Introduction
Radiation therapy represents one of the basic
treatment modalities in the management of breast cancer. Adjuvant
radiotherapy is a standard procedure particularly in women
following breast conserving surgery (1,2).
External-beam radiation therapy with conventional
fractionation usually includes irradiation of the whole breast
followed by a boost dose to the tumor bed. One of the primary
challenges of this type of therapy is an accurate focusing on the
target volume due to breast mobility and limited options for
fixation of the organ. These difficulties are particularly
important when using advanced conformal techniques of radiotherapy,
primarily intensity modulated radiation therapy (IMRT), which is
beneficial for complicated clinical cases, including bilateral
breast carcinoma (3).
Utilization of the image guided radiation therapy
(IGRT) technique constitutes one of the ways to achieve higher
accuracy in target focusing. There are a number of IGRT methods.
Currently, the technique that is used most frequently is the
kilovoltage X-ray imaging of patients immediately before treatment.
The IGRT device is usually integrated into the treatment unit
(4).
For the breast area, it is possible to use the X-ray
contrast metallic markers that were implanted into the tumor bed
during surgery. Such markers may be employed in two ways for
accurate localization of the boost volume during treatment planning
and subsequently for daily monitoring of the patient position using
the selected method of IGRT. Special markers are necessary when the
megavoltage beam is used for the treatment setup, while surgical
clips are adequate in situations where lower beam intensities are
used (5). The implanted clips are
typically stable in the tumor bed throughout the course of
radiation therapy (6).
The benefit of IGRT is superior in its ability to
reduce the safety margin around the target volume, thus decreasing
the irradiated breast volume. It may result in lower risks of the
radiation fibrosis, and lower doses reaching the lungs and heart
(in left-sided tumors). Another advantage is more improved coverage
of the target volume with the planned dose, which increases the
probability of appropriate treatment efficiency (6).
The aim of the present study was to identify the
specific values of the safety margins for the irradiation of the
tumor bed (boost dose) with and without the clip-based IGRT
technique prior to treatment.
Patients and methods
Patients and treatment
The present study did not result in any
interventions related to participating patients. Application of the
IGRT technique with registration of the clips in tumor bed is a
component of standard treatment, more precisely the target volume
localization. The study design enabled a direct comparison of
localization with and without IGRT. There were no ethical issues
regarding the use of various treatment methods because all patients
were treated using IGRT and the setup errors were evaluated based
on standard imaging.
The study population included 184 consecutive
patients who were treated with radiation for breast cancer at our
institution between March 2013 and April 2014. The mean age was 62
years (range, 28–81 years). All patients underwent breast
conserving surgery, and had biopsy-verified invasive breast cancer
stage I or II, experienced surgical staging of the axillary region
(sentinel lymph nodes biopsy or axillary dissection), had metal
clips implanted into the tumor bed and were referred to undergo
postoperative whole breast radiation with boost dose to the tumor
bed. Two women had postoperative radiation of both breasts
simultaneously. Written informed consent was obtained from all
patients.
Radiation was delivered by the linear accelerator
Clinac 2100 C/D (Varian Medical Systems, Inc., Palo Alto,
California, USA) using a 3D conformal radiation therapy with photon
beams (6 MV and/or 18 MV). The whole breast was treated with a dose
of 48.6, 1.8 Gy/fraction. Then, the tumor bed was boosted with a
dose of 9–12.6, 1.8 Gy/fraction.
Computed tomography (CT) simulation was performed
during free breathing on the CT scanner Somatom Definition AS
(Siemens AG, Munich, Germany) with following the parameters: Spiral
mode, slice thickness 5 mm, slice spacing 5 mm, field of view 50 or
78 cm. Each focus in the space was precisely defined using the
Cartesian coordinate system X, Y, Z. This data was transferred to
the online planning system Eclipse 8.6 (Varian Medical Systems,
Inc.). Immobilization was accomplished using the Breastboard fix
board with arm and wrist support on the affected side (Civco
Rabiotherapy, Inc., Orange City, IA, USA).
The clinical target volume (CTV) was defined as the
area of all metal clips and other postoperative changes, while the
planning target volume (PTV) was defined as the CTV plus a margin
of 1 cm (automatic 3D expansion) with elimination of the margin 0.5
cm under the skin surface. Metal markers were delineated as a
separate structure. Next, 3D conformal planning was performed using
a technique of tangential wedged fields with multileaf collimator
shaping. Irradiation was delivered using the linear accelerator
Clinac 2100 C/D. The total boost dose to the tumor bed was 9–12.6
Gy, dose per fraction 1.8 Gy, which was preceded by the whole
breast irradiation with a dose of 48.6 Gy, dose per fraction 1.8
Gy. Localization of the treatment plan isocenter was verified by
checking the position on CT simulator.
Acquisition of image data for IGRT
2D/2D perpendicular kV imaging
Patient imaging was performed daily before every
treatment fraction. Before the boost treatment, patients were
positioned on the isocenter skin marks that were painted at
simulation. Anteroposterior (AP) and laterolateral (LL) scanning on
the kV (kilovoltage) imaging system of the linear accelerator
Clinac 2100 C/D was used. The images obtained were compared with
the AP and LL digitally reconstructed radiographs acquired from the
planning CT. Online correction of the patient position was
performed by a radiation oncologist prior to the treatment start
using metal clips in the breast, thus determining the area of the
tumor bed implanted by the surgeon during the course of surgery.
Overall, 184 women had 1,042 setup errors measurements, which are
the shifts in metal markers in relation to skin marks.
Evaluation of the intra-fraction
movement
The position of the metal markers was checked prior
to and following treatment in 22 patients. Patient imaging was
performed with the AP and LL imaging using the kV imaging device on
the linear accelerator Varian Clinac 2100 C/D. The results of 118
measurements were obtained.
Evaluation of the inter-observer
error
The offline check of the metal markers matching was
performed by another physician in 20 patients. Localization shifts
between the first and second physician were compared and the
resulting differences were used for the calculation of the safety
margin according to the van Herk formula (7). The results of 103 measurements were
obtained.
Breathing excursions
The patients were not instructed to any change of
breathing, both the treatment setup and irradiation thus took place
during free breathing. In a group of ten patients, the magnitude of
the breast movement (metal markers) was monitored. During free
breathing, the maximum amplitudes of the marker movement were
tracked using 4D CT. The difference in the position of markers was
measured at the inspiration/expiration peak.
Quality assurance
An inaccuracy of the presented image in relation to
the isocenter of the on-board imaging system on the linear
accelerator (kV imaging system on the linear accelerator) was
stated to be up to 1.5 mm and the results were monitored regularly.
The measurement on the linear accelerator reported that this
uncertainty was <1 mm. The declared tolerance for the treatment
table shift uncertainty is 1 mm in all directions at the most
(8,9).
Statistical analysis
Microsoft Excel software (Microsoft Corporation,
Redmond, WA, USA) was used to calculate the following parameters:
Mean, median, systematic (∑set-up) and random
(σset-up) error. The safety margins were calculated
according to van Herk formula (2.5Σset-up +
0.7σset-up). The magnitude of the margin between CTV and
PTV, calculated according to the aforementioned formula, ensures
that ≥90% of the CTV is covered by the 95% of the dose (7).
Results
Assessment of the required safety
margin using the setup on skin marks without IGRT
A total of 1,042 measurements in the group of 184
patients were assessed. Using kV 2D/2D imaging, the difference
between setup on skin marks and metal markers in the breast was
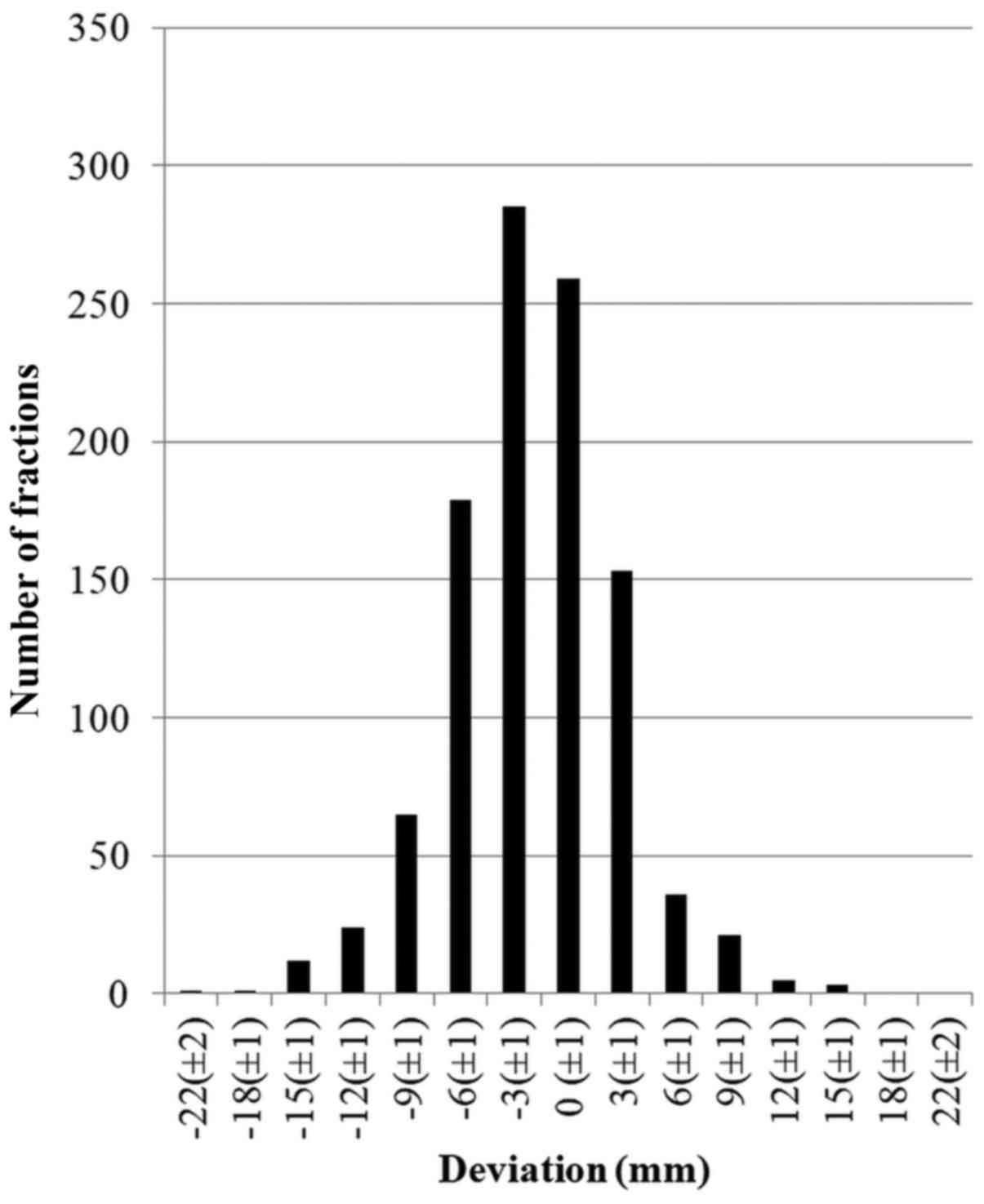
between-21 and +16 mm in the AP direction (mean, −2.0 mm) (Fig. 1). According to van Herk formula, the
calculated margin for the skin mark-based setup was 9.4 mm. In the
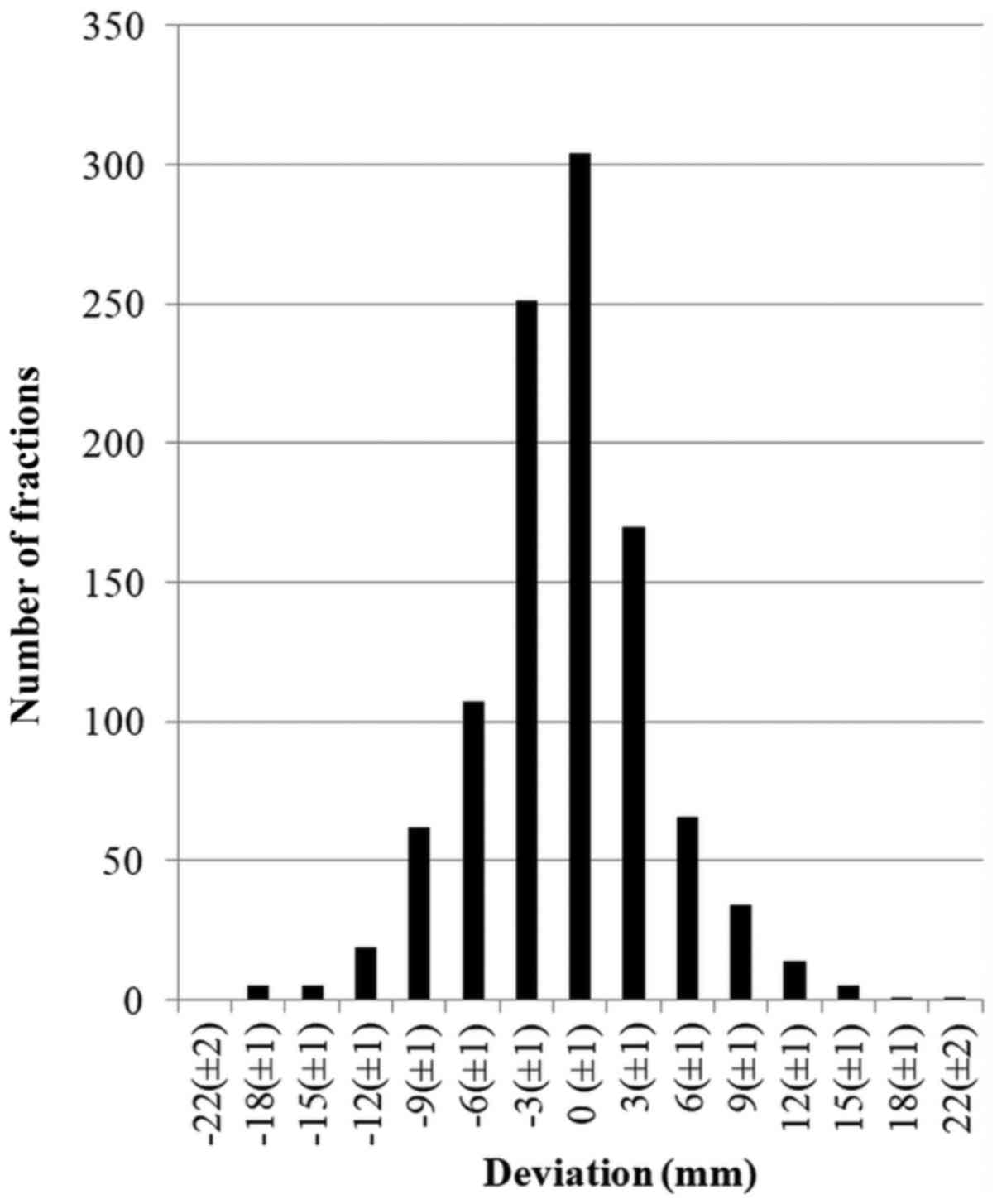
craniocaudal (CC) (Fig. 2) and LL
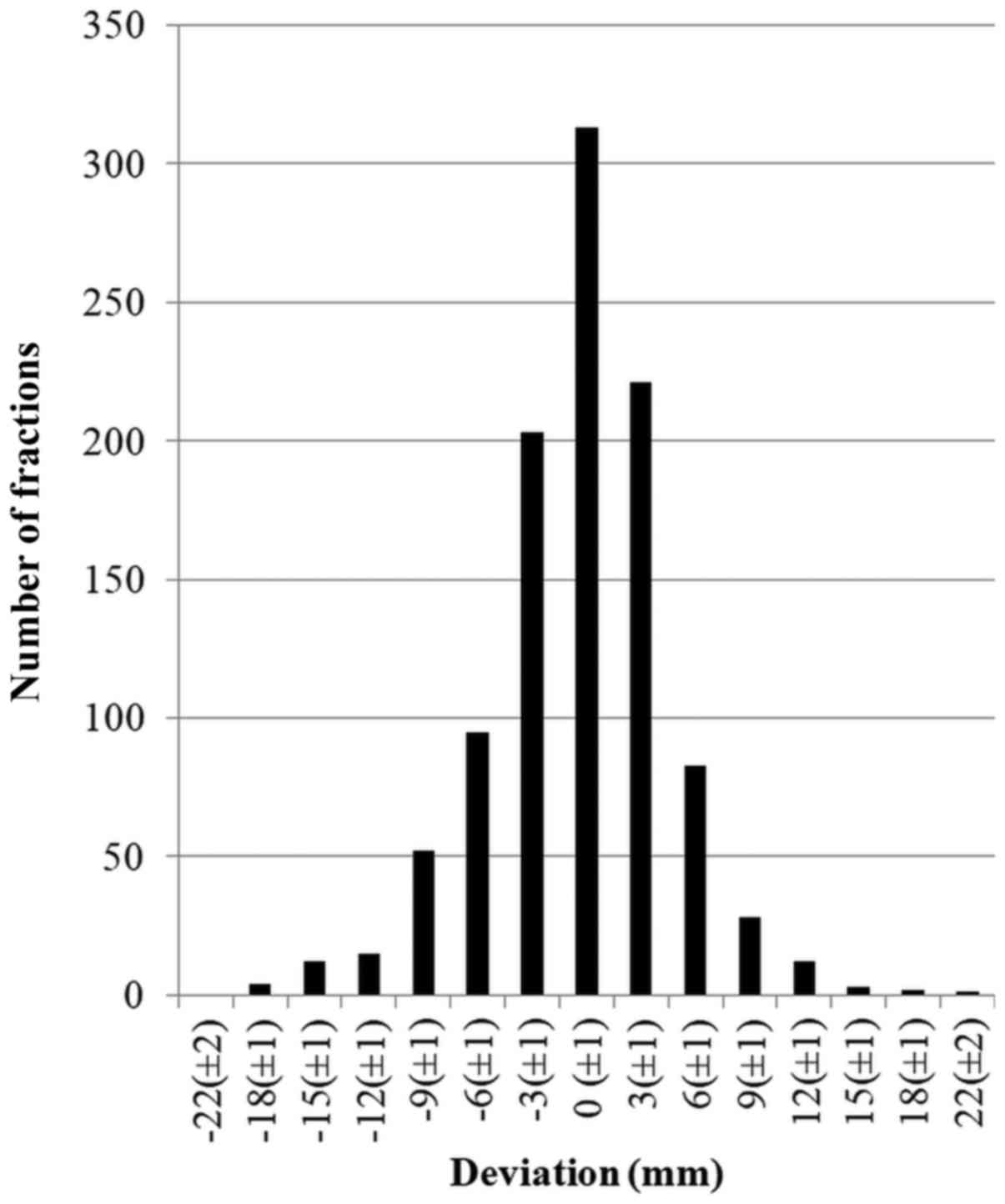
(Fig. 3) directions, the
corresponding values were −18 to 25 mm, mean, −0.9 mm, margin 11.1
mm; and −19 to +21 mm, mean −0.4 mm, margin 11.1 mm, respectively
(Table I). Based on this data, the
most commonly used margin of 10 mm between CTV and PTV was
insufficient.
 | Table I.Differences in setup on skin marks vs.
markers in the breast. |
Table I.
Differences in setup on skin marks vs.
markers in the breast.
| Axis | Maximum deviation -
(mm) | Maximum deviation +
(mm) | Median (mm) | Mean (mm) |
σset-up |
∑set-up | Calculated margin
(mm) |
|---|
| AP | −21 | 16 | −2 | −2.0 | 4.2 | 2.6 | 9.4 |
| CC | −18 | 25 | 1 | −0.9 | 4.2 | 3.3 | 11.1 |
| LL | −19 | 21 | 0 | −0.4 | 4.1 | 3.3 | 11.1 |
A setup error exceeding 10 mm was observed in 12.3%
of all fractions (128/1,042 fractions). When focused on particular
axes, the setup errors exceeding 10 mm in LL, AP and CC directions
were noted in 4.7, 4.5, and 4.8% of all fractions,
respectively.
The setup error exceeding 10 mm observed in at least
one fraction was identified in 40.9% of patients (76/184 patients).
Focusing on this group of 76 patients, the setup error exceeding 10
mm was registered in 29.5% of fractions.
The findings from the 4D CT analysis demonstrated
that the marker movement in the breast during calm and uncontrolled
breathing corresponded to 1–3 mm in all directions, with 95% of
measurements being ≤2.1 mm. The calculated vector magnitude of the
marker movement during breathing did not exceed 3.1 mm.
Evaluation of the interobserver
error
The results of 103 measurements were acquired when
comparing the interobserver differences in setup correction. The
maximum differences in the AP, CC, and LL directions were −1 to +3
mm, −2 to +2 mm, and −3 to +2 mm, respectively. The corresponding
values of the calculated safety margin were 1.1, 1.2 and 1.2 mm,
respectively (Table II).
 | Table II.Interobserver differences in setup on
markers in the breast. |
Table II.
Interobserver differences in setup on
markers in the breast.
| Axis | Maximum deviation -
(mm) | Maximum deviation +
(mm) | Median (mm) | Mean (mm) |
σset-up |
∑set-up | Calculated margin
(mm) |
|---|
| AP | −1 | 3 | 0 | 0.1 | 0.6 | 0.3 | 1.1 |
| CC | −2 | 2 | 0 | −0.1 | 0.7 | 0.3 | 1.2 |
| LL | −3 | 2 | 0 | −0.1 | 0.6 | 0.3 | 1.2 |
Evaluation of the intrafraction
movement
The results of 118 measurements were obtained during
the comparison of the intrafraction movement. The intrafraction
movement, which is the difference in kV 2D/2D imaging between setup
on the metal markers prior to and following irradiation, was −5 to
+4 mm, −7 to +4 mm, and −9 to +5 mm in the AP, CC, and LL
directions, respectively. The corresponding values of the safety
margin calculated according to the van Herk formula were 4.1, 4.5
and 5.4 mm, respectively (Table
III).
 | Table III.Intrafraction differences in setup on
markers in the breast. |
Table III.
Intrafraction differences in setup on
markers in the breast.
| Axis | Maximum deviation -
(mm) | Maximum deviation +
(mm) | Median (mm) | Mean (mm) |
σset-up |
∑set-up | Calculated margin
(mm) |
|---|
| AP | −5 | 4 | −1 | 1.2 | 1.6 | 1.2 | 4.1 |
| CC | −7 | 4 | −1 | −1.2 | 1.6 | 1.4 | 4.5 |
| LL | −9 | 5 | 0 | −0.5 | 1.9 | 1.6 | 5.4 |
Margin calculations using IGRT
The PTV margin for daily online verification of the
marker position in the breast was calculated. The intrafraction
movement (IF), interobserver differences in setup correction (IO)
and respiratory induced movement (RM) of the markers during free
breathing were included in the calculation. The total margin was
calculated as a standard deviation as follows: IF2+IO2+RM2. The resulting margins in AP, CC
and LL directions were 4.7, 5.1, and 5.9 mm, respectively (Table IV) (7).
 | Table IV.Calculated planning target volume
margins for daily online verification of the marker position in the
breast. |
Table IV.
Calculated planning target volume
margins for daily online verification of the marker position in the
breast.
| Axis | IF (mm) | IO (mm) | RM (mm) | Calculated margin
(mm) |
|---|
| AP | 4.1 | 1.1 | 2.1 | 4.7 |
| CC | 4.5 | 1.2 | 2.1 | 5.1 |
| LL | 5.4 | 1.2 | 2.1 | 5.9 |
Discussion
The aim of the present study was to assess the
appropriateness of IGRT technique use, based on the monitoring of
the marker position in the area of tumor bed following surgical
removal of tumors. As is evident from the literature, the implanted
clips are typically stable in the tumor bed throughout the six-week
course of radiation therapy (6). Park
et al (10) investigated the
locations of the clips prior to and following accelerated partial
breast irradiation in 26 patients using 4D CT, and observed
position changes ≤1 mm (10). Weed
et al (11) monitored the
changes in clip locations and resection cavity size in 28 patients
with two CT scans at a mean of 27 days apart. A mean shift of 3 mm
was reported in all three axes with a decreasing volume in the
resection cavity (11). In the
present cohort, no significant movement of the clips was observed.
Nevertheless, tracking of the clips was limited to a period of 6 to
9 days, which was the time between the CT for boost planning and
the last fraction of radiotherapy. Observation was realized a long
time following clip implantation and so the changes in resection
cavity were minor. Thus, the clips are suitable for position
monitoring. More accurate localization of the target volume enabled
a reduction in the magnitude of the safety margin, thus decreasing
the dose delivered to the surrounding healthy tissues. Another
advantage is that precise localization limits the possibility of a
geographical miss, which may result in target volume underdosage.
This is primarily important when using the boost dose (6).
Marker movement in the breast during free breathing,
which was observed in the present study, corresponds with published
data. Wang et al (12)
performed 4D CT in 17 patients and reported a motion vector of
2.09±0.94 mm. Similarly, Richter et al (13) reported a motion vector of 1.8±0.9 mm
using the 4D CT in a group of 10 patients.
Numerous studies were performed examining the proper
magnitude of safety margin when the setup was guided by skin marks.
Yue et al (14) investigated
setup errors in 21 patients using three types of localization:
Setup on skin marks, daily localization with kV portal images of
the anatomic bony structures, and localization of markers implanted
into the tumor bed. The mean interfraction setup error was 9 mm for
skin marks and 7.1 mm for bony structures in relation to metal
markers (14).
Hasan et al (15) compared the registration of the
simulation CT with subsequent CT, and analyzed the position of bony
structures, clips and breast surface. It was demonstrated that
following partial mastectomy the cavity localization using setup on
bony structures was the worst, while the localization using clips
was very stable in relation to anatomic changes (15). The importance of surgical clips for
the IGRT localization was investigated by Topolnjak et al
(16). The authors used cone beam CT
in 21 patients and retrospectively compared the position of clips
in relation to the cavity following surgery. During treatment, the
mean difference between the clip positions in relation to the
cavity center was 1.4 mm, with a maximum distance of 5.8 mm. The
authors concluded that surgical clips are useful for the
localization of the cavity following tumor excision. Harris et
al (17) evaluated position of
the markers implanted into the excision cavity using daily portal
imaging and a CT at the end of treatment. The maximum change of the
position prior to and following treatment was 7 mm (17). Coles et al (18) organized a prospective study in 42
evaluable patients to identify the required safety margin when
using gold seeds implanted into the tumor bed. CT monitoring of the
seed position has been used. An analysis revealed that a margin of
10.1 mm was necessary when no correction was performed (setup
according to skin marks), whereas a margin of 1.4 mm was sufficient
with the correction protocol based on daily localization (18).
Using 4D CT imaging, Latifi et al (6) evaluated migration of markers during the
6-week course of radiation therapy. Only minimal positional changes
have been observed. The authors also investigated the impact of
respiratory movements on the position of markers and seroma,
intrafraction, and interfraction. Such positional uncertainties
were <1 mm (mean value). According to results of the
aforementioned study, a PTV margin of 7 mm was insufficient in the
absence of the marker-based IGRT. A safety margin of at least 9 mm
is necessary for the daily setup using bony structures. In order to
achieve more precise positioning, daily localization of the clips
using IGRT appears to be essential. In this case, the PTV safety
margin may be limited to 6 mm (6). In
2014, Harris et al (19)
published the results of a British multicenter study investigating
the safety margin magnitude in 60 patients with boost irradiation
(sequential boost in 30 patients, simultaneous integrated boost in
30 patients). Two sizes of the safety margin were compared, 5 vs. 8
mm. The authors concluded that a margin of 5 mm was sufficient when
IGRT localization of the surgical clips was utilized (19).
In conclusion, the results of the present study
suggest that the commonly used PTV margin of 10 mm is insufficient
for the setup on skin marks (calculated margin in the present
cohort was 12 mm). It is possible to reduce the safety margin
<10 mm using IGRT localization of the metal markers in the
breast (calculated margin in the present cohort was 6 mm). The
resulting size of the calculated safety margin depends on the
technical equipment and staff quality of a particular
department.
Acknowledgements
The authors would like to thank Mrs. Lenka Jezkova
(Oncology Centre, Multiscan Pardubice) for help with checking the
text and citations.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request
Authors' contributions
AH, JV and KO designed the study. AH, KO, JS, MD,
VU, MV and IK collected the data. ZV digitalized the data. JM
performed statistical data processing. AH, JV and KO wrote the
study. KO made language corrections.
Ethics approval and consent to
participate
There were no ethical issues regarding the use of
various treatment methods because all patients were treated using
IGRT and the setup errors were evaluated based on standard imaging.
Written informed consent was obtained from all patients.
Consent for publication
Consent for data to be published is included in the
written informed consent from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), ; Darby S, McGale P, Correa C, Taylor
C, Arriagada R, Clarke M, Cutter D, Davies C, Ewertz M, et al:
Effect of radiotherapy after breast-conserving surgery on 10-year
recurrence and 15-year breast cancer death: Meta-analysis of
individual patient data for 10,801 women in 17 randomised trials.
Lancet. 378:1707–1716. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fisher B, Anderson S, Bryant J, Margolese
RG, Deutsch M, Fisher ER, Jeong JH and Wolmark N: Twenty-year
follow-up of a randomized trial comparing total mastectomy,
lumpectomy and lumpectomy plus irradiation for the treatment of
invasive breast cancer. N Engl J Med. 347:1233–1241. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fiorentino A, Tebano U, Ruggieri R,
Ricchetti F and Alongi F: Simultaneous integrated bilateral breast
and nodal irradiation with volumetric arc therapy: Case report and
literature review. Tumori J. 102 Suppl 2:S32–S34. 2016. View Article : Google Scholar
|
|
4
|
Vanasek J, Odrazka K, Dolezel M, Dusek L,
Jarkovsky J, Hlavka A, Valentova E and Kolarova I: Searching for an
appropriate image-guided radiotherapy method in prostate
cancer-implications for safety margin. Tumori. 100:518–523. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaikh T, Chen T, Khan A, Yue NJ, Kearney
T, Cohler A, Haffty BG and Goyal S: Improvement in interobserver
accuracy in delineation of the lumpectomy cavity using fiducial
markers. Int J Radiat Oncol. 78:1127–1134. 2010. View Article : Google Scholar
|
|
6
|
Latifi K, Pritz J, Zhang GG, Moros EG and
Harris EER: Fiducial-based image-guided radiotherapy for whole
breast irradiation. J Radiat Oncol. 2:185–190. 2013. View Article : Google Scholar
|
|
7
|
van Herk M, Remeijer P, Rasch C and
Lebesque JV: The probability of correct target dosage:
Dose-population histograms for deriving treatment margins in
radiotherapy. Int J Radiat Oncol Biol Phys. 47:1121–1135. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Varian Medical System: OBI v1.6 and CBCT
v2.1 installation product acceptance. 2015.
|
|
9
|
Varian Medical System: High energy
c-series clinac installation product acceptance. 2016.
|
|
10
|
Park CK, Pritz J, Zhang GG, Forster KM and
Harris EE: Validating fiducial markers for image-guided radiation
therapy for accelerated partial breast irradiation in early-stage
breast cancer. Int J Radiat Oncol Biol Phys. 82:e425–e431. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Weed DW, Yan D, Martinez AA, Vicini FA,
Wilkinson TJ and Wong J: The validity of surgical clips as a
radiographic surrogate for the lumpectomy cavity in image-guided
accelerated partial breast irradiation. Int J Radiat Oncol Biol
Phys. 60:484–492. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang W, Li JB, Hu HG, Li FX, Xu M, Sun T
and Lu J: Correlation between target motion and the dosimetric
variance of breast and organ at risk during whole breast
radiotherapy using 4DCT. Radiat Oncol. 8:1112013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Richter A, Sweeney R, Baier K, Flentje M
and Guckenberger M: Effect of breathing motion in radiotherapy of
breast cancer: 4D dose calculation and motion tracking via EPID.
Strahlenther Onkol. 185:425–430. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yue NJ, Goyal S, Zhou J, Khan AJ and
Haffty BG: Intrafractional target motions and uncertainties of
treatment setup reference systems in accelerated partial breast
irradiation. Int J Radiat Oncol Biol Phys. 79:1549–1556. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hasan Y, Kim L, Martinez A, Vicini F and
Yan D: Image guidance in external beam accelerated partial breast
irradiation: Comparison of surrogates for the lumpectomy cavity.
Int J Radiat Oncol Biol Phys. 70:619–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Topolnjak R, de Ruiter P, Remeijer P, van
Vliet-Vroegindeweij C, Rasch C and Sonke JJ: Image-guided
radiotherapy for breast cancer patients: Surgical clips as
surrogate for breast excision cavity. Int J Radiat Oncol Biol Phys.
81:e187–e195. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harris EJ, Donovan EM, Yarnold JR, Coles
CE and Evans PM; IMPORT Trial Management Group, : Characterization
of target volume changes during breast radiotherapy using implanted
fiducial markers and portal imaging. Int J Radiat Oncol Biol Phys.
73:958–966. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coles CE, Harris EJ, Donovan EM, Bliss P,
Evans PM, Fairfoul J, Mackenzie C, Rawlings C, Syndikus I, Twyman
N, et al: Evaluation of implanted gold seeds for breast
radiotherapy planning and on treatment verification: A feasibility
study on behalf of the IMPORT trialists. Radiother Oncol.
100:276–281. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Harris EJ, Mukesh M, Jena R, Baker A,
Bartelink H, Brooks C, Dean J, Donovan EM, Collette S, Eagle S, et
al: A multicentre observational study evaluating image-guided
radiotherapy for more accurate partial-breast intensity-modulated
radiotherapy: Comparison with standard imaging technique. NIHR
Journals Library; Southampton (UK): 2014
|