Introduction
Osteosarcoma is the most common type of bone cancer
and it usually occurs in children and adolescents. It was also
revealed that metastasis to the lung occurs quickly in patients
with osteosarcoma (1). Although
neoadjuvant chemotherapy in osteosarcoma has greatly enhanced the
survival rate, numerous patients suffer from toxic side effects due
to long-term application of chemotherapeutic drugs. Tumor necrosis
factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a
biological agent that was identified in recent years, has a
selective killing effect on tumor cells without causing damage to
normal cells and has been considered to be a promising novel
biological treatment in the near future (2,3). A large
number of human tumor cells are sensitive to the apoptosis induced
by TRAIL; however, osteosarcoma cells are not sensitive to TRAIL.
Recent studies have suggested that X-linked inhibitor of apoptosis
(XIAP) was frequently overexpressed in cancer cells and served an
important role in the resistance to TRAIL in certain tumor cells
(4–6).
Embelin, identified from the fruit of the Embelia ribes BURM
(Myrsinaceae), was originally discovered by screening a library of
natural products derived from Oriental traditional medicine
(7–9).
Recently, scholars used Embelin as a small-molecule inhibitor of
XIAP to induce the apoptosis of tumor cells and to restore the
sensitivity of certain tumor cells to TRAIL (10). However, the therapeutic effect of
Embelin on TRAIL-induced apoptosis in osteosarcoma cells has not
been investigated. The present study examined whether Embelin may
enhance the susceptibility of human osteosarcoma cells to TRAIL and
investigated the associated mechanism.
Materials and methods
Reagents
The osteosarcoma MG-63 (code no: TCHu 124) and U2OS
(code no: TCHu 88) cell lines were purchased from the Cell Research
Center of the Chinese Academy of Sciences (Shanghai, China).
RPMI-1640 medium, Dulbecco's modified Eagle's high glucose medium
(DMEM) and trypsin were purchased from Gibco; Thermo Fisher
Scientific, Inc. (Waltham, MA, USA). Embelin was obtained from
Abcam (Cambridge, UK). Pan-caspase inhibitor z-VAD-fmk and
caspase-8 inhibitor z-IETD-fmk were purchased from Abcam
(Cambridge, UK). Rabbit antibodies against caspases 3 and 8, death
receptor (DR) 5, XIAP and MMP-9 were purchased from Abcam.
Cell culture and research methods
The U2OS cells were cultured in RPMI-1640 medium
with 10% fetal bovine serum (FBS) and the MG63 cells were cultured
in DMEM with 10% FBS. The two cell lines were cultured in an
incubator in a humidified 5% CO2 atmosphere. The cells
entering the logarithmic growth phase were selected. In the
pre-experiment, U2OS cells (0.8×105/ml) and MG63 cells (1×105/ml)
were inoculated in 96-well plates with culture medium (Gibco;
Thermo Fisher Scientific, Inc.) containing TRAIL with
concentrations of 1, 10 and 100 ng/ml or containing Embelin with
concentrations of 5, 10 and 20 µl/l or experimental control without
drug. Following culture for 12, 24 and 48 h, 20 µl MTT was added
and incubated at 37°C for 4 h, prior to being dissolved in 150 µl
dimethyl sulfoxide. The absorbance was subsequently measured at a
wavelength of 570 nm. According to the pre-experiment on the
concentration of TRAIL and Embelin using an MTT assay,
concentrations of 1, 10 and 100 ng/ml were selected for the TRAIL
group, concentrations of 5, 10 and 20 µl/l for the Embelin group,
and 100 ng/ml TRAIL combined with 20 µl/Embelin for the combined
group. A concentration of 100 ng/ml TRAIL or 20 µmol/l Embelin did
not reach the half maximal inhibitory concentration at 12, 24 and
48 h, according to the pre-experiment. The study was performed
using the following time intervals: 12, 24 and 48 h.
MTT assay
U2OS cells (0.8×105/ml) and MG63 cells
(1×105/ml) were inoculated in 96-well plates, and were
added to the culture medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing reagents of different concentrations
of 100 µl drug or experimental control without drug. Following
culture for 12, 24 and 48 h, 20 µl MTT was added and incubated at
37°C for 4 h, prior to being dissolved in 150 µl dimethyl
sulfoxide. The absorbance was subsequently measured at a wavelength
of 570 nm. The survival rate of tumor cells (%)=experimental group
A value/control group A value ×100 (11).
Detection of the morphology of
apoptotic cells
The apoptotic cells were directly observed under an
inverted phase contrast microscope at the magnification of ×400. A
cover slide was placed in the 6-well plate, and following
apoptosis, cells were fixed using 99.5% absolute ethyl-alcohol at
37°C for 10 min and stained with 0.5 ml Hoechst 33258 staining
solution at 37°C for 5 min. Images were subsequently captured using
a fluorescence microscope at the magnification of ×400 on the
object slide covered by the cover slide and a drop of anti-fading
solution from Hoechst Staining kit (Beyotime Institute of
Biotechnology, Shanghai, China) was added.
Determination of cell apoptosis by
flow cytometry
Following culture for 12, 24 or 48 h, apoptosis was
detected using the Annexin V-fluorescein isothiocyanate (FITC)
Apoptosis Detection kit (BD Biosciences, Franklin Lakes, NJ, USA).
Cells in 6-well plates (5×105/well) were detached by
trypsinization and washed three times in phosphate-buffered saline,
centrifuged at 4°C at 1,000 × g for 5 min and resuspended in 195 µl
Annexin V-FITC binding buffer (BD Biosciences). Annexin V-FITC (5
µl) was added and mixed. Following this, the U2OS and MG63 cells
were stained by Annexin V-FITC in binding buffer in the dark for 10
min at room temperature. The cells were subsequently centrifuged at
4°C at 1,000 × g for 5 min and were resuspended in 190 µl Annexin
V-FITC binding buffer. Finally, 10 µl propidium iodide (PI)
staining solution was added and mixed at 4°C for 15 min. The U2OS
and MG63 cells were maintained on ice in the dark and immediately
subjected to flow cytometric analysis (12). The data were analyzed using the Cell
Quest software (version 7.5.3; BD Biosciences, San Jose, CA,
USA).
Determination of the invasion
ability
The invasive ability of U2OS and MG63 cells was
calculated by the number of cells that passed through a
polycarbonate membrane (8 µmol/l pore). The chamber was washed with
serum-free medium (Gibco; Thermo Fisher Scientific, Inc.), and then
20 µl Matrigel (dilution, 1:8; BD Biosciences) was added to evenly
cover the surface of the polycarbonate membrane. Pre-processed DMEM
(200 µl) containing 2×105 cells with 10% FBS was placed
into the upper Transwell chamber while 600 µl DMEM with 10% FBS was
placed into the lower chamber. The Transwell invasion system was
added to the cell incubator for 24 h. The upper chamber was removed
and the cells were stained with 1% crystal violet for 15 min at
room temperature (13). Invading
cells that attached to the surface of the membrane were observed
under an inverted phase contrast microscope at a magnification of
×400.
Western blot analysis
Since MG-63 cells exhibit certain characteristics of
osteoblasts and U2OS cells are more malignant and stable than MG-63
cells, U2-OS cells were selected for western blot analysis
(14). A total Protein Extraction kit
(Beyotime Institute of Biotechnology, Haimen, China) was used to
extract the total protein from U2OS cells, according to the
manufacturer's protocol. Protein concentration was then determined
using a bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology), according to the manufacturer's protocol. Protein
(40 µg/lane) was separated by 10% SDS-PAGE using an SDS-PAGE Gel
kit (Beyotime Institute of Biotechnology), according to the
manufacturer's protocol, prior to being transferred onto 0.2-µm
pore polyvinylidene fluoride membranes (Beyotime Institute of
Biotechnology). The membrane was blocked with 5% skimmed milk in
TBST at room temperature for 2 h, prior to being incubated with
antibodies against the following: rabbit anti-caspase-3 (dilution,
1:5,000; cat. no. ab32351), rabbit anti-caspase-8 (dilution,
1:5,000; cat. no. ab25901), rabbit anti-DR-5 (dilution, 1:1,000;
cat. no. ab199357), rabbit anti-XIAP (dilution, 1:1,000; cat. no.
ab21278; all Abcam) and rabbit anti-MMP-9 (dilution, 1:1,000; cat.
no. 10375–2-AP; ProteinTech Group, Inc., Chicago, IL, USA) in TBST
containing 1% bovine serum albumin overnight at 4°C. A rabbit
anti-β-actin monoclonal antibody (dilution, 1:5,000; cat. no.
ab8227; Abcam) was used as a control for caspase-3 and caspase-8
while a rabbit anti-GAPDH monoclonal antibody (dilution, 1:5,000;
cat. no. ab181602; Abcam) was used as a control for DR-5, XIAP and
MMP-9. The membranes were subsequently washed three times for 30
min in Tris-buffered saline containing Tween 20 (1×TBST) and were
incubated with the horseradish peroxidase-conjugated AffiniPure
goat anti-rabbit IgG secondary antibody (dilution, 1:50,000; cat.
no. ab182016; Abcam) diluted with TBST for 2 h at room temperature,
prior to being washed again in TBST three times for 30 min. A
BeyoECL Plus kit (Beyotime Institute of Biotechnology) was used to
detect protein bands, according to the manufacturer's protocol.
MF-Chemisis 2.0 (DNR Bio-Imaging Systems, Ltd., Neve Yamin, Israel)
and GelCapture software (version 2.24, DNR Bio-Imaging Systems,
Ltd.) were used to obverse and analyze the protein bands (15,16).
Statistical analysis
Statistical analysis was performed using Windows
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA). Data are
presented as the mean ± standard deviation of three independent
experiments. Data were compared using one-way analysis of variance,
followed by the Student-Newman-Keuls test. P<0.05 was considered
to indicate a statistically significant difference.
Results
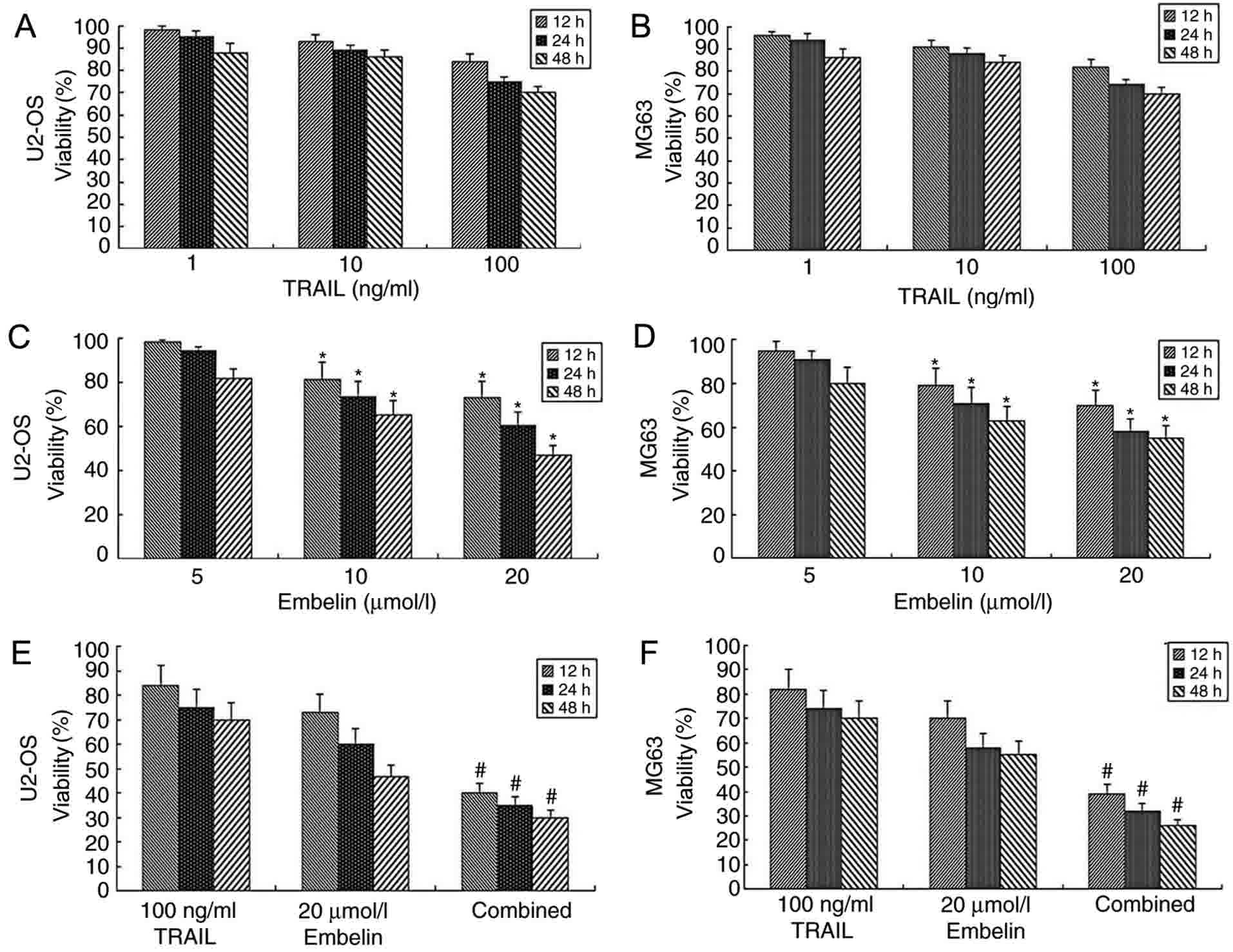
Viability of tumor cells in the
presence of combined TRAIL and Embelin
While concentrations of 1, 10 or 100 ng/ml TRAIL
were used in U2OS or MG63 cells, no significant difference in the
dose-inhibiting effect was observed between different groups
(P>0.05; Fig. 1A and B). This
observation was consistent with the pre-experimental results. In
the pre-experiment, it was revealed that the use of TRAIL alone
with concentrations ranging between 0.1 and 1,000 ng/ml has little
effect on U2OS or MG63 cells. As the purpose of the present study
was to examine and verify the effect of combined application of
Embelin and TRAIL to osteosarcoma cells, appropriate concentrations
were selected. When concentrations of 5 µmol/l Embelin were applied
to U2OS or MG63 cells at 12, 24 or 48 h, no significant difference
in the dose-inhibiting effect was observed compared with at 0 h
(P>0.05; Fig. 1C and D). However,
when concentrations of 10 or 20 µmol/l Embelin were applied to U2OS
or MG63 cells, cell viability was inhibited (P<0.05; Fig. 1C and D). When a combination of 100
ng/ml TRAIL and 20 µmol/l Embelin was applied to U2OS or MG63
cells, the viability rate was significantly lower (P<0.01),
compared with the individual treatment groups (Fig. 1E and F). The results of the present
study demonstrated that the combined application of TRAIL and
Embelin had a strong inhibitory effect on the viability rates of
U2OS and MG63 cells.
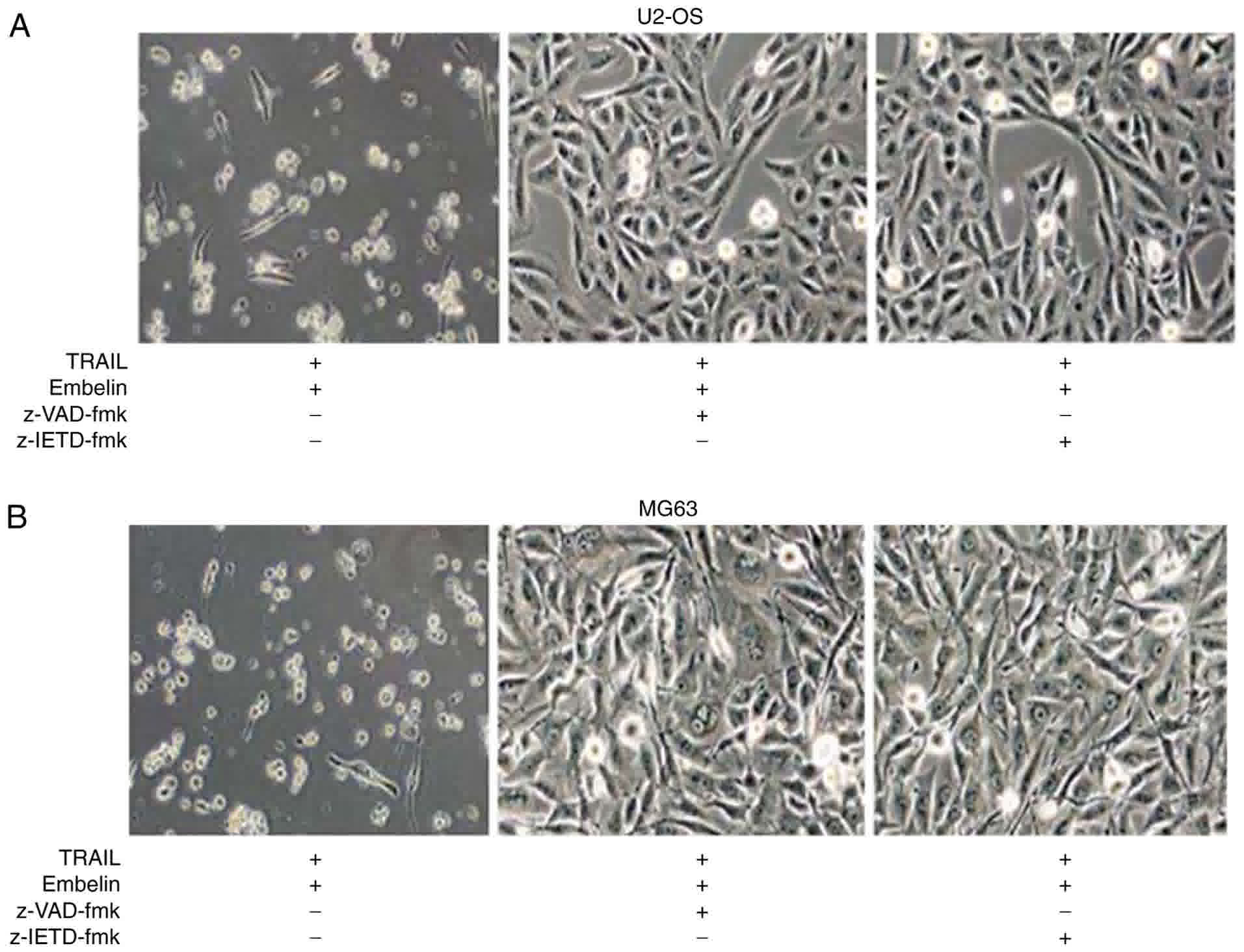
Changes in the apoptosis rate of U2OS
and MG63 cells
U2OS and MG63 cells were added to the surface of a
dish in an angular position (Fig. 2A and
B). Following the application of 100 ng/ml TRAIL or 20 µmol/l
Embelin, few of the osteosarcoma cells were small and round
(Fig. 2A and B). However, with the
combined application of 100 ng/ml TRAIL and 20 µmol/l Embelin,
chromosomes were condensed, and cells became non-adherent and
suspended in the DMEM (Fig. 2A and
B). Cell death was blocked by joint application of TRAIL and
Embelin following the use of 150 µM pan-caspase inhibitor z-VAD-fmk
or 150 µM caspase-8 inhibitor z-IETD-fmk (Fig. 3A and B), which indicated that the
process of cell death requires caspases activation. In a field of
fluorescence microscope, the majority of U2OS and MG63 cells in the
control group were lightly-stained and the morphology of the cells
were condensed and exhibited fluorescence (Fig. 2C and D), while in the cells treated
with the individual application of 100 ng/ml TRAIL or 20 µmol/l
Embelin, only a small number of cells were lightly-stained and
condensed (Fig. 2C and D). With the
combined application of 100 ng/ml TRAIL and 20 µmol/l Embelin, a
large number of cells were condensed and exhibited fluorescence,
indicating the apoptosis of numerous cells (P<0.05) (Fig. 2C and D).
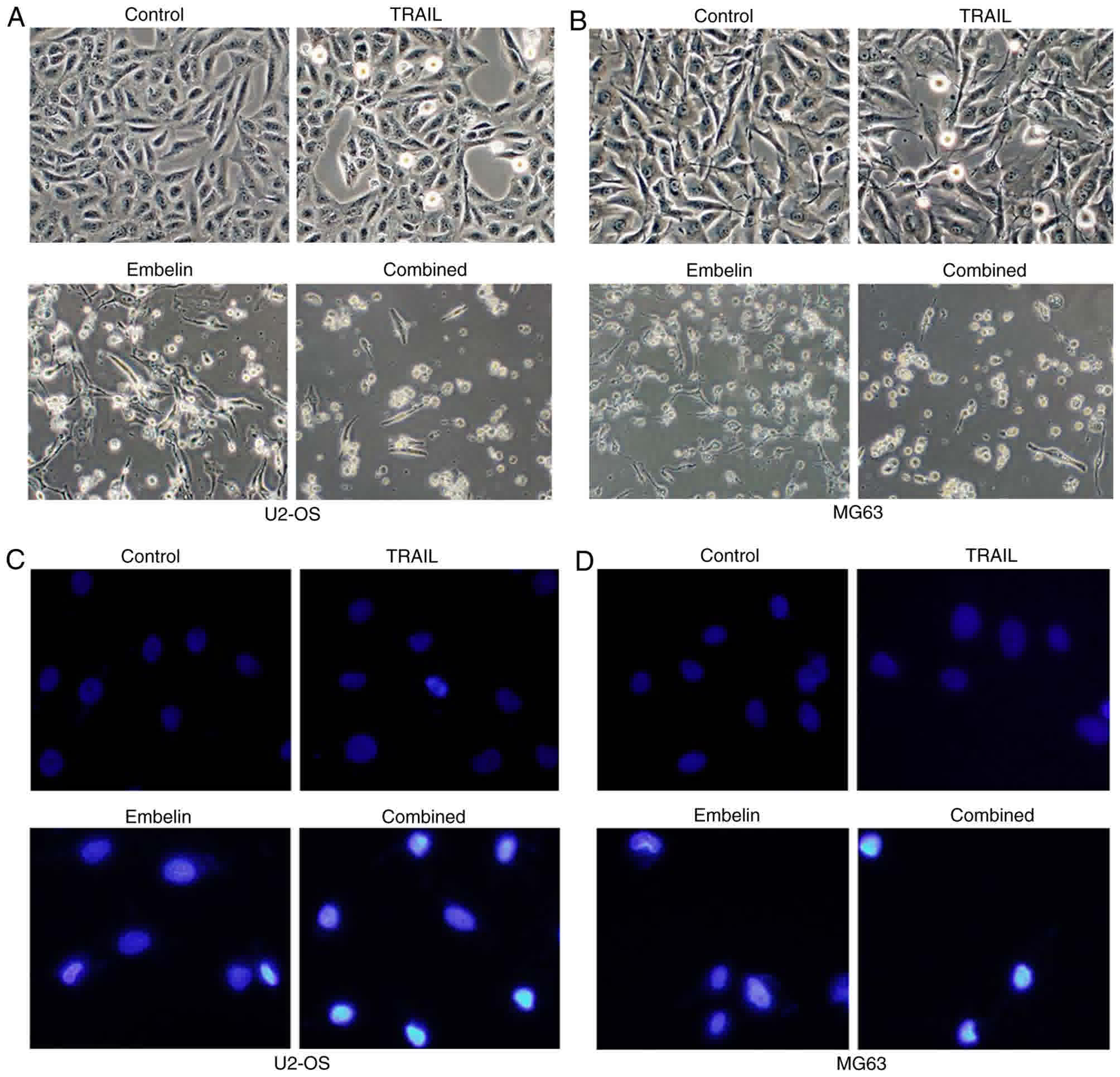 | Figure 2.Morphological changes and fluorescent
staining of U2OS and MG63 cells treated with different drugs after
48 h. (A) The morphological appearance of U2OS cells (control,
treated with 100 ng/ml TRAIL, treated with 20 µmol/l Embelin or
treated with a combination of the two) under an inverted phase
contrast microscope. (B) The morphological appearance of MG63 cells
(control, treated with 100 ng/ml TRAIL, treated with 20 µmol/l
Embelin or treated with a combination of the two) under an inverted
phase contrast microscope. (C) Fluorescent staining of U2OS cells
(control, treated with 100 ng/ml TRAIL, treated with 20 µmol/l
Embelin or a combination of the two) under a fluorescence
microscope. (D) Fluorescent staining of MG63 cells (control,
treated with 100 ng/ml TRAIL, treated with 20 µmol/l Embelin or a
combination of the two) under a fluorescence microscope.
Magnification, ×400. TRAIL, tumor necrosis factor-related
apoptosis-inducing ligand. |
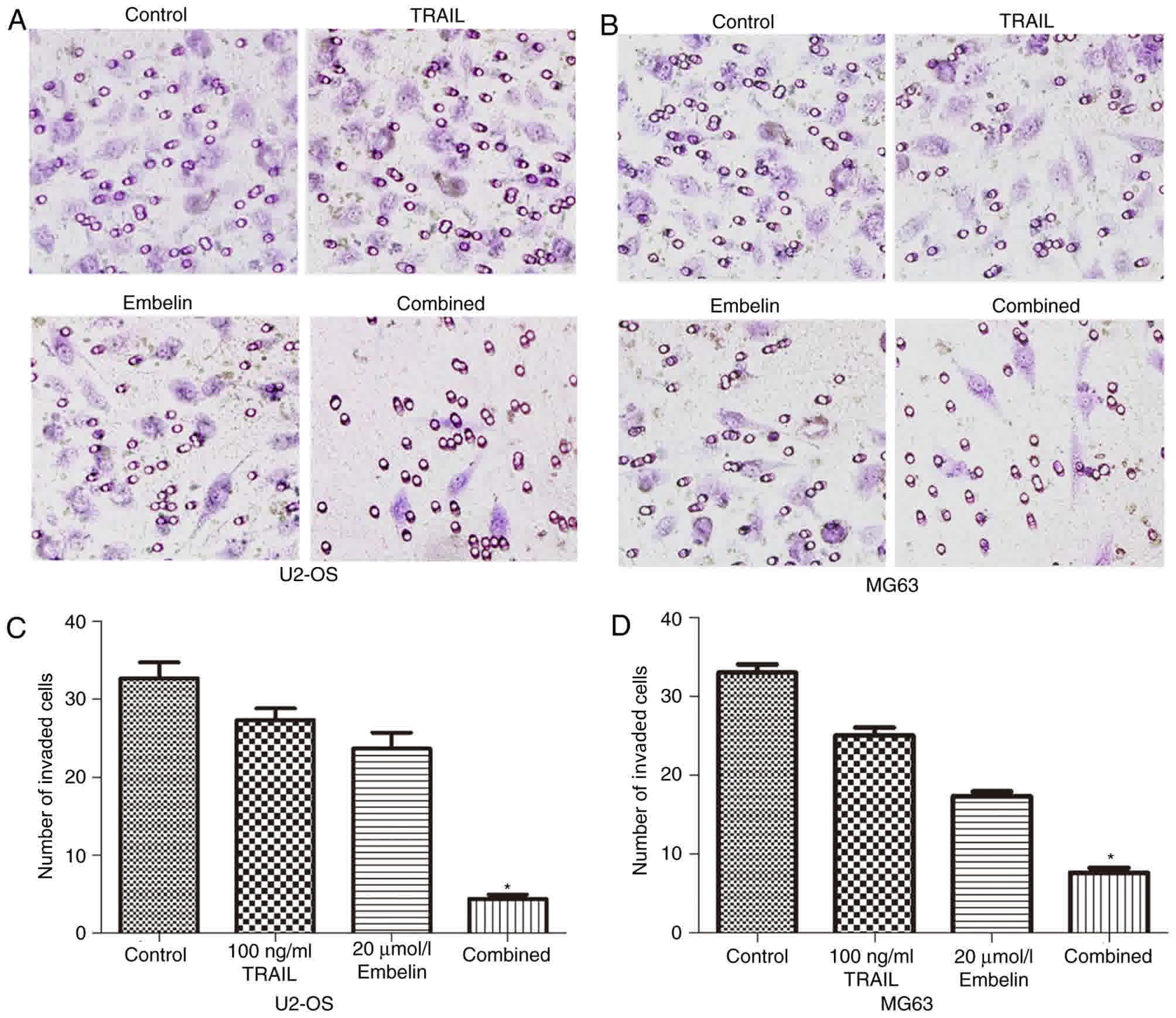
Effect of Embelin on the invasive
ability of U2OS and MG63 cells
The Transwell experiments indicated that the number
of U2OS and MG63 cells in the combined treatment group passing
through the polycarbonate membrane was significantly less than that
in the individual treatment groups and the control group (Fig. 4A and B). Cells that were able to
invade to the lower side of the membrane in the Transwell assays
after 24-h incubation were quantified (Fig. 4C and D). These data indicated that the
combined application of TRAIL and Embelin may diminish the invasion
of U2OS and MG63 cell lines more significantly than the individual
application of either treatment.
Effect of Embelin on the apoptosis of
U2OS and MG63 cells
Annexin V and PI staining experiment results
demonstrated that, with the combined application of 100 ng/ml TRAIL
and 20 µmol/l Embelin, the number of apoptotic cells was more than
that in the control group and the individual treatment groups
(P<0.01; Fig. 5). As Annexin V and
PI staining results demonstrated in Fig.
5A, the early apoptotic rates of the control group, the
TRAIL-only group, the Embelin-only group and the combined treatment
group in U2OS cells were 0.73, 3.54, 12.33 and 20.71%,
respectively. The late apoptotic rates in U2OS cells were 1.87,
6.12, 6.82 and 10.84%, respectively, and the total apoptotic rates
in U2OS cells were 2.60, 9.66, 19.15 and 31.55%, respectively. A
similar trend was also observed in the MG63 cells (Fig. 5B). The early apoptotic rates of the
control group, the TRAIL-only group, the Embelin-only group and the
combined treatment group in MG63 cells were 0.94, 3.51, 5.76 and
10.17%, respectively, the late apoptotic rates in MG63 cells were
2.43, 4.18, 10.64 and 15.14%, respectively, and the total apoptotic
rates in MG63 cells were 3.37, 7.33, 16.40 and 25.31%,
respectively. These data indicated that the combined treatment may
increase the apoptosis rate of U2OS and MG63 cells, compared with
individual treatment with either drug (Fig. 5C and D).
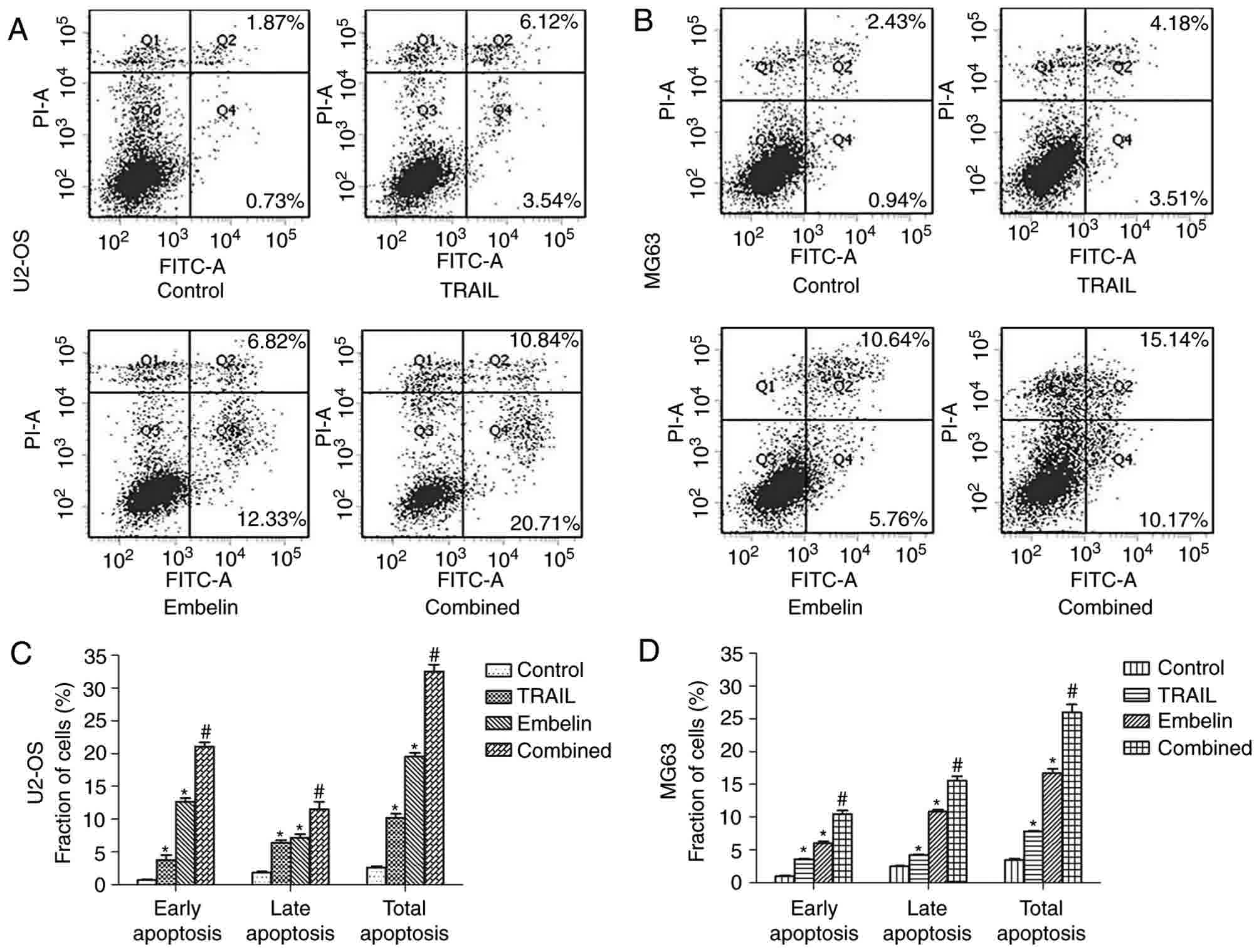 | Figure 5.Apoptosis in U2OS and MG63 cells
treated with different drugs after 24 h. The four groups of (A)
U2OS and (B) MG63 cells (control, treated with 100 ng/ml TRAIL,
treated with 20 µmol/l Embelin or treated with a combination of the
two) were incubated for 24 h. Cells stained with FITC-conjugated
Annexin V and PI were analyzed by flow cytometric analysis. In the
lower right quadrant, early apoptotic cells were observed, and the
necrotic or late apoptotic cells were located in the upper right
quadrant. The percentage of early apoptotic, late apoptotic and
total apoptotic (C) U2OS and (D) MG63 cells (control, treated with
100 ng/ml TRAIL, treated with 20 µmol/l Embelin or treated with a
combination of the two) for 24 h. Data are presented as the mean ±
standard deviation of three independent experiments. *P<0.01 vs.
control group; #P<0.01 vs. TRAIL group or Embelin
group. TRAIL, tumor necrosis factor-related apoptosis-inducing
ligand; FITC, fluorescein isothiocyanate; PI, propidium iodide. |
The expression of caspase-3,
caspase-8, cleaved caspase-3, DR5, XIAP and MMP-9 in U2OS
cells
Following exposure to 100 ng/ml TRAIL, 20 µmol/l
Embelin or the combined application of the two for 48 h, expression
levels of caspase-3, caspase-8, cleaved caspase 3, DR5, XIAP and
MMP-9 in U2OS cells were measured using western blot analysis in
the present study. The levels of caspase-3, caspase-8, cleaved
caspase 3 and DR5 in the combined treatment group were
significantly higher than that of the individual treatment groups
and the control group (P<0.05). By contrast, levels of XIAP and
MMP-9 in the combined treatment group were significantly lower than
in the individual treatments groups and the control group
(P<0.05) (Fig. 6).
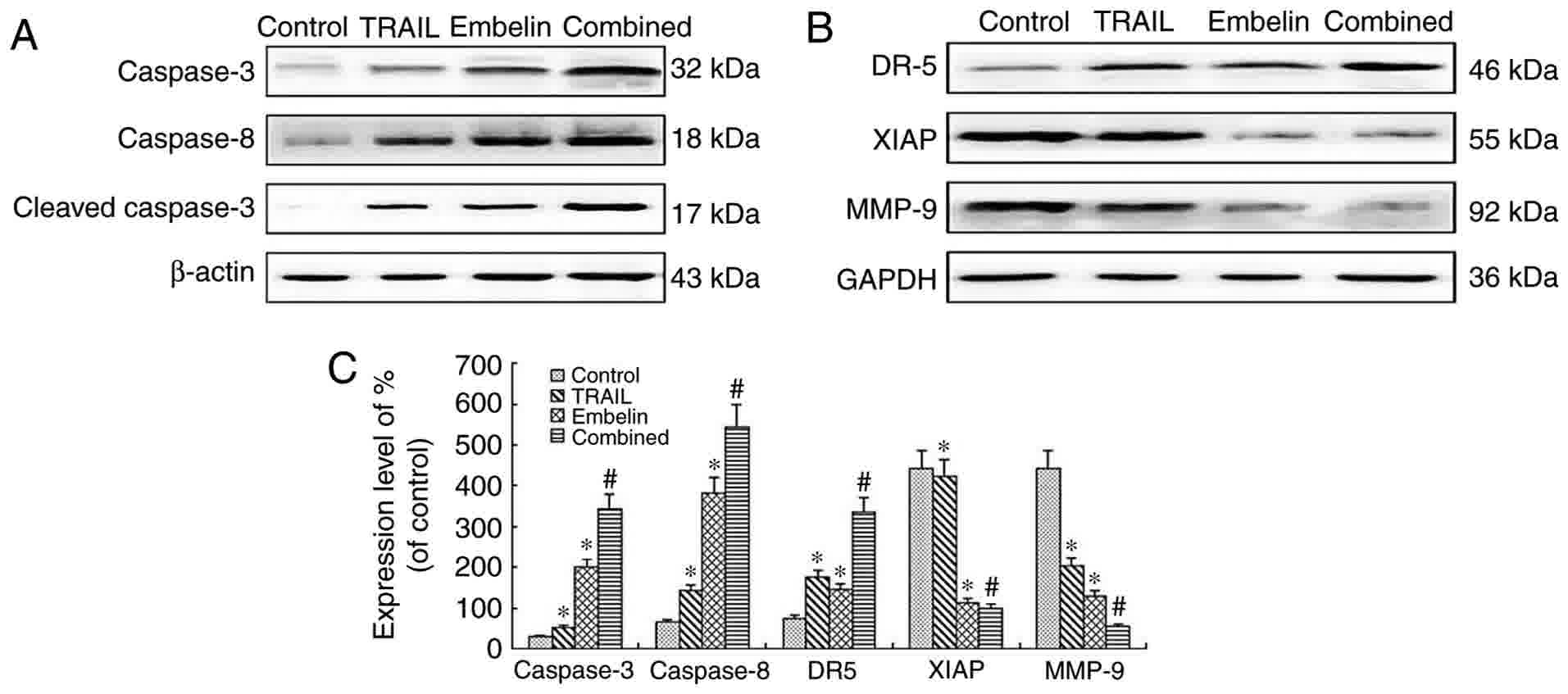 | Figure 6.Western blot analysis of the
expression of proteins after U2OS cells were cultured for 48 h. (A)
The levels of caspase-3 (32 kDa), caspase-8 (18 kDa) and cleaved
caspase-3 (17 kDa) were analyzed by western blot analysis. There
was a marked increase in the expression of caspase-3, cleaved
caspase-3, caspase-8 in the combined treatment group, P<0.01.
(B) The levels of XIAP (55 kDa), MMP-9 (92 kDa) and DR5 (46 KDa)
were analyzed by western blot analysis. There was a marked
downregulation in the expression of XIAP and MMP-9 in the combined
treatment group, P<0.01. However, there was a marked
upregulation in the expression of DR5 in the combination group,
P<0.01. (C) Quantification of relative protein expression. Data
are presented as the mean ± standard deviation of three independent
experiments. *P<0.01 vs. control group; #P<0.01
vs. TRAIL group or Embelin group. TRAIL, tumor necrosis
factor-related apoptosis-inducing ligand; XIAP, X-linked inhibitor
of apoptosis protein; MMP-9, matrix metalloproteinase 9; DR5, death
receptor 5. |
Discussion
TRAIL is a member of the TNF super family of
cytokines (17,18). TRAIL is a potentially important
anticancer agent, since it selectively kills malignant cells while
leaving normal cells unaffected (19,20). TRAIL
induces apoptosis through interactions with its death domain
containing receptors DR4 and DR5 (21,22). Once
activated, TRAIL receptors recruit Fas-associated protein with
death domain as a major cellular adaptor protein, which triggers
the auto-activation of caspase-8 and subsequently leads to the
activation of downstream caspases, including caspases-3, −7 and −9.
This resulted in the cleavage of cellular substrates and ultimately
cell death. However, certain tumor cells, including gastric,
hepatic, lung and osteosarcoma cells are not sensitive to TRAIL
(23–26). The present study also demonstrated
that there was no significant difference in the viability rate of
U2OS and MG63 cells between the TRAIL group and the control group,
which confirmed the tolerance of U2OS and MG63 cells to TRAIL
(Fig. 1A). Although previous studies
have revealed that the combination of TRAIL and certain
chemotherapy drugs may improve the sensitivity of osteosarcoma
cells to TRAIL (27,28), to a certain extent, such treatment
regimens continue to depend upon chemotherapy drugs and side
effects to healthy cells are inevitable. Therefore, identifying a
treatment plan regarding TRAIL that has fewer side effects has
become an aim for numerous researchers worldwide (29). Recent studies regarding biologically
targeted therapy have revealed that the function of TRAIL in
conducting the death signal into cells may be doubled by the high
expression of DR4 or DR5 (30,31). Such
studies may speed up the advancement in application of TRAIL in the
treatment of osteosarcoma and have become a hot topic. Therefore,
the aim of the present study was to identify a drug that may be
used in combination with TRAIL to reduce the resistance of
osteosarcoma cells to TRAIL by activating DR4 or DR5.
XIAP is one of the best characterized and most
potent endogenous inhibitors of the caspases and is therefore
considered a key physiological regulator of cell death (5,6). XIAP
inhibits the upstream caspase-9 by binding it to its BIR3 domain,
and the downstream caspase-3 and caspase-7 by binding them to its
BIR2 domain. XIAP expression is elevated in numerous types of
cancer including lung, ovarian, colon and kidney cancer, and
myeloid leukemia, which was also responsible for resistance to
chemotherapy (32). Embelin was
identified ~50 years ago as an active component of the Embelia
ribes BURM (33). Myrsinaceae, which
has been used as traditional medicine for thousands of years to
treat a diverse range of illnesses, including fever, inflammatory
diseases and various gastrointestinal ailments and therefore, must
pose a low toxicity threat (34).
Until recently, Embelin has been regarded as an inhibitor of XIAP
to induce the proliferation suppression and apoptosis of human
cancer cells in various organs, including pancreatic, colon and
prostate cancer, as well as leukemia (35–37). At,
present, to the best of our knowledge, no reports have stated that
Embelin inhibited the proliferation and invasion of osteosarcoma
cells. Furthermore, a recent study demonstrated that Embelin may
upregulate the expression of DR4 or DR5, and increase the
susceptibility of tumor cells to TRAIL (38). In our pre-experiment, it was revealed
that Embelin posed a killing effect on osteosarcoma cells and,
based on this, further research was performed to investigate
whether or not Embelin may enhance the TRAIL-DR5 pathway, thereby
increasing the sensitivity of osteosarcoma cells in order to
identify a novel strategy for the use of TRAIL.
The results of the present study suggested that the
combined application of TRAIL and Embelin had a synergistic
apoptotic effect compared with that of the individual application
of either treatment. The results of the MTT assay demonstrated that
combined treatment had a stronger inhibitory effect on the
viability rate of U2OS and MG63 cells compared with the individual
application of either agent (Fig. 1).
Furthermore, combined treatment caused more distinct morphological
changes compared with the application of each agent individually
(Fig. 2). The present study also
demonstrated that the killing effect of the combined treatment was
achieved by inducing the apoptosis of osteosarcoma cells, as
revealed by Hoechst staining (Fig. 2C and
D). Furthermore, Annexin V and PI staining results indicated
that the combined application of TRAIL and Embelin also served an
important role in the apoptotic effect on osteosarcoma cells
(Fig. 5). The apoptosis-induced
effects on the combined treatment group were much stronger than
that of the individual treatment groups and the control group.
These results are consistent with those of previous studies on
other types of tumor cell (39). The
results of the Transwell invasion chamber experiments revealed that
the inhibition of invasion in the combined treatment group was more
significant than that in the control group and the individual
treatment groups (Fig. 4A and B).
Furthermore, the expression of MMP-9, which is well known to be the
most important indicator of invasion ability (40), was significantly lower in the combined
treatment group than in the control group and the individual
treatment groups (Fig. 6B). All these
results revealed that the combination of Embelin and TRAIL may
inhibit the invasion of osteosarcoma cells. Similarly to the change
in MMP-9 expression, the expression of XIAP was also downregulated
due to the inhibitory effects of the treatment with Embelin
(Fig. 6B). In addition, the
combination of the two treatments upregulated the expression of
DR5, cleaved-caspase-3 and caspase-8 (Fig. 6A and B). The total caspase-3
expression and the cleaved-caspase-3 expression were also
increased. Based on the aforementioned results, the mechanism
through which Embelin enhances TRAIL-induced apoptosis of
osteosarcoma may be that Embelin upregulates the expression of DR5,
thereby increasing the susceptibility of osteosarcoma cells to
TRAIL, and that Embelin inhibits the expression of XIAP, which may
upregulate the expression of caspases. These two pathways
eventually result in the apoptosis of osteosarcoma cells.
Despite the fact that a number of studies have
reported that the combination of Embelin and TRAIL induced the
apoptosis of cancer cells, the results of the present study exhibit
certain differences. Yang et al (41) demonstrated that Embelin inhibited the
LMP1-mediated upregulation of XIAP and TRAIL resistance in
nasopharyngeal carcinoma cells. The combined application of Embelin
and TRAIL are limited to nasopharyngeal carcinoma cells
overexpressing LMP1 proteins. In the present study, the combined
application of Embelin and TRAIL were not limited to any
over-expressing protein. The study of Yang et al (42) revealed that the underlying mechanism
through which combined application of Embelin and TRAIL suppresses
the NF-κB-dependent survival pathway in human acute myeloid
leukemia cells. By contrast, the present study suggested that
Embelin enhanced the TRAIL-induced apoptosis of osteosarcoma cells
via the activation of the TRAIL-DR5-caspase signal pathway. By
contrast, the indicators and methods used in the present study are
similar to those used in a previously study that employed Embelin
as a small-molecule inhibitor of XIAP to induce the apoptosis of
tumor cells and restore the sensitivity of non-small cell lung
cancer cells to TRAIL (38). However,
in the present study, the therapeutic effect of Embelin on the
TRAIL-induced apoptosis of osteosarcoma cells was investigated.
In conclusion, the results of the present study
suggested that the XIAP inhibitor Embelin enhanced the
TRAIL-induced apoptosis and inhibited the invasion of osteosarcoma
cells, which may be related to the activation of the
TRAIL-DR5-caspase signal pathway. Therefore, Embelin may sensitize
osteosarcoma cells to TRAIL and alleviate the toxic side effects of
the chemotherapy drug on the human body and therefore, the
combination of TRAIL and Embelin may be a promising treatment for
patients with osteosarcoma. However, XIAP siRNA and in vivo
experiments are required in order to further validate the results
of the present study. Since B cell lymphoma 2 (Bcl-2) family
proteins serve an important role in Embelin-enhanced TRAIL-induced
cell apoptosis, whether or not caspase-induced apoptosis depends on
Bcl-2 requires further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Department of Liaoning Province (grant no. 2013225021),
the Science and Technology Department of Shenyang City (grant no.
F14-231-1-48).
Availability of data and materials
All data and materials described in the manuscript
are available upon reasonable request from the corresponding
author.
Authors' contributions
TH conceived and designed the experiments, HQ and YC
performed the experiments; HQ, XL and WZ wrote the paper; TL and GJ
performed the western blot analysis, SC and PL helped perform the
analysis. All authors read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interest
The authors declare that they have no competing
interests.
References
|
1
|
Rasalkar DD, Chu WC, Lee V, Paunipagar BK,
Cheng FW and Li CK: Pulmonary metastases in children with
osteosarcoma: Characteristics and impact on patient survival.
Pediatr Radiol. 41:227–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li X, Huang T, Jiang G, Gong W, Qian H and
Zou C: Proteasome inhibitor MG132 enhances TRAIL-induced apoptosis
and inhibits invasion of human osteosarcoma OS732 cells. Biochem
Biophys Res Commun. 439:179–186. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Somasekharan SP, Koc M, Morizot A, Micheau
O, Sorensen PH, Gaide O, Andera L and Martinou JC: TRAIL promotes
membrane blebbing, detachment and migration of cells displaying a
dysfunctional intrinsic pathway of apoptosis. Apoptosis.
18:324–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ng CP, Zisman A and Bonavida B: Synergy is
achieved by complementation with Apo2L/TRAIL and actinomycin D in
Apo2L/TRAIL-mediated apoptosis of prostate cancer cells: Role of
XIAP in resistance. Prostate. 53:286–299. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gillissen B, Richter A, Richter A,
Overkamp T, Essmann F, Hemmati PG, Preissner R, Belka C and Daniel
PT: Targeted therapy of the XIAP/proteasome pathway overcomes
TRAIL-resistance in carcinoma by switching apoptosis signaling to a
Bax/Bak-independent ‘type I’ mode. Cell Death Dis. 4:e6432013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fakler M, Loeder S, Vogler M, Schneider K,
Jeremias I, Debatin KM and Fulda S: Small molecule XIAP inhibitors
cooperate with TRAIL to induce apoptosis in childhood acute
leukemia cells and overcome Bcl-2-mediated resistance. Blood.
113:1710–1722. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tnah LH, Lee CT, Lee SL, Ng CH and Ng KK:
Development of microsatellites in Labisia pumila (Myrsinaceae), an
economically important Malaysian herb. Appl Plant Sci. 2:pii:
apps.1400019. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ndontsa BL, Tchinda A, Teponno RB, Mpetga
JS, Frédérich M and Tane P: Ardisikivuoside, a new triterpenoid
saponin from Ardisia kivuensis (Myrsinaceae). Nat Prod Commun.
7:515–516. 2012.PubMed/NCBI
|
|
9
|
Karimi E, Jaafar HZ, Aziz MA, Taheri S and
AzadiGonbad R: Genetic relationship among Labisia pumila
(Myrsinaceae) species based on ISSR-PCR. Genet Mol Res.
13:3301–3309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Allensworth JL, Aird KM, Aldrich AJ,
Batinic-Haberle I and Devi GR: XIAP inhibition and generation of
reactive oxygen species enhances TRAIL sensitivity in inflammatory
breast cancer cells. Mol Cancer Ther. 11:1518–1527. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li X, Huang T, Jiang G, Gong W, Qian H and
Zou C: Synergistic apoptotic effect of crocin and cisplatin on
osteosarcoma cells via caspase induced apoptosis. Toxicol Lett.
221:197–204. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong Y, Yin J, Cheng D, Kong Y, Yin J,
Cheng D, Lu Z, Wang N, Wang F and Liang M: Antithrombin III
attenuates AKI following acute severe pancreatitis. Shock. Jul
17–2017.(Epub ahead of print).
|
|
13
|
Song F, Wang H and Wang Y: Myeloid
ecotropic viral integration site 1 inhibits cell proliferation,
invasion or migration in human gastric cancer. Oncotarget.
8:90050–90060. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zou J, Zhang W and Li XL: Effects of SOST
gene silencing on proliferation, apoptosis, invasion, and migration
of human osteosarcoma cells through the Wnt/β-catenin signaling
pathway. Calcif Tissue Int. 100:551–564. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mahmood T and Yang PC: Western blot:
Technique, theory, and trouble shooting. N Am J Med Sci. 4:429–434.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Z, Cheng D, Yin J, Wu R, Zhang G, Zhao
Q, Wang N, Wang F and Liang M: Antithrombin III protects against
contrast-induced nephropathy. EBioMedicine. 17:101–107. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moon MH, Jeong JK, Seo JS, Seol JW, Lee
YJ, Xue M, Jackson CJ and Park SY: Bisphosphonate enhances TRAIL
sensitivity to human osteosarcoma cells via death receptor 5
upregulation. Exp Mol Med. 43:138–145. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Srinivasan S, Kumar R, Koduru S,
Chandramouli A and Damodaran C: Inhibiting TNF-mediated signaling:
A novel therapeutic paradigm for androgen independent prostate
cancer. Apoptosis. 15:153–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Voelkel-Johnson C: An antibody against DR4
(TRAIL-R1) in combination with doxorubicin selectively kills
malignant but not normal prostate cells. Cancer Biol Ther.
2:283–290. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Newsom-Davis T, Prieske S and Walczak H:
Is TRAIL the holy grail of cancer therapy? Apoptosis. 14:607–623.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mitsiades CS, Treon SP, Mitsiades N, Shima
Y, Richardson P, Schlossman R, Hideshima T and Anderson KC:
TRAIL/Apo2L ligand selectively induces apoptosis and overcomes drug
resistance in multiple myeloma: Therapeutic applications. Blood.
98:795–804. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Locklin RM, Federici E, Espina B, Hulley
PA, Russell RG and Edwards CM: Selective targeting of death
receptor 5 circumvents resistance of MG-63 osteosarcoma cells to
TRAIL-induced apoptosis. Mol Cancer Ther. 6:3219–3228. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang WQ, Zhang H, Wang HB, Sun YG, Peng
ZH, Zhou G, Yang SM, Wang RQ and Fang DC: Programmed cell death 4
(PDCD4) enhances the sensitivity of gastric cancer cells to
TRAIL-induced apoptosis by inhibiting the PI3K/Akt signaling
pathway. Mol Diagn Ther. 14:155–161. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
von Pawel J, Harvey JH, Spigel DR, Dediu
M, Reck M, Cebotaru CL, Humphreys RC, Gribbin MJ, Fox NL and
Camidge DR: Phase II trial of mapatumumab, a fully human agonist
monoclonal antibody to tumor necrosis factor-related
apoptosis-inducing ligand receptor 1 (TRAIL-R1), in combination
with paclitaxel and carboplatin in patients with advanced
non-small-cell lung cancer. Clin Lung Cancer. 15:188–196.e2. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng SJ, Wang P, Tsabary G and Chen YH:
Critical roles of TRAIL in hepatic cell death and hepatic
inflammation. J Clin Invest. 113:58–64. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Garnett TO, Filippova M and
Duerksen-Hughes PJ: Bid is cleaved upstream of caspase-8 activation
during TRAIL-mediated apoptosis in human osteosarcoma cells.
Apoptosis. 12:1299–1315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang DS, Miao XD, Ye ZM and Xu YS:
Synergistic induction of apoptosis by the combination of TRAIL and
low dose adriamycin in human osteosarcoma cell line U2OS. Zhonghua
Zhong Liu Za Zhi. 27:595–597. 2005.(In Chinese). PubMed/NCBI
|
|
28
|
Wu J, Zeng T, Wu X, Gao Q, Zhai W and Ding
Z: Ether à go-go 1 silencing in combination with TRAIL
overexpression has synergistic antitumor effects on osteosarcoma.
Cancer Biother Radiopharm. 28:65–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lanuti P, Bertagnolo V, Pierdomenico L,
Bascelli A, Santavenere E, Alinari L, Capitani S, Miscia S and
Marchisio M: Enhancement of TRAIL cytotoxicity by AG-490 in human
ALL cells is characterized by downregulation of cIAP-1 and cIAP-2
through inhibition of Jak2/Stat3. Cell Res. 19:1079–1089. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren YG, Wagner KW, Knee DA, Aza-Blanc P,
Nasoff M and Deveraux QL: Differential regulation of the TRAIL
death receptors DR4 and DR5 by the signal recognition particle. Mol
Biol Cell. 15:5064–5074. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schneider P, Thome M, Burns K, Bodmer JL,
Hofmann K, Kataoka T, Holler N and Tschopp J: TRAIL receptors 1
(DR4) and 2 (DR5) signal FADD-dependent apoptosis and activate
NF-kappaB. Immunity. 7:831–836. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yin D, Wang N, Zhang SL, Jiang Y, Lu YM,
Wei H, Huo NC, Xiao Q and Ou YL: Specific siRNA inhibits XIAP
expression in human endometrial carcinoma cell apoptosis. Cell
Biochem Biophys. 71:161–165. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Poojari R: Embelin-a drug of antiquity:
Shifting the paradigm towards modern medicine. Expert Opin Investig
Drugs. 23:427–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cragg GM, Newman DJ and Yang SS: Natural
product extracts of plant and marine origin having antileukemia
potential. The NCI experience. J Nat Prod. 69:488–498. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dai Y, Qiao L, Chan KW, Yang M, Ye J, Ma
J, Zou B, Gu Q, Wang J, Pang R, et al: Peroxisome
proliferator-activated receptor-gamma contributes to the inhibitory
effects of Embelin on colon carcinogenesis. Cancer Res.
69:4776–4783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu R, Zhu K, Li Y, Yao K, Zhang R, Wang H,
Yang W and Liu Z: Embelin induces apoptosis through down-regulation
of XIAP in human leukemia cells. Med Oncol. 28:1584–1588. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marsh JL, Jackman CP, Tang SN, Shankar S
and Srivastava RK: Embelin suppresses pancreatic cancer growth by
modulating tumor immune microenvironment. Front Biosci (Landmark
Ed). 19:113–125. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jiang L, Hao JL, Jin ML, Zhang YG and Wei
P: Effect of Embelin on TRAIL receptor 2 mAb-induced apoptosis of
TRAIL-resistant A549 non-small cell lung cancer cells. Asian Pac J
Cancer Prev. 14:6115–6120. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hu R, Yang Y, Liu Z, Jiang H, Zhu K, Li J
and Xu W: The XIAP inhibitor Embelin enhances TRAIL-induced
apoptosis in human leukemia cells by DR4 and DR5 upregulation.
Tumour Biol. 36:769–777. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu S, Zhang Z, Chen M, Li C, Liu L and Li
Y: Silibinin inhibits the migration and invasion of human gastric
cancer SGC7901 cells by downregulating MMP-2 and MMP-9 expression
via the p38MAPK signaling pathway. Oncol Lett. 14:7577–7582.
2017.PubMed/NCBI
|
|
41
|
Yang S, Li SS, Yang XM, Yin DH and Wang L:
Embelin prevents LMP1-induced TRAIL resistance via inhibition of
XIAP in nasopharyngeal carcinoma cells. Oncol Lett. 11:4167–4176.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang T, Lan J, Huang Q, Chen X, Sun X, Liu
X, Yang P, Jin T, Wang S and Mou X: Embelin sensitizes acute
myeloid leukemia cells to TRAIL through XIAP inhibition and NF-κB
inactivation. Cell Biochem Biophys. 71:291–297. 2015. View Article : Google Scholar : PubMed/NCBI
|