Introduction
At present, the incidence rate for breast cancer is
1/26 in women aged <65 years in China (1). This situation highlights the urgent
requirement for improvement in prevention and treatment, making it
necessary to search for effective strategies for the early
diagnosis and monitoring of breast cancer. Circulating tumor
(ct)DNA, which carries tumor-specific sequence alterations, is
present in the cell-free fraction of blood, representing a
variable, although generally small, fraction of the total
circulating cell-free (cf)DNA (2,3). The
detection of ctDNA may provide novel strategies for cancer
diagnosis and monitoring. The non-invasive analysis of
tumor-derived DNA may serve an important role in the identification
and analysis of solid tumors, potentially overcoming the
limitations of repeated biopsies (4,5). ctDNA
exhibits a more significant association with tumor burden than
carcinoma antigen 15-3 or circulating tumor cells (6). It has been demonstrated as a potential
tool for monitoring metastatic breast cancer with high sensitivity
(6). ctDNA may also be used to
analyze the mechanisms underlying acquired drug resistance to guide
the strategy of tumor management (7).
The digital droplet polymerase chain reaction can be applied to
monitor ctDNA even in early-stage breast cancer (8). However, the ctDNA detection rate was low
in a previous study, due to the limited number of genes and loci
analyzed (9). A low detection rate
may restrict the feasibility of applying ctDNA analysis.
The aim of the present study was to explore the
potential application of ctDNA. Amplicon sequencing was performed
on DNA extracted from tumor tissues, plasma and peripheral blood
cells in a total of 33 samples from 11 patients with invasive
breast cancer. The ratio of gene mutation between ctDNA and tumor
DNA was analyzed. In addition, its potential role in monitoring the
tumor burden in patients with breast cancer was assessed.
Materials and methods
Sample collection and processing
A total of 11 patients were selected randomly. These
patients were all classified as early-stage tumor node metastasis
(TNM) stage I/II (10). The tumor
tissues were collected through surgical resection of breast tumors
and the matched blood samples were collected before surgery. All
the specimens were stored at Heilongjiang Province Breast
Bio-Sample Bank (Harbin, China). Matched DNA from tumor tissues,
plasma and peripheral blood cells were extracted. The patients
ranged in age from 36–61, with a mean age of 48.8 years. All
patients provided signed informed consent. The study was approved
by the Ethical Committee of Harbin Medical University (Harbin,
China).
Blood collected in EDTA tubes was processed within 1
h of sample collection and centrifuged at 4°C, 820 × g for 10 min
to separate the plasma from the peripheral blood cells. The plasma
was transferred to microcentrifuge tubes and centrifuged at 4°C,
20,000 × g for 10 min to remove cell debris. Cell-free DNA was
extracted from 2-ml aliquots of cell-free plasma using the QIAamp
Ultra Sens Virus kit (Qiagen China Co., Ltd., Shanghai, China)
according to the manufacturer's protocol. The cell pellet from the
initial centrifugation step was used for the isolation of germline
genomic DNA using Pure Link Genomic DNA kits (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA). Matched tumor DNA was
isolated from breast cancer tissues using Pure Link Genomic DNA
kits (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Sequencing and data analyses
The 5 most commonly mutated genes in breast cancer
were identified in The Cancer Genome Atlas (TCGA; http://cancergenome.nih.gov/). The primers used in the
amplification of these genes are included in Table I. The amplification products were
attached to the Ion AmpliSeq™ Adapters (Life
Technologies; Thermo Fisher Scientific, Inc.). Following
purification with Agencount Ampure XP Reagent (Beckman Coulter,
Indianapolis, IN, USA), the products with Adapters were prepared
using the Ion OneTouch™ Template OT2 kit (Life Technologies; Thermo
Fisher Scientific, Inc.). The products were then processed using
the Ion PGM 200 Sequencing v2 kit (Life Technologies; Thermo Fisher
Scientific, Inc.) for sequencing. Sequencing data were analyzed
using the Torrent Mapping Alignment program (Torrent
Suite™ Software 4.4; Life Technologies; Thermo Fisher
Scientific, Inc.). Variants were analyzed in the Torrent variant
caller software (Torrent Suite™ Software 4.4; Life
Technologies; Thermo Fisher Scientific, Inc.).
 | Table I.Primer sequences identified by the
Cancer Genome Atlas. |
Table I.
Primer sequences identified by the
Cancer Genome Atlas.
| Amplicon_ID |
Ion_AmpliSeq_Fwd_Primer* |
Ion_AmpliSeq_Rev_Primer* |
|---|
| AMPL7152996431 |
GGATACGGCCAGGCATTGA |
CCCTGTCATCTTCTGTCCCTT |
| AMPL7152996965 |
CCCAATTGCAGGTAAAACAGTCA |
AGCACTAAGCGAGGTAAGCAAG |
| AMPL7152996966 |
AAGCTATGATGTTCCTTAGATTAGGTGTAT |
CCAGACCAGCTTTCAAAAAGAAAATTGTTA |
| AMPL7152996967 |
TCATAGGGCACCACCACACTAT |
CATGGCCATCTACAAGCAGTCA |
| AMPL7152996968 |
CATTCTGGGAGCTTCATCTGGA |
CGTTCTGGTAAGGACAAGGGTT |
| AMPL7152996970 |
TGTGATGAGAGGTGGATGGGTA |
CCTCATCTTGGGCCTGTGTTAT |
| AMPL7152996971 |
CAGGAAGTCTGAAAGACAAGAGCA |
CCAGGGTTGGAAGTGTCTCATG |
| AMPL7152996972 |
ATTGCAAGCAAGGGTTCAAAGAC |
GCCTTAGGCCCTTCAAAGCATT |
| AMPL7153002870 |
AGTGCTTGTTGCTTGCCAG |
GGATGTGGACCAACGTGAGG |
| AMPL7153002873 |
CCTTCTTGAGCAGCCCTGAAA |
CATGTACGAGATGATGTGCGGT |
| AMPL7153002898 |
GCGTACTCCATGACAAAGCAGA |
GTATCAGGCGACGTGGTATCAA |
| AMPL7153004003 |
CGACCCAGTTACCATAGCAATTTAGT |
GAAAACTGTTCCAATACATGGAAGGATG |
| AMPL7153004013 |
GATCAAGAAATCGATAGCATTTGCAGTATAG |
CTCCTAGAATTAAACACACATCACATACATACA |
| AMPL7153004026 |
CACGACGGGAAGACAAGTTCAT |
CAAAACACCTGCAGATCTAATAGAAAACAA |
| AMPL7153004031 |
GTTAGCTCATTTTTGTTAATGGTGGCTT |
CTCACTCTAACAAGCAGATAACTTTCACTT |
| AMPL7153004036 |
TTTTTCTTATTCTGAGGTTATCTTTTTACCACA |
CCTCTTGTGCCTTTAAAAATTTGCC |
| AMPL7153004059 |
GCCATTTCCATCCTGCAGAAGA |
CCAAACTACGGACATTTTCGCATC |
| AMPL7153007063 |
TCTTCTCCGAGTGCAGGTAGT |
GTTAGGGCTTCTGAGACTTTCCAG |
| AMPL7153007092 |
TCAAATGCACCCGAGAAATAAAAACC |
CCGTGCTTTAAGAGGTTGGCTT |
| AMPL7153007099 |
CACCAGGAAGCCACTCAGATG |
GTGGCTATTGTGAAGGAGGGTT |
| AMPL7153007111 |
CGCCCTACATGGAAAACCG |
CTCACCCAGTGACAACTCAGG |
| AMPL7153007146 |
GGCAGGACTCGGCATCAAG |
GAACCTCATGCTGGACAAGGA |
| AMPL7153007158 |
CCAGTTTTTATCTCCAGCCTCAGTT |
AGTGGACCACTGTCATCGAAC |
| AMPL7153007172 |
AAATGAGGACCAGGCCAGTTT |
GCTTCTTTGCCGGTATCGTGT |
| AMPL7153007194 |
CCACCTCGTCCTGTAAAGCAG |
CTGCCCATAGACCATGAACGA |
| AMPL7153009985 |
CTGTTAGGATCAGATTATAGTGTTACACCA |
TGCCATCATTACTTTGATTACAGGATGAT |
| AMPL7153010057 |
GGACTATGTCCGGGAACACAAA |
ATGGCAAACTCTTGCTATCCCA |
| AMPL7153010068 |
ATGTCAGCCGAGGCAGGGAAT |
GGGCAAAGAATAAAAGGAAGAGAAAATCAA |
| AMPL7153010138 |
CCACGTACCAGATGGATGTGAAC |
GACTCTCCAAGATGGGATACTCCA |
| AMPL7153011255 |
GCAAAGACCTGAAGGTATTAACATCATT |
AGAGCCAAGCATCATTGAGAAAAGATTTA |
| AMPL7153011364 |
ATGTTACTTTTAAAATGAAAAACCTTACA |
ACAGTCCATTGGCAGTTGAGAATAAAGGAA |
|
| AMPL7153011377 |
CTTCCAAATCTACAGAGTTCCCTGTT |
AGTCCTGTACTTCTGGATCTTTAACCATAT |
| AMPL7153017580 |
TGGGATGGTGCTTTGCTGATTA |
GTCGAATGTGCTGTTGACACAG |
| AMPL7153017625 |
TCGCATCACCAATGCCTTCTTTA |
GGCATGTGACAGAACACAGTGA |
| AMPL7153017648 |
ATGATTTTTCTTCTCTCCAATGTAGTGGT |
GGACCCATTAGAACCAACTCCATAAA |
| AMPL7153017672 |
TGCCTGTGGATCCCTAGCTATT |
TGATCACCAATCTCTACCAGTTAAAAAGG |
| AMPL7153017708 |
ATCGGCCTCTTCATGCGAA |
CACATTCAGAGATTCTTTCTGCATCATAAT |
| AMPL7153017718 |
ATCCAACATCCAGACACATAGTGATTTT |
GAGGACAGTCAGAAATGCAGGAA |
| AMPL7153017742 |
ACACCTTCACAATATACCCTCCATGA |
CAGGAAGAAAACTCAAGATTATCTGGGTTA |
| AMPL7153017760 |
GCAGTTGGGCACTTTTGAAGAT |
CCTTTCTCCACTTAGATTTTCTCCAATTTT |
| AMPL7153017833 |
GTGTGTCACTCGTAATTAGGTCCA |
CGAAAGAAAATACTTGCATGTCAGAGGATA |
| AMPL7153017844 |
TCTCTTTAGCAGAACATAAATGCGAAGA |
CATAGGAGCTGGAGGCAGAGATA |
| AMPL7153017850 |
TTTGAAAGAGAAAAGAAAGAGACATGCATG |
TCTCTGAATTTGCAAGAGAGGAAATGTT |
| AMPL7153017856 |
GGGAAATGATCCTACCCTCACTCT |
CCCACAGGAAGTCTTCTGTCCT |
| AMPL7153017910 |
CTGGTAACATCCACCCAGATCA |
GCTGCCAGACATGAGAAAAGGT |
| AMPL7153017922 |
GACTCCGTCCAGTATTGATCGG |
ATCTCAAGGAAACAGGAAAGGACG |
| AMPL7153017944 |
TATAATACAGAGTCCCTGAGAGTCTAGAGT |
TGCCCTATCTTAGCAACTCTCCT |
| AMPL7153019495 |
TTCTTTGTAGATATGATGCAGCCATTGA |
CTTGTGATCCAAAAAGTGTCCAAAATCTAT |
| AMPL7153019513 |
TACTCCTCTTTCAGAATGTTACCTTATGGT |
TGTTCTAACTCAGAGGAATACACAAACAC |
| AMPL7153019555 |
AAGATTGGCCTCCAATCAAACCT |
AGCATAAAACTAGTTAGTGCAGTAGGTTTT |
| AMPL7153019565 |
AGTTTGGGACTTCTTAAGAAGATTCATATGG |
TTTATTTATGTGGACTTTCTGAGAGAAAACAAT |
| AMPL7153019574 |
TCCCTTGAAAAATGAAAGAGAGATGGT |
GCAATTACTTGTTCTGGTACACAGTCAT |
| AMPL7153019590 |
GCTTTCCTGAAGTTTCTTTTGAAGAGT |
GGATAACTTTCAACATACAGGTTGCCTT |
| AMPL7153019600 |
TTAGGAATGGATTCCTAAATAAAAATTGAGGT |
CAATTCAACCACAGTGGCCTTT |
| AMPL7153019614 |
CAGTCTTGCTTCTGTCTCTGAGT |
CATGAAATCTGGTCGCCTCATTT |
| AMPL7153019630 |
GGGACAACCATACATCTAATTCCTTAAAGT |
AAGAAGATTCATCTTGAAGAAGTTGATGGA |
| AMPL7153019653 |
AATATTATGTCTTAGATTGGTTCTTTCCTGTC |
GCTGTTCTTTGTCATTTTCCCTTAATTCAT |
| AMPL7153019708 |
GTTTGATTACACAGACACTCTAGTATCTGG |
ATAAGAGAGAAGGTTTGACTGCCATAAAAA |
| AMPL7153019717 |
TTTGGTTGATCTTTGTCTTCGTGATTTG |
AAAAATTCAATCAGCGGTATAATCAGGAG |
| AMPL7153019725 |
TTCAAAAATGAGTGTTTAAATTGTTTAGCAAAG |
TGCTTCAAATACATCCCACATGCA |
| AMPL7153019740 |
GTAGTCTGATGTCTCCATTGTTATTAGTGG |
AAGGGTTCTCCTCCATGGTAGAT |
| AMPL7152996430 |
ACAACCTCCGTCATGTGCT |
CTTTCAACTCTGTCTCCTTCCTCTTC |
| AMPL7152996432 |
GCTGCCCTGGTAGGTTTTCTG |
CGATATTGAACAATGGTTCACTGAAGAC |
| AMPL7152996963 |
GAGAATGGAATCCTATGGCTTTCCA |
CCGTCATAAAGTCAAACAATTGTAACTTGA |
| AMPL7152996964 |
GGGTTATAGGGAGGTCAAATAAGCA |
GGCCTCTGATTCCTCACTGATTG |
| AMPL7152996969 |
AAGGTGATAAAAGTGAATCTGAGGCAT |
AATGGGACAGGTAGGACCTGAT |
| AMPL7152996973 |
GGGACTGTAGATGGGTGAAAAGA |
AACTGTGAGTGGATCCATTGGAAG |
| AMPL7152996974 |
TCAACTTACGACGAGTTTATCAGGAAG |
CTTCTTGACTGTTTTACCTGCAATTGG |
| AMPL7153002838 |
TTTTTAGGACAAAATGTTTCACTTTTGGG |
TTCCTTGTCATTATCTGCACGCT |
| AMPL7153002851 |
ACGAACTGGTGTAATGATATGTGCATATTT |
CTCAGATCCAGGAAGAGGAAAGGAAAAA |
| AMPL7153004023 |
ATCGTTTTTGACAGTTTGACAGTTAAAGG |
CGGCTGAGGGAACTCAAAGTAC |
| AMPL7153004043 |
GTGTCACATTATAAAGATTCAGGCAATGTT |
ATGTATCTCACTCGATAATCTGGATGACT |
| AMPL7153004047 |
TTGATTGCTGCATATTTCAGATATTTCTTTCC |
TGGCTTAGAAATCTTTTCTAAATGAAAACACAA |
| AMPL7153004054 |
AAGATGAGTCATATTTGTGGGTTTTCATTTTAA |
TTATTTTCATGGTGTTTTATCCCTCTTGATAAA |
| AMPL7153007059 |
CAGCCCGAAGTCTGTGATCTTA |
TATGGCGCTGAGATTGTGTCAG |
| AMPL7153007073 |
CGCCACAGAGAAGTTGTTGAG |
GGGTCTGACGGGTAGAGTGT |
| AMPL7153007080 |
CTTCTCATGGTCCTGGTTGTAGAAG |
GTCCTGAGCACACGCAATG |
| AMPL7153007104 |
GGGTACTAACCTCGTTTGTGCA |
CACATTCAGCTTCCTTTGCTTCTC |
| AMPL7153007116 |
AGGGACACCTCCATCTCTTCAG |
TGGAGCTCCTGATCTGGTACAG |
| AMPL7153007123 |
GCCTCTCTGAGTGTGGAGAGAA |
TGAAGGTCTTGAGCACACTTGAG |
| AMPL7153007132 |
CCTGGAAAGTCTCAGAAGCCCTA |
GCCCTGAAGTACTCTTTCCAGA |
| AMPL7153007163 |
CTCAGGAGTCTCCACATGGAAG |
CTCTGTGAGGCGAGAAACTGAG |
| AMPL7153007177 |
GCACCTTCTTCTCGTACACGT |
GGCCCTACATCACAGGAGGAA |
| AMPL7153007184 |
CTCACGTGCCCAAGAAGACAGGA |
CATGAAGATCCTCAAGAAGGAAGTCA |
| AMPL7153007197 |
CAGCAGCTTCAGGTACTCAAAC |
CAGCCATGTGTTCCCTGTAAG |
| AMPL7153011276 |
TCAGAAGTTAAGGCAGTGTTTTAGATGG |
GTCCAGAAGTTCCATAGCCTGTTC |
| AMPL7153011349 |
ATTTTACAGAGTAACAGACTAGCTAGAGACA |
GAAAAAGAAACAGAGAATCTCCATTTTAGCA |
| AMPL7153017587 |
CCGAGTATCTCAACACTGTCCA |
CTGGATTTTTAGGGCTCATACTATCCTC |
| AMPL7153017599 |
AGTCACAGGTTCAGTTGCTTGT |
CAGAAGAAATGTTTTTATTCCAAGGGAACA |
| AMPL7153017611 |
CCCAACTCCTTGACCATTACCTC |
CCATCATCACTGTTCGGCTTCT |
| AMPL7153017641 |
ACCTACCATCATTGGAAAGCAGT |
CTGACGACTGCAAGAGAAAACTG |
| AMPL7153017661 |
GCATGAACATGACCCTGAATTCG |
CAGGAAAATGCTGGCTGACCTA |
| AMPL7153017684 |
TGTGATTGTTCATTCATGATCCCACT |
GCCTCAGCTGTTTGGCTAAGAG |
| AMPL7153017693 |
GCCAAGAAGTGGAATAGCATCTCT |
GAGTAGACACAGCTTGAGAGAGAGA |
| AMPL7153017702 |
TTTTTCTCCTTTTAGAAGCTACATAGTGTC |
TGCGCTTCCGAACGATGT |
| AMPL7153017730 |
CCTCAAAAGAGAAATCACGCATTTATGT |
CTGCTGTCTGCATTTATGAACCC |
| AMPL7153017753 |
GAGTCTCTGTGTGGAGAGAGTGA |
ATCCTCTGGAGGCTGAGAAAATG |
| AMPL7153017791 |
AAAGCCATGTTATTCTGCCTTTTTAAACT |
GAACAGACACGTGAAGGCATG |
| AMPL7153017821 |
GCCTGCCCAACCTACTAATCAG |
GGTTGAGGAGCAGGACTGTTTC |
| AMPL7153017863 |
GTACGTATTTTGAAACTCAAGATCGCAT |
CAGGTACTGGGAGCCAATATTGTC |
| AMPL7153017872 |
GTAGCCAGCATGTCTGTGTCA |
CCAGAAGGTTGCACTTGTCCA |
| AMPL7153017881 |
GGGAGGTCCAGAAAGTGATTGG |
CCATATGTCAAATGAATAGTGTCAGGACT |
| AMPL7153017889 |
GGTGACCCTTGTCTCTGTGTTC |
GGAAATATACAGCTTGCAAGGACTCT |
| AMPL7153017902 |
CCACCGTCATCACCTTCCTTTC |
GAAGAGGAAGATGTGTTCCTTTGGA |
| AMPL7153017917 |
GCCAGCCAAACAATCAGAGAATAAG |
CCAATCACCTAAGCAAGTGAAGGA |
| AMPL7153017929 |
GCTGGGTTTTCCCACACTAGTG |
GGTGGCACCAAAGCTGTATTTG |
| AMPL7153019490 |
CTTTGGTCAGGGAACATCTGGA |
CAGCACATGAACGTGTAAACAGG |
| AMPL7153019505 |
GGAAAGGCAGTAAAGGTCATGCA |
CACAGTCACCGATTGACAGACA |
| AMPL7153019527 |
CGACAGCATGCCAATCTCTTCA |
CCAGAGTGAGCTTTCATTTTCTCAGTTA |
| AMPL7153019540 |
TTTCTTTAGATCGGCCATGCAGAA |
ACTCTTCCAGCCAAACATAAACAAAAGTATATA |
| AMPL7153019581 |
ACAAGCAGAAGTATACTCTGAAAATCA |
ACAGATACTCATCCTCAATGTGATTACTTTACC |
| AMPL7153019606 |
GAGTGTCGAATTATGTCCTCTGCAA |
AAATGGCTTTCAGTAGTTTTCATGGTTC |
| AMPL7153019620 |
TCAGGTACAGATGAAGTTTTTAGTTGAGC |
GTAGGAGAATGAGAGAGAGAAGCATAAATT |
| AMPL7153019634 |
GCATGAACTATTTAAAGAAGCAAGAAAATACCC |
GAATAGGATATTGTATCATACCAATTTCTCGA |
| AMPL7153019643 |
TTATGGCAGTCAAACCTTCTCTCTTATG |
GGATCCTTTTCCATAGAGAAAGTATCTACC |
| AMPL7153019670 |
TGGACACTTTTTGGATCACAAGAAGA |
GATTGTTTCTAATAGAGCAGCCAGAACT |
| AMPL7153019681 |
ATTTGATGTTGATGGCTAAAGAAAGCC |
AAATAATAAGCATCAGCATTTGACTTTACC |
| AMPL7153019694 |
GGCATGCCAGTGTGTGAATTTG |
ACAGGTAGAAGACTGCACTATAGTAATGAT |
| AMPL7153019702 |
GGGAAAAAGGAAAGAATGGGCTT |
TGGCCAAAGATTCAAAGCCATTTTT |
| AMPL7153019732 |
CCTGCTTTTGGAGTCCTATTGTCG |
AAAAGAGTCTCAAACACAAACTAGAGTCA |
| AMPL7153019748 |
TTTTTCTTTTAGATCTATGTTCGAACAGGT |
CTGGCCAGTGCCTAGCTAATTT |
Statistical analysis
All analyses were performed using statistical
software (SPSS 17.0; SPSS, Inc., Chicago, IL, USA). An unpaired
Student's t-test was performed for comparisons of detection rates
across molecular subtypes in Table
II. A Mann-Whitney test was performed to analyze constituent
ratios of gene mutations in Table
III. An unpaired Student's t-test was performed to compare the
amount of DNA released from tumor tissues into the circulating
blood across molecular subtypes in Table
IV. Linear-regression analysis was used to evaluate the
association between ctDNA concentration and tumor volume in
basal-like breast cancer. P<0.05 was considered to indicate a
statistically significant difference.
 | Table II.SNV component of cfDNA. |
Table II.
SNV component of cfDNA.
|
| cfDNA SNV
number |
|
|
|---|
|
|
|
|
|
|---|
| Patient | Total SNV | SNP, n (%) | Mutation, n
(%) | Unknown source, n
(%) | Subtype | P-value |
|---|
| 2 | 29 | 16 (55) | 10 (35) | 3 (10) | Basal-like |
|
| 6 | 31 | 17 (55) | 13 (42) | 1 (3) | Basal-like |
|
| 7 | 18 | 16 (89) | 0 (0) | 2 (11) | Basal-like |
|
| 3 | 27 | 18 (67) | 7 (26) | 2 (7) | Her2 | 0.033 |
| 4 | 24 | 19 (79) | 5 (21) | 0 (0) | Her2 |
|
| 5 | 22 | 13 (59) | 9 (41) | 0 (0) | Her2 |
|
| 8 | 23 | 16 (70) | 7 (30) | 0 (0) | Luminal A |
|
| 1 | 11 | 8 (73) | 2 (18) | 1 (9) | Luminal B
Her2− |
|
| 9 | 26 | 22 (85) | 3 (11) | 1 (4) | Luminal B
Her2− |
|
| 10 | 30 | 19 (63) | 7 (23) | 4 (14) | Luminal B
Her2+ |
|
| 11 | 12 | 9 (75) | 2 (17) | 1 (8) | Luminal B
Her2+ |
|
| Total | 253 | 173 (68) | 65 (26) | 15 (6) | – |
|
 | Table III.Constituent ratio of gene mutation in
plasma and tumor tissue. |
Table III.
Constituent ratio of gene mutation in
plasma and tumor tissue.
|
| Mutation rate,
% | Constituent
ratio |
|---|
|
|
|
|
|---|
| Gene | Plasma | Tissue | Plasma | Tissue |
|---|
| TP53 | 22.92 | 28.43 | 0.27 | 0.21 |
|
Phosphatidylinositol-4,5-biphosphate
3-kinase catalytic subunit α | 14.63 | 21.46 | 0.17 | 0.16 |
| Phosphatase and
tensin homolog | 6.97 | 17.27 | 0.08 | 0.13 |
| Epidermal growth
factor receptor | 9.79 | 21.56 | 0.12 | 0.16 |
| AKT1 | 29.63 | 41.76 | 0.35 | 0.32 |
| Total | 83.94 | 130.48 | 1 | 1 |
 | Table IV.Comparison of the average mutation
rate per patient with breast cancer. |
Table IV.
Comparison of the average mutation
rate per patient with breast cancer.
|
| Average mutation
rate, % |
|
|
|---|
|
|
|
|
|
|---|
| Patient | Plasma | Tissue | Tissue-plasma | Subtype | P-value |
|---|
| 2 | 17.69 | 23.14 | 5.45 | Basal-like |
|
| 6 | 42.11 | 46.76 | 4.65 | Basal-like | 0.048 |
| 7 | 43.07 | 6.02 | −37.05 | Basal-like |
|
| 3 | 12.75 | 20.40 | 7.65 | Her2 |
|
| 4 | 8.04 | 26.53 | 18.49 | Her2 |
|
| 5 | 35.00 | 46.12 | 11.12 | Her2 |
|
| 8 | 29.97 | 42.31 | 12.34 | Luminal A |
|
| 9 | 10.54 | 19.16 | 8.62 | Luminal B
Her2− |
|
| 10 | 21.43 | 24.94 | 3.51 | Luminal B
Her2+ |
|
| 11 | 6.38 | 17.13 | 10.75 | Luminal B
Her2+ |
|
| 1 | 4.24 | 22.31 | 18.07 | Luminal B
Her2− |
|
Results
Amplicon sequencing
Phosphatidylinositol-4,5-biphosphate 3-kinase
catalytic subunit α, TP53, epidermal growth factor receptor, Akt
and phosphatase and tensin homolog were identified as the most
commonly mutated in breast cancer, according to TCGA. Primers were
designed for these genes and the amplicon-based method for
whole-exon sequencing was performed on DNA samples from 11 patients
with invasive breast cancer from the Heilongjiang Province Breast
Bio-Sample Bank, including from tumor tissues, plasma and
peripheral blood cells. Ion torrent sequencing was performed; this
achieved 98.95% coverage based on a mean sequencing depth of 4,920
reads/sample. The sequenced DNA fragments were 25.2 kb, and the
amplicon length was 125–275 bp (coverage >10, quality >100,
strand bias <0.8).
Association between the ctDNA mutation
rate and clinical data
Subsequent to excluding single-nucleotide
polymorphisms (SNPs) detected in the peripheral blood cells, the
mean mutation frequencies of the 5 genes for each patient (tested
in plasma and tissues) are listed in Table IV. A total of 3/11 patients exhibited
the basal-like subtype. Of the patients with the basal-like
subtype, the difference in the mutation frequencies between tumor
tissues and plasma samples (tissue-plasma) was the lowest in
patients 2 and 6, whereas the mutation frequency in plasma was
higher than in the tumor tissue of patient 7. Therefore, it was
demonstrated that the amount of DNA released from tumor tissues to
circulating blood in the basal-like subtype was generally increased
compared with that in other subtypes (P=0.048). We hypothesized
that this was caused by the high proliferative activity and
elevated level of necrosis and apoptosis associated with the
basal-like subtype (11).
The effect on cell survival and growth of particular
mutations lead to an alteration in the mutation compositions
between plasma and tumor tissues. To further elucidate this issue,
the constituent ratios of gene mutations in plasma and tumor
tissues were analyzed (Table III).
However, no significant difference in the constituent ratios of
gene mutations were observed between plasma and tumor tissues
(P=0.917, Mann-Whitney test). On account of the similarity in the
constituent ratios identified in tumor tissues and plasma in the
present study, ctDNA may be useful to track the clonal evolution of
tumors.
Comparison of the ctDNA concentration
with clinical data
The concentration of ctDNA was previously
demonstrated as a biomarker for the assessment of cancer burden.
This was verified in the present study. Multiplying the
concentration of cfDNA, as determined by a previously determined
method (12), by the mutation
frequency of ctDNA, yielded the concentration of ctDNA. The mean
value for the mutation frequencies of the genes was used as the
ctDNA mutation frequency. The clinical data and ctDNA
concentrations of the 11 patients are summarized in Table V. The scatter plot comparing the ctDNA
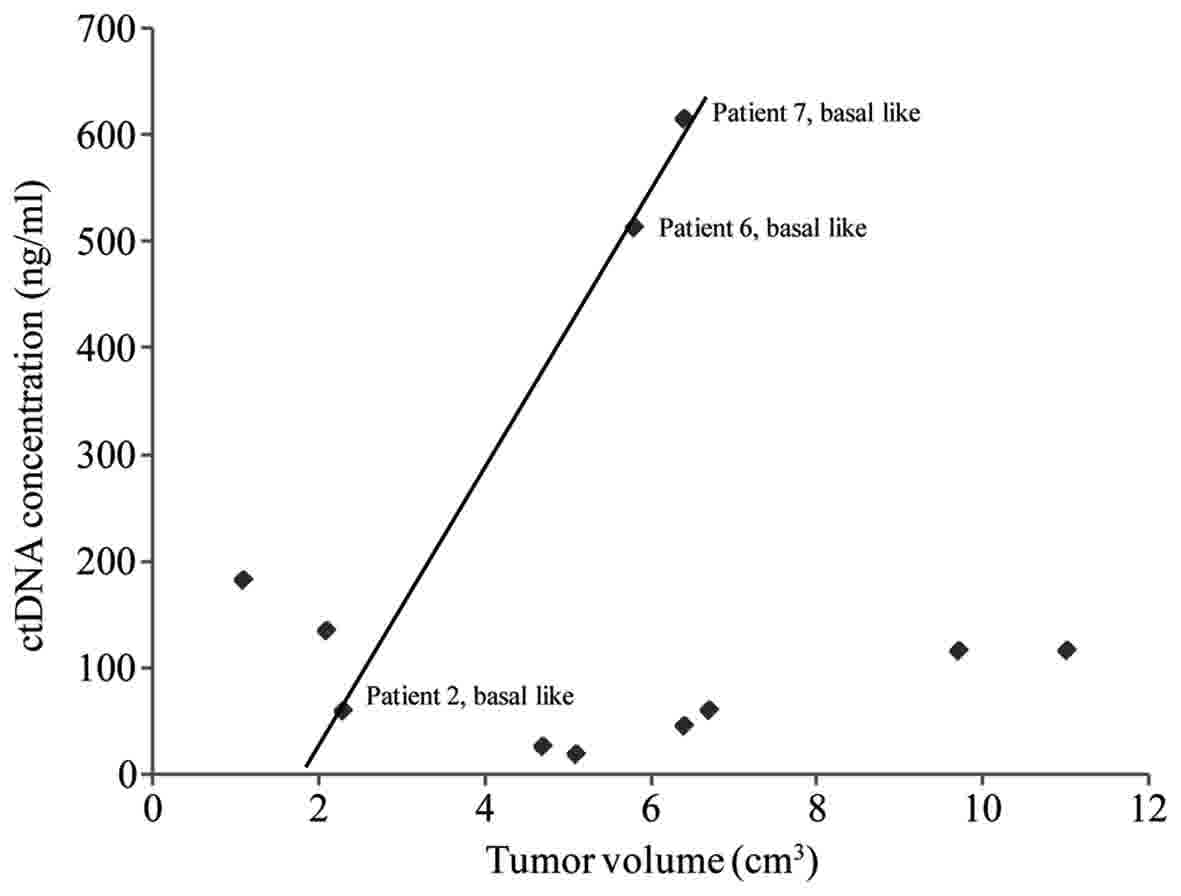
concentration with tumor volume demonstrated a non-linear
association for the majority of the patients (Fig. 1). However, the ctDNA concentration vs.
tumor volume demonstrated a highly linear association in the 3
patients with basal-like breast cancer (R2=0.999,
P=0.018). Therefore, ctDNA may provide an effective assessment
regarding tumor burden in patients with basal-like breast
cancer.
 | Table V.Clinical data of patients and ctDNA
concentration. |
Table V.
Clinical data of patients and ctDNA
concentration.
| Patient | Age, years | Tumor size, cm | Grade | P53 status | TNM | ctDNA (ng/ml) |
|---|
| 1 | 40 | 2.6×1.3 | 2 | – | IIB | 45.79 |
| 2 | 47 | 1.4×1.2 | 2 | – | IIA | 59.79 |
| 3 | 36 | 1.5×1.1 | 3 | 2+ | IIA | 135.15 |
| 4 | 53 | 2.0×1.4 | 2 | – | I | 26.37 |
| 5 | 42 | 2.1×2.2 | 2 | 2+ | IIA | 116.20 |
| 6 | 55 | 2.0×1.6 | 2 | 3+ | I | 513.74 |
| 7 | 43 | 2.6×1.3 | 3 | – | IIA | 615.90 |
| 8 | 60 | 1.1×1.0 | 2 | – | I | 182.81 |
| 9 | 45 | 2.1×1.7 | 2 | – | IIB | 60.49 |
| 10 | 55 | 2.5×2.0 | 3 | 2+ | IIB | 116.57 |
| 11 | 61 | 2.0×1.5 | 1 | – | I | 19.14 |
Function of circulating blood cell
samples and tumor tissue samples in ctDNA detection
SNPs in the cfDNA were excluded by collectively
examining the mutations identified in the blood cell samples. Tumor
tissues were used to verify the reliability of ctDNA and exclude
the possibility of an unknown source of cfDNA contributing sequence
variants that are not SNPs and that do not exist in tumor tissue.
SNPs constituted 68% (173/253) of all the single nucleotide
variants (SNVs) in cfDNA. Somatic mutations derived from tumor
tissues accounted for 26% (65/253). The remaining variants were
SNVs of unknown source (15/253, 6%; Table II). As SNPs accounted for most of the
SNVs in cfDNA, the detection of germline SNPs should be coupled
with ctDNA detection to exclude SNPs from the assessment of cancer
burden or prediction of drug sensitivity. There were a limited
number of unknown source SNVs, which may have affected the result,
even though the number was limited. Therefore, the simultaneous
detection of variation between tumor tissue samples and plasma
samples was determined to be necessary for accurate data
analysis.
Effect of the mutation frequency in
tumor tissue on the ctDNA detection rate
The present study identified a similarity in the
mutation frequency between tumor tissues and ctDNA in basal-like
breast cancer. To determine whether a higher mutation detection
rate in ctDNA was observed in patients with basal-like breast
cancer, the detection rates across molecular subtypes were compared
(Table II). It was demonstrated that
basal-like and Her2-positive breast cancer exhibited a markedly
increased mutation detection rate compared with the luminal subtype
(P=0.033). It was hypothesized that the cause may be the increased
frequency of mutations in the two molecular subtypes. To further
examine this hypothesis, the distribution of mutations derived from
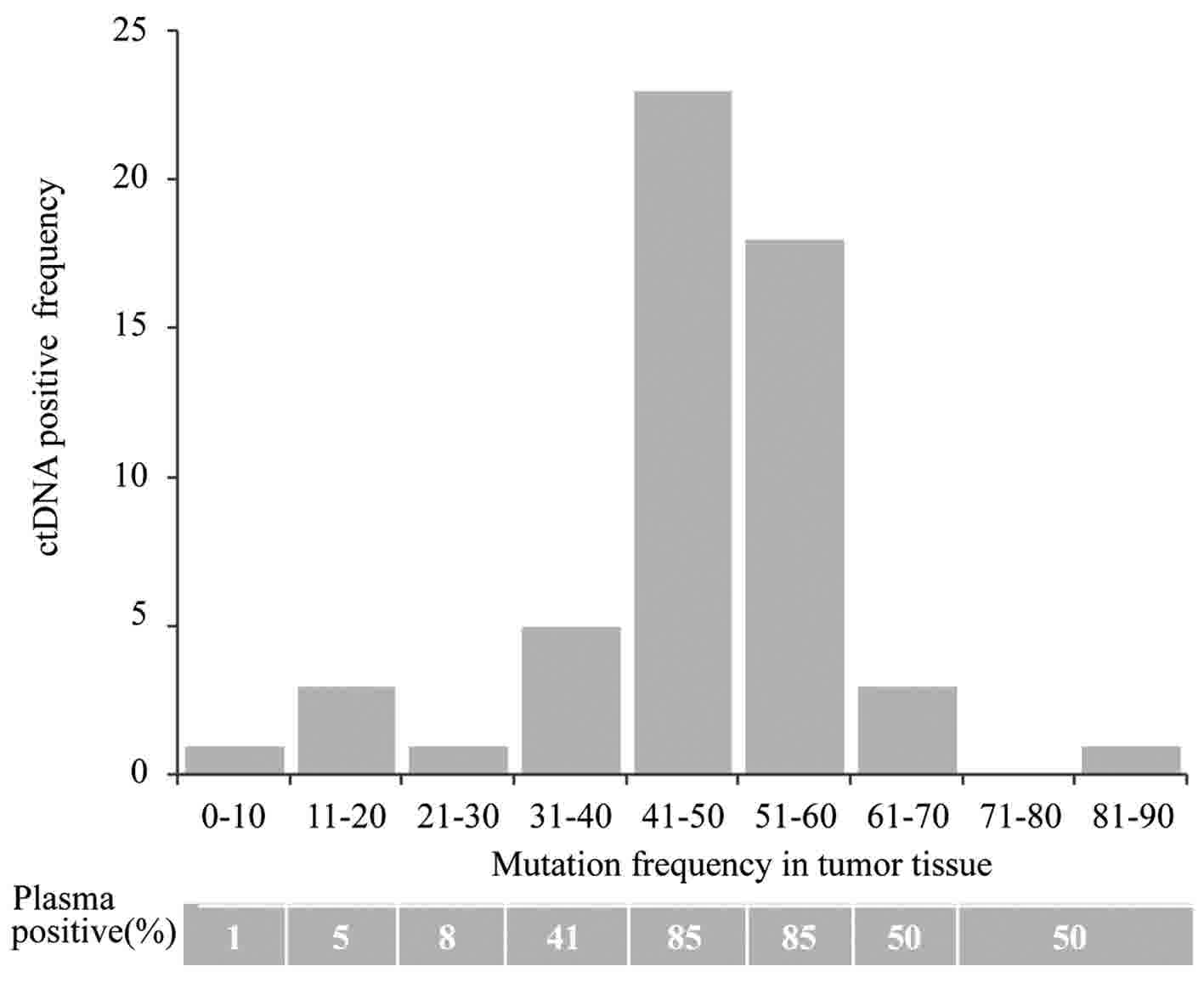
tumor tissue in circulating DNA was analyzed (Fig. 2).
A mutant locus with a mutation frequency of >30%
in tissues presented a detection rate of >40% in plasma. An
additional locus with a <10% mutation frequency in tissue
samples exhibited a detection rate of ≤1% in the plasma. A low
positive detection rate may be limited by the sensitivity of the
detection methods and the number of detected loci. Therefore, the
detection rate may be increased by improving the detection
sensitivity or increasing the number of detected genes.
Discussion
To the best of our knowledge, the present study is
the first to identify a distinction between ctDNA across molecular
subtypes. The mutation frequency differed the least between ctDNA
and tissue DNA in basal-like breast cancer. Additionally, the
association between ctDNA concentration and tumor volume was linear
in basal-like breast cancer. Based on these results, ctDNA
detection appears to be a promising technique for the assessment of
cancer burden in basal-like breast cancer. It has been suggested
that ctDNA is a highly sensitive biomarker of metastatic breast
cancer (6). However, there is little
evidence concerning the significance of tumor burden assessment in
primary breast cancer, particularly early-stage breast cancer; the
present study demonstrated that ctDNA may allow the assessment of
tumor burden in the basal-like subtype of early-stage breast
cancer, not in other subtypes.
Understanding the factors that influence the ctDNA
detection rate is beneficial to ensure the accuracy of the results.
The present study demonstrated that the ctDNA detection rate
depended on the mutation frequency in the corresponding tumor
tissues. In a previous study, ctDNA was detectable in 50% of
samples from patients with localized breast cancer, as assessed by
digital PCR of a relatively small number of loci (13). ctDNA was detected in all patients in
the present study, due to the greater number of detected loci vs.
the previous study. However, the concentration of circulating DNA
varied among patients. Therefore, an increased number of mutant
loci are required to accurately assess the concentration of ctDNA
and improve the reliability of ctDNA measurement in future
studies.
In the present study, the mutations identified in
tumor tissues exhibited a similar constituent ratio to the
mutations identified in plasma. ctDNA may theoretically be used to
track tumor subclonal evolution in order to correctly reflect tumor
progression. Therefore, liquid biopsy analysis of clonal evolution
may be applicable to analyze the origin of cancer. However, SNPs
may seriously affect the mutation frequency and concentration of
ctDNA as they accounted for almost 70% of all the SNVs in cfDNA in
the present study. Therefore, tumor burden assessment required the
detection and exclusion of SNPs. In predictions of sensitivity,
tissue samples and SNPs should be detected concurrently to
accurately identify mutant loci.
In conclusion, the present study indicated that
ctDNA may reflect the mutations observed in breast cancer. In
basal-like breast cancer, ctDNA may be a particularly sensitive
biomarker for the assessment of mutation frequency and cancer
burden. Although the detection of circulating DNA was previously
reported as a promising method, there are a number of unsolved
problems concerning the detection methods, analysis approach and
application fields. Previously published ctDNA detection methods
employing Tam-Seq (14) or CAPP-Seq
(15) may be highly sensitive for the
detection and monitoring of ctDNA. However, to further expand the
potential clinical applications of ctDNA, additional investigation
into the association of mutations with the physical characteristics
of each type of cancer is required. The present study suggests that
the analysis of ctDNA could be applied clinically to detect and
monitor diverse types of malignancy.
Acknowledgements
The authors would like to thank Dr Guangwen Zhang
from Heilongjiang Province Breast Bio-Sample Bank (Harbin, China)
for assistance with the collection of tissue and blood samples.
Funding
The present study was supported by the Project of
Heilongjiang Province Applied Technology Research and Development
(grant no., GA13C201).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Author's contributions
WW, XZ and DP conceived and designed the study,
participated in the analysis and interpretation of the data, and
coordinated and drafted the manuscript. SS performed the
statistical analysis, participated in the interpretation of the
data and assisted in drafting the manuscript. BX participated in
the design of the study, statistical analysis, interpretation of
the data, coordination and helped in drafting the manuscript. XL
participated in the design of the study, interpretation of the
data, coordination and assisted in drafting the manuscript. YC and
SG participated in the interpretation of the data and assisted in
drafting the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
Harbin Medical University. All patients provided written informed
consent.
Consent for publication
All patients provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ctDNA
|
circulating tumor DNA
|
|
cfDNA
|
circulating cell-free DNA
|
|
SNPs
|
single-nucleotide polymorphisms
|
|
SNVs
|
single nucleotide variants
|
References
|
1
|
Hao J, Zhao P and Chen WQ: Chines cancer
registry annual report. 2012.
|
|
2
|
Schwarzenbach H, Hoon DS and Pantel K:
Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev
Cancer. 11:426–437. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gormally E, Caboux E, Vineis P and Hainaut
P: Circulating free DNA in plasma or serum as biomarker of
carcinogenesis: Practical aspects and biological significance.
Mutat Res. 635:105–117. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Whiteside TL: The potential of
tumor-derived exosomes for noninvasive cancer monitoring. Expert
Rev Mol Diagn. 15:1293–1310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nakamura T, Sueoka-Aragane N, Iwanaga K,
Sato A, Komiya K, Abe T, Ureshino N, Hayashi S, Hosomi T, Hirai M,
et al: A noninvasive system for monitoring resistance to epidermal
growth factor receptor tyrosine kinase inhibitors with plasma DNA.
J Thorac Oncol. 6:1639–1648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dawson SJ, Tsui DW, Murtaza M, Biggs H,
Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B,
et al: Analysis of circulating tumor DNA to monitor metastatic
breast cancer. N Engl J Med. 368:1199–1209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murtaza M, Dawson SJ, Tsui DW, Gale D,
Forshew T, Piskorz AM, Parkinson C, Chin SF, Kingsbury Z, Wong AS,
et al: Non-invasive analysis of acquired resistance to cancer
therapy by sequencing of plasma DNA. Nature. 497:108–112. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Beaver JA, Jelovac D, Balukrishna S,
Cochran R, Croessmann S, Zabransky DJ, Wong HY, Toro PV, Cidado J,
Blair BG, et al: Detection of cancer DNA in plasma of patients with
early-stage breast cancer. Clin Cancer Res. 20:2643–2650. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perrone F, Lampis A, Bertan C, Verderio P,
Ciniselli CM, Pizzamiglio S, Frattini M, Nucifora M, Molinari F,
Gallino G, et al: Circulating free DNA in a screening program for
early colorectal cancer detection. Tumori. 100:115–121. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
NCCN Clinical Practice Guidelines in
Oncology. Breast Cancer. https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
|
|
11
|
Rakha EA, Reis-Filho JS and Ellis IO:
Impact of basal-like breast carcinoma determination for a more
specific therapy. Pathobiology. 75:95–103. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Diehl F, Schmidt K, Choti MA, Romans K,
Goodman S, Li M, Thornton K, Agrawal N, Sokoll L, Szabo SA, et al:
Circulating mutant DNA to assess tumor dynamics. Nat Med.
14:985–990. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bettegowda C, Sausen M, Leary RJ, Kinde I,
Wang Y, Agrawal N, Bartlett BR, Wang H, Luber B, Alani RM, et al:
Detection of circulating tumor DNA in early- and late-stage human
malignancies. Sci Transl Med. 6:224ra242014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Forshew T, Murtaza M, Parkinson C, Gale D,
Tsui DW, Kaper F, Dawson SJ, Piskorz AM, Jimenez-Linan M, Bentley
D, et al: Noninvasive identification and monitoring of cancer
mutations by targeted deep sequencing of plasma DNA. Sci Transl
Med. 4:136ra682012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Newman AM, Bratman SV, To J, Wynne JF,
Eclov NC, Modlin LA, Liu CL, Neal JW, Wakelee HA, Merritt RE, et
al: An ultrasensitive method for quantitating circulating tumor DNA
with broad patient coverage. Nat Med. 20:548–554. 2014. View Article : Google Scholar : PubMed/NCBI
|