Introduction
Ovarian cancer is one of the leading causes of
cancer-associated mortality in females. As epithelial ovarian
cancer accounts for ~90% of all ovarian cancer cases worldwide, it
is considered to be one of the most common types of gynecological
tumor (1,2). Due to the lack of effective means for
early diagnosis, the majority of patients are diagnosed at an
advanced stage of the disease, and the 5-year survival rate of
advanced ovarian cancer remains unsatisfactory, at only 20–25%
(3). Cisplatin-mediated chemotherapy
has been used to improve the prognosis and survival patients with
advanced stage ovarian cancer (2).
However, the clinical application of this therapeutic agent is
limited by chemoresistance. Therefore, it is necessary to
investigate the mechanism underlying the development of
chemoresistance, which may assist in developing novel treatment
strategies for ovarian cancer patients with chemoresistance.
MicroRNAs (miRNAs or miRs) are a class of small
(18–25 nucleotides), endogenous, non-coding RNAs, which function by
directly binding to the 3′-untranslated region (3′UTR) of their
target mRNAs, causing translational inhibition of proteins and mRNA
degradation (4). miRNAs have been
documented to be involved in various biological processes,
including tumorigenesis through the regulation of cell
proliferation, apoptosis, differentiation, migration, invasion,
epithelial-mesenchymal transition and chemoresistant phenotype
formation (5–11). Several previous studies have reported
that miR-149 serves crucial roles in the progression of various
tumors. For instance, Chen et al (12) demonstrated that downregulated
expression of miR-149 promoted apoptosis in side population cells
that were sorted from the TSU prostate cancer cell lines. In
addition, miR-149-3p-Wnt-1 signaling was observed to be involved in
18β-glycyrrhetinic acid-mediated gastric cancer suppression
(13). However, the mechanism
underlying the effect of miR-149 in ovarian cancer remains
unclear.
In the present study, miR-149 expression was
observed to be downregulated in ovarian cancer tissues and cell
lines. Low miR-149 expression was associated with a poor patient
prognosis, whereas forced expression of miR-149 increased the
sensitivity of ovarian cancer cell to cisplatin treatment.
Furthermore, it was demonstrated that X-linked inhibitor of
apoptosis (XIAP) was a target of miR-149 and was involved in the
function of miR-149 in ovarian cancer.
Patients and methods
Patients
A total of 58 human epithelial ovarian tumor tissues
and adjacent normal ovarian tissues were obtained from the patients
who received chemotherapy for ovarian cancer in the Department of
Gynecology, Affiliated Hospital of Qingdao University (Qingdao,
China) between September 2010 and October 2015. Patients were
between 25 and 85 years of age, with a mean age of 44.5±3.4.
According to the criteria of the International Federation of
Gynecology and Obstetrics, International Gynecologic Cancer Society
(14), these patients were divided
into three stages (stage I, n=12; stage II, n=28; stage III, n=18).
The exclusion criteria of samples are as follows: i) Patients who
were pregnant when they were afflicted with ovarian cancer, ii)
patients who were simultaneously afflicted with ovarian cancer and
other malignant tumor types and iii) patients whose case or data
were incomplete. According to the radiologic Response Evaluation
Criteria in Solid Tumors (RECIST) guidelines, the 58 clinical
tissues were divided into two groups immediately following
administration of the chemotherapy regime (15). According to their response to
chemotherapy, they were divided into the ‘sensitive’ (complete or
partial response) and ‘insensitive’ (stable or progressive disease)
groups. The research protocol was reviewed and approved by the
Ethical Committee and Institutional Review Board of the Department
of Gynecology, Affiliated Hospital of Qingdao University. Written
informed consent was obtained from each patient included into the
current study. Following detection of the expression level of
miR-149 in all samples, the mean value of miR-149 was used as the
cut-off value. Thus, the ovarian cancer samples were divided into
two groups in accordance with the level of miR-149 expression (the
miR-149 high expression group and miR-149 low expression
group).
Cell lines
In total, four ovarian cancer cell lines (HG-SOC,
HO8910, SKOV-3 and ES2) and the immortalized normal fallopian tube
epithelial FTE187 cell line were purchased from the Cell Bank of
the Type Culture Collection of Chinese Academy of Sciences
(Shanghai, China). The cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS, Gibco; Thermo Fisher
Scientific, Inc.), 100 µl ampicillin and 100 µl streptomycin at
37°C in a humidified atmosphere with 95% air and 5%
CO2.
Cell transfection
HO8910 and SKOV-3 cells were preserved in RPMI-1640
(Invitrogen; Thermo Fisher Scientific, Inc.), supplemented with 100
µl penicillin/streptomycin and 10% fetal bovine serum until
transfection. Cells were incubated until 80% confluence was
reached, and then transfected for 15 min at 37°C. For
overexpression and knockdown of XIAP, cells were separately
transfected with pcDNA3.1-XIAP or small interfering RNA
(siRNA)-XIAP vectors (both from GenePharma Co., Ltd., Shanghai,
China). At the same time, the negative controls (the pcDNA3.1 empty
vector and si-NC) were transfected into cells for comparison. The
negative controls were obtained from GenePharma Co., Ltd. For
overexpression and knockdown of miR-149, cells were separately
transfected with miR-149 mimics and miR-149 inhibitors and miR-NC
(Shanghai GenePharma Co., Ltd., Shanghai, China). All transfections
were performed using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from the cells or tissues was extracted
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The concentration of RNA
was quantified using a NanoDrop ND-1000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc., Wilmington, DE, USA).
RNA concentration was determined using the following formula;
optical density (OD)260 nm × dilution × ratio 0.04 ug/ul. cDNA
synthesized from total RNA was reverse transcribed with the
Transcriptor High Fidelity cDNA synthesis kit (Roche Applied
Science, Mannheim, Germany) using the miRNA-specific primer or
oligo (dT) and the random hexamer-primers. The relative expression
level of mRNA was detected by SYBR-Green qRT-PCR assay (Bio-Rad
Laboratories Inc, Hercules, CA, USA). qPCR was then performed to
measure the miR-149 and XIAP expression levels using SYBR-Green PCR
Master Mix on an ABI 7500 PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) following the manufacturer's instructions.
The primers sequences used for this experiment were as follows:
miR-149 forward, 5′-GGCTCTGGCTCCGTGTCTT-3′, and reverse,
CAGTGCAGGGTCCGAGGTATT; U6 forward, 5′-CAAATTCGTGAAGCGTTCCATA-3′ and
reverse, 5′-AGTGCAGGGTCCGAGGTATTC-3′; GAPDH forward,
CCTGTACGCCAACACAGTGC and reverse, ATACTCCTGCTTGCTGATCC. qPCR
conditions were as follows; pre-denaturation at 95°C for 10 min
followed by 45 cycles at 95°C for 15 sec and 40 cycles at 60°C for
1 min. The miR-149 or XIAP levels were calculated with the
2−ΔΔCq method (16), and
were normalized to U6 RNA or GAPDH mRNA levels, respectively.
Control levels were defined as 1.0, and the miRNA or mRNA
expression levels were presented relative to the fold change of the
corresponding control. All assays were performed in triplicate.
Bioinformatics analysis
By employing the online miRNA target binding site
analysis miRanda software (www.microrna.org/microrna/home.do), ~8,955 prediction
targets of miR-149 were identified. Among these 8,955 prediction
targets, the current study focused on investigating the role of
XIAP, which is the study object of our research group.
Dual-luciferase activity assay
A XIAP 3′-UTR luciferase reporter gene plasmid was
constructed by inserting it into the pGL3 vector (Promega
Corporation, Madison, WI, USA), and the fragment containing
putative binding sites for miR-149 was amplified. The plasmids
pXIAP-wild-type (WT) and pXIAP-mutated (MUT) and miR-149
overexpressing vector were generated via subcloning the downstream
luciferase vector using Fugene (Promega Corporation).
Dual-luciferase reporter experiments were then performed. Briefly,
human ovarian cancer cells (SKOV-3, 5×104) seeded into
96-well plates and then co-transfected with 100 ng pXIAP-WT or
pXIAP-MUT and with miR-149 mimics or scramble oligonucleotide in
the presence of 50 nM Lipofectamine 2000 (Life Technologies,
Carlsbad, USA). After 48 h, cells were assayed using the Dual-Glo
Luciferase Assay kit (Promega Corp., Madison, WI, USA), according
to the manufacturer's instructions. The SpectraMax M5 microplate
reader (Molecular Devices, LLC, Sunnyvale, CA, USA) was used to
analyze the results.
Western blot analysis
Total proteins were extracted from the cells or
tissues using radioimmunoprecipitation assay lysis buffer (Thermo
Fisher Scientific, Inc.). Protein concentration was determined
using the following formula: [(1.45 × (OD280 nm −0.74 × OD260 nm) ×
dilution factor]. Next, the total protein samples were separated by
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
electrophoretically transferred to polyvinylidene difluoride
membranes (Roche Diagnostics, Basel, Switzerland). The membranes
were then blocked using 3% non-fat milk for ~30 min at 4°C,
followed by incubation with primary antibodies against XIAP
(1:5,000; cat. no. ab21278; Santa Cruz Biotechnology, Inc., Dallas,
TX, USA) and GAPDH (1:5,000; cat. no. ab9485; Abcam, Cambridge, MA
USA) at 4°C overnight. Subsequently, the membranes were probed with
horseradish peroxidase-labeled secondary antibody (1:5,000; cat.
no. ab205718; Abcam), and the signals were visualized using an
enhanced chemiluminescence detection system (cat. no. NEL100001EA;
PerkinElmer, Inc., Waltham, MA, USA). All antibodies were incubated
at room temperature for 1–2 h. Quantity One 1-D analysis software
(version 4.6.5; Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
used to quantify results. GAPDH was used as the control.
In vitro chemosensitivity assay
An MTT assay (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) was conducted to examine the chemosensitivity of cells to
cisplatin. Briefly, cells (500 cells/well) were cultured in 96-well
plates and then treated with cisplatin at different concentrations
(0, 2.5, 5, 10, 20, 40, 80 µM). Cells were then incubated for 1 h
at room temperature. After 48 h of incubation, MTT solution (5
mg/ml; 20 µl) was added to each well for 4 h, followed by removal
of the media and addition of 100 µl dimethyl sulfoxide to each
well. The relative number of surviving cells was subsequently
assessed by measuring the optical density of the cell lysates at a
wavelength of 560 nm. All assays were conducted in triplicate.
Colony formation assay
In order to examine the colony formation ability of
cells, 500 cells per well were seeded into 6-well plates and
incubated in RPMI 1640 supplemented with 10% FBS at 37°C. After 2
weeks, the cells were fixed in 4% paraformaldehyde for 15 min and
stained with 0.1% crystal violet. Subsequently, the number of
visible colonies in the plates was counted manually.
Flow cytometric analysis of
apoptosis
In order to investigate cell apoptosis, flow
cytometry was conducted using an Annexin V-FITC Apoptosis Detection
kit (BD Biosciences, Franklin Lakes, NJ, USA), according to the
manufacturer's protocol. All samples were assayed in
triplicate.
Statistical analysis
All data are expressed as the mean ± standard
deviation. Student's t-test or one-way analysis of variance was
used to determine any statistically significant differences among
the groups using SPSS version 17.0 software (SPSS, Inc., Chicago,
IL, USA). Log-rank test and Kaplan-Meier was used for survival
analysis. All tests performed were two-sided. The correlation
between miR-149 and XIAP expression levels in the 58 cases of
ovarian tumor tissues was analyzed by Spearman's correlation
analysis. P<0.05 was considered to indicate a difference that
was statistically significant. All the experiments were repeated at
least three times with each sample examined in triplicate.
Results
miR-149 expression is downregulated in
chemosensitive and chemo-insensitive ovarian cancer tissues, and
ovarian cancer cell lines
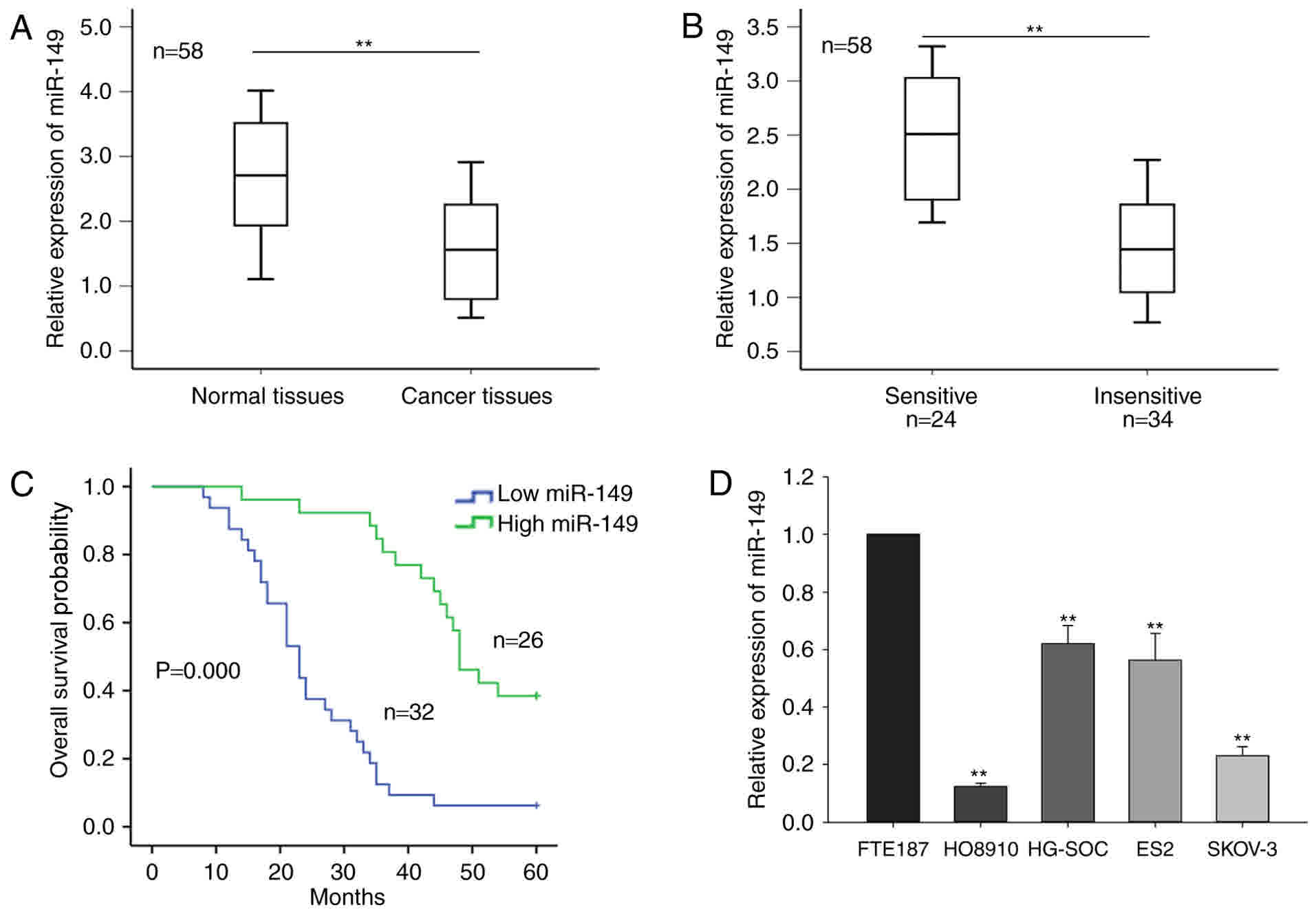
In the current study, the level of miR-149 in 58
ovarian tumor tissues and the corresponding normal tissues was
initially measured. As shown in Fig.
1A, miR-149 expression was significantly downregulated in
cancer tissues compared with that in the normal tissues
(P<0.01). Furthermore, based on the radiologic RECIST
guidelines, the 58 cases of ovarian tumor were classified as
sensitive (complete or partial response) and insensitive (stable or
progressive disease) to cisplatin-based chemotherapy. As
demonstrated in Fig. 1B, a
significantly higher level of miR-149 expression was observed in
the chemosensitive group (n=24) compared with the chemo-insensitive
group (n=34), as detected by RT-qPCR. The mean value of the miR-149
expression level of the samples was regarded as the cut-off value,
and the ovarian cancer samples were divided into two groups
according to the level of miR-149 expression (miR-149 high
expression group and miR-149 low expression group). Furthermore,
survival analysis indicated that a low level of miR-149 was
associated with poor prognosis of the patients (Fig. 1C).
Furthermore, the current study determined the level
of miR-149 in four ovarian cancer cell lines, namely HG-SOC,
HO8910, SKOV-3 and ES2, and the immortalized normal fallopian tube
epithelial FTE187 cell line. As presented in Fig. 1D, miR-149 expression was significantly
reduced in the four ovarian cancer cell lines, as compared with
that in the normal cells. In addition, the level of miR-149 in
HO8910 and SKOV-3 cells was relatively lower in comparison with
that in the other two cancer cell lines; thus, the HO8910 and
SKOV-3 cell lines were selected for use in subsequent assays. These
results indicated that miR-149 may be involved in the progression
of ovarian cancer.
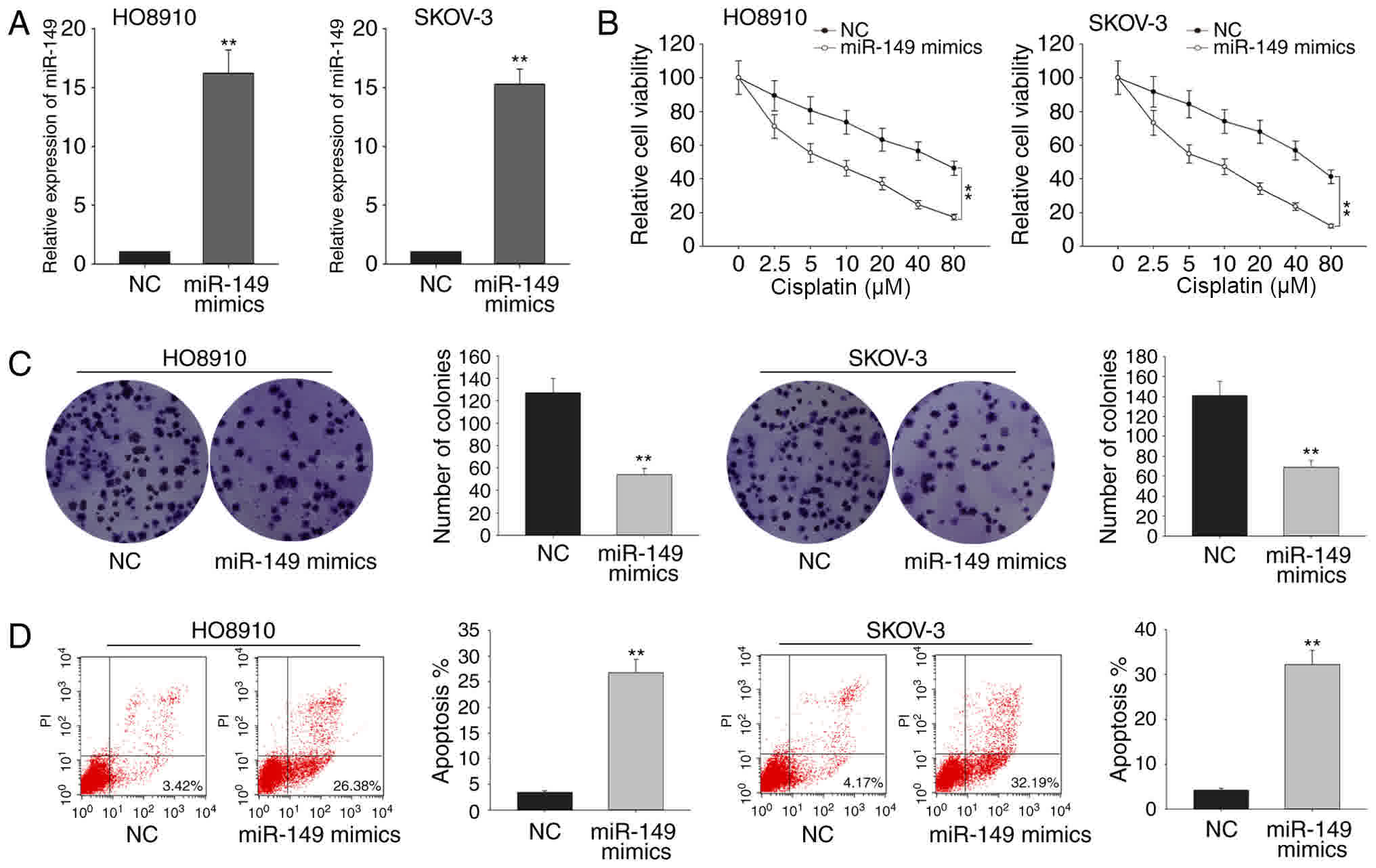
Ectopic expression of miR-149
increases the sensitivity of ovarian cancer cells to cisplatin,
inhibits cell proliferation and induces cell apoptosis
To determine the function of miR-149 in ovarian
cells, the two cell lines HO8910 and SKOV-3 were selected as model
cells, since they exhibited the lowest expression levels in RT-qPCR
assay. These cells were transfected with miR-149 mimics to induce
overexpression of this miRNA, or with miR-negative control (NC),
which served as the negative control group (Fig. 2A). The results of the MTT assay
revealed that the overexpression of miR-149 significantly increased
the sensitivity of HO8910 and SKOV-3 cells to cisplatin with the
increasing concentration of cisplatin, compared with that of the
control transfected cells (Fig. 2B).
Colony formation assays also revealed that ectopic expression of
miR-149 significantly inhibited the HO8910 and SKOV-3 cell
proliferation as compared with that of miR-NC-transfected cells
(Fig. 2C). Additionally, flow
cytometry analysis indicated that overexpression of miR-149
markedly increased the apoptosis rate in the two cell lines
(Fig. 2D). These findings revealed
that miR-149 may function as a tumor suppressor in ovarian
cancer.
XIAP is a direct target of miR-149 in
the ovarian cancer cells
Based on the results of bioinformatics analysis
using the miRanda software, 8,955 predicted targets of miR-149 were
identified. Among these predicted targets, XIAP was observed to be
a potential target gene of miR-149 (Fig.
3A), and this gene was selected for further investigation.
Next, the level of XIAP in the four ovarian cancer cell lines
(HG-SOC, HO8910, SKOV-3 and ES2) and the immortalized normal
fallopian tube epithelial cell line (FTE187) was detected. As
illustrated in Fig. 3B, the mRNA
levels of XIAP was significantly increased in the four ovarian
cancer cells, particularly in HO8910 and SKOV-3 cells.
Subsequently, the interaction between miR-149 and XIAP was measured
by a dual-luciferase reporter assay in SKOV-3 cells. As
demonstrated in Fig. 3C, miR-149
overexpression by mimic transfection significantly inhibited the
luciferase activity of the XIAP-WT reporter, but not of the
XIAP-MUT 3′UTR reporter. Furthermore, forced expression of miR-149
significantly inhibited XIAP expression at the mRNA and protein
levels in SKOV-3 cells (Fig. 3D).
Furthermore, the level of XIAP in the ovarian cancer tissues was
measured. It was revealed that XIAP was significantly upregulated
in ovarian cancer tissues compared with that in the corresponding
normal tissues (Fig. 3E). A lower
level of XIAP was observed in the chemosensitive group when
compared with that in the chemo-insensitive group (Fig. 3F). Additionally, XIAP expression was
inversely correlated with miR-149 expression in ovarian cancer
tissues in accordance with the Pearson correlation results.
Collectively, all results indicate that miR-149 negatively
regulated XIAP in ovarian cancer cells (Fig. 3G).
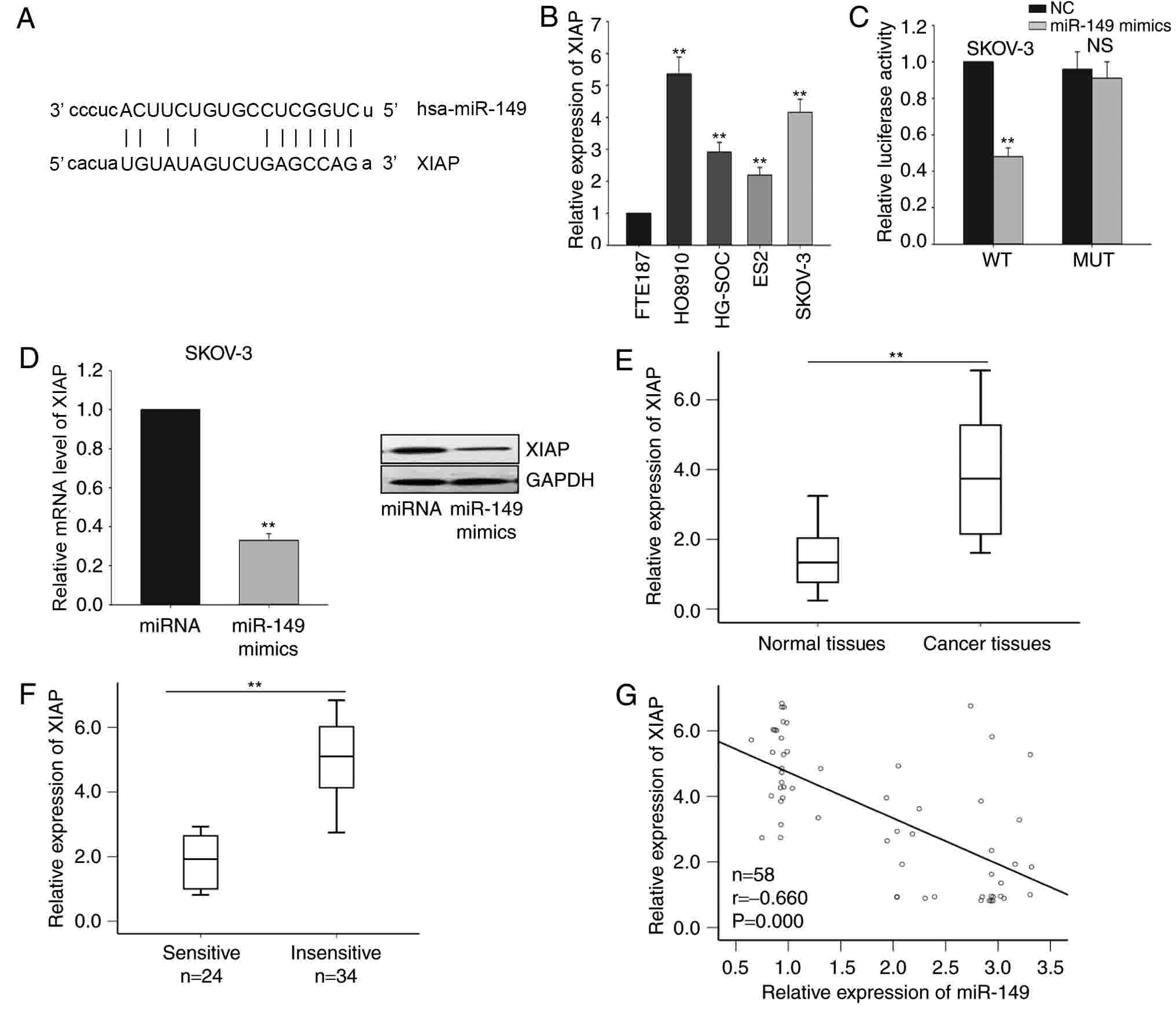 | Figure 3.XIAP is a direct target of miR-149 in
ovarian cancer cells. (A) Bioinformatics analysis was used to
predict the binding site for miR-149 in the 3′-untranslated region
of XIAP. (B) RT-qPCR was performed to measure the level of XIAP in
four ovarian cancer cells (HG-SOC, HO8910, SKOV-3 and ES2) and one
immortalized normal fallopian tube epithelial (FTE187) cell lines.
(C) Dual-luciferase reporter assay was performed to confirm the
interaction between miR-149 and XIAP. (D) mRNA and protein levels
of XIAP in response to forced expression of miR-149 in SKOV-3
cells. The mRNA levels of XIAP in (E) ovarian cancer and
corresponding normal tissues, as were as in (F) sensitive and
insensitive ovarian cancer tissues, were measured by RT-qPCR. (G)
The correlation between miR-149 and XIAP was analyzed by Spearman's
correlation analysis. Error bars represent the mean ± standard
deviation of at least three independent experiments. **P<0.01
vs. corresponding control group. XIAP, X-linked inhibitor of
apoptosis; miR, microRNA; NC, negative control; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; WT,
wild-type; MUT, mutated; NS, non-significant. |
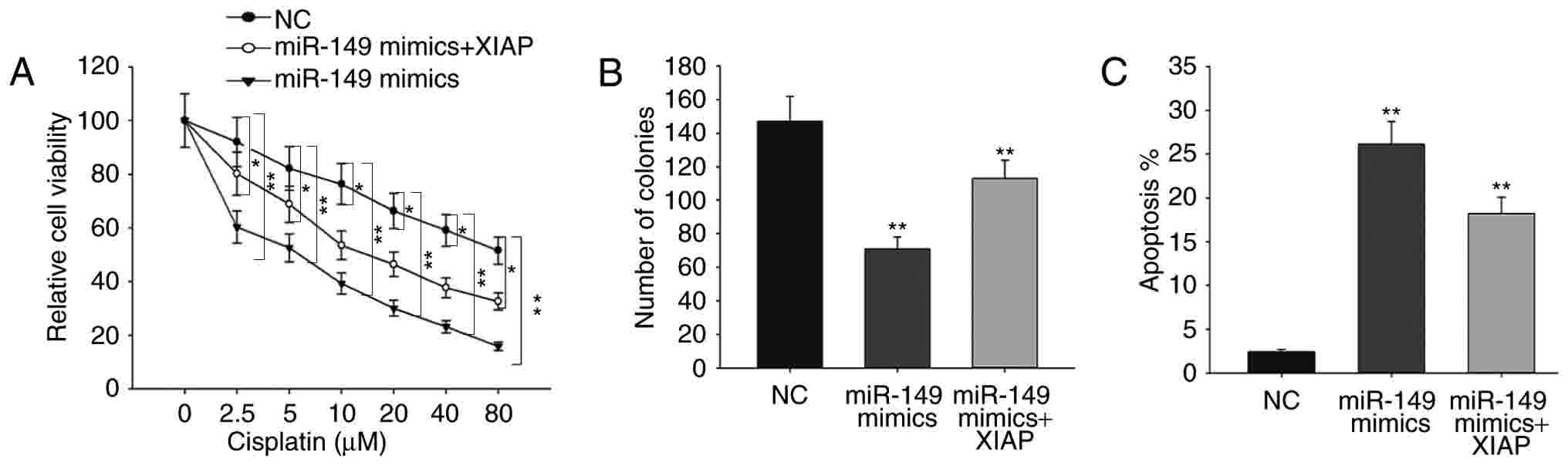
miR-149 increases the sensitivity of
SKOV-3 cells to cisplatin and inhibits ovarian cancer cell
proliferation in a XIAP-dependent manner
To further confirm the role of XIAP in ovarian
cancer, rescue assays were performed. As shown in Fig. 4A, forced expression of XIAP was able
to abolish the responses of miR-149-overexpressing ovarian cancer
cells to cisplatin. The results of the colony formation assay
revealed that miR-149 mimics decreased cell proliferation in
comparison with NC, while the decreased proliferation was increased
by XIAP (Fig. 4B). Furthermore, flow
cytometry analysis demonstrated that the increased apoptosis rate
caused by miR-149 mimics was reversed by XIAP overexpression
(Fig. 4C). All these findings
indicated that the function of miR-149 in ovarian cancer was
exerted in an XIAP-dependent manner.
Discussion
Drug resistance is identified as a major obstacle in
tumor chemotherapy, and miRNAs have been reported to serve critical
roles in this process (17–23). To date, several studies have
identified that miR-149 exerts a critical function in numerous
types of tumors. For instance, Jin et al (24) demonstrated that the tumor suppressor
miR-149-5p was associated with cellular migration, proliferation
and apoptosis in renal cell carcinoma. Fan et al (25) also reported that miR-149 promoted cell
proliferation and suppressed apoptosis by mediating JunB in T-cell
acute lymphoblastic leukemia. In addition, Luo et al
(26) indicated that miR-149
repressed the metastasis of hepatocellular carcinoma by targeting
protein phosphatase 1F, an actin-regulatory protein. Despite the
existence of numerous studies investigating miR-149 in several
tumors, the biological function and molecular mechanism of miR-149
in ovarian cancer remain unclear to date.
In the present study, miR-149 expression was
demonstrated to be significantly downregulated in chemosensitive
and chemo-insensitive ovarian cancer tissues, as well as in ovarian
cancer cell lines. Furthermore, miR-149 overexpression suppressed
the ovarian cancer cell proliferation and increased the sensitivity
of these cells to cisplatin treatment. XIAP, as a member of the
inhibitors of apoptosis family, has been reported in numerous types
of human cancer, and is associated with chemical or radiation
resistance (27–30). Recent studies indicated that
expression of XIAP may be regulated by miRNAs (31–34). In
the current study, it was observed that XIAP is a direct target
gene of miR-149 in ovarian cancer. Ectopic expression of miR-149
inhibited the luciferase activity of the XIAP-WT reporter, but not
of the XIAP-MUT reporter, suggesting that miR-149 was able to
directly regulate the XIAP expression. Furthermore, miR-149
overexpression suppressed XIAP expression at the mRNA and protein
levels. It was also observed that XIAP was overexpressed in ovarian
cancer tissues and cells, and that its expression was negatively
correlated with that of miR-149 expression in ovarian cancer
tissues. Additionally, rescue assays revealed that overexpression
of XIAP was able to abolish the increased cisplatin sensitivity and
weaken the proliferative ability of ovarian cancer cells
transfected with miR-149 mimics. These findings indicated that
miR-149 functioned as a tumor suppressor in ovarian cancer and
increased the sensitivity of ovarian cancer to cisplatin, at least
partially, through targeting XIAP. However, certain limitations
remain in the current study. For example, the present study only
elucidated the effects of miR-149 on proliferation and apoptosis of
ovarian cancer cells, the metastatic conditions still need to be
explored. We performed experiments in only four ovarian cancer cell
lines due to some limitations. We will make experiments in more
cell lines in future. Future investigations should examine the role
of other target genes of miR-149 and the upstream regulatory
pathway of miR-149.
In conclusion, the findings of the current study
revealed that miR-149 was downregulated in ovarian cancer cells and
tissues. Forced expression of miR-149 suppressed the ovarian cancer
cell proliferation and promoted the response of ovarian cancer
cells to cisplatin via targeting XIAP. These data suggested that
the miR-149/XIAP signaling pathway may be a potential therapeutic
target for patients with ovarian cancer.
Acknowledgements
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tian S, Zhang M, Chen X, Liu Y and Lou G:
MicroRNA-595 sensitizes ovarian cancer cells to cisplatin by
targeting ABCB1. Oncotarget. 7:87091–87099. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Flavin R, Smyth P, Barrett C, Russell S,
Wen H, Wei J, Laios A, O'Toole S, Ring M, Denning K, et al: miR-29b
expression is associated with disease-free survival in patients
with ovarian serous carcinoma. Int J Gynecol Cancer. 19:641–647.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miao Y, Zhang LF, Guo R, Liang S, Zhang M,
Shi S, Shang-Guan CF, Liu MF and Li B: (18)F-FDG PET/CT for
monitoring the response of breast cancer to miR-143-based
therapeutics by targeting tumor glycolysis. Mol Ther Nucleic Acids.
5:e3572016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai M, Wang Z, Zhang J, Zhou H, Jin L, Bai
R and Weng Y: Adam17, a Target of Mir-326, Promotes Emt-Induced
Cells Invasion in Lung Adenocarcinoma. Cell Physiol Biochem.
36:1175–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Perry MM, Baker JE, Gibeon DS, Adcock IM
and Chung KF: Airway smooth muscle hyperproliferation is regulated
by microRNA-221 in severe asthma. Am J Respir Cell Mol Biol.
50:7–17. 2014.PubMed/NCBI
|
|
8
|
O'Leary L, Sevinc K, Papazoglou IM, Tildy
B, Detillieux K, Halayko AJ, Chung KF and Perry MM: Airway smooth
muscle inflammation is regulated by microRNA-145 in COPD. FEBS
Lett. 590:1324–1334. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baldwin S, Deighan C, Bandeira E, Kwak KJ,
Rahman M, Nana-Sinkam P, Lee LJ and Paulaitis ME: Analyzing the
miRNA content of extracellular vesicles by fluorescence
nanoparticle tracking. Nanomedicine. 13:765–770. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goeppert B, Ernst C, Baer C, Roessler S,
Renner M, Mehrabi A, Hafezi M, Pathil A, Warth A, Stenzinger A, et
al: Cadherin-6 is a putative tumor suppressor and target of
epigenetically dysregulated miR-429 in cholangiocarcinoma.
Epigenetics. 11:780–790. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xi S, Inchauste S, Guo H, Shan J, Xiao Z,
Xu H, Miettenen M, Zhang MR, Hong JA, Raiji MT, et al: Cigarette
smoke mediates epigenetic repression of miR-217 during esophageal
adenocarcinogenesis. Oncogene. 34:5548–5559. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Zhao J, Luo Y, Wang Y and Jiang Y:
Downregulated expression of miRNA-149 promotes apoptosis in side
population cells sorted from the TSU prostate cancer cell line.
Oncol Rep. 36:2587–2600. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cao D, Jia Z, You L, Wu Y, Hou Z, Suo Y,
Zhang H, Wen S, Tsukamoto T, Oshima M, et al: 18β-glycyrrhetinic
acid suppresses gastric cancer by activation of miR-149-3p-Wnt-1
signaling. Oncotarget. 7:71960–71973. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Assem H, Rambau PF, Lee S, Ogilvie T,
Sienko A, Kelemen LE and Köbel M: High-grade endometrioid carcinoma
of the ovary: A clinicopathologic study of 30 cases. Am J Surg
Pathol. Jan 5–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guenther LM, Rowe RG, Acharya PT, Swenson
DW, Meyer SC, Clinton CM, Guo D, Sridharan M, London WB, Grier HE,
et al: Response evaluation criteria in solid tumors (RECIST)
following neoadjuvant chemotherapy in osteosarcoma. Pediatr Blood
Cancer. Dec 18–2017.(Epub ahead of print). PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Sun M, Guo J, Ma L, Jiang H, Gu L,
Wen H, Liao S, Chen J, Zeng B, et al: 3-O-(Z)-coumaroyloleanolic
acid overcomes Cks1b-induced chemoresistance in lung cancer by
inhibiting Hsp90 and MEK pathways. Biochem Pharmacol. 135:35–49.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan CW, Jin X, Zhao Y, Pan Y, Yang J,
Karnes RJ, Zhang J, Wang L and Huang H: AKT-phosphorylated FOXO1
suppresses ERK activation and chemoresistance by disrupting
IQGAP1-MAPK interaction. EMBO J. 36:995–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ma H, Yokoyama S, Saiki I and Hayakawa Y:
Chemosensitizing effect of saikosaponin B on B16F10 melanoma cells.
Nutr Cancer. 69:505–511. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
D'Angelo D, Mussnich P, Arra C, Battista S
and Fusco A: Critical role of HMGA proteins in cancer cell
chemoresistance. J Mol Med (Berl). 95:353–360. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Huang J, Liu Z, Liang Q, Zhang N
and Jin Y: FAM83A is amplified and promotes cancer stem cell-like
traits and chemoresistance in pancreatic cancer. Oncogenesis.
6:e3002017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pu Y, Zhao F, Wang H and Cai S: MiR-34a-5p
promotes multi-chemoresistance of osteosarcoma through
down-regulation of the DLL1 gene. Sci Rep. 7:442182017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu Y, Zhang B, Shi T and Qin H: miR-182
promotes tumor growth and increases chemoresistance of human
anaplastic thyroid cancer by targeting tripartite motif 8. Onco
Targets Ther. 10:1115–1122. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin L, Li Y, Liu J, Yang S, Gui Y, Mao X,
Nie G and Lai Y: Tumor suppressor miR-149-5p is associated with
cellular migration, proliferation and apoptosis in renal cell
carcinoma. Mol Med Rep. 13:5386–5392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan SJ, Li HB, Cui G, Kong XL, Sun LL,
Zhao YQ, Li YH and Zhou J: miRNA-149* promotes cell proliferation
and suppresses apoptosis by mediating JunB in T-cell acute
lymphoblastic leukemia. Leuk Res. 41:62–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo G, Chao YL, Tang B, Li BS, Xiao YF,
Xie R, Wang SM, Wu YY, Dong H, Liu XD and Yang SM: miR-149
represses metastasis of hepatocellular carcinoma by targeting
actin-regulatory proteins PPM1F. Oncotarget. 6:37808–37823. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Riley A, Jordan LE and Holcik M: Distinct
5′ UTRs regulate XIAP expression under normal growth conditions and
during cellular stress. Nucleic Acids Res. 38:4665–4674. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Deveraux QL, Takahashi R, Salvesen GS and
Reed JC: X-linked IAP is a direct inhibitor of cell-death
proteases. Nature. 388:300–304. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tamm I, Kornblau SM, Segall H, Krajewski
S, Welsh K, Kitada S, Scudiero DA, Tudor G, Qui YH, Monks A, et al:
Expression and prognostic significance of IAP-family genes in human
cancers and myeloid leukemias. Clin Cancer Res. 6:1796–1803.
2000.PubMed/NCBI
|
|
30
|
Pardo OE, Lesay A, Arcaro A, Lopes R, Ng
BL, Warne PH, McNeish IA, Tetley TD, Lemoine NR, Mehmet H, et al:
Fibroblast growth factor 2-mediated translational control of IAPs
blocks mitochondrial release of Smac/DIABLO and apoptosis in small
cell lung cancer cells. Mol Cell Biol. 23:7600–7610. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding WB, Wang YX and Dong CW: microRNA15b
induced SMCC7721 apoptosis via down-regulation of XIAP. Eur Rev Med
Pharmacol Sci. 21:542–548. 2017.PubMed/NCBI
|
|
32
|
Li X, Chen W, Zeng W, Wan C, Duan S and
Jiang S: microRNA-137 promotes apoptosis in ovarian cancer cells
via the regulation of XIAP. Br J Cancer. 116:66–76. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Han J, Liu Z, Wang N and Pan W:
MicroRNA-874 inhibits growth, induces apoptosis and reverses
chemoresistance in colorectal cancer by targeting X-linked
inhibitor of apoptosis protein. Oncol Rep. 36:542–550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu Q, Yan H, Tao SQ, Wang XN, Mou L, Chen
P, Cheng XW, Wu WY and Wu ZS: XIAP 3′-untranslated region as a
ceRNA promotes FSCN1 function in inducing the progression of breast
cancer by binding endogenous miR-29a-5p. Oncotarget. 8:16784–16800.
2017.PubMed/NCBI
|