Introduction
Cholangiocarcinoma is a common primary biliary
malignancy that originates from bile duct epithelial cells and has
presented difficulties in diagnosis and treatment (1). Cholangiocarcinoma, including
intrahepatic, perihilar, and distal cholangiocarcinoma, accounts
for ~10–15% of total hepatobiliary malignancies (2). The incidence of cholangiocarcinoma
increased from 1832 cases in 2010 to 1964 cases in 2013 in England,
and the majority of cholangiocarcinoma patients are >60 years
old (3). Early diagnosis, operative
treatment and chemotherapy for cholangiocarcinoma remain
ineffective at treating cholangiocarcinoma (4). Cancer recurrence and metastasis have
remained as the important mortality factors for patients with
cholangiocarcinoma (5); therefore,
the identification of an effective approach to treat human
cholangiocarcinoma is required.
Solamargine is an alkaloid that is primarily derived
from the Solanum nigrum plant. S. nigrum may exhibit
heat-clearing and detoxifying effects, according to the theory of
traditional Chinese medicine (6).
Solamargine is an effective active ingredient of S. nigrum
that may inhibit the proliferation and induce the apoptosis of
multiple types of cancer cell, particularly human hepatocellular
carcinoma cells (7–10). In addition, solamargine may enhance
the susceptibility of human lung cancer and breast cancer to
chemotherapeutic drugs (11–13). However, the effect of solamargine on
human cholangiocarcinoma QBC939 cells and the underlying molecular
mechanism remain unknown.
Apoptosis serves an important function in tumor
formation and metastasis and is typically repressed in the tumor
microenvironment (14,15). Therefore, increasing the apoptosis
induced by drugs is an effective method to inhibit cancer (16). In the present study, the effect of
solamargine on the viability of cholangiocarcinoma QBC939 cells and
associated molecular mechanisms was investigated and may provide
experimental evidence of cholangiocarcinoma treated by
solamargine.
Materials and methods
Materials
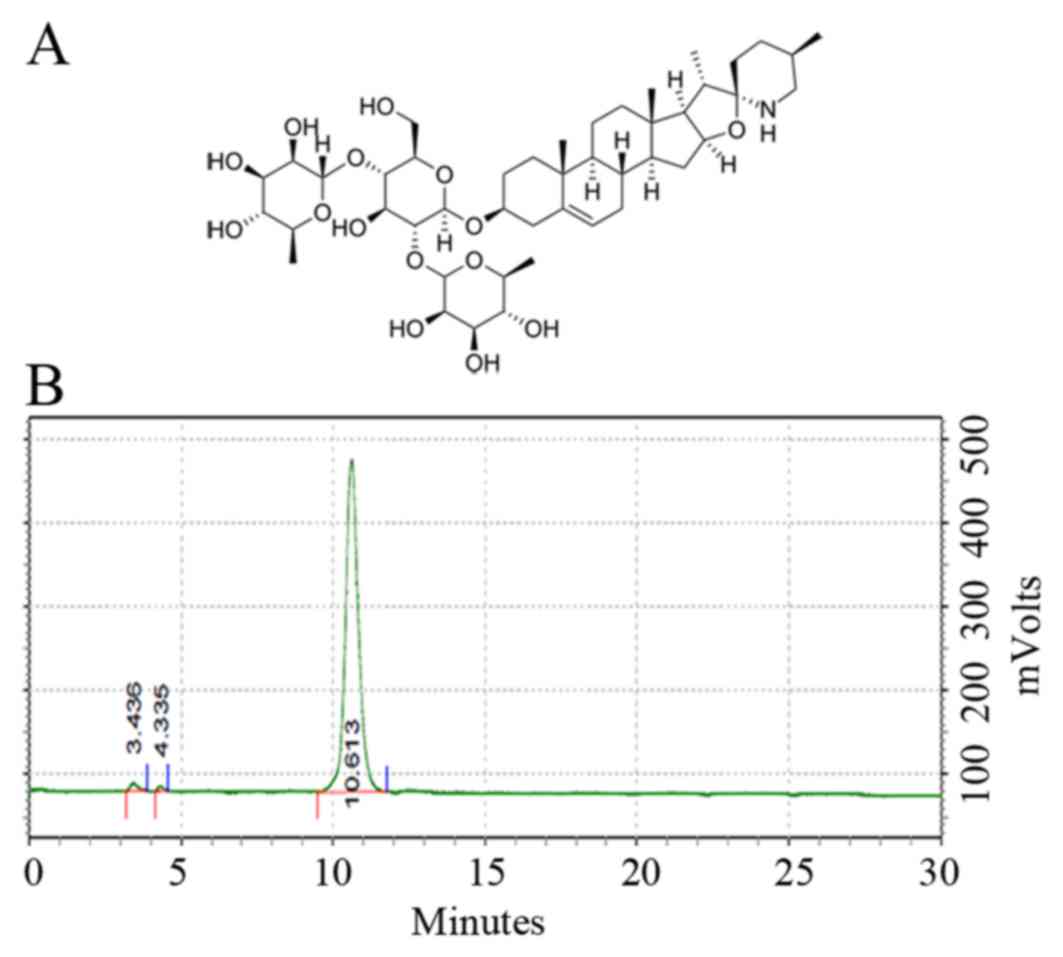
Solamargine (Fig. 1A),
also known as (22R, 25R)-3β-(β-D-Glucopyranosyloxy) spirosol-5-ene
or solasodine 3-glucoside, was purchased from Chendu Must
Bio-Technology Co., Ltd. (Chendu, China) and dissolved in dimethyl
sulfoxide (DMSO). The QBC939 cell line was obtained from Nanjing
Chinese Medical University (Nanjing, China). RPMI 1640 medium,
fetal bovine serum (FBS), 0.25% Trypsin-EDTA, penicillin and
streptomycin were purchased from Gibco; Thermo Fisher Scientific,
Inc. (Waltham, MA, USA). The mitochondrial membrane potential assay
kit with JC-1, MTT and radioimmunoprecipitation assay (RIPA)
protein lysis buffer were purchased from Beyotime Institute of
Biotechnology (Haimen, China). The Annexin V-fluorescein
isothiocyanate (FITC) apoptosis detection kit was purchased from BD
Biosciences (Franklin Lakes, NJ, USA). The first cDNA synthesis kit
for reverse transcription-quantitative polymerase chain reaction
(RT-qPCR), the SYBR Green/ROX qPCR master mix and the protein
ladder were obtained from Thermo Scientific, Inc. Caspase3 (catalog
no. 9662), caspase7 (catalog no. 12827), X-linked inhibitor of
apoptosis protein (XIAP) (catalog no. 2042), poly ADP ribose
polymerase (PARP) (catalog no. 9542), B-cell lymphoma-2 (Bcl-2)
(catalog no. 2876), Bcl-2-associated X protein (Bax) (catalog no.
2772) and β-actin (catalog no. 4970) antibodies were purchased from
Cell Signaling Technology, Inc. (Danvers, MA, USA). Dylight
800-labeled goat anti-rabbit immunoglobulin G (H+L) fluorescence
antibody (catalog no. 072-07-15-06) was purchased from KPL, Inc.
(Gaithersburg, MD, USA).
High-performance liquid chromatography (HPLC). 0.02
mg/ml solamargine is preparaed in 80% ethanol and detected by HPLC
Agilent 1100 series (Agilent Technologies, Inc., Santa Clara, CA,
USA). Chromatographic condition are displayed below.
Chromatographic column: SinoChrom ODS-BP (C18), 5 µm, 250×4.6 mm
(catalog no. 31110006, Dalian Elite Analytical Instruments Co.,
Ltd, Liaoning, China). Column temperature: 30°C. Mobile phrase
consists of acetonitrile and 0.1% ammonium hydroxide. The content
of acetonitrile in gradient mobile phrase varies as below: From 25
to 45% in 0–20 min; from 45 to 75% in 20–30 min, flow rate is 1
ml/min, detected at wavelength 203 nm, sample loading volumn is 5
µl.
Cell culture
Human cholangiocarcinoma QBC939 cells were cultured
in RPMI-1640 medium supplemented with 10% (v/v) FBS, 100 U/ml
penicillin and 100 µg/ml streptomycin. Cells were maintained at
37°C in a humidified environment containing 5% CO2. For
all the experiments, cells were serum-starved and treated with
solamargine for the specified times.
MTT assay and morphologic
observation
QBC939 cells, in the period of logarithmic phase,
were seeded in 96-well plate at a density of 1×104
cells/well in 100 µl RPMI-1640 medium, in triplicate, and cultured
at 37°C overnight in an atmosphere containing 5% CO2.
Cells were allowed to culture to 70% confluence/well and were
treated with solamargine at the indicated concentration (0, 2, 4,
6, 8, 10, 12 and 14 µM) for 24 h at 37°C. The morphology of QBC939
cells was observed by using inverted microscopy (magnification,
×200) (Olympus Corporation, Tokyo, Japan). Subsequently, 10 µl MTT
(5 mg/ml) was added to cells. After 4 h incubation at 37°C, the
cell medium was removed completely and 100 µl DMSO was added in
cells to resolve the blue formazan crystals of live cells. The
optical density of cells/well was measured at absorbance wavelength
570 nm using the Multiskan Spectrum Microplate Reader (Tecan Group,
Ltd., Mannedorf, Switzerland). Finally, cell viability in the
different treated groups (0, 2, 4, 6, 8, 10, 12 and 14 µM
solamargine) was calculated as a proportion, using the formula:
Cell viability (%)=(OD570 nm-OD630
nm)treated/(OD570 nm-OD630
nm)untreatedx100%.
Flow cytometry for detecting
apoptosis
QBC939 cells, in the period of logarithmic phase,
were seeded in 6-well plates (3×105 cells/well, in 2 ml
RPMI-1640 medium) and cultured overnight at 37°C in an atmosphere
containing 5% CO2. Cells were allowed to culture to 70%
confluence/well and were treated with solamargine at the indicated
concentration (0, 2, 4, 6, 8 and 10 µM) for 24 h at 37°C. Cells
were digested using 0.25% Trypsin-EDTA at 37°C, washed with PBS and
resuspended in 100 µl 1X Binding Buffer (included in the Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit). Cells
in each group (0, 2, 4, 6, 8 and 10 µM) were stained with 5 µl
propidium iodide (PI) and 5 µl Annexin V-FITC, according to the
Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit
(BD Biosciences, Franklin Lakes, NJ, USA) protocol and incubated
for 15 min at room temperature in the dark. An aliquot of 400 µl 1X
Binding Buffer was added and the apoptosis of QBC939 cells was
detected by Guava easyCyte 6–2L flow cytometer (Merck KGaA,
Darmstadt, Germany) and analyzed by the GuavaSoft software (version
2.7; Merck KGaA, Darmstadt, Germany).
Detecting the mitochondrial membrane
potential by JC-1 staining buffer
QBC939 cells in the mid-log phase were seeded in
12-well plates (2×105 cells/well in 1 ml RPMI 1640
medium) and cultured overnight at 37°C. Cells were treated with
solamargine at 2, 4, 6, 8 and 10 µM for 24 h at 37°C. The treated
cells were washed with PBS two times and digested with 0.25%
Trypsin-EDTA. Carbonyl cyanide 3-chlorophenylhydrazone (from the
mitochondrial membrane potential assay kit) was added into the
positive control well and incubated at 37°C for 20 min. A total of
1 ml JC-1 staining buffer was added to the wells and incubated for
20 min at 37°C in the dark. The supernatant was removed at 600 × g
for 3 min at room temperature. Cells were washed with JC-1 washing
buffer (1X) two times and then suspended in washing buffer. Flow
cytometry was conducted to detect JC-1 fluorescence and analyze the
change in mitochondrial membrane potential (MMP) in QBC939
cells.
RT-qPCR
QBC939 cells in the mid-log phase were seeded in
6-well plates (3×105 cells/well in 2 ml RPMI 1640
medium) and cultured overnight at 37°C. Cells were allowed to
culture to 70% confluence and were treated with 0, 2, 4, 6, 8 and
10 µM solamargine for 24 h at 37°C. The treated cells were washed
with PBS twice and total RNA in cells was extracted using the
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.).
According to the manufacturer's protocol of the first cDNA
synthesis kit for RT-qPCR, mRNA was reverse transcribed to cDNA by
using the reaction system (Reaction Mix 4 µl, Maxima Enzyme Mix 2
µl, Template RNA 500 ng, and add nuclease-free Water to 20 µl) and
the reaction procedure (25°C for 10 min, 50°C for 15 min, 85°C for
5 min). The reaction system included (Maxima SYBR Green/ROX qPCR
Master Mix (Thermo Fisher Scientific, Inc.) 10 µl, forward primer
0.3 µM, reverse primer 0.3 µM, Template DNA 300 ng, and
nuclease-free water until a final volume of 25 µl), the
thermocycling conditions were 95°C for 10 min, 1 cycle; 95°C for 15
sec, 60°C for 30 sec and 72°C for 30 sec, 40 cycles of qPCR, the
genes (Bax, Bcl-2, Bcl-xL, XIAP) mRNA relative expression were
detected by fluorescence quantitative PCR equipment (Applied
Biosystems, Thermo Fisher Scientific, Inc.) and analyzed by the
2−∆∆Cq method. GAPDH mRNA expression was used as the
control. Primers used in the experiments were synthesized by
GenScript Biotech (Nanjing, China) and are listed in Table I.
 | Table I.Primers used for quantitative
polymerase chain reaction. |
Table I.
Primers used for quantitative
polymerase chain reaction.
|
| Sequence (5′-3′) |
|---|
|
|
|
|---|
| Name | Forward | Reverse |
|---|
| GAPDH |
GCAAATTCCATGGCACCGTC |
GACTCCACGACGTACTCAGC |
| Bax |
GAACCATCATGGGCTGGACA |
GCGTCCCAAAGTAGGAGAGG |
| Bcl-2 |
GAACTGGGGGAGGATTGTGG |
CCGTACAGTTCCACAAAGGC |
| XIAP |
TGGCAGATTATGAAGCACGGA |
GGTCTTCACTGGGCTTCCAA |
| Bcl-xL |
ACTCTTCCGGGATGGGGTAA |
ACAAAAGTATCCCAGCCGCC |
Western blot
QBC939 cells in the period of logarithmic phase were
seeded in 6-well plates (3×105 cells/well in 2 ml RPMI
1640 medium) and cultured overnight at 37°C in an atmosphere
containing 5% CO2. Cells were allowed to culture to 70%
confluence/well and were treated with solamargine at the indicated
concentration (0, 2, 4, 6, 8 and 10 µM) for 24 h at 37°C. Cells
were washed with ice cold PBS three times and digested using RIPA
lysis buffer with protease phosphatase inhibitor cocktail (Thermo
Scientific, Inc., Waltham, MA, USA) on ice for 5 min. Cell lysates
were selected and centrifuged (12,000 × g, 10 min, 4°C) to remove
the cell debris. Total protein concentration was determined using
the BCA Protein Assay kit (Beyotime Institute of Biotechnology) and
detected using the Multiskan Spectrum Microplate Reader. Cell
lysates are mixed with 2X Sample Buffer and heated in water at
100°C for 5 min. Prepared protein (~30 µg per lane) was separated
using SDS-PAGE (12% gels) and transferred onto polyvinylidene
difluoride membranes (Merck KGaA). After the proteins were
transferred to the PVDF membrane, protein was blocked using
Tris-Buffered Saline (TBS) containing 5% non-fat milk for 1 h at
room temperature and incubated with primary antibody (Bax, Bcl-2,
caspase3, caspase7, XIAP, PARP and β-actin, all antibodies come
from Cell Signaling Technology Incorporation, antibodies were
diluted by 1:1,000) overnight at 4°C. The PVDF membrane was washed
with TBS-0.1% Tween 20 and incubated with the Dylight 800-labeled
goat anti-rabbit immunoglobulin G (H+L) fluorescence antibody
(dilution, 1:10,000; catalog no. 072-07-15-06, KPL, Inc.,
Gaithersburg, MD, USA) for 1 h at room temperature. The blot
membrane was exposed and scanned by the Odyssey infrared imaging
system (LI-COR Biosciences, Lincoln, NE, USA).
Statistical analysis
Data are expressed as the mean ± standard deviation
of 3 independent experiments. The difference between different
groups was analyzed using a one-way analysis of variance (with
Tukey's post-hoc test) or a Student's t-test. P<0.05 was
considered to indicate a statistically significant difference. The
data were analyzed using SPSS software (version 16.0; SPSS, Inc.,
Chicago, IL, USA) and graphs were plotted using GraphPad Prism
software (version 5; GraphPad Software, Inc., La Jolla, CA,
USA).
Results
Solamargine inhibits the viability and
alters the morphology of cholangiocarcinoma QBC939 cells
To determine the precision of experiment and the
quality of solamargine, HPLC was used to determine the purity of
solamargine. The results revealed that the purity of solamargine
was >98% (Fig. 1B).
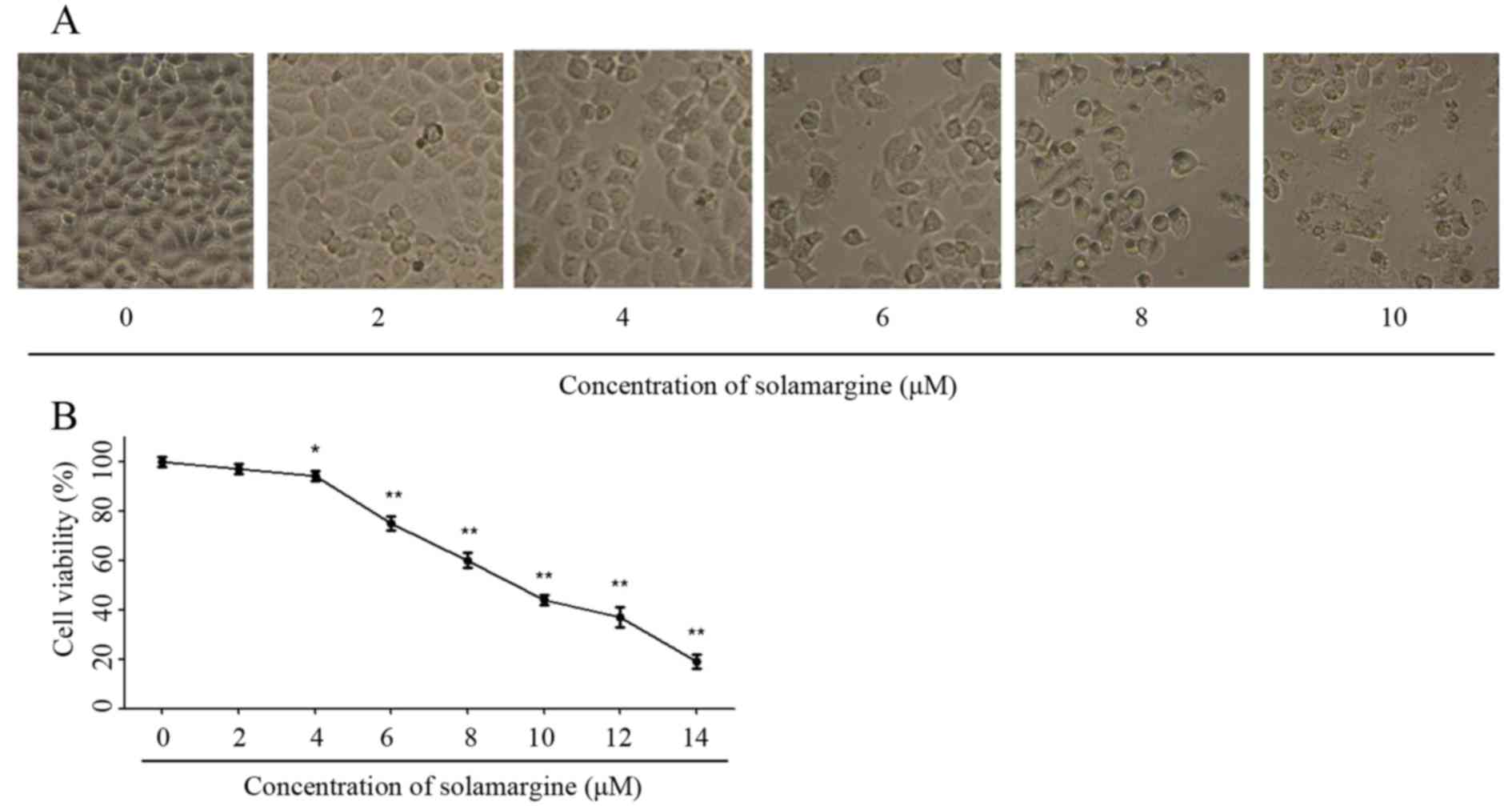
The effect of solamargine on QBC939 cells was
observed using light microscopy (magnification, ×200) at first. As
the concentration of solamargine increased (0, 2, 4, 6, 8 and 10
µM), the morphology of cholangiocarcinoma cells changed markedly at
24 h. Solamargine may cause shrinkage, irregularity and inhibit the
viability of cells (Fig. 2A). The
viability of human cholangiocarcinoma QBC939 cells was analyzed
using an MTT assay after 24 h treatment with solamargine (0, 2, 4,
6, 8, 10, 12 µM). Solamargine treatment significantly inhibited the
viability of QBC939 cells in dose-dependent manner (concentration,
>4 µM; Fig. 2B). The half-maximal
inhibitory concentration (IC50) value (9.81 µM) of
solamargine on QBC939 cells is analyzed by SPSS software.
Solamargine induces apoptosis of
cholangiocarcinoma QBC939 cells
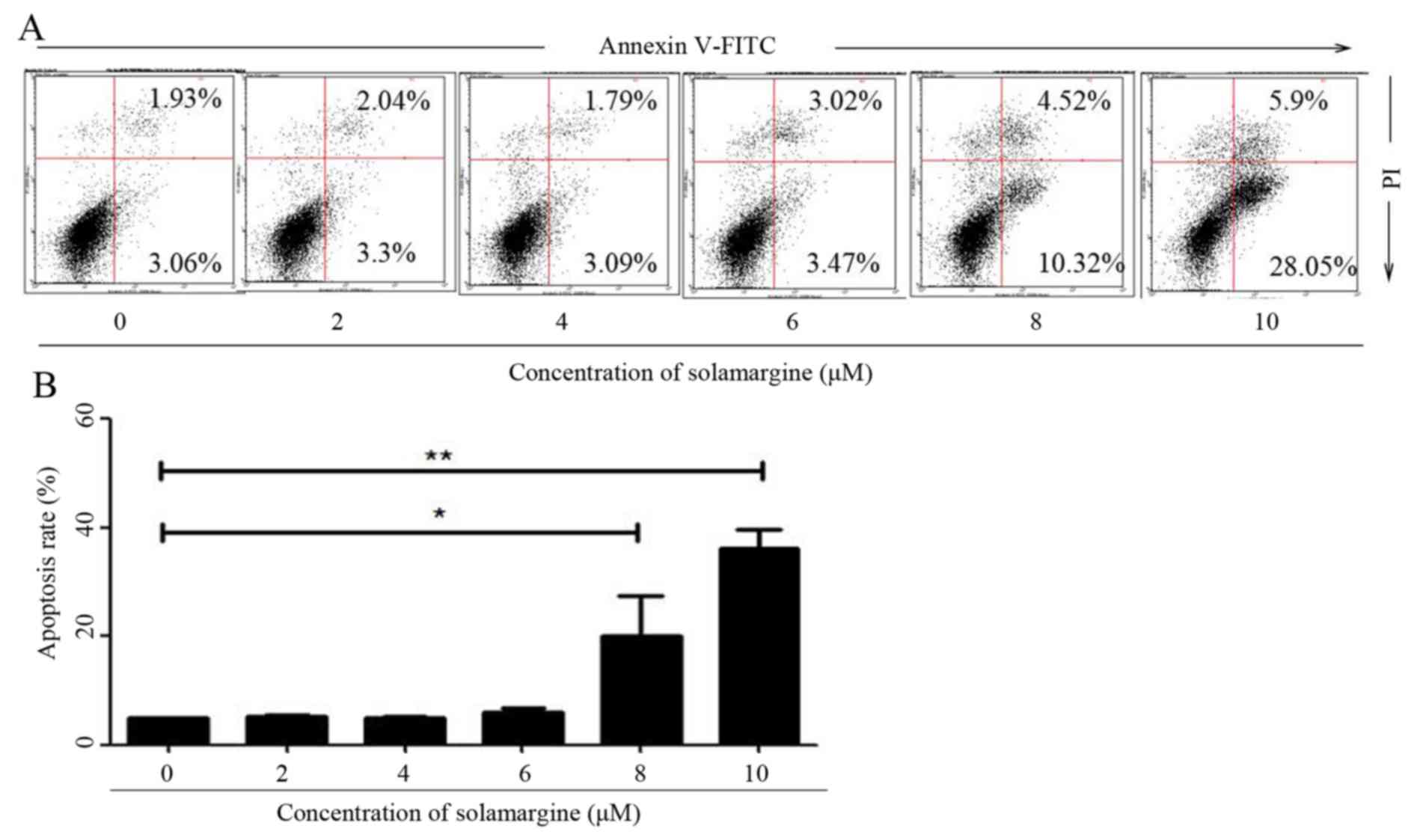
To validate whether solamargine inhibits the
viability of QBC939 cells by inducing apoptosis, the apoptosis of
QBC939 cells, after 24 h treatment with different concentrations of
solamargine (0, 2, 4, 6, 8 and 10 µM), was determined using flow
cytometry. After QBC939 cells were digested and harvested, cells
were stained with Annexin V-FITC and PI. Cells positive for Annexin
V-FITC only represented early apoptotic cells, whereas cells
positive for Annexin V-FITC and PI represented late apoptotic
cells. Flow cytometric analysis revealed that solamargine induced
apoptosis of cholangiocarcinoma QBC939 cells significantly in a
dose-dependent manner. Solamargine significantly induced apoptosis
at >6 µM and could primarily induced early apoptosis (Fig. 3).
Solamargine alters the mitochondrial
membrane potential in QBC939 cells
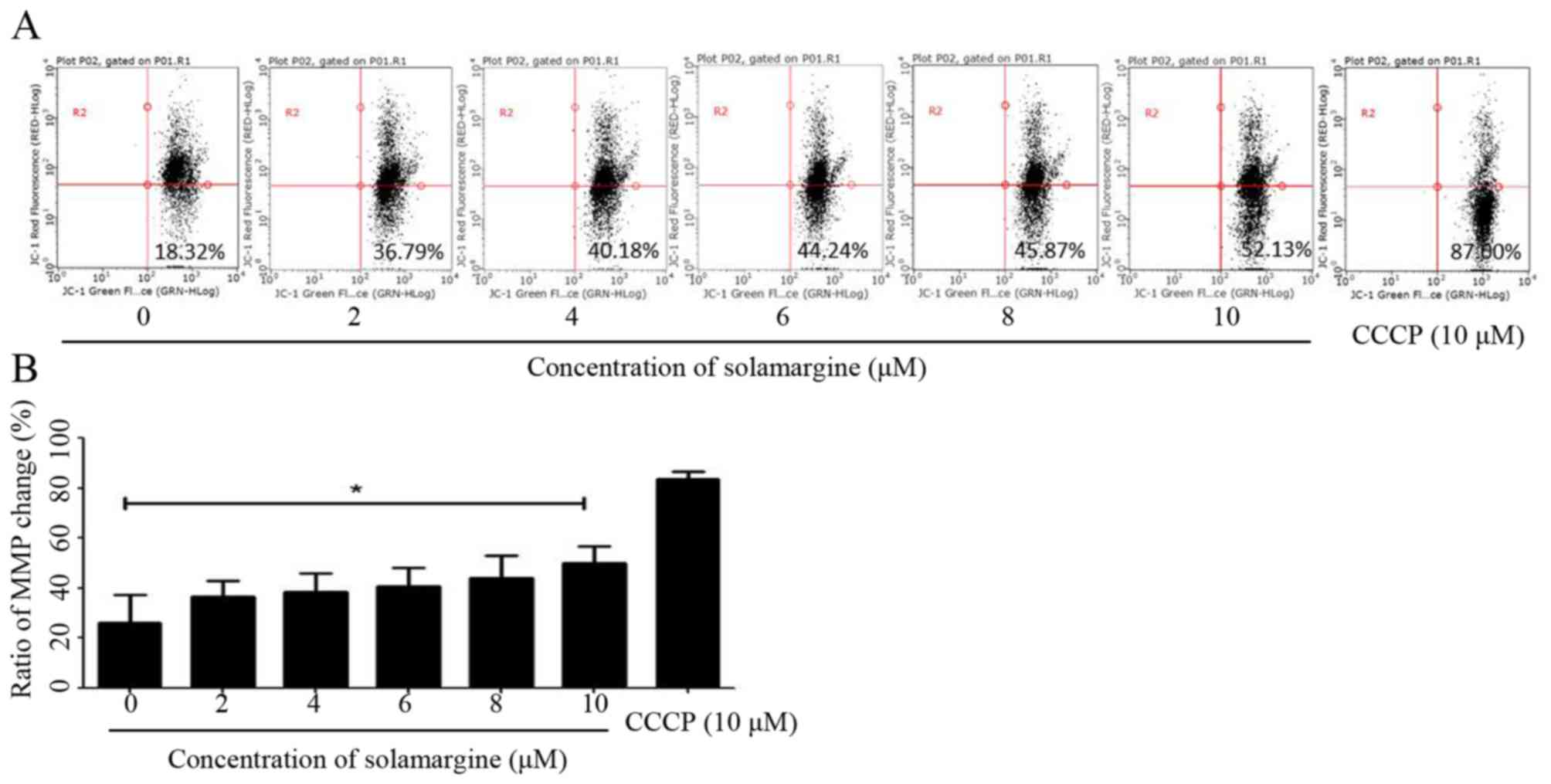
The present study identified that solamargine may
induce the pro-apoptosis of human cholangiocarcinoma cells
significantly. The alteration in mitochondrial membrane potential
may lead to early apoptosis (14).
Therefore, in the present study, QBC939 cells were treated with
different concentrations of solamargine (0, 2, 4, 6, 8 and 10 µM)
for 24 h and stained with JC-1 staining buffer for 20 min in the
dark. Subsequently, the MMP of QBC939 cells was determined using
flow cytometry. As presented in Fig.
4A, compared with the untreated group, the results demonstrated
an increase in green fluorescence and a decrease in red
fluorescence, which indicated that solamargine caused a depolarized
MMP in QBC939 cells (Fig. 4B).
Solamargine alters the expression and
activation of apoptosis-associated proteins in QBC939 cells
Based on the above results, the molecular mechanism
of apoptosis effect of solamargine on human cholangiocarcinoma
QBC939 cells was subsequently investigated. The present study
assessed the expression of apoptosis-associated proteins in QBC939
cells, after 24 h treatment with different concentrations of
solamargine (0, 2, 4, 6, 8 and 10 µM), using RT-qPCR and western
blot analysis. The total RNA of QBC939 cells was extracted using
TRIzol reagent and the mRNA was transcribed into cDNA. The relative
expression of Bax, Bcl-2, Bcl-extra-large (Bcl-xL) and XIAP mRNA
was determined using RT-qPCR (using GAPDH as the reference gene).
The results revealed that solamargine significantly inhibited Bcl-2
and XIAP mRNA levels but significantly increased the mRNA level of
Bax (Fig. 5A). The total protein of
QBC939 cells was extracted using RIPA cell lysis buffer, after 24 h
incubation, and quantitated using the BCA protein assay kit. The
protein levels of Bax, Bcl-2, caspase 3, cleaved-caspase 3, caspase
7, XIAP, PARP and cleaved PARP were determined using western blot
analysis. As presented in Fig. 5B,
solamargine increased the protein expression of Bax, caspase 3,
cleaved-caspase 3, caspase 7 and cleaved PARP, but decreased the
protein expression of Bcl-2, XIAP and PARP. Therefore, solamargine
may influence apoptosis-associated proteins to inhibit the
viability of QBC939 cells.
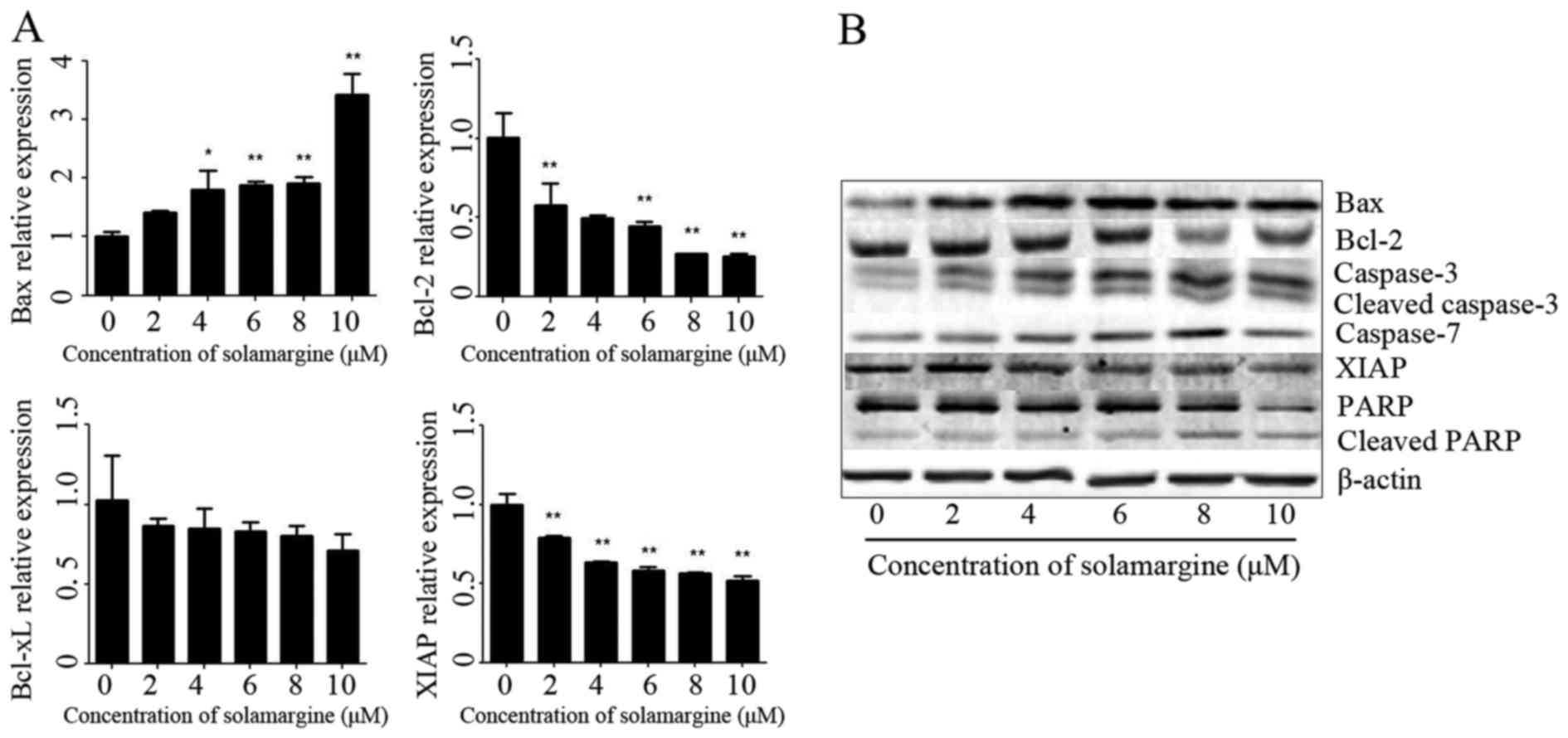 | Figure 5.Effect of solamargine on the
expression and activation of apoptosis-associated proteins. (A) The
expression of Bax increased with solamargine treatment; whereas the
expression of Bcl-2, Bcl-xL and XIAP decreased with solamargine
treatment. Determined using quantitative polymerase chain reaction,
with GAPDH used as the internal control. (B) Western blot analysis
indicated that solamargine treatment increased expression of Bax,
caspase-3, cleaved caspase-3, caspase-7 and cleaved PARP but
decreased the expression of Bcl-2, XIAP and PARP. β-actin was used
as the internal control. *P<0.05, **P<0.01 vs. control.
Bcl-2, B-cell lymphoma-2; Bcl-xL, Bcl-extra-large; Bax,
Bcl-2-associated X protein; XIAP, X-linked inhibitor of apoptosis
protein; PARP, poly ADP ribose polymerase. |
Discussion
Cholangiocarcinoma is a type of aggressive and
refractory malignancy, and characterized by late diagnosis and poor
outcomes; therefore, identifying novel therapeutic drugs is
required (2,17). Solamargine, a type of alkaloid derived
from S. nigrum, may inhibit the proliferation of and induce
apoptosis in multiple types of tumor cell lines (including human
breast cancer, lung cancer and hepatocellular carcinoma cell lines)
(7–13). The present study explored the
therapeutic effect of solamargine on human cholangiocarcinoma
QBC939 cells preliminarily. The present study revealed that
alkaloid solamargine treatment on QBC939 cells can result in the
emergence of cell shrinkage, irregularity and apoptotic bodies,
which were observed by using a light microscope. An MTT assay
revealed that solamargine inhibited the cell viability of QBC939
cells in the dose-dependent manner and the value of IC50
was 9.81 µM. The results of the present study suggested that
solamargine may be a chemotherapeutic drug for the treatment of
human cholangiocarcinoma. Therefore, future studies may focus on
exploring how solamargine inhibits the viability of QBC939 cells.
Apoptosis is a type of programmed cell death and could serve as an
important antitumor target (15,18).
Induction of apoptosis in the tumor microenvironment may inhibit
excessive cell proliferation and is an effective therapeutic
strategy against cancer (19). The
present study demonstrated that solamargine induced apoptosis of
QBC939 cells significantly in a dose-dependent manner, as
determined using flow cytometry. A previous study revealed that the
alteration in MMP is an early event of pro-apoptosis and may result
in the release of cytochrome c in mitochondria, which may
induce the activation of caspase 9 (14). The alterations in MMP induced by
solamargine, determined using flow cytometry in the present study,
demonstrated that solamargine could change MMP in QBC939 cells.
Apoptosis is associated with multiple pro-apoptotic proteins
(including Bax and caspase 3) and anti-apoptotic proteins
(including Bcl-2 and XIAP) and is determined by the ratio of pro-to
anti-apoptotic proteins (20). To
evaluate the underlying molecular mechanism of solamargine-induced
apoptosis, the alterations in apoptosis-associated gene and protein
expression were detected using RT-qPCR and western blot analysis.
The results indicated that solamargine increased the expression of
Bax, caspase 3, cleaved caspase 3, caspase 7 and cleaved PARP and
decreased the expression of Bcl-2, Bcl-xL, XIAP and PARP.
Therefore, solamargine may be an effective chemotherapeutic agent
against cholangiocarcinoma by inducing apoptosis.
The results of the present study indicated that
solamargine may induce apoptosis significantly in human
cholangiocarcinoma QBC939 cells via the MMP pathway. Solamargine is
the one member of natural compounds (luteolin, matrine, berberine
and so on) against human cholangiocarcinoma (21,22), and
the results of the present study suggested that solamargine may be
an effective drug candidate against cholangiocarcinoma. Since the
present study only included in vivo experiments, additional
in vitro assays are required to validate whether solamargine
may be a therapeutic agent for the treatment of
cholangiocarcinoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Research
Innovation Program for Academic Degree Postgraduate of Jiangsu
Province General University (grant no. 2014965) and the Key Project
supported by the Medical Science and Technology Development
Foundation, Nanjing Department of Health (grant no. YKK14176).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
Cell culture and apoptosis detection were conducted
by XZ and ZY. Western blotting and RT-qPCR were performed by TX and
ZA. HPLC was analyzed by MH. Experimental data were analyzed by WC
and XW. FZ and ZY were the major contributors to study design and
wrote the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Plentz RR and Malek NP: Clinical
presentation, risk factors and staging systems of
cholangiocarcinoma. Best Pract Res Clin Gastroenterol. 29:245–252.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bergquist A and von Seth E: Epidemiology
of cholangiocarcinoma. Best Pract Res Clin Gastroenterol.
29:221–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rizvi S and Gores GJ: Pathogenesis,
diagnosis, and management of cholangiocarcinoma. Gastroenterology.
145:1215–1229. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rizvi S, Borad MJ, Patel T and Gores GJ:
Cholangiocarcinoma: Molecular pathways and therapeutic
opportunities. Semin Liver Dis. 34:456–464. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhu AX: Future directions in the treatment
of cholangiocarcinoma. Best Pract Res Clin Gastroenterol.
29:355–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ding X, Zhu F, Yang Y and Li M:
Purification, antitumor activity in vitro of steroidal
glycoalkaloids from black nightshade (Solanum nigrum L.). Food
Chem. 141:1181–1186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Munari CC, de Oliveira PF, Campos JC,
Martins Sde P, Da Costa JC, Bastos JK and Tavares DC:
Antiproliferative activity of Solanum lycocarpum alkaloidic extract
and their constituents, solamargine and solasonine, in tumor cell
lines. J Nat Med. 68:236–241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ding X, Zhu FS, Li M and Gao SG: Induction
of apoptosis in human hepatoma SMMC-7721 cells by solamargine from
Solanum nigrum L. J Ethnopharmacol. 139:599–604. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sani IK, Marashi SH and Kalalinia F:
Solamargine inhibits migration and invasion of human hepatocellular
carcinoma cells through down-regulation of matrix
metalloproteinases 2 and 9 expression and activity. Toxicol In
Vitro. 29:893–900. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie X, Zhu H, Yang H, Huang W, Wu Y, Wang
Y, Luo Y, Wang D and Shao G: Solamargine triggers hepatoma cell
death through apoptosis. Oncol Lett. 10:168–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shiu LY, Chang LC, Liang CH, Huang YS,
Sheu HM and Kuo KW: Solamargine induces apoptosis and sensitizes
breast cancer cells to cisplatin. Food Chem Toxicol. 45:2155–2164.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Tang Q, Wu J, Zheng F, Yang L and
Hann SS: Inactivation of PI3-K/Akt and reduction of SP1 and p65
expression increase the effect of solamargine on suppressing EP4
expression in human lung cancer cells. J Exp Clin Cancer Res.
34:1542015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang CH, Shiu LY, Chang LC, Sheu HM, Tsai
EM and Kuo KW: Solamargine enhances HER2 expression and increases
the susceptibility of human lung cancer H661 and H69 cells to
trastuzumab and epirubicin. Chem Res Toxicol. 21:393–399. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koff JL, Ramachandiran S and
Bernal-Mizrachi L: A time to kill: Targeting apoptosis in cancer.
Int J Mol Sci. 16:2942–2955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fulda S: Targeting apoptosis for
anticancer therapy. Semin Cancer Biol. 31:84–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Su Z, Yang Z, Xu Y, Chen Y and Yu Q:
Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol
Cancer. 14:482015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Razumilava N and Gores GJ:
Cholangiocarcinoma. Lancet. 383:2168–2179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fulda S: Targeting extrinsic apoptosis in
cancer: Challenges and opportunities. Semin Cell Dev Biol.
39:20–25. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lopez J and Tait SW: Mitochondrial
apoptosis: Killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldar S, Khaniani MS, Derakhshan SM and
Baradaran B: Molecular mechanisms of apoptosis and roles in cancer
development and treatment. Asian Pac J Cancer Prev. 16:2129–2144.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aneknan P, Kukongviriyapan V, Prawan A,
Kongpetch S, Sripa B and Senggunprai L: Luteolin arrests cell
cycling, induces apoptosis and inhibits the JAK/STAT3 pathway in
human cholangiocarcinoma cells. Asian Pac J Cancer Prev.
15:5071–5076. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang N, Han F, Cui H, Huang J, Wang T,
Zhou Y and Zhou J: Matrine suppresses proliferation and induces
apoptosis in human cholangiocarcinoma cells through suppression of
JAK2/STAT3 signaling. Pharmacol Rep. 67:388–393. 2015. View Article : Google Scholar : PubMed/NCBI
|