Introduction
Cellular adhesion is controlled by adherent
molecules in epithelial tissues, which are down-regulated in many
cancers to promote transformation and might profoundly affect
cellular migration and invasiveness (1–5). Since
cancer metastasis decreases the patient survival rates, regional
lymph node metastasis (RLNM) is one of the most adverse prognostic
factors (6–12) for almost all cancers, including oral
squamous cell carcinoma (OSCC). Therefore, elucidation of the
molecular mechanisms involved in cancer metastasis clearly is
needed to improve the prognosis (6,13). We
reported previously that endothelial cell-specific tyrosine-protein
kinase receptor (Tie2) is related closely to OSCC metastasis using
overexpressed Tie2 (oeTie2) cells and its neutralization technique
(14).
Tie2 and its ligand, angiopoietin 1 (Ang1), are
essential for vascular maturation and blood vessel remodeling
during embryonic angiogenesis (15–25). Ang1
regulates endothelial cell survival (16), anti-inflammatory actions (26–28), and
radiation-induced endothelial-cell damage (29). Ang1, produced by many types of cells,
has been described as a transcriptionally regulated molecule in
several tumors (30,31). However, the Tie2/Ang1 interaction is
poorly understood.
In the current study, we showed that the Tie2/Ang1
interaction promotes RLNM in OSCCs by controlling cellular
adhesion. Thus, our results indicated that Tie2 and Ang1 are
biomarkers for therapeutic targets in patients with OSCC.
Materials and methods
Ethics statement
The Ethics Committee of Chiba University approved
our study protocol (approval no. 236), which was performed
according to the tenets of the Declaration of Helsinki. All
patients provided written informed consent.
oeTie2 cells and tissue specimens
oeTie2 cells, which were established in our previous
study (14), were grown in Dulbecco's
modified Eagle medium (DMEM) (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) supplemented with 10% fetal bovine serum (Sigma-Aldrich;
Merck KGaA) and 50 units/ml of penicillin and streptomycin
(Sigma-Aldrich; Merck KGaA). We performed histopathological
diagnosis of each OSCC sample according to the World Health
Organization criteria at the Department of Pathology of Chiba
University Hospital (32). The
clinicopathological stages were determined based on the TNM
classification of the International Union against Cancer (33). Twenty (10 cases each, RLNM-positive,
RLNM-negative) pairs of primary OSCCs and patient-matched normal
oral epithelia were obtained during surgical resections performed
at Chiba University Hospital. The resected tissues were fixed in
20% buffered formaldehyde solution for pathologic diagnosis and
immunohistochemistry (IHC).
mRNA expression analysis
Total RNA was isolated using TRIzol Reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA),
according to the manufacturer's instructions. cDNA was generated
from 5 µg of total RNA using Ready-To-Go You-Prime First-Strand
Beads (GE Healthcare Life Sciences, Little Chalfont, UK) and oligo
(dT) primers (Hokkaido System Science Co., Ltd., Sapporo, Japan).
As described previously (14),
real-time quantitative reverse transcriptase-polymerase chain
reaction (qRT-PCR) was performed using the LightCycler 480
apparatus (Roche Diagnostics GmbH, Mannheim, Germany). Primers were
designed using the Universal Probe Library Assay Design Center
(http://lifescience.roche.com/), which
specifies the most suitable set. The primer sequences used for
qRT-PCR were: Tie2, forward, 5′-CCCCTATGGGTGTTCCTGT-3′; reverse,
5′-GCTTACAATCTGGCCCGTAA-3′; and probe, no. 10; and
glyceraldehyde-3-phosphate dehydrogenase (GAPDH), forward,
5′-AACATCATCCCTGCCTCTACTGG-3′; reverse,
5′-TTGAAGTCAGAGGAGACCACTG-3′; and probe, no. 61. The transcript
amount was estimated from the respective standard curves and
normalized to the GAPDH transcript amount determined in
corresponding samples. All samples were analyzed in triplicate, and
three independent preparations of RNA were analyzed from the
cells.
Immunoblot analysis
The cells were washed twice with cold
phosphate-buffered saline (PBS) and centrifuged briefly. The
cellular pellets were incubated at 4°C for 30 min in a lysis buffer
(7 M urea, 2 M thiourea, 4% (w/v) CHAPS, and 10 mM Tris). The
protein concentration was measured using a commercial Bradford
reagent (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Immunoblot
analysis was performed as described previously (14,34–37).
Briefly, protein extracts (20 µg) were electrophoresed on 4–12%
Bis-Tris gel (Invitrogen; Thermo Fisher Scientific, Inc.),
transferred to polyvinylidene fluoride membranes (Invitrogen;
Thermo Fisher Scientific, Inc.), and blocked for 1 h at room
temperature in Blocking One (Nacalai Tesque Inc., Kyoto, Japan).
The membranes were washed three times with 0.1% Tween-20 in
Tris-buffered saline (TBS-T) and incubated with affinity-purified
rabbit anti-Tie2 polyclonal antibody (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) or mouse anti-GAPDH monoclonal antibody
(Santa Cruz Biotechnology, Inc.) overnight at 4°C. The membranes
were washed with TBS-T and incubated with horseradish
peroxidase-conjugated anti-rabbit or anti-mouse IgG as a secondary
antibody (Promega Corporation, Madison, WI, USA) for 1 h at room
temperature. Finally, the membranes were detected using
Super-Signal West Pico Chemiluminescent substrate (Thermo Fisher
Scientific, Inc.), and immunoblot analyses were visualized by
exposing the membranes to the ChemiDoc XRS system (Bio-Rad
Laboratories, Inc.). The signal intensities were quantitated using
Image Lab software (Bio-Rad Laboratories, Inc.). Densitometric Tie2
protein data were normalized to the GAPDH protein levels.
Cellular aggregation assay
To investigate the effect of Tie2 and cartilage
oligomeric matrix protein, Ang1, a ligand for Tie2, on cell-cell
adhesion, we performed cellular aggregation assays as described
previously (38,39). The oeTie2 and Mock cells were
incubated for 30 min at 37°C in PBS containing 1 mM
ethylenediaminetetraacetic acid, detached by gentle agitation,
washed, and mechanically dissociated to obtain a single-cell
suspension. The 3×105 single cells in 1 ml of the
serum-free DMEM were transferred to 12-well tissue culture plates
and rotated at 60 rotations/min for 30 min at room temperature
supplemented with and without human Ang1 (1 µg/ml) (R&D
Systems, Inc., Minneapolis, MN, USA) or heat-inactivated Ang1 (1
µg/ml). Three random fields, each containing 200 cells, were viewed
at ×200 magnification for the presence of single and adherent
cells. The percentage of adherent cells was calculated for each
field and averaged (38,39).
Cellular adhesion assay
An adhesion assay was performed as described
previously (14,40). Briefly, the cells were seeded in
collagen I-coated 96-well plates, incubated for 1 h at 37°C at a
density of 2×104 cells/well, and incubated for 1 h in
DMEM, washed once with PBS, fixed in methanol, stained with crystal
violet, and photographed. The numbers of the stained cells were
measured using a microplate spectrophotometer (absorbance at 540 nm
and at 405 nm to subtract the background). Before the adhesion
assay, collagen I-coated 96-well plates were treated with and
without Ang1 (1 µg/ml) or heat-inactivated Ang1 (1 µg/ml) for 1 h,
and the assay was performed.
Multiplex IHC
Multiplex IHC was performed on 4-µm sections of
paraffin-embedded specimens using rabbit anti-Tie2 polyclonal
antibody (Santa Cruz Biotechnology, Inc.) and mouse anti-Ang1
polyclonal antibody (LifeSpan BioSciences, Inc., Seattle, WA, USA).
Briefly, after deparaffinization and hydration, the endogenous
peroxidase activity was quenched by a 3-min incubation in a mixture
of 0.3% hydrogen peroxide solution in 100% methanol. The sections
were blocked for 2 h at room temperature with 1.5% blocking serum
(Santa Cruz Biotechnology, Inc.) in PBS before reaction with the
anti-Tie2 and anti-Ang1 antibodies at 4°C in a moist chamber
overnight. For all washing steps, 0.1% Tween-20 in PBS was used.
After primary antibody incubations, the Envision G/2 Double Stain
System, Rabbit/Mouse (DAB+/Permanent Red) (Agilent Technologies,
Inc., Santa Clara, CA, USA) was used according to the
manufacturer's instructions. The slides were counterstained lightly
with hematoxylin, dehydrated with ethanol, cleaned with xylene, and
mounted. As a negative control, triplicate sections were
immunostained without exposure to primary antibodies, which
confirmed the staining specificity. To quantify the status of the
Tie2 and Ang1 protein expression levels, we used the IHC scoring
systems described previously (14,41–45). The
mean percentages of positively stained cells were determined in at
least three random fields at ×400 magnification in each
section.
Statistical analysis
To compare the Tie2 expression levels and the
cell-cell and cell-extracellular matrix (ECM) adhesive capacities,
statistical significance was evaluated using the Mann-Whitney
U-test. P<0.05 was considered to indicate a statistically
significant difference.
Results
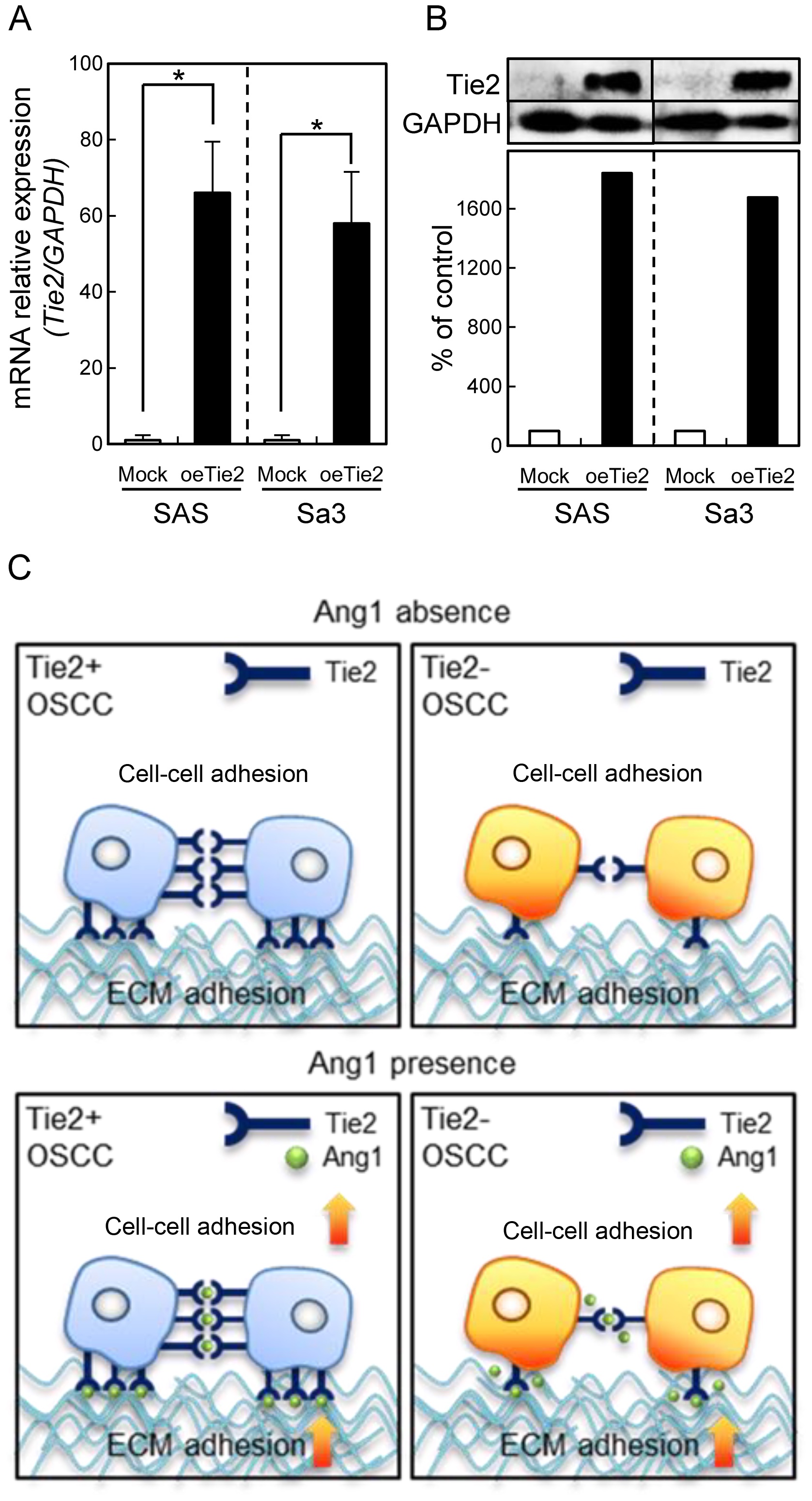
Expression level of Tie2 in its
overexpressed cells
Since frequent down-regulation of Tie2 was observed
in OSCC in vitro and in vivo, we previously
established oeTie2 cells derived from two OSCC cell lines, SAS and
Sa3 (14). To confirm the expression
level of Tie2 in the oeTie2 cells, we performed qRT-PCR and
immunoblot analyses. Consistent with our previous study, the Tie2
mRNA and protein expression levels in oeTie2 cells were
significantly (P<0.05) higher than that in the Mock cells
(Fig. 1A and B). Our previous study
also showed that Tie2 plays an important role in cellular adhesion.
In the current study, we hypothesized that not only Tie2 but also
Ang1, the specific ligand for Tie2, regulate cell-cell and cell-ECM
interactions (Fig. 1C).
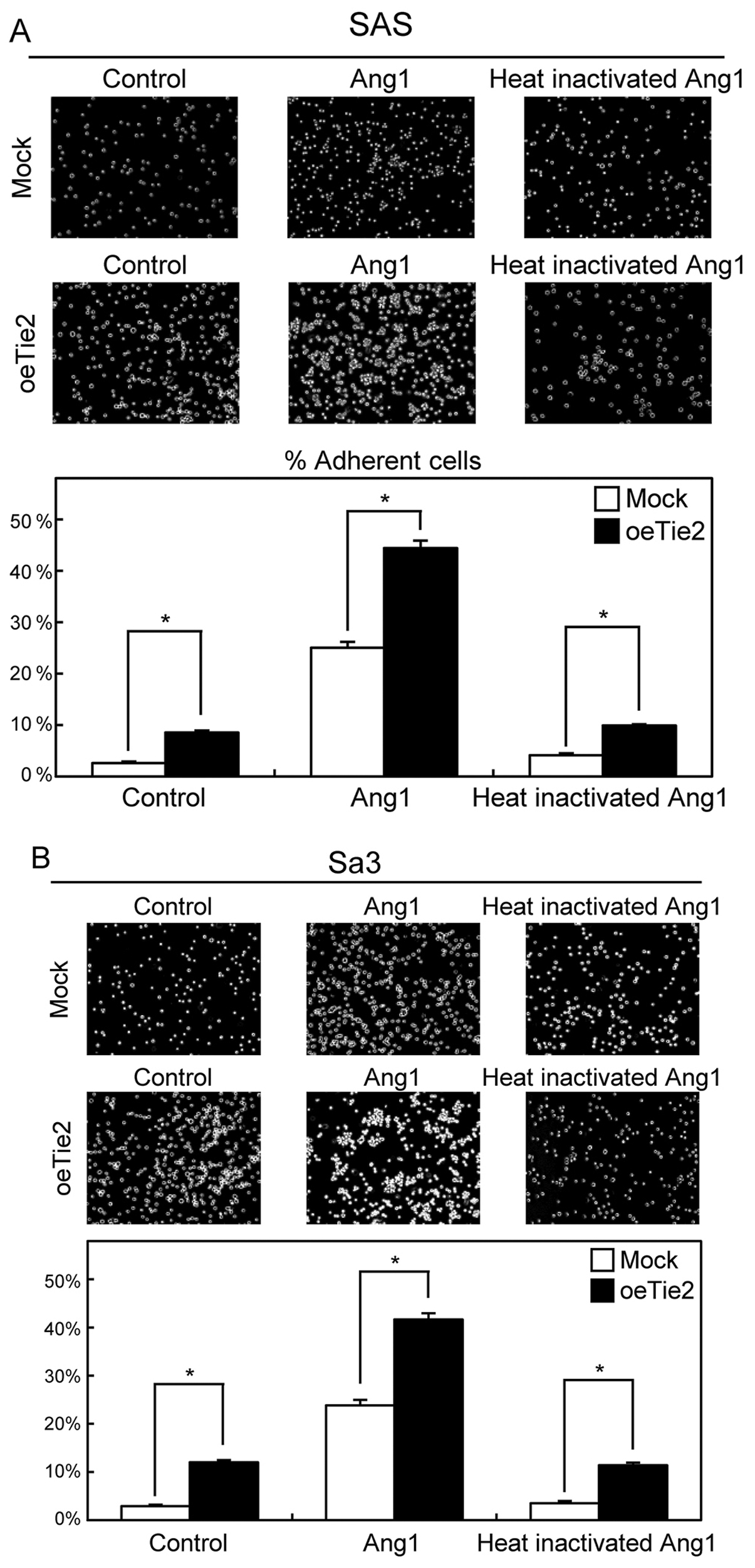
Functional analyses of oeTie2
cells
To evaluate the effect of Tie2 overexpression on
cell-cell adhesion activity, we performed the cellular aggregation
assay. The cell-cell adhesion activity of oeTie2 cells increased
significantly (P<0.05) compared with Mock cells (control)
(Fig. 2A and B). We then examined
whether Ang1 regulates cell-cell adhesion activity. After treatment
with Ang1, the number of aggregated cells increased dramatically
compared with control cells and the cells treated with
heat-inactivated Ang1 (Ang1 and heat-inactivated Ang1) (Fig. 2A, B).
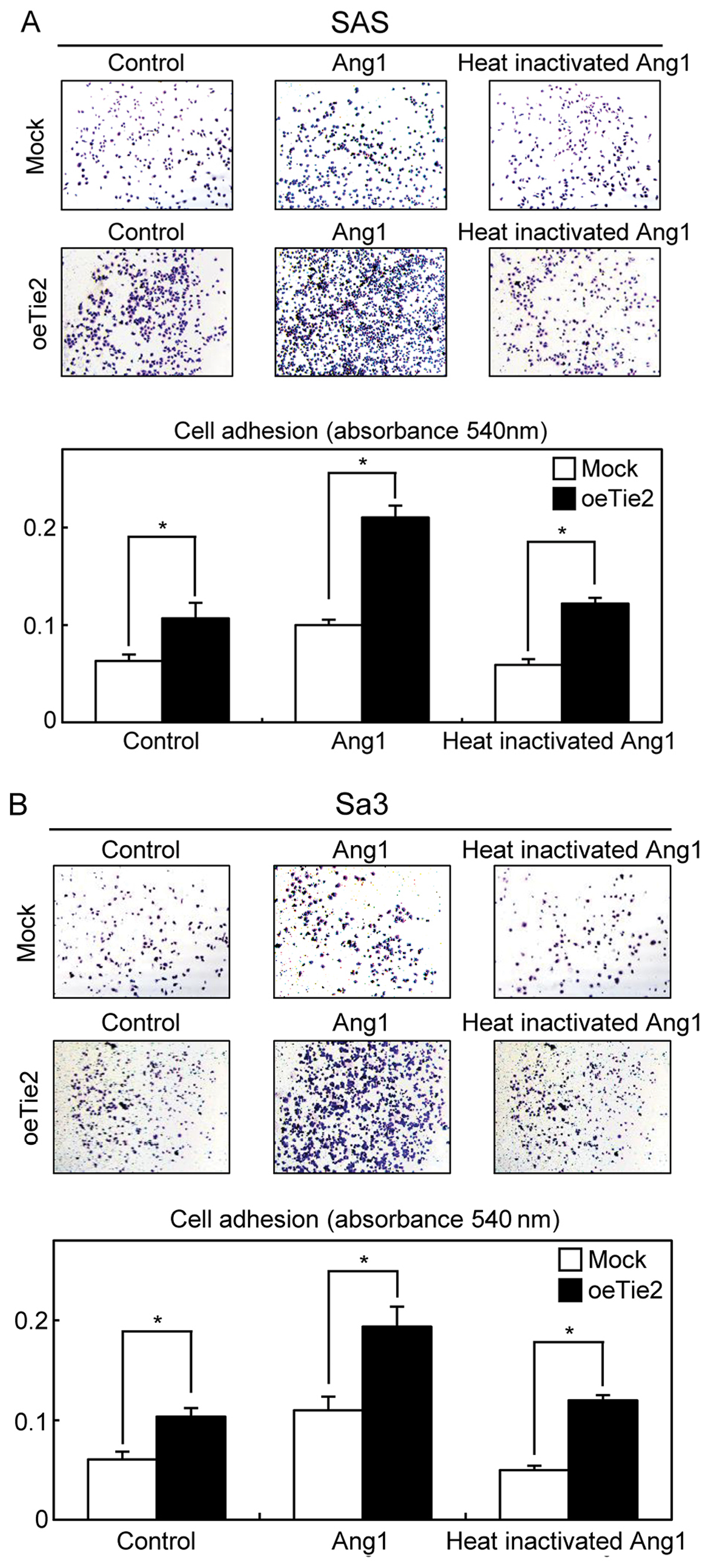
We then performed a cellular adhesion assay to
determine the biologic effects of Tie2 and Ang1 on cell-ECM
interactions. The cell-ECM adhesion in the oeTie2 cells increased
significantly (P<0.05) compared with Mock cells (control)
(Fig. 3A and B). In addition, the
cell-ECM adhesion activity of the cells treated with Ang1 increased
significantly compared with the control cells and the cells treated
with heat-inactivated Ang1 (Ang1 and heat-inactivated Ang1)
(Fig. 3A and B), suggesting that not
only Tie2 but also Ang1 might be critical molecules for cell-cell
and cell-ECM adhesions.
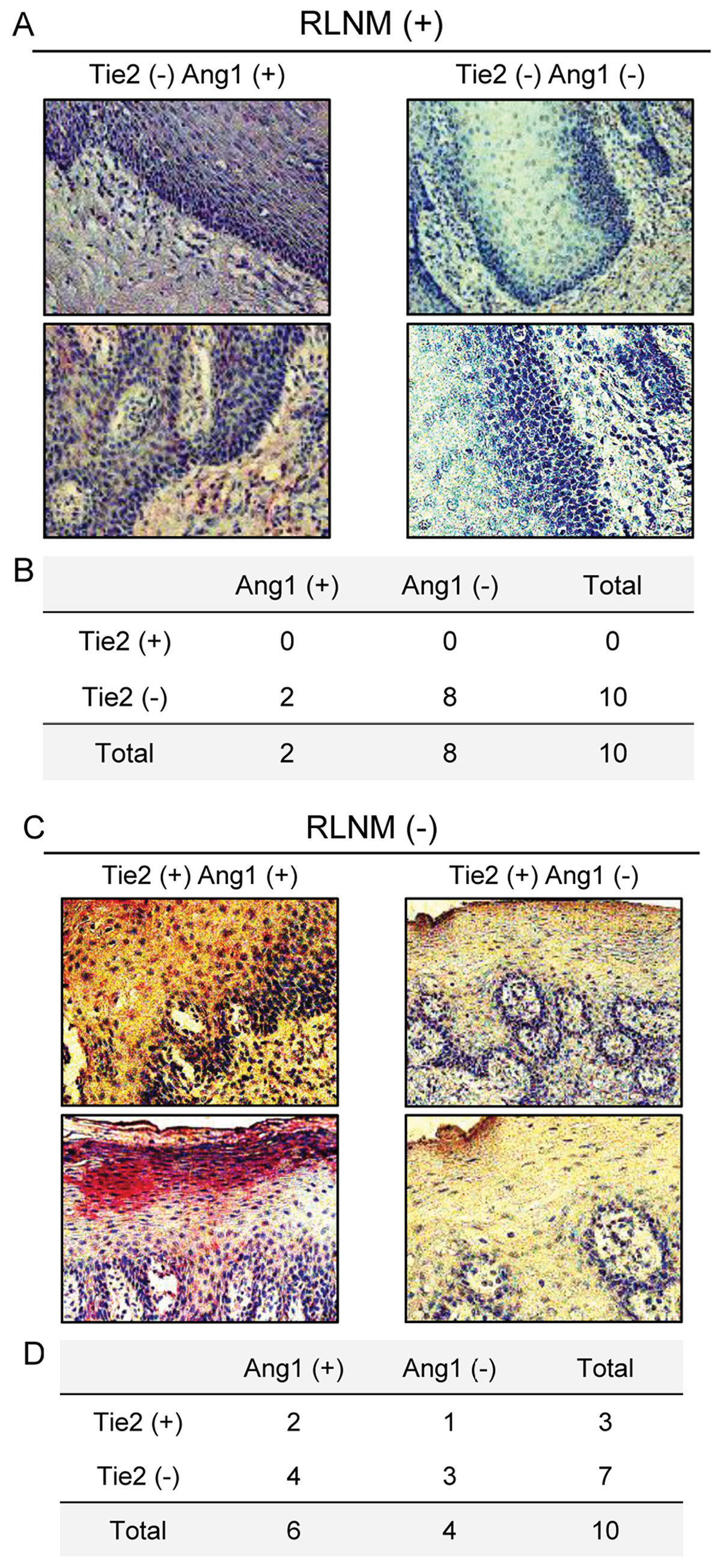
Evaluation of Tie2 and Ang1 expression
levels in primary OSCCs and the clinical correlations with
RLNM
We analyzed the Tie2 and Ang1 protein expression
levels in 20 cases of primary OSCCs, RLNM-positive (n=10 cases) and
RLNM-negative (n=10 cases), using the IHC scoring system.
Representative IHC results for the Tie2 and Ang1 proteins in
primary OSCC are shown in Fig. 4A and
B. In the RLNM-positive cases, all cases (100%) were Tie2
negative, and eight (80%) cases were Ang1 negative (double negative
expression, 8/10 cases), whereas three (30%) of the 10
RLNM-negative cases were negative for both Tie2 and Ang1 (Fig. 4C and D).
Discussion
In addition to our previous finding that Tie2 is in
part a key modulator of OSCC tumor adhesion and invasion (14), the current findings indicated that the
ligand of Tie2, Ang1, enhances the Tie2 functions in OSCC
progression. Although cancer cells show that Tie2 is related
closely to cancer metastasis (14,46,47),
little is known about Ang1 function in cancer research.
Ang1 is thought to support endothelial cell adhesion
and vascular integrity while inhibiting vascular permeability
(18,19,48,49). Ang1
also induces phosphorylation of Tie2 and promotes endothelial cell
migration and survival (23,50–52). The
Tie2/Ang1 signaling pathway is thought to regulate proliferation
and osteogenic differentiation of mesenchymal stem cells through
activation of the p38 MAPK and Akt pathways (53,54). The
Tie2/Ang1 interaction has different functions during angiogenesis
and differentiation, suggesting that the Tie2/Ang1 signaling
pathway differs at the molecular level in several types of cells.
Since Kim et al reported a novel Ang1 function as a cell
primer (55), we speculated that Ang1
increases cell-cell and cell-ECM adhesion activities through the
Tie2/Ang1 interaction (Fig. 1C).
Consistent with our hypothesis, patients with OSCC with low
expression of Tie2 and Ang1 have high risk for RLNM (Fig. 4).
In conclusion, these data provide new insight that
the Tie2/Ang1 interaction seems to have complex regulatory
mechanisms, especially considering our finding that the Tie2/Ang1
interaction controls critical behaviors in metastatic OSCCs. While
further studies using large cohort specimens are needed to study
the Tie2/Ang1 interaction, the current data suggested that the
Tie2/Ang1 interaction plays an important role in cellular adhesion
and might be a potential biomarker for RLNM in OSCCs.
Acknowledgements
The authors would like to thank Ms. Lynda C.
Charters for editing this manuscript.
References
|
1
|
Jamora C and Fuchs E: Intercellular
adhesion, signalling and the cytoskeleton. Nat Cell Biol.
4:E101–E108. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Green KJ and Simpson CL: Desmosomes: New
perspectives on a classic. J Invest Dermatol. 127:2499–2515. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dusek RL and Attardi LD: Desmosomes: New
perpetrators in tumour suppression. Nat Rev Cancer. 11:317–323.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
South AP, Wan H, Stone MG,
Dopping-Hepenstal PJ, Purkis PE, Marshall JF, Leigh IM, Eady RA,
Hart IR and McGrath JA: Lack of plakophilin 1 increases
keratinocyte migration and reduces desmosome stability. J Cell Sci.
116:3303–3314. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yin T and Green KJ: Regulation of
desmosome assembly and adhesion. Semin Cell Dev Biol. 15:665–677.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takes RP: Staging of the neck in patients
with head and neck squamous cell cancer: Imaging techniques and
biomarkers. Oral Oncol. 40:656–667. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Karatzanis AD, Waldfahrer F, Psychogios G,
Hornung J, Zenk J, Velegrakis GA and Iro H: Resection margins and
other prognostic factors regarding surgically treated glottic
carcinomas. J Surg Oncol. 101:131–136. 2010.PubMed/NCBI
|
|
8
|
Fan S, Tang QL, Lin YJ, Chen WL, Li JS,
Huang ZQ, Yang ZH, Wang YY, Zhang DM, Wang HJ, et al: A review of
clinical and histological parameters associated with contralateral
neck metastases in oral squamous cell carcinoma. Int J Oral Sci.
3:180–191. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lea J, Bachar G, Sawka AM, Lakra DC,
Gilbert RW, Irish JC, Brown DH, Gullane PJ and Goldstein DP:
Metastases to level IIb in squamous cell carcinoma of the oral
cavity: A systematic review and meta-analysis. Head Neck.
32:184–190. 2010.PubMed/NCBI
|
|
10
|
Okura M, Aikawa T, Sawai NY, Iida S and
Kogo M: Decision analysis and treatment threshold in a management
for the N0 neck of the oral cavity carcinoma. Oral Oncol.
45:908–911. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenberg JS, Fowler R, Gomez J, Mo V,
Roberts D, El Naggar AK and Myers JN: Extent of extracapsular
spread: A critical prognosticator in oral tongue cancer. Cancer.
97:1464–1470. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sano D and Myers JN: Metastasis of
squamous cell carcinoma of the oral tongue. Cancer Metastasis Rev.
26:645–662. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Casiglia J and Woo SB: A comprehensive
review of oral cancer. Gen Dent. 49:72–82. 2001.PubMed/NCBI
|
|
14
|
Kitajima D, Kasamatsu A, Nakashima D,
Miyamoto I, Kimura Y, Saito T, Suzuki T, Endo-Sakamoto Y, Shiiba M,
Tanzawa H and Uzawa K: Tie2 regulates tumor metastasis of oral
squamous cell carcinomas. J Cancer. 7:600–607. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Dumont DJ, Gradwohl G, Fong GH, Puri MC,
Gertsenstein M, Auerbach A and Breitman ML: Dominant-negative and
targeted null mutations in the endothelial receptor tyrosine
kinase, tek, reveal a critical role in vasculogenesis of the
embryo. Genes Dev. 8:1897–1909. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suri C, Jones PF, Patan S, Bartunkova S,
Maisonpierre PC, Davis S, Sato TN and Yancopoulos GD: Requisite
role of angiopoietin-1, a ligand for the TIE2 receptor, during
embryonic angiogenesis. Cell. 87:1171–1180. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sato TN, Tozawa Y, Deutsch U,
Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T,
Wolburg H, Risau W and Qin Y: Distinct roles of the receptor
tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature.
376:70–74. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peters KG, Kontos CD, Lin PC, Wong AL, Rao
P, Huang L, Dewhirst MW and Sankar S: Functional significance of
Tie2 signaling in the adult vasculature. Recent Prog Horm Res.
59:51–71. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brindle NP, Saharinen P and Alitalo K:
Signaling and functions of angiopoietin-1 in vascular protection.
Circ Res. 98:1014–1023. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eklund L and Olsen BR: Tie receptors and
their angiopoietin ligands are context-dependent regulators of
vascular remodeling. Exp Cell Res. 312:630–641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pfaff D, Fiedler U and Augustin HG:
Emerging roles of the Angiopoietin-Tie and the ephrin-Eph systems
as regulators of cell trafficking. J Leukoc Biol. 80:719–726. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Davis S, Aldrich TH, Jones PF, Acheson A,
Compton DL, Jain V, Ryan TE, Bruno J, Radziejewski C, Maisonpierre
PC and Yancopoulos GD: Isolation of angiopoietin-1, a ligand for
the TIE2 receptor, by secretion-trap expression cloning. Cell.
87:1161–1169. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jones N, Iljin K, Dumont DJ and Alitalo K:
Tie receptors: New modulators of angiogenic and lymphangiogenic
responses. Nat Rev Mol Cell Biol. 2:257–267. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Maisonpierre PC, Suri C, Jones PF,
Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J,
Aldrich TH, Papadopoulos N, et al: Angiopoietin-2, a natural
antagonist for Tie2 that disrupts in vivo angiogenesis. Science.
277:55–60. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gamble JR, Drew J, Trezise L, Underwood A,
Parsons M, Kasminkas L, Rudge J, Yancopoulos G and Vadas MA:
Angiopoietin-1 is an antipermeability and anti-inflammatory agent
in vitro and targets cell junctions. Circ Res. 87:603–607. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeon BH, Khanday F, Deshpande S, Haile A,
Ozaki M and Irani K: Tie-ing the antiinflammatory effect of
angiopoietin-1 to inhibition of NF-kappaB. Circ Res. 92:586–588.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ramsauer M and D'Amore PA: Getting Tie(2)d
up in angiogenesis. J Clin Invest. 110:1615–1617. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cho CH, Kammerer RA, Lee HJ, Yasunaga K,
Kim KT, Choi HH, Kim W, Kim SH, Park SK, Lee GM and Koh GY:
Designed angiopoietin-1 variant, COMP-Ang1, protects against
radiation-induced endothelial cell apoptosis. Proc Natl Acad Sci
USA. 101:5553–5558. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stratmann A, Risau W and Plate KH: Cell
type-specific expression of angiopoietin-1 and angiopoietin-2
suggests a role in glioblastoma angiogenesis. Am J Pathol.
153:1459–1466. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sugimachi K, Tanaka S, Taguchi K, Aishima
S, Shimada M and Tsuneyoshi M: Angiopoietin switching regulates
angiogenesis and progression of human hepatocellular carcinoma. J
Clin Pathol. 56:854–860. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pindborg JJ, Reichart PA, Smith CJ and van
der Waal I: Histological typing of cancer and precancer of the oral
mucosaWorld Health Organization classification of tumours. 2nd
edition. Springer-Verlag; Berlin Heidelberg:
|
|
33
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumors. 7th edition. New York:
Wiley-Liss; 2009
|
|
34
|
Minakawa Y, Kasamatsu A, Koike H, Higo M,
Nakashima D, Kouzu Y, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H
and Uzawa K: Kinesin family member 4A: A potential predictor for
progression of human oral cancer. PLoS One. 8:e859512013.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamatoji M, Kasamatsu A, Kouzu Y, Koike H,
Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H and Uzawa K:
Dermatopontin: A potential predictor for metastasis of human oral
cancer. Int J Cancer. 130:2903–2911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Uchida F, Uzawa K, Kasamatsu A, Takatori
H, Sakamoto Y, Ogawara K, Shiiba M, Tanzawa H and Bukawa H:
Overexpression of cell cycle regulator CDCA3 promotes oral cancer
progression by enhancing cell proliferation with prevention of G1
phase arrest. BMC Cancer. 12:3212012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Unozawa M, Kasamatsu A, Higo M, Fukumoto
C, Koyama T, Sakazume T, Nakashima D, Ogawara K, Yokoe H, Shiiba M,
et al: Cavin-2 in oral cancer: A potential predictor for tumor
progression. Mol Carcinog. 55:1037–1047. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ramanathan R, Wilkemeyer MF, Mittal B,
Perides G and Charness ME: Alcohol inhibits cell-cell adhesion
mediated by human L1. J Cell Biol. 133:381–390. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tripathi V, Popescu NC and Zimonjic DB:
DLC1 induces expression of E-cadherin in prostate cancer cells
through Rho pathway and suppresses invasion. Oncogene. 33:724–733.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kasamatsu A, Uzawa K, Nakashima D, Koike
H, Shiiba M, Bukawa H, Yokoe H and Tanzawa H: Galectin-9 as a
regulator of cellular adhesion in human oral squamous cell
carcinoma cell lines. Int J Mol Med. 16:269–273. 2005.PubMed/NCBI
|
|
41
|
Endo Y, Uzawa K, Mochida Y, Shiiba M,
Bukawa H, Yokoe H and Tanzawa H: Sarcoendoplasmic reticulum Ca(2+)
ATPase type 2 downregulated in human oral squamous cell carcinoma.
Int J Cancer. 110:225–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lombardi DP, Geradts J, Foley JF, Chiao C,
Lamb PW and Barrett JC: Loss of KAI1 expression in the progression
of colorectal cancer. Cancer Res. 59:5724–5731. 1999.PubMed/NCBI
|
|
43
|
Shimada K, Uzawa K, Kato M, Endo Y, Shiiba
M, Bukawa H, Yokoe H, Seki N and Tanzawa H: Aberrant expression of
RAB1A in human tongue cancer. Br J Cancer. 92:1915–1921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Baba T, Sakamoto Y, Kasamatsu A, Minakawa
Y, Yokota S, Higo M, Yokoe H, Ogawara K, Shiiba M, Tanzawa H and
Uzawa K: Persephin: A potential key component in human oral cancer
progression through the RET receptor tyrosine
kinase-mitogen-activated protein kinase signaling pathway. Mol
Carcinog. 54:608–617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ishige S, Kasamatsu A, Ogoshi K, Saito Y,
Usukura K, Yokoe H, Kouzu Y, Koike H, Sakamoto Y, Ogawara K, et al:
Decreased expression of kallikrein-related peptidase 13: Possible
contribution to metastasis of human oral cancer. Mol Carcinog.
53:557–565. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Saharinen P, Eklund L, Miettinen J,
Wirkkala R, Anisimov A, Winderlich M, Nottebaum A, Vestweber D,
Deutsch U, Koh GY, et al: Angiopoietins assemble distinct Tie2
signalling complexes in endothelial cell-cell and cell-matrix
contacts. Nat Cell Biol. 10:527–537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Buehler D, Rush P, Hasenstein JR, Rice SR,
Hafez GR, Longley BJ and Kozak KR: Expression of angiopoietin-TIE
system components in angiosarcoma. Mod Pathol. 26:1032–1040. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wong AL, Haroon ZA, Werner S, Dewhirst MW,
Greenberg CS and Peters KG: Tie2 expression and phosphorylation in
angiogenic and quiescent adult tissues. Circ Res. 81:567–574. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Thurston G, Suri C, Smith K, McClain J,
Sato TN, Yancopoulos GD and McDonald DM: Leakage-resistant blood
vessels in mice transgenically overexpressing angiopoietin-1.
Science. 286:2511–2514. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Koblizek TI, Weiss C, Yancopoulos GD,
Deutsch U and Risau W: Angiopoietin-1 induces sprouting
angiogenesis in vitro. Curr Biol. 8:529–532. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Papapetropoulos A, García-Cardeña G,
Dengler TJ, Maisonpierre PC, Yancopoulos GD and Sessa WC: Direct
actions of angiopoietin-1 on human endothelium: Evidence for
network stabilization, cell survival, and interaction with other
angiogenic growth factors. Lab Invest. 79:213–223. 1999.PubMed/NCBI
|
|
52
|
Saharinen P, Kerkelä K, Ekman N, Marron M,
Brindle N, Lee GM, Augustin H, Koh GY and Alitalo K: Multiple
angiopoietin recombinant proteins activate the Tie1 receptor
tyrosine kinase and promote its interaction with Tie2. J Cell Biol.
169:239–243. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim S, Lee JC, Cho ES and Kwon J:
COMP-Ang1 promotes chondrogenic and osteogenic differentiation of
multipotent mesenchymal stem cells through the Ang1/Tie2 signaling
pathway. J Orthop Res. 31:1920–1928. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kook SH, Lim SS, Cho ES, Lee YH, Han SK,
Lee KY, Kwon J, Hwang JW, Bae CH, Seo YK and Lee JC:
COMP-angiopoietin 1 increases proliferation, differentiation, and
migration of stem-like cells through Tie-2-mediated activation of
p38 MAPK and PI3K/Akt signal transduction pathways. Biochem Biophys
Res Commun. 455:371–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim MS, Lee CS, Hur J, Cho HJ, Jun SI, Kim
TY, Lee SW, Suh JW, Park KW, Lee HY, et al: Priming with
angiopoietin-1 augments the vasculogenic potential of the
peripheral blood stem cells mobilized with granulocyte
colony-stimulating factor through a novel Tie2/Ets-1 pathway.
Circulation. 120:2240–2250. 2009. View Article : Google Scholar : PubMed/NCBI
|