Introduction
Colorectal cancer (CRC) is one of the most common
types of gastrointestinal cancer. Almost two million new cases of
CRC are diagnosed every year, making CRC the third most common
cancer and the fourth most common cancer-associated cause of
mortality in the world (1–3). The risk of CRC increases in certain
populations and is associated with risk factors including:
Hereditary nonpolyposis CRC; inflammatory bowel disease; a family
history of CRC; being of African American descent (4–6). Patients
with CRC are typically asymptomatic and therefore it is difficult
to the disease diagnose until advanced stages, where the disease
becomes incurable. Early diagnosis and therapy is able to decrease
the risk of CRC in this asymptomatic population; however, early
diagnosis of CRC remains a challenge in clinical practice. Hence,
identification of novel non-invasive diagnostic methods for early
tumor detection in CRC is required (7,8).
The tumor protein (TP)53 gene is the most widely
used tumor biomarker in detecting a potential tumor as p53
mutations are the most commonly observed mutations in different
types of cancer. Furthermore, the majority of anti-p53
autoantibodies are produced in response to p53 mutation (9). In healthy cells a p53 nuclear
phosphoprotein is expressed as a protein product of the p53 gene.
In contrast with p53 protein, anti-p53 antibodies are rarely
detected in the serum of healthy controls (10). Mutated p53 leads to the accumulation
of nonfunctional protein due to the increased stability and
increased half-life (several h) compared with wild type p53 (20
min) (10). The accumulated protein
acts as an antigen, leading to the subsequent development of
anti-p53 antibodies, which are detectable within tissues and blood
(10,11). Due to the expanding field of molecular
biotechnology, numerous studies on the potential diagnostic value
of s-p53 antibody in CRC have been conducted (11,12). The
primary aim of the present meta-analysis was to determine whether
the s-p53 antibody may be a potential biomarker for the diagnosis
of CRC, and confirmed the accuracy of s-p53 antibody for CRC
diagnosis.
Materials and methods
Systematic review strategies
The present study systematically searched the
following databases; Cochrane Library (http://www.cochranelibrary.com/), PubMed (https://www.ncbi.nlm.nih.gov/pubmed) and EmBase
(https://www.elsevier.com/solutions/embase-biomedical-research)
accessed on and prior to 31 July 2016. Articles investigating s-p53
antibodies being used for the detection of CRC were taken into
account in the present study. The following combined search terms
were used: ‘Colorectal cancer’, ‘colorectal carcinoma’, ‘blood OR
serum’, ‘seropositive OR serum antibody’ and ‘p53 OR tp53’. PubMed
was used to identify associated articles, which were searched
manually for associated functions and references (11). The study was conducted according to
the meta-analysis of observational studies in epidemiology (MOOSE)
guidelines (12,13) for systemic reviews and meta-analysis
was conducted using PRISMA.
Inclusion criteria
All articles were evaluated carefully and eligible
articles were included if they met the following criteria: i)
Studies investigating the diagnostic value of serum (s)-p53
antibody in CRC; ii) articles that diagnosed CRC using the
established gold standard (pathological examination of biopsies)
and also tested patient serum for the detection of anti-p53 prior
to any treatment and used controls without any other cancer; iii)
the results of the included articles on diagnostic accuracy were
able to be summarized in a 2×2 table.
Exclusion criteria
The exclusion criteria were as follows: i) If the
same author reported results of patients in several publications,
only the most relevant one was selected; ii) if available data for
analysis was not complete, the study was excluded; iii) all
systematic reviews, meta-analyses, case reports and editorials were
excluded.
Data extraction, data synthesis and
quality assessment
All articles that were included in the present study
were assessed by two different authors, any disagreements between
the two authors were resolved by discussion. All the included
studies were assessed using the quality assessment for studies of
diagnostic accuracy (QUADAS) guidelines (14). The Cochrane Collaboration Methods
group on screening and diagnostic tests recommended the 11 items of
QUADAS. A QUADAS score of ≥7 was considered as good quality and
score of <7 was considered as suboptimal quality. The following
data were extracted from the included studies: First author's name,
year of publication, country of publication, golden criteria of
diagnosis, threshold value(s), number of TP (true positive), FP
(false positive), FN (false negative), TN (true negative)
diagnoses, diagnosis time (the period of time between admission to
hospital and diagnosis), stage of tumor (TNM; Japanese
classification of colorectal carcinoma, 8th Edition) (15) and research methods. To avoid
transcriptional errors all data were reviewed twice.
Statistical analysis
Meta DiSc statistical software (version 1.4;
Universidad Complutense, Madrid, Spain) and STATA (version 14.0,
Stata Corporation, College Station, TX, USA) were used to analyze
the extracted data. The present study adopted standard methods
previously recommended for the meta-analysis of diagnostic test
evaluations (16). The sensitivity,
specificity, positive and negative likelihood ratio (PLR and NLR),
and 95% confidence intervals (CIs) were calculated with a random
effects model according to the Mantel-Haensed method, as previously
described (17). The diagnostic odds
ratio (DOR) was determined using Moses' constant of linear method
and was used to measure the accuracy, which indicates the change in
diagnostic performance of the test under study per unit increase in
the covariant (18). Summary receiver
operating characteristic curves (SROC) were used to summarize
overall test performance, and the area under the curve (AUC) was
calculated. Additionally, the issue associated of sensitivities and
specificities of 100% were solved by the default method of adding
0.5 to all cells within the diagnostic 2×2 table (16,19).
The χ2 test was applied to detect
heterogeneity in the included studies. Inter-study heterogeneity
was assessed using the I2 test according to the
following formula: I2=100% × (Cochran Q-degrees of
freedom)/Cochran Q (20). Meta
regression was conducted to detect the heterogeneity between
studies. In order to detect cut-off threshold effects, Spearman's
correlation coefficient was used to evaluate the association
between sensitivity and specificity. A scatter plot of the inverse
square root of the effective sample size (1/ESS1/2) vs. the
diagnostic log odds ratio (lnDOR) was used to visually assess the
publication bias is, and should demonstrate a symmetrical funnel
shape (21).
Results
Literature search
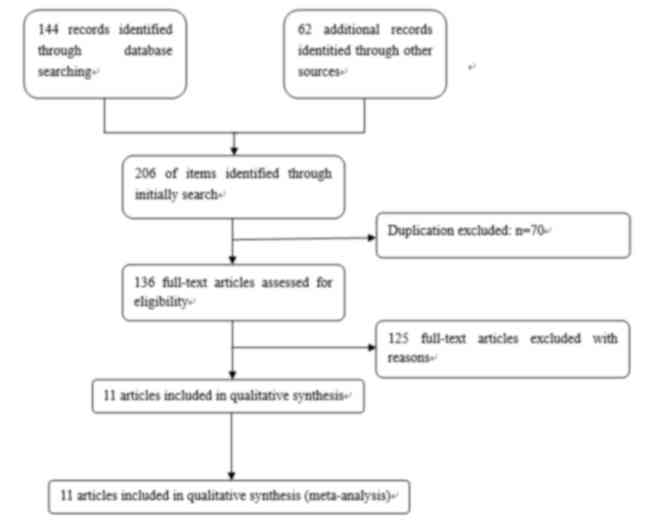
A total of 206 abstracts and titles were identified
following all search strategies as described in Fig. 1. A total of 136 records were retained
in the present study following rejection of replication errors in
the included records. In total, 94 articles were excluded, as they
did not meet the aforementioned inclusion criteria on the basis of
the titles and abstracts. Of the remaining 42 studies, 18
additional articles were excluded due to the lack of a study
control, 13 studies were excluded from the meta-analysis due to the
lack of s-p53 detection in serum. The final 11 articles included in
the present study met all inclusion criteria and were of
good quality (Tables I and II).
 | Table I.Main characteristics and results of
the 11 eligible studies. |
Table I.
Main characteristics and results of
the 11 eligible studies.
| Author/year | Country | Ref. standard | Assay method | Cut-off | TP | FP | FN | TN |
|---|
| Shimada et
al, 2003 | Japan | Unknown | ELISA | 1.3 U/ml | 46 | 23 | 146 | 371 |
| Yoshizawa et
al, 2007 | Japan | Histology | ELISA | mean+2SD | 43 | 44 | 39 | 305 |
| Tagi et al,
2010 | Japan | Histology | ELISA | Unknown | 30 | 2 | 100 | 23 |
| Hammel et
al, 1997 | France | Histology | ELISA | Unknown | 14 | 0 | 40 | 24 |
| Kojima et
al, 2009 | Japan | Histology | ELISA | 6 u/ml | 9 | 0 | 36 | 22 |
| Takeda et
al, 2001 | Japan | Histology | ELISA | Index≥1.1 | 17 | 1 | 10 | 37 |
| Lechpammer et
al, 2003 | America | Histology | ELISA | Index≥0 Abs | 40 | 0 | 180 | 42 |
| Broll et al,
2001 | Germany | Histology | ELISA | Index≥0 Abs | 20 | 0 | 110 | 44 |
| Shiota et
al, 2000 | Japan | Histology | ELISA | Unknown | 18 | 1 | 53 | 17 |
| Wu et al,
2011 | China | Histology | ELISA | index>1.7 | 24 | 20 | 52 | 180 |
| Tang et al,
2001 | China | Histology | ELISA | 10 u/Ul | 130 | 2 | 868 | 209 |
 | Table II.Main characteristics of the 11
eligible studies. |
Table II.
Main characteristics of the 11
eligible studies.
| Author, year | Time of specimen
collection | Stage I, %
(proportion) | QUADAS |
Consecutive/random | (Refs.) |
|---|
| Shimada et
al, 2003 | Unknown | Unknown | 6 | Unknown | (22) |
| Yoshizawa et
al, 2007 | Unknown | Unknown | 7 | Consecutive | (23) |
| Tagi et al,
2010 | Unknown | 15% (19/130) | 8 | Unknown | (24) |
| Hammel et
al, 1997 | Prior to
treatment | 19% (10/54) | 6 | Unknown | (30) |
| Kojima et
al, 2009 | Unknown | 36% (16/45) | 7 | Unknown | (26) |
| Takeda et
al, 2001 | Prior to
treatment | Unknown | 8 | Consecutive | (27) |
| Lechpammer et
al, 2003 | Prior to
treatment | 12.7% (28/220) | 9 | Consecutive | (28) |
| Broll et al,
2001 | Prior to
treatment | 32% (41/130) | 8 | Unknown | (29) |
| Shiota et
al, 2000 | Prior to
treatment | Unknown | 8 | Consecutive | (25) |
| Wu et al,
2011 | Prior to
treatment | (2/67) | 8 | Consecutive | (31) |
| Tang et al,
2001 | Before
treatment | 25% (252/998) | 7 | Consecutive | (32) |
Characteristics of included
research
A total of 11 clinical trials presenting the
diagnostic value of s-p53 antibody for CRC diagnosis were
investigated in the present study, demonstrating different
characteristics. For example, studies included in the meta-analyses
were conducted in different countries; 6 studies were conducted in
Japan (22–27), 1 was conducted in the USA (28), 1 was conducted in Germany (29), 1 was conducted in France (30) and 2 were conducted in China (31,32). The
year of publication ranged between 1997 and 2011. All 11 studies
were retrospective; however, 4 studies did not provide TNM stage
data (22,23,25,27). In
total, 10 studies included health volunteers as a control and 1
used patients with benign disease as the control (32).
Threshold effect
Computation of the Spearman correction coefficient
for the logit of sensitivity and logit of 1-specificity of s-p53
antibody calculated by metadisc was 0.518 (P=0.102) (data not
shown), indicating that there was no threshold effect and positive
correlations were not statistically significant.
Outcome of meta-analysis
The random-effects model was applied to pooled data
as there were distinct definitions of outcome and differing patient
baseline characteristics between trials. Results presented in
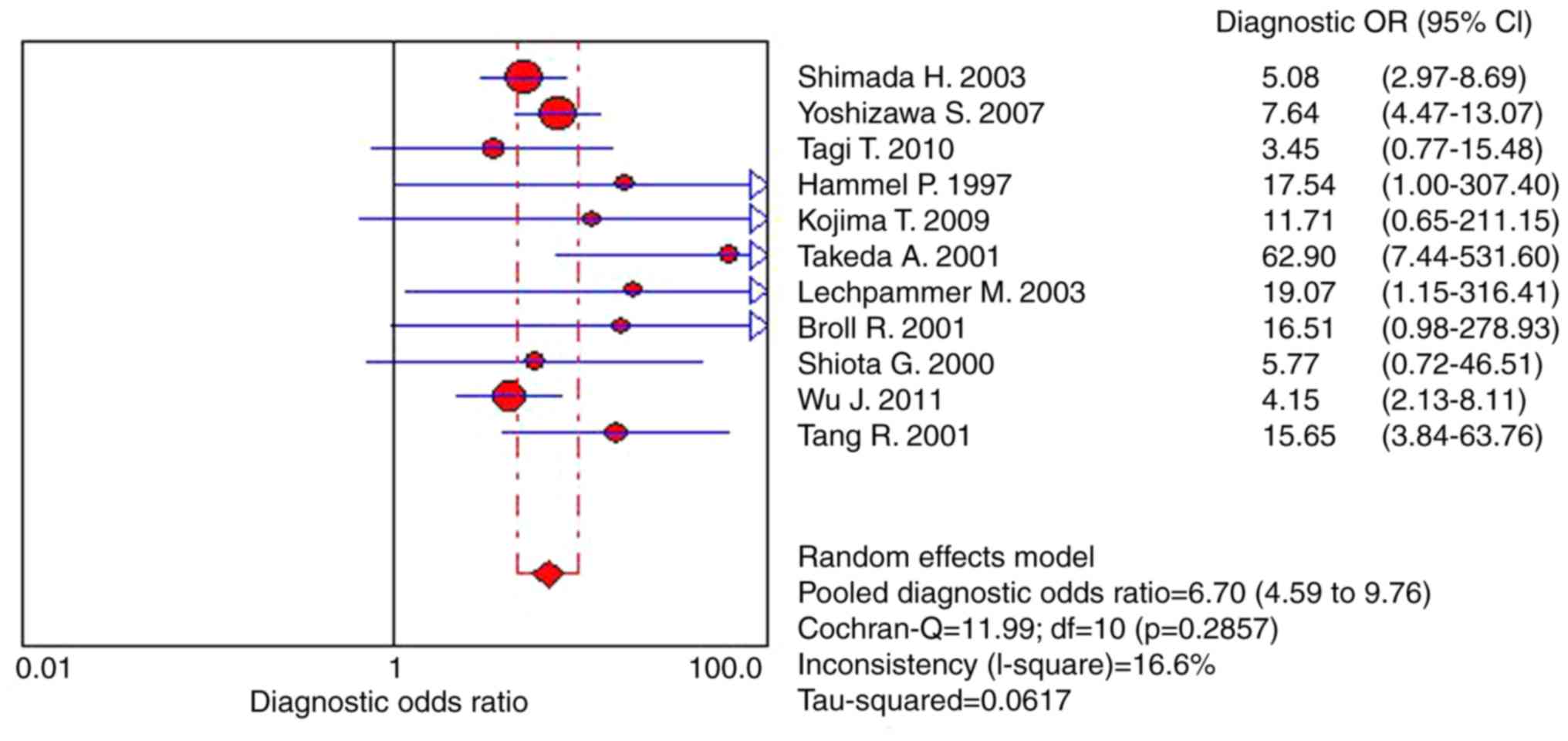
Fig. 2 demonstrate that the pooled
DOR was 6.70 (95% CI, 4.59–9.76), heterogeneity χ2
=11.99 (P=0.286) and I2=16.60%. In the present study,
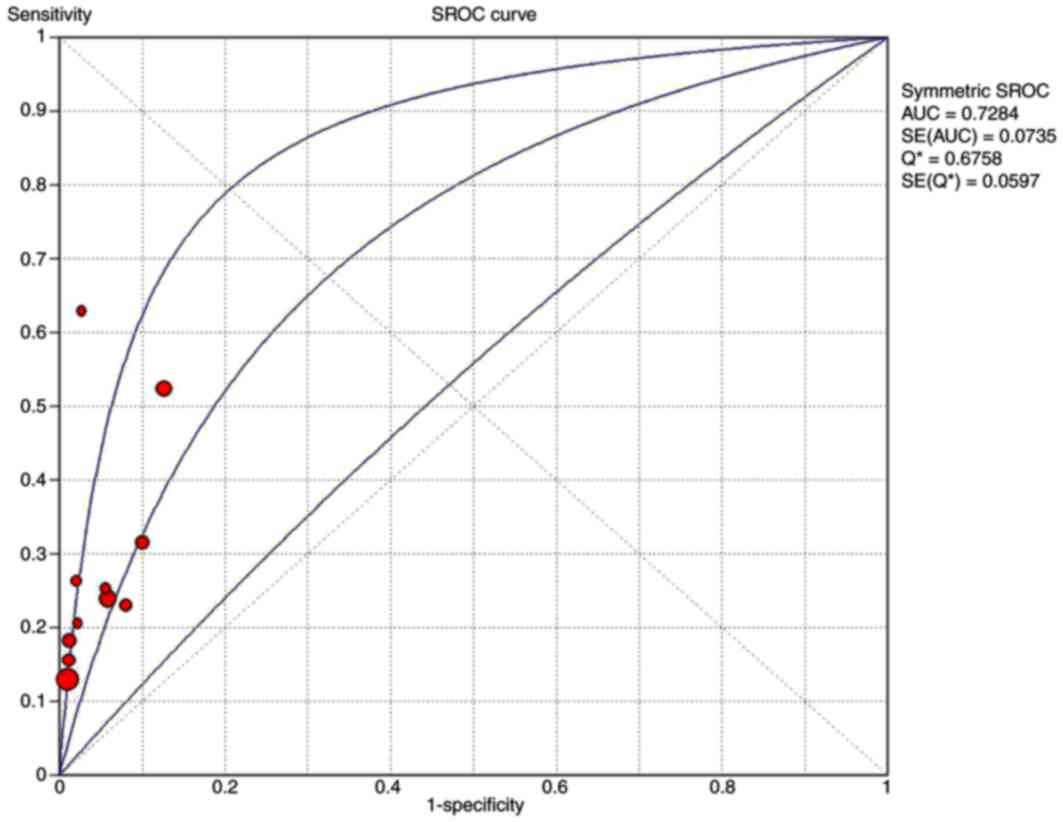
the symmetrical SROC of P-53 was 0.73 (Fig. 3). Thus, according to results of the
present study, s-p53 antibody exhibited reasonable accuracy in
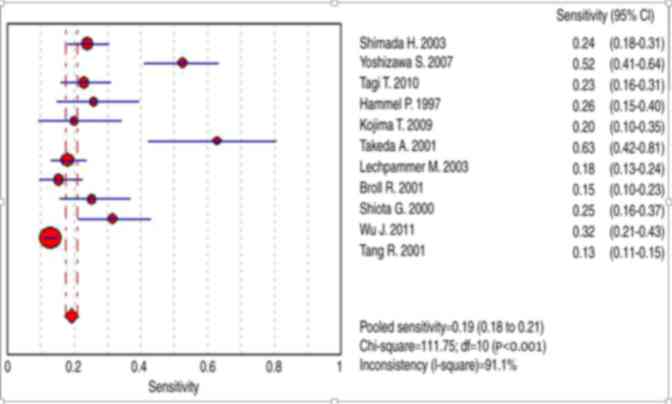
terms of differential diagnosis in cases of CRC. The range of the
sensitivity was between 13 and 63%, pooled sensitivity was 0.19
(95% CI, 0.18–0.21; Fig. 5),
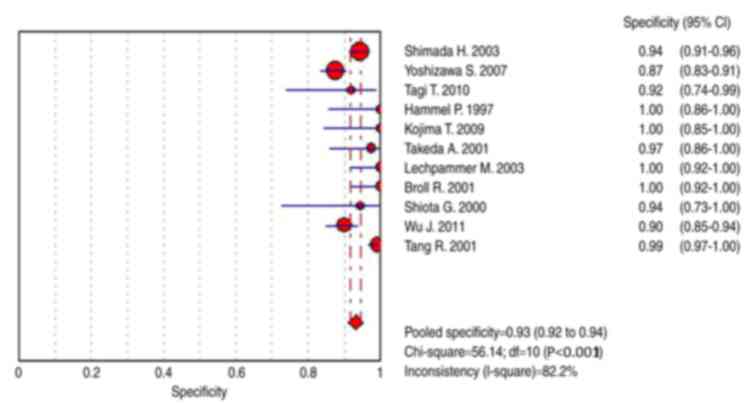
specificity was between 87 and 100% and pooled specificity was 0.93
(95% CI, 0.92–0.94) (Figs. 4 and
5). In the present study, results
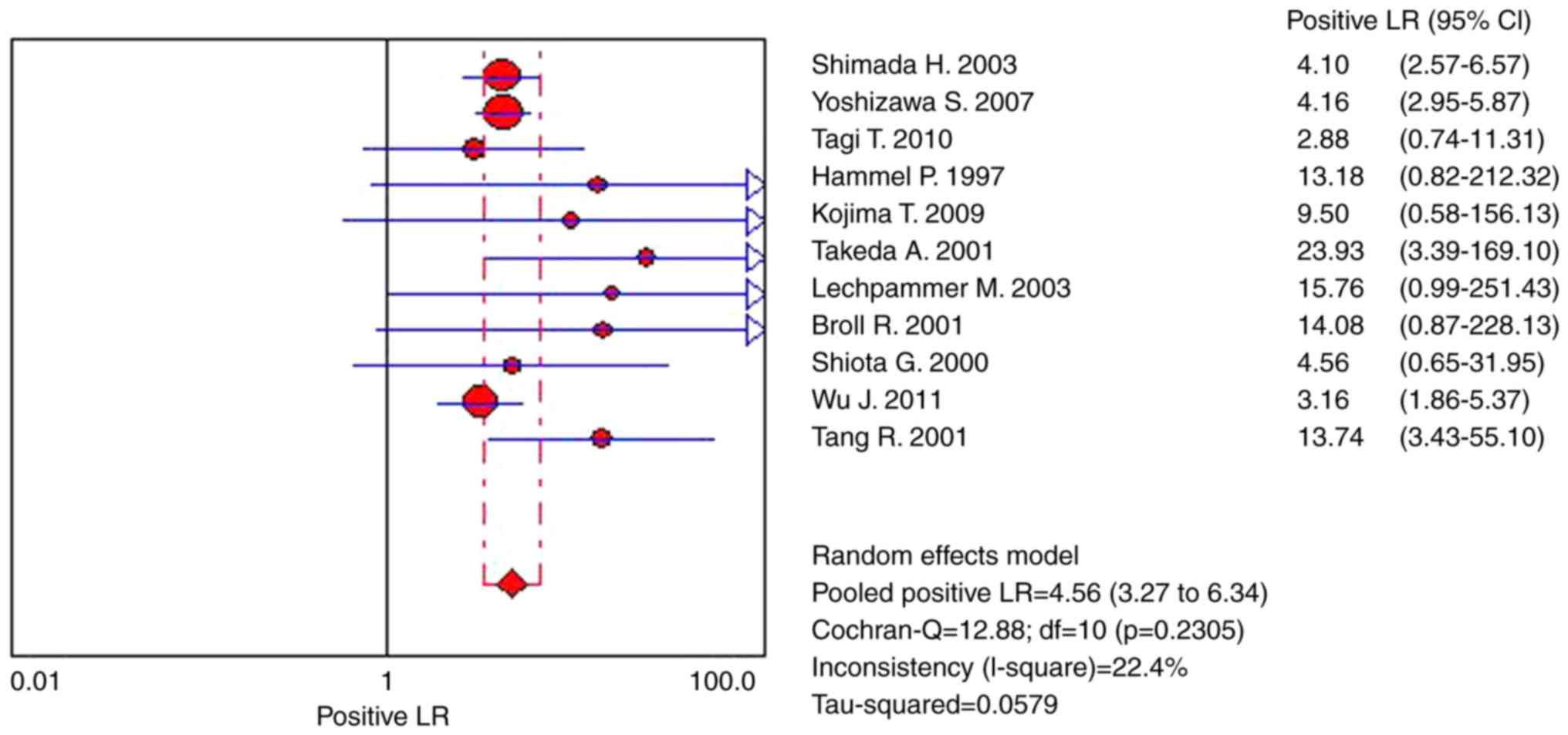
demonstrated a pooled PLR of 4.56 (95% CI, 3.27–6.34), suggesting
that patients with CRC exhibited 4.56-fold increased chance of
testing positive for s-p53 antibody compared with patients without
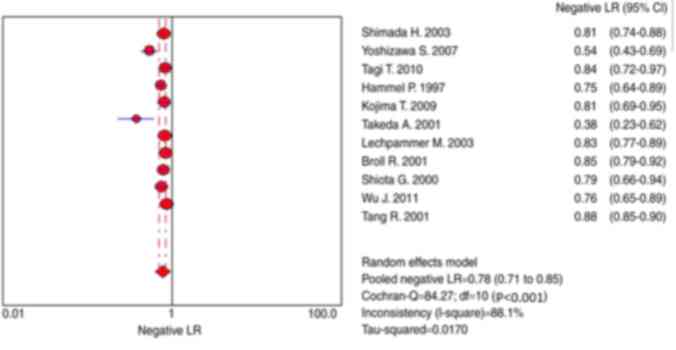
CRC (Fig. 6). Furthermore, the NLR
was 0.78 (95% CI, 0.71–0.85; Fig. 7).
Significant heterogeneity was identified for all eligible studies.
Heterogeneity χ2 =84.27 (P<0.001) and
I2=88.10%.
Possible sources of heterogeneity
The meta-regression was adopted to explore the
possible sources of heterogeneity, which included: Variation in the
quality of methodology (QUADAS), study design, sample size, assay
method, staging system (stage I %) (TNM) and the time taken to
collect and fix the sample. Meta-regression indicated that study
design (pre- or post-treatment) [relative diagnostic odds ratio,
1.68; 95% (0.65–4.36)] was the probable source of
heterogeneity.
Publication bias evaluation and
sensitivity analysis
Despite the studies included in the meta-analysis
exhibiting some heterogeneity, the results of the present study
demonstrated that no publication bias was detected by using Egger's
test (P=0.72). Furthermore, the funnel plots (Fig. 6) used to detect publication bias also
demonstrated no asymmetry upon visual inspection. Sensitivity
analysis was conducted in terms of statistical analysis methods,
study design and sample size. The results of sensitivity analysis
produced no obvious changes. When the studies without matched cases
and control sample size were excluded, results were not
affected.
Discussion
The p53 gene comprises ~20,000 base pairs spread
over 11 exons located on 17 p13 (33–36).
Discovered in 1979, it serves a critical function as a
tumor-suppressor gene (37,38). As previously established, the s-p53
antibody is not a specific biomarker for CRC (34,35).
However, positive associations have been reported between p53
immunoreactivity and the presence of s-p53 antibodies in patients
with other types of cancer, including gastric carcinoma (39), esophageal carcinoma (40) and ovarian carcinoma (41). Previous studies on the molecular
biology of malignant tumors have emphasized the importance of a
number of proto-oncogenes and tumor suppressor genes in human
malignancy. Thus, the identification of biomarkers that are capable
of providing a definitive diagnosis in various types of malignancy
is of high importance in order to provide improved management for
patients (11).
CRC is one of the most commonly diagnosed cancers
globally (17,33). However, early detection of CRC remains
challenging in clinical practice. To the best of our knowledge,
there is currently no diagnostic biomarker for CRC. Recently, the
s-p53 antibody has been widely utilized in clinical practice as a
tumor biomarker for CRC; however, results vary between studies and
no large-scale studies have been conducted which investigate the
use of S-p53Ab as a diagnostic tool for patients with CRC (4,20). The
meta-analysis conducted in the present study was used to summarize
the potential diagnostic value of s-p53 antibody for the early
detection of CRC. The conclusions drawn from the meta-analysis are
as follows: i) The pooled sensitivity was 0.19 (95% CI, 0.18–0.21)
and the pooled specificity was 0.93 (95% CI, 0.92–0.94); ii)
patients with CRC have an increased chance of exhibiting a positive
s-p53 test compared with patients without CRC; iii) the odd ratio
for positive test for CRC was increased 5-fold compared with the
odds ratio for positive test in non CRC. In brief, s-p53-antibody
may be useful for the detection and diagnosis of CRC; however, it
is important to recognize that s-p53-antibody exhibits low
sensitivity levels.
The results of the present study reveal that s-p53
may serve a significant function in screening for cancer, offering
a convenient, noninvasive, low costs biomarker with the assumption
that future research focuses on the following: i) Improve the
sensitivity and specificity by combining additional serum tumor
biomarkers; ii) use sputum, serum or other samples that are easy to
acquire to improve sensitivity; iii) standardize the detection
method and threshold values (lower threshold value leads to
increased sensitivity and decreased specificity); iv) conduct
normative diagnostic tests or collect samples from cases prior to
biopsy in order to improve sensitivity. Collectively, these
conditions may decrease the heterogeneity among the included
studies, enabling future studies to conduct an accurate
meta-analysis to identify the diagnostic value of the s-p53
antibody.
It is important to appreciate the limitations of the
present study including: i) Lack of calculation for diagnostic
accuracy for early stage (stage I–II) CRC due to lack of access to
raw data; additionally, only five studies with small patient
cohorts described the different stages of CRC; ii) all 11 included
studies lacked the appropriate matching of age, location, and
methods of obtaining and handling of the samples between case and
control. Furthermore, large-scale studies are required to examine
the association between s-p53 antibodies, disease staging and
prognosis for patients with CRC. Collectively, this will assist in
improving the treatment options for patients with CRC.
In conclusion, according to the results of the
present study, s-p53 antibody demonstrated a potential diagnostic
value with low sensitivity. Furthermore, patients with CRC have an
increased chance of exhibiting a positive result for the p-53
antibody test compared with patients without CRC. Due to its high
specificity, s-p53 antibody may be useful for monitoring residual
tumor cells and aiding in the diagnosis of patients who have CRC.
Larger scale research is required to identify the patterns of
multiple biomarkers to further increase the power of CRC
detection.
Acknowledgements
The authors wish to thank Wu Liang (Department of
Oncology, Chengdu 363 Hospital) for analyzing the data.
References
|
1
|
International Agency for Research on
Cancer (IARC), . World Cancer Report 2014. Stewart B and Wild CP:
IARC; Lyon: 2014, http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014
|
|
2
|
Brody H: Colorectal cancer. Nature.
521:S12015. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mármol I, Sánchez-de-Diego C, Pradilla
Dieste A, Cerrada E and Rodriguez Yoldi MJ: Colorectal carcinoma: A
general overview and future perspectives in colorectal cancer. Int
J Mol Sci. 18(pii): E1972017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Komaki Y, Komaki F, Micic D, Ido A and
Sakuraba A: Risk of colorectal cancer in chronic liver diseases; a
systematic review and meta-analysis. Gastrointest Endosc.
86:S93–S104.e5. 2017. View Article : Google Scholar
|
|
5
|
El-Shami K, Oeffinger KC, Erb NL, Willis
A, Bretsch JK, Pratt-Chapman ML, Cannady RS, Wong SL, Rose J,
Barbour AL, et al: American cancer society colorectal cancer
survivorship care guidelines. CA Cancer J Clin. 65:428–455. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giardiello FM, Allen JI, Axilbund JE,
Boland CR, Burke CA, Burt RW, Church JM, Dominitz JA, Johnson DA,
Kaltenbach T, et al: Guidelines on genetic evaluation and
management of Lynch syndrome: A consensus statement by the US
multi-society task force on colorectal cancer. Gastroenterol.
147:502–526. 2014. View Article : Google Scholar
|
|
7
|
Corley DA, Levin TR and Doubeni CA:
Adenoma detection rate and risk of colorectal cancer and death. N
Engl J Med. 370:25412014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Loberg M, Kalager M, Holme Ø, Hoff G,
Adami HO and Bretthauer M: Long-term colorectal-cancer mortality
after adenoma removal. N Engl J Med. 371:799–807. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Levine AJ, Momand J and Finlay CA: The p53
tumour suppressor gene. Nature. 351:453–456. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu M, Mao C, Chen Q, Cu XW and Zhang WS:
Serum p53 protein and anti-p53 antibodies are associated with
increased cancer risk: A case-control study of 569 patients and 879
healthy controls. Mol Biol Rep. 37:339–343. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang J, Xv Z, Wu X and Li K: Potential
diagnostic value of serum p53 antibody for detecting esophageal
cancer: A meta-analysis. PLoS One. 7:e528962012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stroup DF, Berlin JA, Morton SC, Olkin I,
Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA and Thacker
SB: Meta-analysis of observational studies in epidemiology: A
proposal for reporting. Meta-analysis of observational studies in
epidemiology (MOOSE) group. JAMA. 283:2008–2012. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Moher D, Liberati A, Tetzlaff J and Altman
DG; PRISMA Group, : Preferred reporting items for systematic
reviews and metaanalyses: The PRISMA statement. Int J Surg.
8:336–341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Smidt N, Deeks J and Moore T: Guide to the
contents of a Cochrane review and protocol. Cochrane handbook for
systematic reviews of diagnostic test accuracy. 2011.
|
|
15
|
Japanese Society for Cancer of the Colon
and Rectum Japanese classification of colorectal carcinoma. The.
8th. Kanehara & CO., LTD; Tokyo: 2013
|
|
16
|
Devillé WL, Buntinx F, Bouter LM, Montori
VM, de Vet HC, van der Windt DA and Bezemer PD: Conducting
systematic reviews of diagnostic studies: Didactic guidelines. BMC
Med Res Methodol. 2:92002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu P, Huang G, Chen Y, Zhu C, Yuan J and
Sheng S: Diagnostic utility of pleural fluid carcinoembryonic
antigen and CYFRA 21-1 in patients with pleural effusion: A
systematic review and meta-analysis. J Clin Lab Anal. 21:398–405.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang J, Zhu Z, Liu Y, Jin X, Xu Z, Yu Q
and Li K: Diagnostic value of multiple tumor markers for patients
with esophageal carcinoma. PLoS One. 10:e01169512015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dinnes J, Deeks J, Kirby J and Roderick P:
A methodological review of how heterogeneity has been examined in
systematic reviews of diagnostic test accuracy. Health Technol
Assess. 9:1–133, iii. 2005. View
Article : Google Scholar
|
|
21
|
Deeks JJ, Macaskill P and Irwig L: The
performance of tests of publication bias and other sample size
effects in systematic reviews of diagnostic test accuracy was
assessed. J Clin Epidemiol. 58:882–893. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shimada H, Ochiai T and Nomura F:
Titration of serum p53 antibodies in 1,085 patients with various
types of malignant tumors: A multiinstitutional analysis by the
Japan p53 Antibody Research Group. Cancer. 97:682–689. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yoshizawa S, Matsuoka K, Inoue N, Takaishi
H, Ogata H, Iwao Y, Mukai M, Fujita T, Kawakami Y and Hibi T:
Clinical significance of serum p53 antibodies in patients with
ulcerative colitis and its carcinogenesis. Inflamm Bowel Dis.
13:865–873. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tagi T, Matsui T, Kikuchi S, Hoshi S,
Ochiai T, Kokuba Y, Kinoshita-Ida Y, Kisumi-Hayashi F, Morimoto K,
Imai T, et al: Dermokine as a novel biomarker for early-stage
colorectal cancer. J Gastroenterol. 45:1201–1211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiota G, Ishida M, Noguchi N, Oyama K,
Takano Y, Okubo M, Katayama S, Tomie Y, Harada K, Hori K, et al:
Circulating p53 antibody in patients with colorectal cancer:
Relation to clinicopathologic features and survival. Dig Dis Sci.
45:122–128. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kojima T, Yoshikawa K, Matsui T, Kodera Y
and Kojima H: Titration of serum CEA, p53 antibodies and CEA-IgM
complexes in patients with colorectal cancer. Mol Med Rep.
2:477–480. 2009.PubMed/NCBI
|
|
27
|
Takeda A, Shimada H, Nakajima K, Yoshimura
S, Suzuki T, Asano T, Ochiai T and Isono K: Serum p53 antibody as a
useful marker for monitoring of treatment of superficial colorectal
adenocarcinoma after endoscopic resection. Int J Clin Oncol.
6:45–49. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lechpammer M, Lukač J, Lechpammer S,
Kovacević D, Loda M and Kusić Z: Humoral immune response to p53
correlates with clinical course in colorectal cancer patients
during adjuvant chemotherapy. Int J Colorectal Dis. 19:114–120.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Broll R, Duchrow M, Oevermann E, Wellm C,
Schwandner O, Schimmelpenning H, Roblick UJ, Bruch HP and Windhövel
U: p53 autoantibodies in sera of patients with a colorectal cancer
and their association to p53 protein concentration and p53
immunohistochemistry in tumor tissue. Int J Colorectal Dis.
16:22–27. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hammel P, Boissier B, Chaumette MT,
Piedbois P, Rotman N, Kouyoumdjian JC, Lubin R, Delchier JC and
Soussi T: Detection and monitoring of serum p53 antibodies in
patients with colorectal cancer. Gut. 40:356–361. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu J, Qiu T, Pan P, Yu D, Ju Z, Qu X, Gao
X, Mao C and Wang L: Detection of serum anti-P53 antibodies from
patients with colorectal cancer in China using a combination of
P53-and phage-ELISA: Correlation to clinical parameters. Asian Pac
J Cancer Prev. 12:2921–2924. 2011.PubMed/NCBI
|
|
32
|
Tang R, Ko MC, Wang JY, Chen HH, Chen JS,
Hsu KC, Chiang JM and Hsieh LL: Humoral response to p53 in human
colorectal tumors: A prospective study of 1,209 patients. Int J
Cancer. 94:859–863. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
International Agency for Research on
Cancer, . World Health Organisation: IARC TP53 Database. Version
R18. IARC; Lyon: 2016, http://p53.iarc.fr/April. 2016
|
|
34
|
Petitjean A, Mathe E, Kato S, Ishioka C,
Tavtigian SV, Hainaut P and Olivier M: Impact of mutant p53
functional properties on TP53 mutation patterns and tumor
phenotype: Lessons from recent developments in the IARC TP53
database. Hum Mutat. 28:622–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suppiah A and Greenman J: Clinical utility
of anti-p53 auto-antibody: Systematic review and focus on
colorectal cancer. World J Gastroenterol. 19:4651–4670. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Soussi T, Dehouche K and Béroud C: p53
website and analysis of p53 gene mutations in human cancer: Forging
a link between epidemiology and carcinogenesis. Hum Mutat.
15:105–113. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Levine AJ: The common mechanisms of
transformation by the small DNA tumor viruses: The inactivation of
tumor suppressor gene products: p53. Virology. 384:285–293. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Staples OD, Steele RJ and Lain S: p53 as a
therapeutic target. Surgeon. 6:240–243. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maehara Y, Kakeji Y, Watanabe A, Baba H,
Kusumoto H, Kohnoe S and Sugimachi K: Clinical implications of
serum anti-p53 antibodies for patients with gastric carcinoma.
Cancer. 85:302–308. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ralhan R, Arora S, Chattopadhyay TK,
Shukla NK and Mathur M: Circulating p53 antibodies, p53 gene
mutational profile and product accumulation in esophageal
squamous-cell carcinoma in India. Int J Cancer. 85:791–795. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Angelopoulou K, Rosen B, Stratis M, Yu H,
Solomou M and Diamandis EP: Circulating antibodies against p53
protein in patients with ovarian carcinoma. Correlation with
clinicopathologic features and survival. Cancer. 78:2146–2152.
1996. View Article : Google Scholar : PubMed/NCBI
|