Introduction
Thyroid cancer is the most frequently occurring
tumor in the endocrine system and accounts for ~1% of all newly
diagnosed cancer cases in the United States (1). The incidence of thyroid cancer,
particularly differentiated-type thyroid cancer, is rapidly
increasing (2–5). Thyroid cancer is primarily derived from
two thyroid cell types: Follicular thyroid cells and parafollicular
cells. The follicular thyroid cell-derived tumors account for the
majority of thyroid cancer cases, including anaplastic thyroid
cancer, poorly differentiated thyroid cancer, differentiated
papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC).
Medullary thyroid cancer (MTC), derived from parafollicular C
cells, accounts for a relatively small proportion of thyroid
cancers (6). Genetic and epigenetic
alterations, including mutations, copy number variation and
abnormal methylation, are the primary causative factors of the
pathogenesis of thyroid cancer (1,6).
Well-differentiated PTC and FTC can be effectively treated by
surgery followed by radioiodine therapy. Tumors without
differentiation are candidates for molecularly targeted therapy, as
they are associated with a less favorable prognosis (7).
Quaking (QKI) is an RNA-binding protein that belongs
to the signal transduction and activation of RNA (STAR) protein
family (8). QKI encodes a diverse set
of transcript variants through alternative splicing, of which the
most well-documented isoforms are QKI 5, 6 and 7 (9). QKI has been associated with regulating
various cellular processes, including apoptosis, the cell cycle,
differentiation and cell fate determination (10–12). The
decreased expression of QKI has been implicated in the pathogenesis
of many types of human disease, including a number of types of
cancer (8,13). Yang et al (14) reported that the levels of QKI5/6 were
significantly reduced or absent in colorectal cancer (CRC) cells.
It has also been observed that the downregulation of QKI in colon
cancers may be partially due to the hypermethylation of the QKI
promoter region. The induced expression of QKI decreases the cell
proliferation ability and increases the differentiation ability of
colon cancer cells (14).
MicroRNAs (miRNA) are small noncoding RNAs that
function in RNA splicing and the post-transcriptional regulation of
gene expression via targeting complementary sequences within mRNA
to induce mRNA degradation (15–17). The
dysregulation of miRNAs and their associated target genes may
participate in tumorigenesis (18,19).
miR-574-5p is a potential oncogenic miRNA that has been
demonstrated to be significantly upregulated in thyroid cancer
cells (18,19).
Wnt/β-catenin signaling is constitutively activated
in cancer cells. Phosphorylated β-catenin may be degraded by
proteasomes. However, non-phosphorylated β-catenin is transferred
into the nucleus and protected from degradation, thereby activating
the expression of genes associated with cell proliferation
(20). Thus, the phosphorylation
level of β-catenin indicates the activity of the Wnt/β-catenin
pathway and promotes cancer cell proliferation.
Previous studies have demonstrated that miR-574-5p
regulates CRC tumorigenesis and progression by targeting QKI and
subsequently activating the Wnt/β-catenin pathway (8). However, it remains unclear the mechanism
by which miR-574-5p regulates QKI and functions as an oncogene in
thyroid cancer.
In the present study, miR-574-5p-specific small
interfering RNA (siRNA) was utilized to decrease the miR-574-5p
level, and the influence of miR-574-5p on cell proliferation, the
cell cycle and apoptosis was analyzed in BCPAP and FTC133 thyroid
cancer cells. The expression of QKI and crucial factors of the
Wnt/β-catenin pathway following miR-574-5p knockdown in thyroid
cell lines were also detected. The results of the present study
suggested that miR-574-5p induced cell proliferation and cell cycle
progression, and repressed the apoptosis of thyroid cancer cells
via Wnt/β-catenin signaling by targeting QKIs.
Materials and methods
Cell culture and siRNA
transfection
The human thyroid cells BCPAP and FTC133 (American
Type Culture Collection, Manassas, VA, USA) were cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) with 10% fetal bovine serum
(FBS; Gibco, Thermo Fisher Scientific, Inc.) at 37°C with 5%
CO2 in an incubator. A total of 2×105 BCPAP
or FTC133 cells were plated into 6-well dishes. At 24 h after
seeding, siRNA targeting miRNA-574 or QKI, or negative control
siRNAs were transfected into cells using Lipofectamine®
2000 (Invitrogen, Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Cells transfected with the miR-574-5p
siRNA or negative control siRNA were used for proliferation, cell
cycle, apoptosis, reverse transcription-quantitative polymerase
chain reaction (RT-qPCR) and western blotting assays. Cells
transfected with miR-574-5p siRNA and QKI siRNA were used for
apoptosis and western blotting assays. The miR-574-5p-specific
siRNA was purchased from Ambion (Thermo Fisher Scientific, Inc.;
sequence 5′-UGAGUGUGUGUGUGUGAGUGUGU-3′), a scrambled sequence, as
previously described (8), was used as
negative control. The siRNA for pan-QKI knockdown was synthesized
by Shanghai Sangon Pharmaceutical Co., Ltd. (Shanghai, China) with
the sequence 5′-CCUUGAGUAUCCUAUUGAACCUAGU-3′, whereas the negative
control sequence was 5′-UUCUCCGAACGUGUCACGUTT-3′.
Cell proliferation assay
Cell proliferation was detected by an MTT assay
(Sigma-Aldrich, Merck KGaA, Darmstadt, Germany) according to the
manufacture's protocol, with some modification, as previously
described (21). At 24, 48, and 72 h
after transfection, BCPAP and FTC133 cells were treated with MTT (5
mg/ml in 1X PBS), in an amount equal to 10% of the culture volume,
for 4 h. Absorbance at 570 nm was then detected. Three independent
experimental repeats were performed.
Cell cycle assay
BCPAP and FTC133 cells transfected with miR-574-5p
or negative control siRNA were collected, washed twice with 1X PBS,
and fixed in 70% ethanol at −20°C. After 24 h of fixation, cells
were incubated with RNase A (Takara Bio, Inc., Otsu, Japan) at 100
µg/ml in 1X PBS for 30 min at 37°C. Cells were then stained with
propidium iodide (PI; BD Biosciences, San Jose, CA, USA) at 50
µg/ml for 30 min at room temperature. Subsequently, cells were
analyzed for DNA content using a BD FACSCalibur™ flow cytometer (BD
Biosciences). Three independent experimental repeats were
performed.
Apoptosis analysis
BCPAP and FTC133 cells transfected with miR-574-5p,
QKI or negative control siRNA were collected for apoptosis
analysis. An Annexin V-FITC PI staining assay kit (BD Biosciences)
was used for the detection of apoptotic cells according to the
manufacturer's protocol. Three independent experimental repeats
were performed.
RNA extraction and RT-qPCR analyses
for miRNA and mRNAs
The total RNA of BCPAP and FTC133 cells transfected
with miR-574-5p siRNA or negative controls was extracted using an
RNeasy Mini kit (Qiagen, Valencia, CA, USA) according to the
manufacturer's protocol. For mRNA quantification, RT was performed
with the PrimeScript Reverse Transcriptase (Takara Bio) using a
random primer mix (Invitrogen). qPCR assays were performed with the
SYBR Premix Ex Taq™ (Takara Bio) on an ABI Prism 7500 Real-time PCR
system (Thermo Fisher Scientific). DNA was denatured at 94°C for 10
min, followed by initial denaturation with 30 cycles at 94°C for 1
min, 60°C for 1 min and 72°C for 2 min, and finally ended up with
an extension step at 72°C for 5 min. The relative expression of
genes was calculated by 2−ΔΔCt method (22). Each assay was performed three
times.
For miR-574-5p quantification, RT was performed with
the PrimeScript Reverse Transcriptase (Takara Bio) using the
miR-574-5p-specific RT primer (Shanghai Sangon Pharmaceutical,
Shanghai, China). qPCR assays were performed using the previously
described universal primer (Shanghai Sangon Pharmaceutical) and
miR-574-5p-specific reverse locked nucleic acid primers (LNA;
Shanghai Sangon Pharmaceutical) (8,23).
The primers were as follows: Pan-QKI forward:
5′-CATCAGCTGCATCTTCTTCAG-3′, pan-QKI reverse:
5′-CACTGTGGAAGATGCTCAGAA-3′; QKI5 forward:
5′-GCCCTACCATAATGCCTTTGA-3′, QKI5 reverse:
5′-AACTTTAGTAGCCACCGCAACC-3′; QKI6 forward:
5′-GCCCGAAGCTGGTTTAATCTATA-3′, QKI6 reverse:
5′-TCGTTGGGAAAGCCATACCTAAT-3′; QKI7 forward:
5′-GCTGGTTTAATCTATACACCCTATGA-3′, QKI7 reverse:
5′-GACTGGCATTTCAATCCACTCTA-3′; miR-574-5p-specific RT primer:
5′-GTCGTATCCAGTGCGTGTCGTGGAGTCGGCAATTGCACTGGATACGACTACACAC-3′;
universal primer, GGGGTGAGTGTGTGTGTG-3′; miR-574-5p-specific
reverse LNA, TGCGTGTCGTGGAGTC-3′.
Western blotting
Total protein extracts from BCPAP and FTC133 cells
with or without miR-574-5p knockdown were used for western blotting
analysis, as previously described (8). The primary antibodies used for western
blot analysis were as follows: Anti-pan-QKI (cat. no. sc103851;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA); anti-QKI5 (cat.
no. AB9904); anti-QKI6 (cat. no. AB9906); anti-QKI7 (cat. no.
AB9908; all EMD Millipore, Billerica, MA, USA); anti-α-Tubulin
(cat. no. 2125); anti-β-catenin (cat. no. 8480);
anti-phospho-β-catenin (Ser33/37/41; cat. no. 9561); anti-cyclin D1
(cat. no. 2978); and anti-survivin (cat. no. 2808; all Cell
Signaling Technology, Danvers, MA, USA). Each western blotting
assay was performed three times.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical
evaluation was performed using Student's t test (two-tailed)
between two groups, or one-way analysis of variance followed by
Tukey's post hoc test for multiple comparisons. P<0.05 was
considered to indicate a statistically significant difference.
Results
Inhibition of miR-574-5p suppresses
the proliferation of BCPAP and FTC133 cells
To investigate the role of miR-574-5p in thyroid
cancer, siRNA was used to knockdown miR-574-5p in the thyroid
carcinoma-derived cell lines BCPAP and FTC133. The reduction of
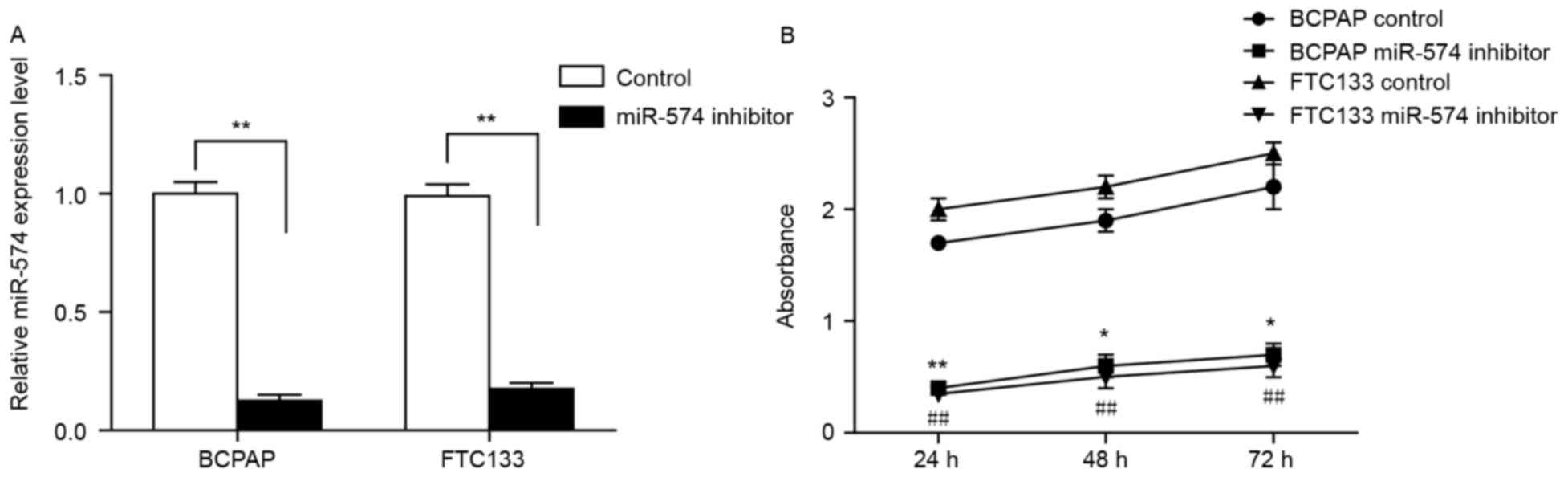
miR-574-5p expression in miR-574-5p siRNA-transfected cells was
confirmed by RT-qPCR (Fig. 1A).
Subsequently, an MTT assay was conducted to investigate any changes
in cell growth following miR-574-5p knockdown. As demonstrated in
Fig. 1B, the viability of BCPAP and
FTC133 cells transfected with miR-574-5p siRNA was significantly
reduced when compared with the negative control, indicating that
miR-574-5p may promote thyroid cancer cell growth.
Inhibition of miR-574-5p leads to the
G1/S-phase arrest of BCPAP and FTC133 cells
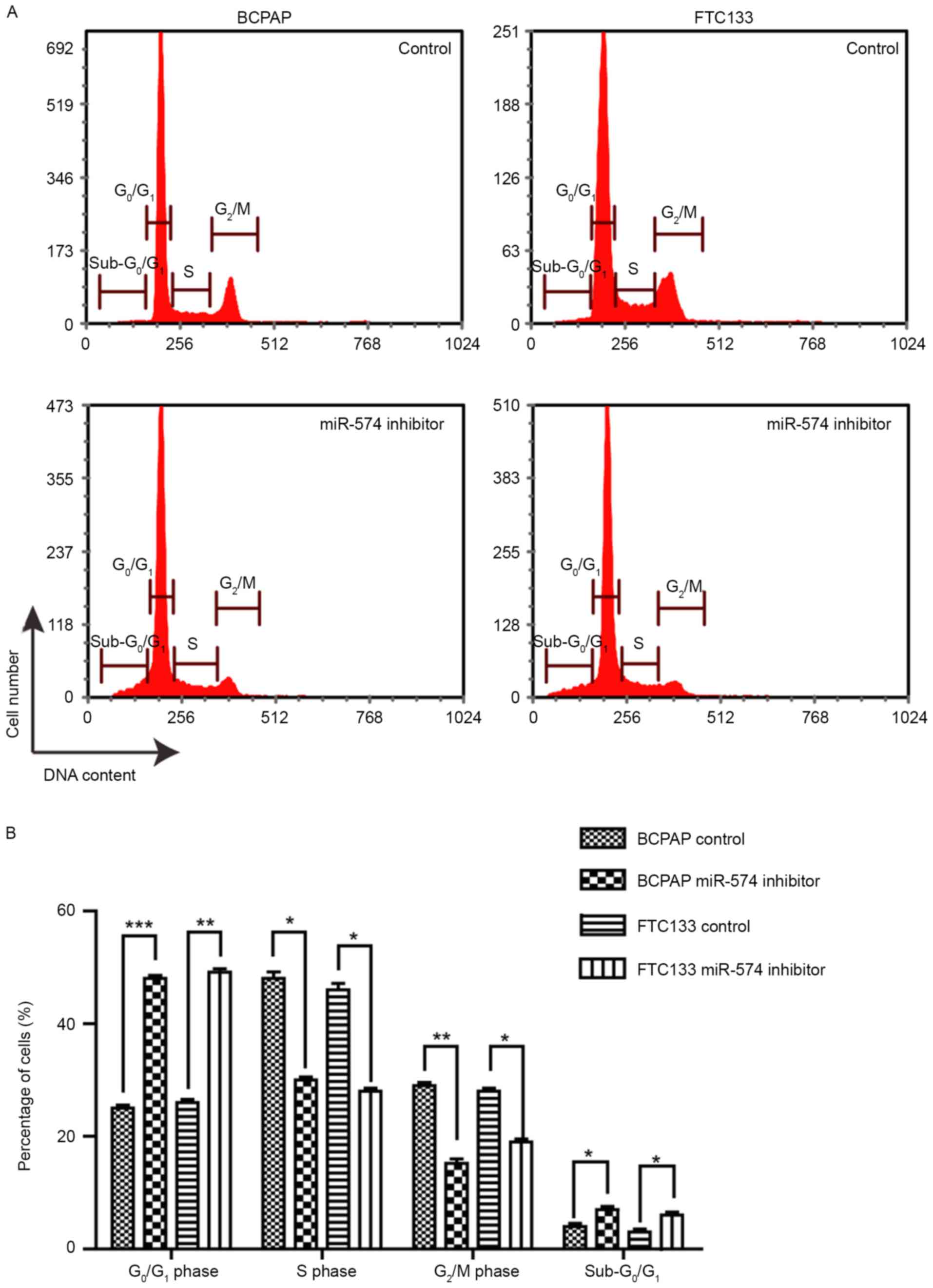
The effect of miR-574-5p knockdown on the cell cycle
distribution was investigated. As demonstrated by Fig. 2, following miR-574-5p knockdown, the
proportion of G0/G1-phase cells was
significantly increased, from ~25 to 50% (BCPAP) and from ~26 to
51% (FTC133), whereas the proportion of S-and G2/M-phase
cells was significantly decreased. This result implied that the
knockdown of miR-574-5p led to cell cycle arrest at the
G1/S phase, suggesting that miR-574-5p promoted the cell
cycle progression of thyroid cancer cells. Furthermore, the
proportion of sub-G0/G1 phase cells was
significantly increased following miR-574-5p knockdown, indicating
the induction of apoptosis.
Inhibition of miR-574-5p induces the
apoptosis of BCPAP and FTC133 cells
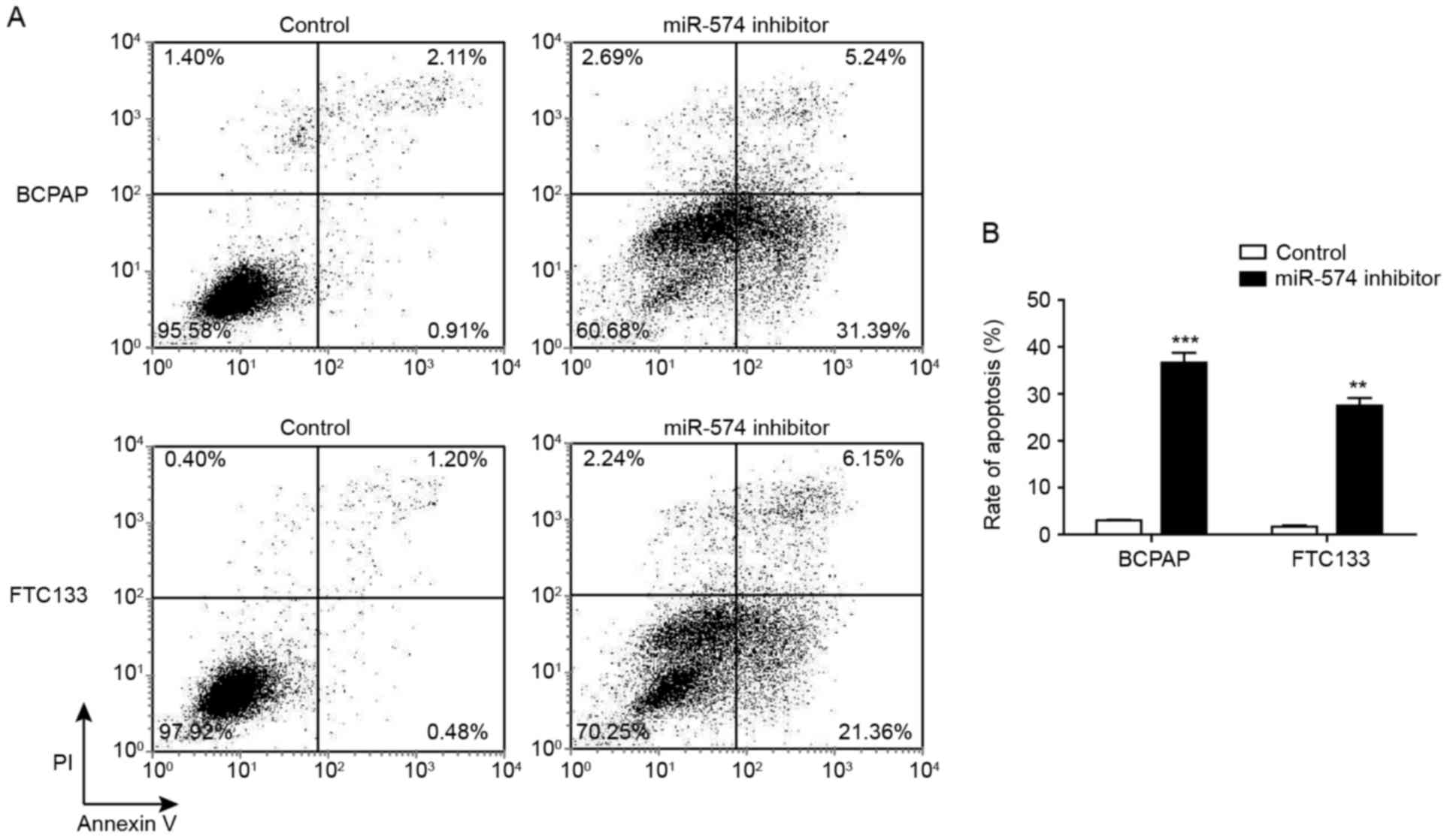
The cell cycle analysis indicated that the
inhibition of miR-574-5p may affect the apoptotic status of thyroid
carcinoma cells. Thus, the proportion of early and late apoptotic
cells following miR-574-5p knockdown were determined through
Annexin V-FITC/PI staining. Fig. 3
demonstrated that, following miR-574-5p knockdown, the proportion
of early apoptotic cells (Annexin V+/PI−) was
increased from 0.91 to 31.39% for BCPAP cells, and from 0.48 to
21.36% for FTC133 cells. Furthermore, the proportion of late
apoptotic and necrotic (Annexin V+/PI+) cells
following miR-574-5p knockdown was increased from 2.11 to 5.24% for
BCPAP cells, and from 1.20 to 6.15% for FTC133 cells.
Inhibition of miR-574-5p upregulates
the level of pan-QKI and QKI5/6/7 in BCPAP and FTC133 cells
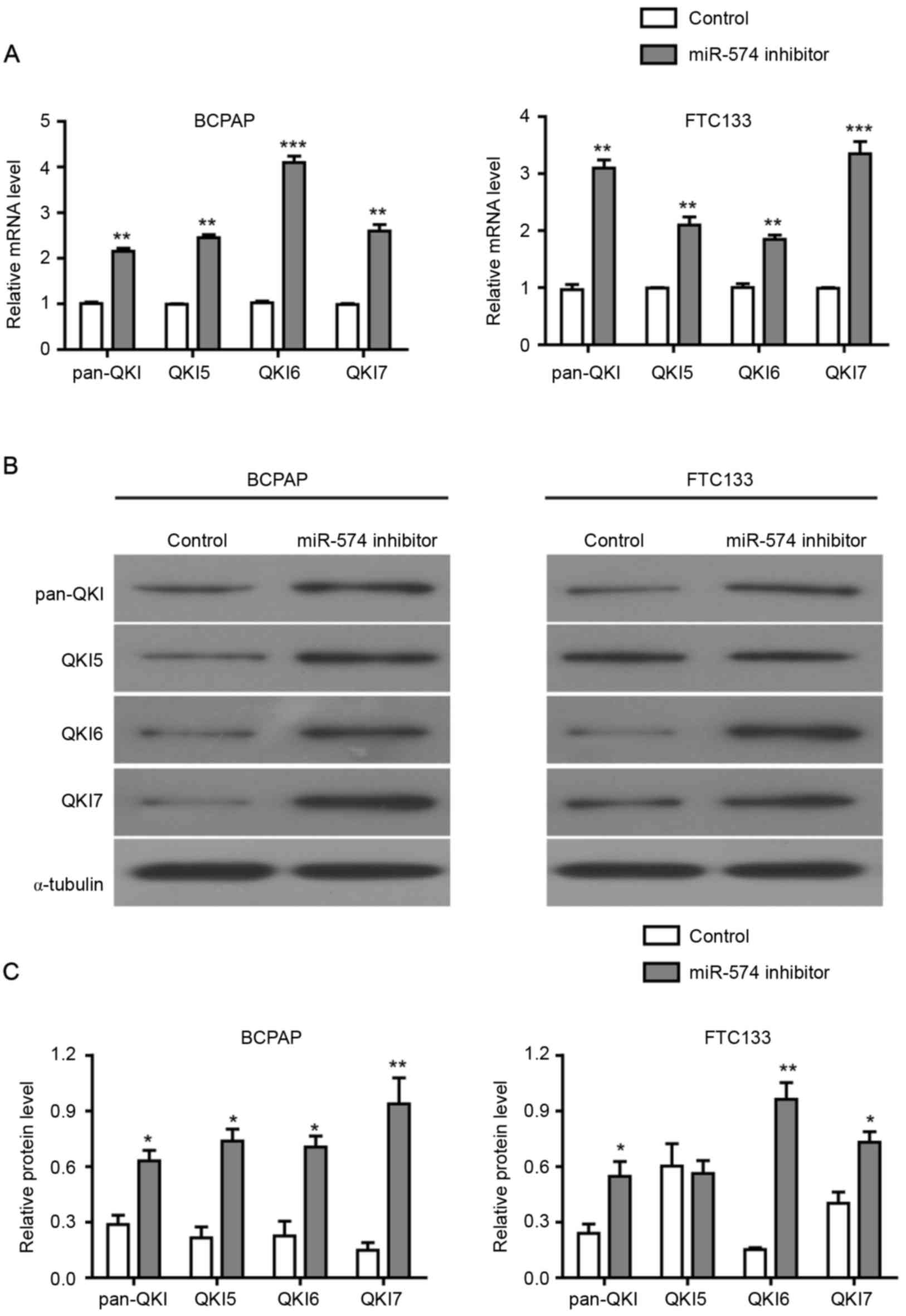
The mechanism of miR-574-5p in the development of
thyroid carcinoma has yet to be characterized. A previous study
demonstrated that the inhibition of miR-574-5p in CRC suppressed
tumor growth by the activation of QKI proteins (8). Therefore, the present study further
investigated whether the expression levels of QKIs were influenced
by miR-574-5p in thyroid carcinoma.
Initially, an RT-qPCR assay was performed to detect
the level of pan-QKI and QKI5/6/7 mRNAs prior to and following the
inhibition of miR-574-5p. As demonstrated in Fig. 4A, the mRNA levels of pan-QKI and
QKI5/6/7 were significantly higher in the miR-574-5p
siRNA-transfected BCPAP and FTC133 cells compared with the negative
control. Subsequently, western blotting assays were conducted to
assess the protein level of pan-QKI and QKI5/6/7. As demonstrated
in Fig. 4B and C, the protein levels
of pan-QKI and QKI5/6/7 were also increased following the
miR-574-5p knockdown in BCPAP and FTC133 cells, with the exception
of QKI5 protein in FTC133 cells. Collectively, these results
indicated that miR-574-5p repressed the expression of QKIs at the
mRNA and protein levels, suggesting that the influence of
miR-574-5p on cell proliferation, cell cycle distribution and
apoptosis may be mediated by the repression of QKIs.
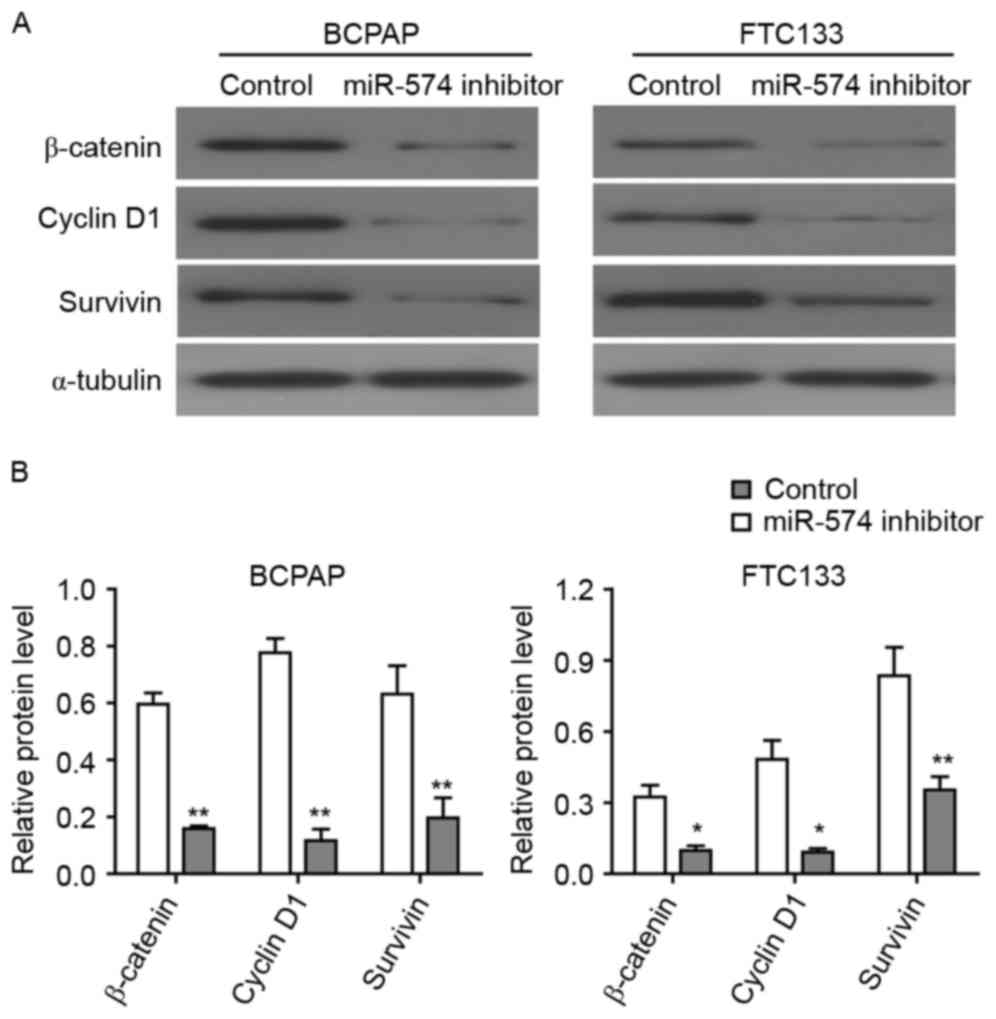
Inhibition of miR-574-5p represses the
Wnt/β-catenin pathway
β-catenin is a core factor of the Wnt/β-catenin
signaling pathway and a downstream target of QKI proteins (24). As the expression of QKIs may be
regulated by miR-574-5p, it was investigated in the present study
how miR-574-5p affected the Wnt/β-catenin signaling pathway through
the western blot analysis of thyroid carcinoma cells. The knockdown
of miR-574-5p led to the significant downregulation of β-catenin
protein in BCPAP and FTC133 cells (Fig.
5), indicating that miR-574-5p may regulate Wnt/β-catenin
pathway via affecting the QKI proteins. Furthermore, the expression
levels of two Wnt/β-catenin target genes, cyclin D1 and survivin,
were detected following miR-574-5p knockdown. As demonstrated in
Fig. 5, the levels of cyclin D1,
which regulated the cell cycle, and survivin, which regulated
apoptosis, were significantly lower in miR-574-5p siRNA-treated
BCPAP and FTC133 cells as compared with control siRNA-treated
cells. Collectively, these results indicated that the depletion of
miR-574-5p may lead to the repression of the Wnt/β-catenin
pathway.
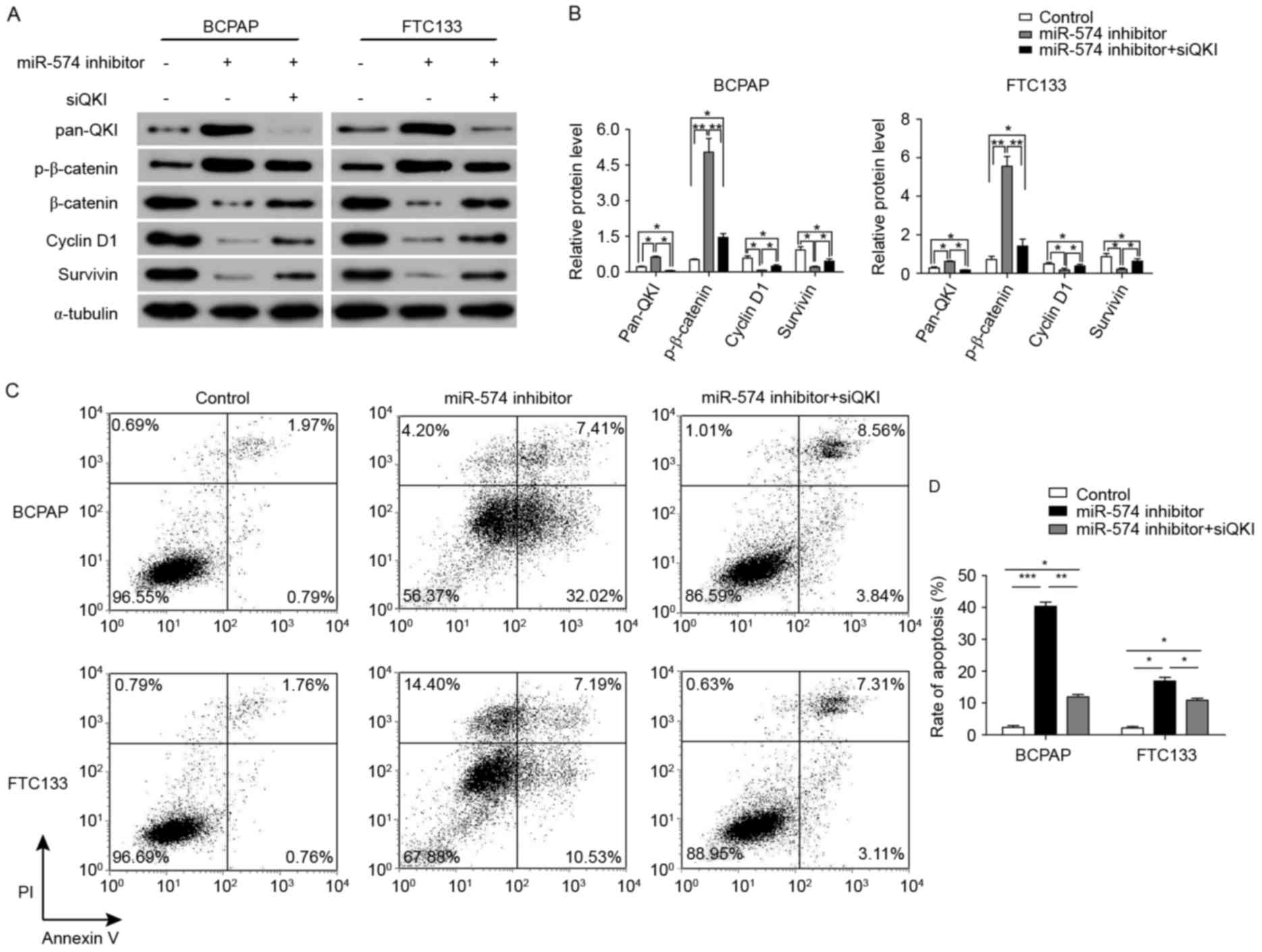
miR-574-5p may regulate the
Wnt/β-catenin pathway by repressing QKIs
The results of Fig. 4
demonstrate that QKIs, which have been reported to repress the
Wnt/β-catenin pathway, were repressed by miR-574-5p. In order to
identify whether the repression of the Wnt/β-catenin pathway
subsequent to miR-574-5p knockdown was mediated by QKIs, the
further knockdown of QKI expression was induced through siRNAs
against all QKI isoforms. As illustrated in Fig. 6A and B, the expression levels of
β-catenin, cyclin D1 and survivin were significantly increased
following QKI knockdown in combination with miR-574-5p knockdown.
Furthermore, the level of phosphorylated β-catenin was
significantly upregulated following miR-574-5p knockdown in BCPAP
and FTC133 cells. However, the level of phosphorylated β-catenin
was decreased following QKI knockdown in miR-574-5p-knockdown BCPAP
and FTC133 cells.
The biological effects of further QKI knockdown in
miR-574-5p-knockdown BCPAP and FTC133 cells were also examined.
Cells with the co-knockdown of QKIs and miR-574-5p exhibited a
significant decrease in the rate of apoptosis when compared with
the cells with only miR-574-5p knockdown (from 39.43 to 12.40% in
BCPAP cells, and from 17.72 to 10.42% in FTC133 cells; Fig. 6C and D). Collectively, these results
indicated that miR-574-5p may positively activate the Wnt/β-catenin
signaling pathway through repressing QKIs, and thus regulate the
cell cycle and apoptosis.
Discussion
Thyroid cancer accounts for ~1% of all newly
diagnosed cancers in the United States, and is the most frequently
occurring tumor in the endocrine system (1). miR-574-5p, a potential oncogenic miRNA,
is upregulated in thyroid cancer (18). In the present study, it was
demonstrated that miR-574-5p induced cell proliferation and cell
cycle progression, and repressed the apoptosis of thyroid cancer
cells via Wnt/β-catenin signaling by downregulating QKIs. The
inhibition of miR574-5p repressed proliferation and induced the
apoptosis of thyroid cancer cells. These data may enhance the
understanding of the association between the miR-574-5p and
Wnt/β-catenin pathway. Additionally, miR-574-5p may provide a novel
potential therapeutic target for the treatment of thyroid
cancer.
miRNAs may participate in tumorigenesis by acting as
oncogenes or tumor suppressors (16,25).
miR-574-5p has been identified as upregulated in thyroid cancer
cells (18). The present study
demonstrated that the inhibition of miR-574-5p reduced the
oncogenic behavior of thyroid cancer cells. Knockdown of miR-574-5p
repressed the proliferation of thyroid cancer cells, led to cell
cycle arrest at the G1/S phase, and increased the number
of cells undergoing apoptosis. An increasing amount of evidence has
indicated the oncogenic role of miR-574-5p. For example, miR-574-5p
has been identified as aberrantly upregulated in CRC, and the
inhibition of miR-574-5p suppressed the growth of tumors in murine
models (8). Furthermore, miR-574-5p
was identified as one of the most commonly upregulated miRNAs in
lung cancer, and the repression of miR-574-5p significantly
abrogated the tumor progression induced by Toll-like receptor 9
signaling (26). The present study
provided evidence to support the oncogenic potential of miR-574-5p
and the association between miR-574-5p and thyroid cancer.
QKI is associated with the determination of cell
fate, including apoptosis, cell cycle progression and
differentiation (13,27,28). The
suppression of QKI, as may be induced by DNA methylation in the QKI
promoter region (29), has been
implicated in numerous cancer types (8,30,31). In addition, miR-574-5p has been
indicated to affect the regulation of QKI. For example, Yang et
al (14) demonstrated that
miR-574-5p negatively regulated QKI6/7 in CRC. In the present
study, qPCR and western blotting assays showed that pan-QKI and
QKI5/6/7 were significantly repressed by miR-574-5p in thyroid
cancer. Furthermore, since QKI is a negative regulator of the
Wnt/β-catenin pathway, the influence of miR-574-5p on the
Wnt/β-catenin pathway was also investigated. The data indicated
that miR-574 activated the expression of β-catenin through
repressing QKIs. As a result, the knockdown of miR-574-5p led to
the downregulation of total β-catenin and the upregulation of
phosphorylated β-catenin. The expression of cyclin D1 and survivin,
two important targets of the Wnt/β-catenin pathway (32,33), was
also repressed by the knockdown of miR-574-5p. As a consequence,
the repression of miR-574-5p led to the cell cycle arrest and
apoptosis of the thyroid cancer cells. In summary, the present
findings support the hypothesis that the oncogenic function of
miR-574-5p is achieved through an effect on QKIs.
The primary contribution of the present study was to
demonstrate the association between miR-574-5p and the oncogenic
behavior of thyroid cancer cells. It was identified that the
aberrantly high expression of miR-574-5p in thyroid cancer cells
promoted cell proliferation and the cell cycle progression of
thyroid cancer cells. Furthermore, the function of miR-574-5p in
the cell cycle and apoptosis of thyroid cancer cells may be
mediated via Wnt/β-catenin signaling by the repression of QKIs.
Thus, from a clinical perspective, these findings suggest that
miR-574-5p may be a candidate therapeutic target for the treatment
and prevention of thyroid cancer.
Acknowledgements
Not applicable.
Funding
This study was supported by National Natural Science
Foundation of China (Grant number, 81372860).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Study concepts and design, ZJZ and ZMW; Literature
research, XYL; Experimental studies and data analysis, ZJZ and QX;
Manuscript editing and review, ZJZ, XYL, QX and ZMW.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nikiforov YE: Thyroid carcinoma: Molecular
pathways and therapeutic targets. Mod Pathol. 21 Suppl 2:S37–S43.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Ward E, Murray T, Xu J
and Thun MJ: Cancer statistics, 2007. CA Cancer J Clin. 57:43–66.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pacini F, Schlumberger M, Dralle H, Elisei
R, Smit JW and Wiersinga W; European Thyroid Cancer Taskforce, :
European consensus for the management of patients with
differentiated thyroid carcinoma of the follicular epithelium. Eur
J Endocrinol. 154:787–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zheng T, Holford TR, Chen Y, Ma JZ,
Flannery J, Liu W, Russi M and Boyle P: Time trend and
age-period-cohort effect on incidence of thyroid cancer in
Connecticut, 1935–1992. Int J Cancer. 67:504–509. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu S, Semenciw R, Ugnat AM and Mao Y:
Increasing thyroid cancer incidence in Canada, 1970–1996: Time
trends and age-period-cohort effects. Br J Cancer. 85:1335–1339.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xing M: Molecular pathogenesis and
mechanisms of thyroid cancer. Nat Rev Cancer. 13:184–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Begum S, Rosenbaum E, Henrique R, Cohen Y,
Sidransky D and Westra WH: BRAF mutations in anaplastic thyroid
carcinoma: Implications for tumor origin, diagnosis and treatment.
Mod Pathol. 17:1359–1363. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ji S, Ye G, Zhang J, Wang L, Wang T, Wang
Z, Zhang T, Wang G, Guo Z, Luo Y, et al: miR-574-5p negatively
regulates Qki6/7 to impact beta-catenin/Wnt signalling and the
development of colorectal cancer. Gut. 62:716–726. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo T, Furuta T, Mitsunaga K, Ebersole
TA, Shichiri M, Wu J, Artzt K, Yamamura K and Abe K: Genomic
organization and expression analysis of the mouse qkI locus. Mamm
Genome. 10:662–669. 1999.PubMed/NCBI
|
|
10
|
Pilotte J, Larocque D and Richard S:
Nuclear translocation controlled by alternatively spliced isoforms
inactivates the QUAKING apoptotic inducer. Genes Dev. 15:845–858.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Biedermann B, Hotz HR and Ciosk R: The
Quaking family of RNA-binding proteins: Coordinators of the cell
cycle and differentiation. Cell Cycle. 9:1929–1933. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Larocque D, Galarneau A, Liu HN, Scott M,
Almazan G and Richard S: Protection of p27(Kip1) mRNA by quaking
RNA binding proteins promotes oligodendrocyte differentiation. Nat
Neurosci. 8:27–33. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chénard CA and Richard S: New implications
for the QUAKING RNA binding protein in human disease. J Neurosci
Res. 86:233–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang G, Fu H, Zhang J, Lu X, Yu F, Jin L,
Bai L, Huang B, Shen L, Feng Y, et al: RNA-binding protein quaking,
a critical regulator of colon epithelial differentiation and a
suppressor of colon cancer. Gastroenterology. 138:231–240.e1-5.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fan M, Li X, Jiang W, Huang Y, Li J and
Wang Z: A long non-coding RNA, PTCSC3, as a tumor suppressor and a
target of miRNAs in thyroid cancer cells. Exp Ther Med.
5:1143–1146. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Melo SA and Esteller M: Dysregulation of
microRNAs in cancer: Playing with fire. FEBS Lett. 585:2087–2099.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Barker N and Clevers H: Mining the Wnt
pathway for cancer therapeutics. Nat Rev Drug Discov. 5:997–1014.
2006. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Futaki S, Suzuki T, Ohashi W, Yagami T,
Tanaka S, Ueda K and Sugiura Y: Arginine-rich peptides. An abundant
source of membrane-permeable peptides having potential as carriers
for intracellular protein delivery. J Biol Chem. 276:5836–5840.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Raymond CK, Roberts BS, Garrett-Engele P,
Lim LP and Johnson JM: Simple, quantitative primer-extension PCR
assay for direct monitoring of microRNAs and short-interfering
RNAs. RNA. 11:1737–1744. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Galarneau A and Richard S: Target RNA
motif and target mRNAs of the Quaking STAR protein. Nat Struct Mol
Biol. 12:691–698. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shenouda SK and Alahari SK: MicroRNA
function in cancer: Oncogene or a tumor suppressor? Cancer
Metastasis Rev. 28:369–378. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li Q, Li X, Guo Z, Xu F, Xia J, Liu Z and
Ren T: MicroRNA-574-5p was pivotal for TLR9 signaling enhanced
tumor progression via down-regulating checkpoint suppressor 1 in
human lung cancer. PLoS One. 7:e482782012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Noveroske JK, Lai L, Gaussin V, Northrop
JL, Nakamura H, Hirschi KK and Justice MJ: Quaking is essential for
blood vessel development. Genesis. 32:218–230. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Novikov L, Park JW, Chen H, Klerman H,
Jalloh AS and Gamble MJ: QKI-mediated alternative splicing of the
histone variant MacroH2A1 regulates cancer cell proliferation. Mol
Cell Biol. 31:4244–4255. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bian Y, Wang L, Lu H, Yang G, Zhang Z, Fu
H, Lu X, Wei M, Sun J, Zhao Q, et al: Downregulation of tumor
suppressor QKI in gastric cancer and its implication in cancer
prognosis. Biochem Biophys Res Commun. 422:187–193. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zong FY, Fu X, Wei WJ, Luo YG, Heiner M,
Cao LJ, Fang Z, Fang R, Lu D, Ji H and Hui J: The RNA-binding
protein QKI suppresses cancer-associated aberrant splicing. PLoS
Genet. 10:e10042892014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He Z, Yi J, Liu X, Chen J, Han S, Jin L,
Chen L and Song H: MiR-143-3p functions as a tumor suppressor by
regulating cell proliferation, invasion and epithelial-mesenchymal
transition by targeting QKI-5 in esophageal squamous cell
carcinoma. Mol Cancer. 15:512016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Miyoshi K, Rosner A, Nozawa M, Byrd C,
Morgan F, Landesman-Bollag E, Xu X, Seldin DC, Schmidt EV, Taketo
MM, et al: Activation of different Wnt/beta-catenin signaling
components in mammary epithelium induces transdifferentiation and
the formation of pilar tumors. Oncogene. 21:5548–5556. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wieczorek M, Paczkowska A, Guzenda P,
Majorek M, Bednarek AK and Lamparska-Przybysz M: Silencing of Wnt-1
by siRNA induces apoptosis of MCF-7 human breast cancer cells.
Cancer Biol Ther. 7:268–274. 2008. View Article : Google Scholar : PubMed/NCBI
|