ | +++ | Positive
number | Positive rate
(%) | P-value |
|---|
| p110α |
|
|
|
|
|
| <0.001 |
| Colon
cancer | 13 | 22 | 22 | 8 | 52 | 80.00 |
|
| Colonic
adenoma | 12 | 2 | 1 | 0 | 3 | 20.00 |
|
| p-Akt Ser473 |
|
|
|
|
|
| 0.001 |
| Colon
cancer | 17 | 30 | 14 | 4 | 48 | 73.85 |
|
| Colonic
adenoma | 10 | 5 | 0 | 0 | 5 | 33.33 |
|
| Cyclin D1 |
|
|
|
|
|
| <0.001 |
| Colon
cancer | 20 | 26 | 13 | 6 | 45 | 69.23 |
|
| Colonic
adenoma | 13 | 2 | 0 | 0 | 2 | 13.33 |
|
| CDK4 |
|
|
|
|
|
| 0.005 |
| Colon
cancer | 24 | 26 | 11 | 4 | 41 | 63.08 |
|
| Colonic
adenoma | 12 | 2 | 1 | 0 | 3 | 20.00 |
|
| p-mTOR Ser2448 |
|
|
|
|
|
| 0.002 |
| Colon
cancer | 14 | 22 | 16 | 13 | 51 | 78.46 |
|
| Colonic
adenoma | 11 | 1 | 2 | 1 | 4 | 26.67 |
|
| NF-κβ |
|
|
|
|
|
| 0.021 |
| Colon
cancer | 22 | 17 | 17 | 9 | 43 | 66.15 |
|
| Colonic
adenoma | 10 | 3 | 1 | 1 | 5 | 33.33 |
|
| RAS |
|
|
|
|
|
|
|
| Colon
cancer | 25 | 16 | 15 | 9 | 40 | 61.54 | 0.022 |
| Colonic
adenoma | 11 | 2 | 1 | 1 | 4 | 26.67 |
|
| ERK1/2 |
|
|
|
|
|
| 0.019 |
| Colon
cancer | 19 | 25 | 19 | 2 | 46 | 70.77 |
|
| Colonic
adenoma | 11 | 1 | 2 | 1 | 4 | 26.67 |
|
Association between target proteins
and clinicopathological characteristics in CRC
Statistical analysis results indicated that p110α,
p-Akt Ser473 and p-mTOR Ser2448 expression were not significantly
associated with sex, age, degree of differentiation, lymphatic
metastasis or TNM staging of patients with CRC (P>0.05; Table II). No significant association was
identified between cyclin D1 or CDK4 expression and sex, age and
lymphatic metastasis (P>0.05; Table
III). The positive expression of cyclin D1 and CDK4 in stage I
and stage II of CRC were significantly increased compared with in
stage III and stage IV (P<0.05; Table III). Tumors in poorly differentiated
CRC presented a significantly increased positive expression of
cyclin D1 or CDK4 compared with tumors in moderately differentiated
CRC (P<0.05; Table III).
 | Table II.Association between the expression of
p110α, p-Akt Ser473 and p-mTOR Ser2448 and clinicopathological
characteristics. |
Table II.
Association between the expression of
p110α, p-Akt Ser473 and p-mTOR Ser2448 and clinicopathological
characteristics.
|
|
| p110α |
| p-Akt Ser473 |
| p-mTOR Ser2448 |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Parameters | n | − | + | ++ | +++ | P-value | − | + | ++ | +++ | P-value | − | + | ++ | +++ | P-value |
|---|
| Sex |
|
|
|
|
| 0.081 |
|
|
|
| 0.628 |
|
|
|
| 0.445 |
|
Male | 34 | 8 | 15 | 7 | 4 |
| 9 | 17 | 6 | 2 |
| 8 | 11 | 11 | 4 |
|
|
Female | 31 | 5 | 7 | 15 | 4 |
| 8 | 13 | 8 | 2 |
| 6 | 11 | 5 | 9 |
|
| Age, years |
|
|
|
|
| 0.831 |
|
|
|
| 0.988 |
|
|
|
| 0.494 |
|
≥60 | 41 | 7 | 16 | 14 | 4 |
| 11 | 19 | 7 | 4 |
| 10 | 14 | 9 | 8 |
|
|
<60 | 24 | 6 | 6 | 8 | 4 |
| 6 | 11 | 7 | 0 |
| 4 | 8 | 7 | 5 |
|
| Degree of
differentiation |
|
|
|
|
| 0.494 |
|
|
|
| 0.385 |
|
|
|
| 0.191 |
|
Moderate | 36 | 7 | 10 | 15 | 4 |
| 9 | 15 | 9 | 3 |
| 7 | 10 | 10 | 9 |
|
|
Poor | 29 | 6 | 12 | 7 | 4 |
| 8 | 15 | 5 | 1 |
| 7 | 12 | 6 | 4 |
|
| Lymphatic
metastasis |
|
|
|
|
| 0.07 |
|
|
|
| 0.498 |
|
|
|
| 0.728 |
|
Yes | 33 | 5 | 8 | 16 | 4 |
| 12 | 15 | 8 | 4 |
| 5 | 15 | 5 | 8 |
|
| No | 32 | 8 | 14 | 6 | 4 |
| 9 | 15 | 6 | 0 |
| 9 | 7 | 11 | 5 |
|
| TNM staging |
|
|
|
|
| 0.053 |
|
|
|
| 0.102 |
|
|
|
| 0.263 |
|
I+II | 28 | 7 | 13 | 5 | 3 |
| 9 | 14 | 5 | 0 |
| 9 | 6 | 10 | 3 |
|
|
III+IV | 37 | 6 | 9 | 17 | 5 |
| 8 | 16 | 9 | 4 |
| 5 | 16 | 6 | 10 |
|
 | Table III.Association between the expression of
cyclin D1, CDK4 and p65 and clinicopathological
characteristics. |
Table III.
Association between the expression of
cyclin D1, CDK4 and p65 and clinicopathological
characteristics.
|
|
| Cyclin D1 |
| CDK4 |
| p65 |
|
|---|
|
|
|
|
|
|
|
|
|
|---|
| Parameter | n | − | + | ++ | +++ | P-value | − | + | ++ | +++ | P-value | − | + | ++ | +++ | P-value |
|---|
| Sex |
|
|
|
|
| 0.48 |
|
|
|
| 0.823 |
|
|
|
| 0.163 |
|
Male | 34 | 11 | 14 | 8 | 1 |
| 12 | 14 | 6 | 2 |
| 14 | 9 | 7 | 4 |
|
|
Female | 31 | 9 | 12 | 5 | 5 |
| 12 | 12 | 5 | 2 |
| 8 | 8 | 10 | 5 |
|
| Age, years |
|
|
|
|
| 0.487 |
|
|
|
| 0.701 |
|
|
|
| 0.329 |
|
≥60 | 41 | 14 | 15 | 10 | 2 |
| 16 | 16 | 6 | 3 |
| 14 | 14 | 8 | 5 |
|
|
<60 | 24 | 6 | 11 | 3 | 4 |
| 8 | 10 | 5 | 1 |
| 8 | 3 | 9 | 4 |
|
| Degree of
differentiation |
|
|
|
|
| 0.01 |
|
|
|
| 0.008 |
|
|
|
| 0.69 |
|
Moderate | 36 | 16 | 12 | 7 | 1 |
| 18 | 13 | 4 | 1 |
| 14 | 8 | 8 | 6 |
|
|
Poor | 29 | 4 | 14 | 6 | 5 |
| 6 | 13 | 7 | 3 |
| 8 | 9 | 9 | 3 |
|
| Lymphatic
metastasis |
|
|
|
|
| 0.562 |
|
|
|
| 0.067 |
|
|
|
| 0.008 |
|
Yes | 33 | 7 | 12 | 9 | 5 |
| 9 | 14 | 7 | 3 |
| 6 | 11 | 8 | 8 |
|
| No | 32 | 13 | 14 | 4 | 1 |
| 15 | 12 | 4 | 1 |
| 16 | 6 | 9 | 1 |
|
| TNM staging |
|
|
|
|
| 0.002 |
|
|
|
| 0.034 |
|
|
|
| 0.006 |
|
I+II | 28 | 13 | 12 | 2 | 1 |
| 14 | 10 | 4 | 0 |
| 15 | 5 | 7 | 1 |
|
|
III+IV | 37 | 7 | 14 | 11 | 5 |
| 10 | 16 | 7 | 4 |
| 7 | 12 | 10 | 8 |
|
The expression of p65 was significantly increased in
cancer tissues with lymph node metastasis compared with in cancer
tissues without lymph node metastasis (P<0.05; Table III). The expression of p65
significantly decreased in stage I and stage II of CRC compared
with stage III and stage IV of CRC (P<0.05; Table III). However, the expression of p65
was not associated with sex, age and degree of differentiation
(P>0.05; Table III). The
positive expression of Ras was significantly increased in poorly
differentiated CRC compared with moderately differentiated CRC
(P<0.05; Table IV). However, the
expression of RAS was not associated with sex, age, TNM staging or
lymphatic metastasis (P>0.05; Table
IV). The expression of ERK1/2 was significantly increased in
lymphatic metastasized tissues compared with non-lymphatic
metastasized tissues (P<0.05; Table
IV). The expression of ERK1/2 significantly decreased in stage
I and stage II of CRC compared with stage III and stage IV of CRC
(P<0.05; Table IV). There was no
significant association indicated between the expression of ERK1/2
and sex, age or degree of differentiation (P>0.05; Table IV).
 | Table IV.Association between the expression of
Ras and ERK1/2 and clinicopathological characteristics. |
Table IV.
Association between the expression of
Ras and ERK1/2 and clinicopathological characteristics.
|
|
| Ras |
| ERK1/2 |
|
|---|
|
|
|
|
|
|
|
|---|
| Parameter | n | − | + | ++ | +++ | P-value | − | + | ++ | +++ | P-value |
|---|
| Sex |
|
|
|
|
| 0.962 |
|
|
|
| 0.527 |
|
Male | 34 | 13 | 9 | 7 | 5 |
| 13 | 9 | 11 | 1 |
|
|
Female | 31 | 12 | 7 | 8 | 4 |
| 6 | 16 | 8 | 1 |
|
| Age, years |
|
|
|
|
| 0.820 |
|
|
|
| 0.977 |
|
≥60 | 41 | 16 | 8 | 12 | 5 |
| 12 | 16 | 11 | 2 |
|
|
<60 | 24 | 9 | 8 | 3 | 4 |
| 7 | 9 | 8 | 0 |
|
| Degree of
differentiation |
|
|
|
|
| 0.002 |
|
|
|
| 0.308 |
|
Moderate | 36 | 17 | 6 | 9 | 4 |
| 9 | 14 | 11 | 2 |
|
|
Poor | 29 | 8 | 10 | 6 | 5 |
| 10 | 11 | 8 | 0 |
|
| Lymphatic
metastasis |
|
|
|
|
| 0.426 |
|
|
|
| 0.033 |
|
Yes | 33 | 11 | 12 | 8 | 1 |
| 6 | 13 | 13 | 1 |
|
| No | 32 | 14 | 4 | 7 | 8 |
| 13 | 12 | 6 | 1 |
|
| TNM staging |
|
|
|
|
| 0.260 |
|
|
|
| 0.036 |
|
I+II | 28 | 12 | 3 | 6 | 8 |
| 13 | 8 | 6 | 1 |
|
|
III+IV | 37 | 13 | 13 | 9 | 1 |
| 6 | 17 | 13 | 1 |
|
Discussion
In the present study, the expression of the
associated factors and key components of the PI3K signaling pathway
in CRC tissues and colonic adenoma tissues were evaluated, and
their clinical significances analyzed. These components include
p110α, p-Akt Ser473, p-mTOR Ser2448, cyclin D1, CDK4, p65, Ras and
ERK1/2. The expression of the target proteins was all significantly
increased in CRC tissues compared with in colonic adenoma tissues
(P<0.05). The expression of cyclin D1, CDK4 and Ras were
significantly increased in poorly differentiated CRC compared with
moderately differentiated CRC (P<0.05). The expression of p65
and ERK1/2 were significantly increased in cancer tissues with
lymph node metastasis compared with in cancer tissues without lymph
node metastasis (P<0.05).
p110α protein is the catalytic subunit of PI3K. The
amplification of the genes encoding p110α have been demonstrated in
ovarian, breast and pancreatic cancer cells (14). A previous study demonstrated that
clustered regions of point mutations in the p110α catalytic subunit
occurred in 20–30% of the breast, colon, brain and gastric tumors
examined (10). Additionally, the
overexpression of the regulatory subunit of PI3K (p85) protein and
mutations in the gene encoding p85 have been identified in CRC
(13,21). The aforementioned previous studies
provide direct evidence for the alteration of PI3K in human cancer
types. In the present study, it was revealed that the expression of
p110α is significantly increased in CRC tissues compared with in
colonic adenoma tissues (P<0.05; Table
I). However, its expression was not associated with the sex,
age, degree of differentiation, lymphatic metastasis or TNM staging
of patients with CRC (P>0.05; Table
II). The results of the present study suggest that p110α may be
involved in the tumorigenesis of CRC.
Akt is the downstream effector of PI3K and regulates
the effects of PI3K on tumor growth and progression (9,10). The
complete activation of Akt requires phosphorylation at Serine 473
in its C-terminus (11,12). Akt phosphorylation has been observed
in patients with non-small cell lung cancer (22,23), and
Akt phosphorylation in human CRC was associated with the
clinicopathological parameters of patients (24,25). In
the present study, it was revealed that the expression of p-Akt
Ser473 was significantly increased in CRC tissues compared with in
colonic adenoma tissues (P<0.05; Table
I). However, its expression was not associated with the sex,
age, degree of differentiation, lymphatic metastasis or TNM staging
of patients with CRC (P>0.05; Table
II). The results of the present study suggest that p-Akt Ser473
may be involved in the tumorigenesis of CRC.
mTOR is the downstream target of Akt signaling and
Akt may directly phosphorylate its Serine 2448 (10). The abnormal expression of p-mTOR
Ser2448 has been linked to tumor progression in a number of types
of human cancer, including CRC (13,26). In
the present study, it was revealed that the expression of p-mTOR
Ser2448 was significantly increased in CRC tissues compared with in
colonic adenoma tissues (P<0.05; Table
I). However, its expression was not associated with the sex,
age, degree of differentiation, lymphatic metastasis or TNM staging
of patients with CRC (P>0.05; Table
II). The results of the present study suggest that p-mTOR
Ser2448 may be involved in the tumorigenesis of CRC.
Cyclin D1 is a key cell cycle regulatory protein,
and may form a complex with CDK4 in order to govern the cell cycle
and its progression (7,14). Akt may indirectly regulate the levels
of cyclin D1 and the assembly of cyclin D1-CDK4 complexes (14,15). The
altered expression of cyclin D1 and CDK4 has been demonstrated in a
variety of tumor types, including colon tumors (27–29). They
may be involved in early events in gastric tumorigenesis (29). In the present study, it was revealed
that the expression levels of cyclin D1 and CDK4 were significantly
increased in CRC tissues compared with those in colonic adenoma
tissues (P<0.05; Table I). The
expression levels of cyclin D1 and CDK4 were significantly
increased in poorly differentiated CRC compared with moderately
differentiated CRC (P<0.05). The results of the present study
suggest that cyclin D1 and CDK4 may participate in the
tumorigenesis and progression of CRC.
The NF-κβ signaling pathway serves important
functions in oncogenesis by regulating the expression of genes
associated with the development and progression of cancer (20). Akt may affect cell survival by
indirectly regulating the NF-κβ signaling pathway (7). p65, the subunit of activated NF-κβ, has
been demonstrated to regulate the phosphorylation and expression of
Akt (16). An abnormal expression of
p65 was observed in pancreatic cancer, in which a high expression
of p65 indicated poor patient survival (30). In the present study, it was revealed
that the expression of p65 was significantly increased in CRC
tissues compared with that in colonic adenoma tissues (P<0.05;
Table I). The expression of p65 was
significantly increased in cancer tissues with lymph node
metastasis compared with in cancer tissues without lymph node
metastasis (P<0.05; Table III).
These results suggest that p65 may be associated with the
tumorigenesis and progression of CRC.
Ras proteins may regulate the phosphorylation of the
Raf/MEK/ERK and the PI3K/Akt/mTOR signaling pathways (17). They exhibit essential functions in
controlling a number of the malignant characteristics of
transformed cells, for example in breast cancer (17,18). The
activated mutations in the Ras genes themselves or alterations in
upstream or downstream signaling components occurred in various
types of human cancer, including CRC (10,33). KRAS
proto-oncogene, GTPase (KRAS) is a member of the Ras family, and
its mutations are predictors for resistance to cetuximab therapy in
CRC (34). KRAS expression was
significantly increased in carcinomatous lesions of the pancreas
compared with in normal ducts or ductal hyperplasia (35). The antibody used in the present study
may detect the expression of the three members of the Ras family
(namely, HRAS proto-oncogene, GTPase, KRAS and NRAS proto-oncogene,
GTPase). It was revealed that the expression of Ras was
significantly increased in CRC tissues compared with in colonic
adenoma tissues (P<0.05; Table I).
The positive expression rate of Ras was significantly increased in
poorly differentiated CRC compared with that in moderately
differentiated CRC (P<0.05; Table
IV). The results of the present study suggest that Ras may
participate in the tumorigenesis and progression of CRC.
ERK1 and ERK2 are MAP kinases and serve important
functions in normal physiological development and carcinogenesis
(17,36). ERK1/2 may mediate the phosphorylation
of the essential scaffolding protein of mTORC1 in order to promote
mTOR signaling (18). ERK1 expression
may be an early marker of cervical carcinogenesis (37). In the present study, it was revealed
that the expression of ERK1/2 was significantly increased in CRC
tissues compared with in colonic adenoma tissues (P<0.05;
Table I). The expression of ERK1/2
was significantly increased in lymphatic metastasized tissues
compared with non-lymphatic metastasized tissues (P<0.05;
Table IV). The results of the
present study suggest that ERK1/2 may participate in the
tumorigenesis and progression of CRC.
In conclusion, the results of the present study
demonstrated that p110α, p-Akt Ser473, p-mTOR Ser2448, cyclin D1,
CDK4, p65, Ras and ERK1/2 may all participate in the tumorigenesis
of CRC. Additionally, cyclin D1, CDK4, Ras, p65 and ERK1/2 may be
important in the progress of CRC. These results may provide novel
predictive factors and therapeutic targets for CRC.
Acknowledgements
The present study was supported by the Science and
Technology Plan of Shandong (grant no. 2016GSF118008).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics. 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stein U, Walther W, Arlt F, Schwabe H,
Smith J, Fichtner I, Birchmeier W and Schlag PM: MACC1, a newly
identified key regulator of HGF-MET signaling, predicts colon
cancer metastasis. Nat Med. 15:59–67. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Board RE and Valle JW: Metastatic
colorectal cancer: Current systemic treatment options. Drugs.
67:1851–1867. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferlay J, Autier P, Boniol M, Heanue M,
Colombet M and Boyle P: Estimates of the cancer incidence and
mortality in Europe in 2006. Ann Oncol. 18:581–592. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hansen JD, Kumar S, Lo WK, Poulsen DM,
Halai UA and Tater KC: Ursodiol and colorectal cancer or dysplasia
risk in primary sclerosing cholangitis and inflammatory bowel
disease: A meta-analysis. Dig Dis Sci. 58:3079–3087. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vivanco I and Sawyers CL: The
phosphatidylinositol 3-Kinase Akt pathway in human cancer. Nat Rev
Cancer. 2:489–501. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yang SX, Polley E and Lipkowitz S: New
insights on PI3K/Akt pathway alterations and clinical outcomes in
breast cancer. Cancer Treat Rev. 45:87–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ciruelos Gil EM: Targeting the
PI3K/Akt/mTOR pathway in estrogen receptor-positive breast cancer.
Cancer Treat Rev. 40:862–871. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sarbassov DD, Guertin DA, Ali SM and
Sabatini DM: Phosphorylation and regulation of Akt/PKB by the
rictor-mTOR complex. Science. 307:1098–1101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Badve S and Nakshatri H: Role of Akt
isotypes in breast cancer. J Pathol. 229:e12013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Johnson SM, Gulhati P, Rampy BA, Han Y,
Rychahou PG, Doan HQ, Weiss HL and Evers BM: Novel expression
patterns of PI3K/Akt/mTOR signaling pathway components in
colorectal cancer. J Am Coll Surg. 210:767–778. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo J, Manning BD and Cantley LC:
Targeting the PI3K-Akt pathway in human cancer: Rationale and
promise. Cancer Cell. 4:257–262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Dowbenko D and Lasky LA: AKT/PKB
phosphorylation of p21Cip/WAF1 enhances protein stability of
p21Cip/WAF1 and promotes cell survival. J Biol Chem.
277:11352–11361. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Meng F and D'Mello SR: NF-kappaB
stimulates Akt phosphorylation and gene expression by distinct
signaling mechanisms. Biochim Biophys Acta. 1630:35–40. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saini KS, Loi S, de Azambuja E,
Metzger-Filho O, Saini ML, Ignatiadis M, Dancey JE and
Piccart-Gebhart MJ: Targeting the PI3K/Akt/mTOR and Raf/MEK/ERK
pathways in the treatment of breast cancer. Cancer Treat Rev.
39:935–946. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carriere A, Romeo Y, Acosta-Jaquez HA,
Moreau J, Bonneil E, Thibault P, Fingar DC and Roux PP: ERK1/2
phosphorylate Raptor to promote Ras-dependent activation of mTOR
complex 1 (mTORC1). J Biol Chem. 286:567–577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okkenhaug K, Graupera M and Vanhaesebroeck
B: Targeting PI3K in Cancer: Impact on tumor cells, their
protective Stroma, Angiogenesis, and Immunotherapy. Cancer Discov.
6:1090–1105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dolcet X, Llobet D, Pallares J and
Matias-Guiu X: NF-κB in development and progression of human
cancer. Virchows Arch. 446:475–482. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bader AG, Kang S, Zhao L and Vogt PK:
Oncogenic PI3K deregulates transcription and translation. Nat Rev
Cancer. 5:921–929. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee SH, Kim HS, Park WS, Kim SY, Lee KY,
Kim SH, Lee JY and Yoo NJ: Non-small cell lung cancers frequently
express phosphorylated Akt; an immunohistochemical study. APMIS.
110:587–592. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cappuzzo F, Magrini E, Ceresoli GL,
Bartolini S, Rossi E, Ludovini V, Gregorc V, Ligorio C, Cancellieri
A, Damiani S, et al: Akt phosphorylation and gefitinib efficacy in
patients with advanced non-small-cell lung cancer. J Natl Cancer
Inst. 96:1133–1141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Khaleghpour K, Li Y, Banville D, Yu Z and
Shen SH: Involvement of the PI 3-kinase signaling pathway in
progression of colon adenocarcinoma. Carcinogenesis. 25:241–248.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Itoh N, Semba S, Ito M, Takeda H, Kawata S
and Yamakawa M: Phosphorylation of Akt/PKB is required for
suppression of cancer cell apoptosis and tumor progression in human
colorectal carcinoma. Cancer. 94:3127–3134. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Müller J, Ehlers A, Burkhardt L, Sirma H,
Steuber T, Graefen M, Sauter G, Minner S, Simon R, Schlomm T, et
al: Loss of pSer2448-mTOR expression is linked to adverse prognosis
and tumor progression in ERG-fusion-positive cancers. Int J Cancer.
132:1333–1340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McKay JA, Douglas JJ, Ross VG, Curran S,
Murray GI, Cassidy J and McLeod HL: Cyclin D1 protein expression
and gene polymorphism in colorectal cancer. Aberdeen Colorectal
Initiative. Int J Cancer. 88:77–81. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Motohashi M, Wakui S, Muto T, Suzuki Y,
Shirai M, Takahashi H and Hano H: Cyclin D1/cdk4, estrogen
receptors α and β, in N-methyl-N'-nitro-N-nitrosoguanidine-induced
rat gastric carcinogenesis: Immunohistochemical study. J Toxicol
Sci. 36:373–378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee KH, Lee HE, Cho SJ, Cho YJ, Lee HS,
Kim JH, Nam SY, Chang MS, Kim WH and Lee BL: Immunohistochemical
analysis of cell cycle-related molecules in gastric carcinoma:
Prognostic significance, correlation with clinicopathological
parameters, proliferation and apoptosis. Pathobiology. 75:364–372.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Weichert W, Boehm M, Gekeler V, Bahra M,
Langrehr J, Neuhaus P, Denkert C, Imre G, Weller C, Hofmann HP, et
al: High expression of RelA/p65 is associated with activation of
nuclear factor-kappaB-dependent signaling in pancreatic cancer and
marks a patient population with poor prognosis. Br J Cancer.
97:523–530. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Uzgare AR, Kaplan PJ and Greenberg NM:
Differential expression and/or activation of P38MAPK, erk1/2, and
jnk during the initiation and progression of prostate cancer.
Prostate. 55:128–139. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Noone AM, Schussler N, Negoita S, Adamo M,
Cronin K, Cyr J, Gress D, Grove C, Kosary C, Liu B, et al:
Availability of TNM staging data elements in the medical record and
training needs assessment: Results from the 2014 SEER Training
Needs Assessment for TNM study. J Registry Manag. 42:40–47.
2015.PubMed/NCBI
|
|
33
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lievre A, Bachet JB, Le Corre D, Boige V,
Landi B, Emile JF, Coté JF, Tomasic G, Penna C, Ducreux M, et al:
KRAS mutation status is predictive of response to cetuximab therapy
in colorectal cancer. Cancer Res. 66:3992–3995. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Apple SK, Hecht JR, Lewin DN, Jahromi SA,
Grody WW and Nieberg RK: Immunohistochemical evaluation of K-ras,
p53, and HER-2/neu expression in hyperplastic, dysplastic, and
carcinomatous lesions of the pancreas: Evidence for multistep
carcinogenesis. Hum Pathol. 30:123–129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Poulikakos PI and Solit DB: Resistance to
MEK inhibitors: Should we co-target upstream? Sci Signal.
4:pe162011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Branca M, Ciotti M, Santini D, Bonito LD,
Benedetto A, Giorgi C, Paba P, Favalli C, Costa S, Agarossi A, et
al: Activation of the ERK/MAP kinase pathway in cervical
intraepithelial neoplasia is related to grade of the lesion but not
to high-risk human papillomavirus, virus clearance, or prognosis in
cervical cancer. Am J Clin Pathol. 122:902–911. 2004. View Article : Google Scholar : PubMed/NCBI
|