Introduction
Gastric cancer is the third leading cause of
cancer-associated mortalities worldwide (1), with a particularly high incidence rate
in China (2). Surgical resection is
the principal method of treatment, however, the prognosis of
patients with gastric cancer is poor (3). The post-operative recurrence rate is as
high as 50–70%, with a 5-year survival rate of 20–50%.
Consequently, novel methods other than surgery are being
investigated (4,5). In recent years, traditional Chinese
medicines, including licorice (Glycyrrhiza glabra) and
Xanthium bitter ginseng, are gaining more attention in the
treatment of tumors, as they have less severe side effects
(6). Licorice is primarily cultivated
in Iran, China, Russia, Spain and India. In traditional Chinese
medicine, the roots and rhizomes of a variety species of the
perennial herb licorice are used for the treatment of a number of
conditions, including fatigue, asthma and excessive phlegm
production, and for relieving drug toxicity (7). One study demonstrated that Chinese
licorice inhibits the growth of HepG2 cells by arresting cell
proliferation and the subsequent induction of apoptosis (8).
Glabridin is an active isoflavane located in the
hydrophobic fraction of licorice root (9,10); in
humans and mice, it can be easily incorporated into gut cells and
released to the basolateral surface in an aglycone form (11,12).
Glabridin exhibits a number of biological activities, including
modulation of the quantity and function of lymphocytes, inhibition
of the antibody formation of IgE, effects against inflammatory
mediator and proinflammatory cytokines, and induction of
pharmacologic activities against inflammation and allergy (9–19). Studies
have reported that glabridin also exhibits properties of growth
inhibition against a number of types of human cancer, including
breast and liver cancer, and hepatocellular carcinoma (13–19).
Enhanced cancer chemotherapy efficiency via inhibition of
P-glycoprotein and multidrug resistance protein 1 synthesis has
also been demonstrated (20).
In the present study, the gastric cancer MKN-45 cell
line was used to investigate the effects of glabridin, either alone
or in conjunction with the commonly administered gastric cancer
chemotherapeutic 5-fluorouracil (5-FU). The effects of glabridin
and 5-FU on MKN-45 cells were evaluated, including cell
proliferation, invasion, colony formation and the number of cells
undergoing apoptosis. Therefore, our aim is to further determine
the effect of glabridin in combination with 5-FU on the
proliferation, invasion and apoptosis of MKN-45 cells and to
further investigate the intrinsic mechanism by which glabridin plus
5-FU affects MKN-45 cells. Hope to explore new ways for the
clinical treatment of gastric cancer.
Materials and methods
Reagents
Cell culture reagents were purchased from Gibco;
Thermo Fisher Scientific Inc. (Waltham, MA, USA). Unless otherwise
stated, all other reagents were from Sigma-Aldrich; Merck KGaA
(Darmstadt, Germany). The cell counting kit-8 (CCK-8) for assessing
cell proliferation was purchased from Dojindo Molecular
Technologies, Inc. (Kumamoto, Japan). Annexin V-FITC/PI Apoptosis
Detection Kit and binding buffer, were obtained from Nanjing KeyGen
Biotech. Co. Ltd. (Nanjing, China). The TRIzol® reagent
was purchased from Thermo Fisher Scientific, Inc. (Waltham, MA,
USA) and the ReverTra Ace® qPCR RT kit was purchased
from Toyobo Co., Ltd. (Osaka, Japan). Primers were synthesized by
Beijing Genomics Institute (Shenzhen, China). 5-FU was purchased
from Sigma-Aldrich; Merck KGaA. Glabridin was obtained from Wako
Pure Chemical Industries, Ltd. (Osaka, Japan).
Cell culture
Human gastric cancer MKN-45 cells, purchased from
the Chinese Academy of Sciences Cell Bank (Shanghai, China) were
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum, 100 µg/ml ampicillin, and 0.1 mg/ml streptomycin at 37°C in
5% CO2.
Cell proliferation assay
Cells were plated in 96-well plates (Falcon; BD
Biosciences, Franklin Lakes, NJ, USA) at a density of
1×104 cells per well. After 24 h, different
concentrations of glabridin (0, 6, 12, 25, 30 and 40 µM) and 5-FU
(0, 0.01, 0.05, 0.1 0.2 and 1 mM) were added and the cells were
cultured for a further 48 h. The CCK-8 staining solution was then
diluted (1:10), added to the 96-well plate and cultured for 1–2 h
in an incubator at 37°C, according to the manufacturer's protocol.
The intensity of the color developed was detected using a
microplate reader at 570 nm. All assays were performed with five
replicates. And empty culture medium was used as a blank control
group.
Cell colony formation assay
A total of 500 MKN-45 cells were seeded in a 6-well
plate and treated with glabridin (25 µM), 5-FU (0.1 mM) or
glabridin combined with 5-FU for 10 days. The cell culture medium
was replaced every 2–3 days. When a visible colony appeared in the
6-well plate, the culture was terminated and the cells were washed
twice in PBS. Next, the cells were fixed with methanol for 15 min
and stained with Giemsa Stain Solution (cat. no. RFT200-WHI;
Biomart, Co., Ltd., Beijing, China) for 10 min at room temperature.
The number of cell clones was counted under the microscope (>50
clones validated; Leica, Germany), and 4 independent experiments
were performed.
Cell apoptosis assay
In total, 1×106 MKN-45 cells were treated
with glabridin (25 µM), 5-FU (0.1 mM) or glabridin combined with
5-FU for 3 days. The drug-treated cells were digested with trypsin
and washed twice with PBS. Next, 100 µl Binding Buffer and 10 µl
fluorescein isothiocyanate (FITC)-labeled Annexin-V (20 µg/ml) were
added and incubated at room temperature for 30 min. Propidium
iodide (5 µl; 50 µg/ml) was added and the cells were incubated for
5 min in the dark. Next, 400 µl Binding Buffer was added and the
results were immediately quantified by FACScan flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). A total of 3 independent
experiments were performed.
Cell invasion assays
A total of 1×105 MKN-45 cells were
suspended in 200 µl serum-free RPMI-1640 medium, and seeded into
the Matrigel pre-coated Transwell upper chamber. A total of 500 µl
RPMI-1640 medium containing 10% FBS with or without glabridin (25
µM), 5-FU (0.1 mM) or glabridin combined with 5-FU was added to the
lower chamber of a 24-well plate fitted with insert and cultured in
an incubator at 37°C in 5% CO2. The culture medium was
discarded following a 72-h incubation and the Matrigel and the
cells that did not pass through the upper surface of the insert
were removed with a wet cotton swab. Cells were subsequently
stained with 0.1% crystal violet for 10 min and observed using a
microscope (Leica, Microsystems GmbH, Wetzlar, Germany), and the
number of cells penetrating the artificial basement membrane were
counted. Each experiment was repeated 3 times and averaged as the
experimental results for statistical analysis.
Caspase assays
A total of 1×106 MKN-45 cells were
incubated in a 6-well plate with or without glabridin (25 µM), 5-FU
0.1 mM) or glabridin combined with 5-FU for 3 days. The cells from
the different treatment groups were harvested and washed twice with
PBS. The cells were subsequently lysed on ice in a 50-µl lysis
buffer for 30 min. The cells were centrifuged at 4°C, 12,000 × g,
for 10 min, and the supernatant protein concentration was
quantified using the BCA protein assay kit (cat. no. 23252; Thermo
Fisher Scientific, Inc.). The fluorescence substrates of caspase-3
(BF3100), −8 (BF4100) and −9 (BF10100) (all R&D Systems China,
Co., Ltd.) were added to the protein samples and incubated at 37°C
for 1 h. The absorbance was detected using a microplate reader at
405 nm. A standard curve provided by the kit was used to calculate
the activity of caspase-3, −8 and −9.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
A total of 1×106 MKN-45 cells were
collected and total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The optical
density (OD)260 and OD280 values in the
extracted RNA were determined using a spectrophotometer and the
ratio was between 1.8–2.1. cDNA was obtained by 3 µg RNA reverse
transcription using a ReverTra Ace qPCR RT kit (Toyobo Co. Ltd.)
according to the manufacturer's protocol. Furthermore, qPCR of the
cDNA was performed to analyze the gene expression levels of
glyceraldehyde 3-phosphate dehydrogenase (GADPH), vascular
endothelial growth factor (VEGF), apoptosis regulator BAX (Bax),
apoptosis regulator Bcl-2 (Bcl-2), cyclin D1, epidermal growth
factor receptor (EGFR), proliferation marker protein Ki-67 (Ki-67),
matrix metalloproteinase (MMP)-9, MMP-2, metalloproteinase
inhibitor 2 (TIMP-2) and E-cadherin using the ReverTra Ace qPCR RT
kit with SYBR-Green Supermix (Bio-Rad Laboratories, Inc., Hercules,
CA, USA) in a GFX96 Real-time system (Bio-Rad Laboratories, Inc.).
The mixture consisted of 10 µl SYBR-Green Mix, 1 µl forward, 1 µl
reverse primers, 1 µl diluted cDNA and 7 µl nuclease-free waters.
The reaction process was performed at 95°C for 2 min, followed by
40 cycles of 95°C for 10 sec and 61°C for 30 sec. All the primers
used are shown in Table I and GAPDH
was used as the control gene. The 2−ΔΔCq (14) method was used to calculate the
relative mRNA expression of target genes.
 | Table I.Primer sequences used for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer | Reverse primer | Product length,
bp |
|---|
| GAPDH |
5′-TGAACGGGAAGCTCACTGG-3′ |
5′-TCCACCACCCTGTTGCTGTA-3′ | 307 |
| VEGF |
5′-ATTATGCGGATCAAACCTC-3′ |
5′-ATTTCTTGCGCTTTCGTT-3′ | 157 |
| Bax |
5′-CCCGAGAGGTCTTTTTCCGAG-3′ |
5′-CCAGCCCATGATGGTTCTGAT-3′ | 155 |
| Bcl-2 |
5′-CCTGGGCAATTCCGCATT-3′ |
5′-AACAGGCCACGTAAAGCAAC-3′ | 158 |
| Cyclin D1 |
5′-GCTGCGAAGTGGAAACCATC-3′ |
5′-CCTCCTTCTGCACACATTTGAA-3′ | 135 |
| EGFR |
5′-AGGCACGAGTAACAAGCTCAC-3′ |
5′-ATGAGGACATAACCAGCCACC-3′ | 177 |
| Ki-67 |
5′-AGAAGACCTGCTACTCCAAAGA-3′ |
5′-AGTTTGCGTGGCCTGTACTAA-3′ | 70 |
| MMP-9 |
5′-ACTACTGTGCCTTTGAGTCC-3′ |
5′-AGAATCGCCAGTACTTCCCA-3′ | 115 |
| MMP-2 |
5′-ACTCTGGACTTAGACCGCTTG-3′ |
5′-ACAGGTTGCAGCTCTCCTTG-3′ | 217 |
| TIMP-2 |
5′-ACCCCTGTTCGCTTCCTGT-3′ |
5′-GGGTCAAATGCTTCCACGAT-3′ | 196 |
| E-cadherin |
5′-GCTAACGTCGTAATCACCAC-3′ |
5′-AATGCCATCGTTGTTCACTG-3′ | 141 |
Western blot analysis
A total of 1×106 MKN-45 cells were
incubated with indicated concentrations of glabridin (25 µM), 5-FU
(0.1 mM) or glabridin combined with 5-FU for 3 days. The cell
culture medium was discarded and the cells were washed twice with
PBS. Next, the cells were lysed in 200 µl radioimmunoprecipitation
assay buffer (cat. no. P0013B; Beyotime Institute of Biotechnology,
Haimen, China). The concentration of the extracted protein was
determined using the bicinchoninic acid protein quantification
method and 10% SDS-PAGE was performed with 40 µg of protein sample
and transferred to a polyvinylidene difluoride (PVDF) membrane, and
then 5% skimmed milk powder was added and incubation occurred for 1
h at room temperature. The PVDF membrane was then incubated at 4°C
overnight with anti-cyclin-dependent kinase inhibitor 2A
(CDKN2A)/p16INK4a antibody (cat. no. ab108349; dilution, 1:1,000),
anti-Bax antibody (cat. no. ab32503; dilution, 1:1,000), anti-Bcl-2
antibody (cat. no. ab32124; dilution, 1:1,000), anti-E-cadherin
antibody (cat. no. ab76055; dilution, 1:500), anti-N-cadherin
antibody (cat. no. ab76057; dilution, 1:500) and anti-β-actin
antibody (cat. no. ab8226; dilution, 1:1,000) (all Abcam,
Cambridge, UK), and washed with Tris-buffered saline plus Tween-20
(TBST) 3–5 times. The corresponding horseradish
peroxidase-conjugated secondary antibodies (cat. nos. ab6721 and
ab6789; dilution, 1:2,000; both Abcam) were used and incubated for
1 h at room temperature, respectively. The membranes were washed
three times with TBST, incubated with ECL solution (cat. no. 34580;
Thermo Fisher Scientific Inc. Waltham, MA, USA) and analyzed using
a gel imaging system (Biox-vision, Co., Ltd., Anhui, China).
Western blotting quantification was estimated using ImageJ software
(version 1.42q; National Institutes of Health, Bethesda. MD,
USA).
Statistical analysis
One-way analysis of variance (ANOVA) and the
Kruskal-Wallis test were used for data analysis, and Dunnett's
t-test was used following ANOVA. P<0.05 was considered to
indicate a statistically significant difference. All data were
analyzed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla,
CA, USA) and had a minimum of three independent experimental
repeats.
Results
Glabridin combined with 5-FU inhibits
MKN-45 cell proliferation
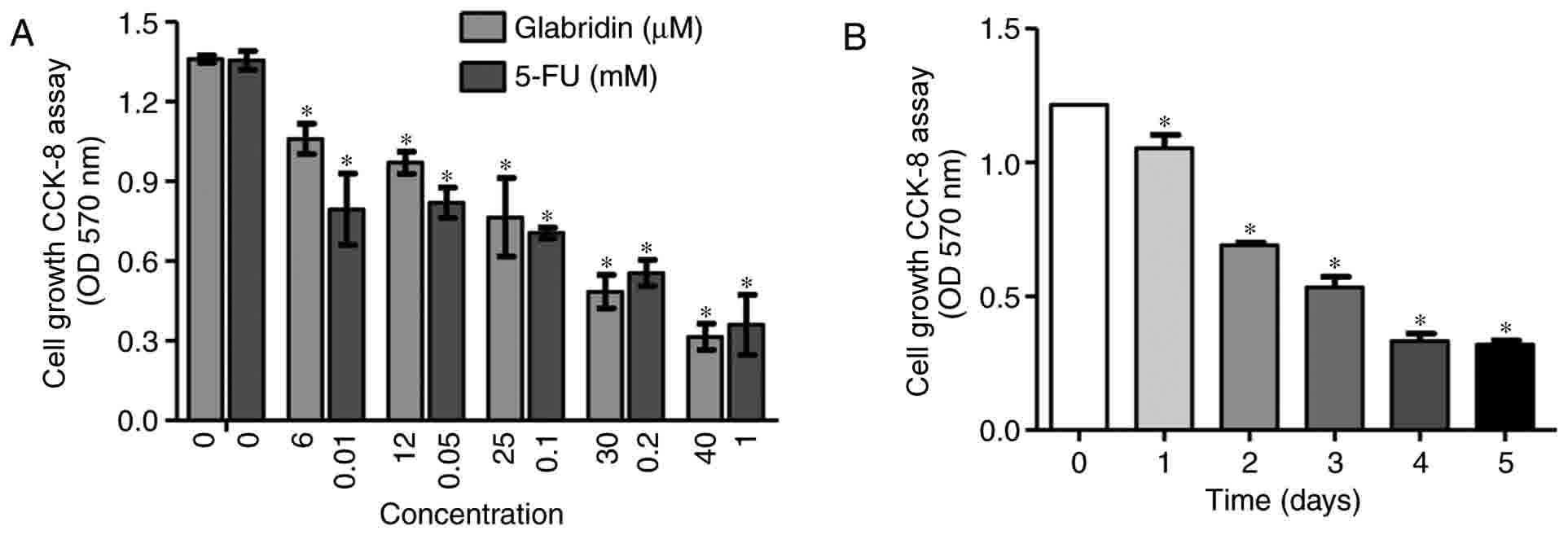
The present study examined the effects of glabridin
and 5-FU on gastric cancer cell proliferation. MKN-45 cells were
treated with different concentrations of glabridin (0, 6, 12, 25,
30 and 40 µM) and 5-FU (0, 0.01, 0.05, 0.1 0.2 and 1 mM) for 48 h
(Fig. 1A). MKN-45 cells also were
treated with 25 µM glabridin alone for 1–5 days (Fig. 1B). The results demonstrated that
glabridin and 5-FU inhibited the proliferation of MKN-45 cells in a
dose-and time-dependent manner. Based on these preliminary
experimental results, it was confirmed that glabridin may inhibit
gastric cancer cell proliferation. As demonstrated in Fig. 1B, at the end of the 5-day incubation
period, glabridin was effective in significantly inhibiting the
growth of the MKN-45 cells. This is supported by a number of
studies where 5-FU or glabridin was applied to tumor cells, such as
in the study by Khazraei-Moradian et al (21), which demonstrated that licorice
protein fractions are capable of inhibiting the proliferation of
gastrointestinal cancer cell lines. Additionally, Zhou et al
(22) identified that curcumin
enhances the effects of 5-FU and oxaliplatin in inducing the
apoptosis of gastric cancer cells in vitro and in
vivo (22). Although 5-FU or
glabridin was able to inhibit the proliferation and induce the
apoptosis of the gastric cancer cell lines cited in the previous
studies, 5-FU and glabridin used at varying concentrations
(14,15,17).
Therefore, following analysis of these studies and the preliminary
experimental results of the present study (Fig. 1), a concentration of 0.1 mM 5-FU and
25 µM glabridin were used in the subsequent experiments. In the
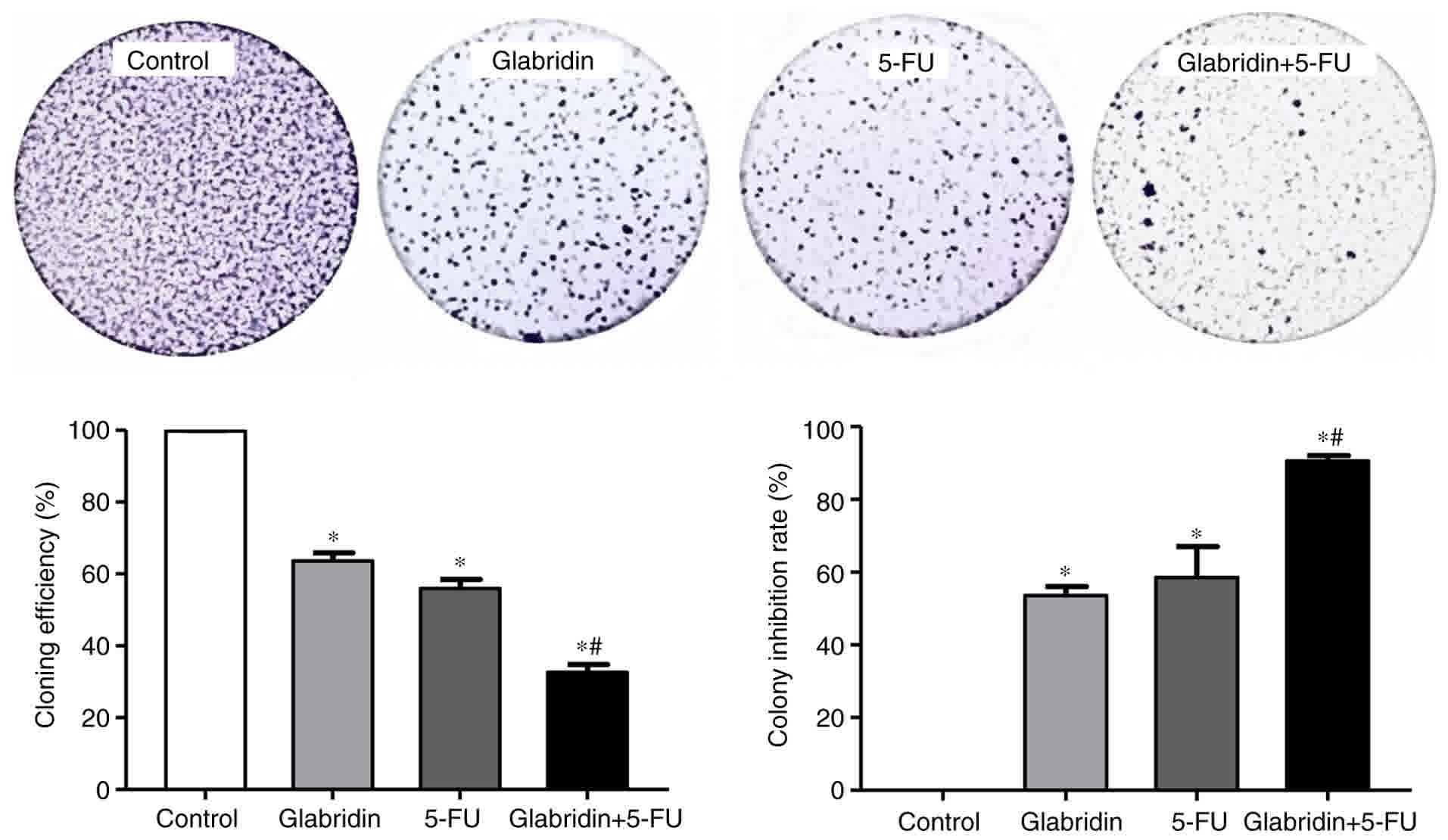
colony formation assay, the results also demonstrated that a
combination of glabridin and 5-FU treatment could significantly
inhibit colony formation when compared with glabridin or 5-FU alone
(Fig. 2).
Glabridin combined with 5-FU promotes
MKN-45 cell apoptosis
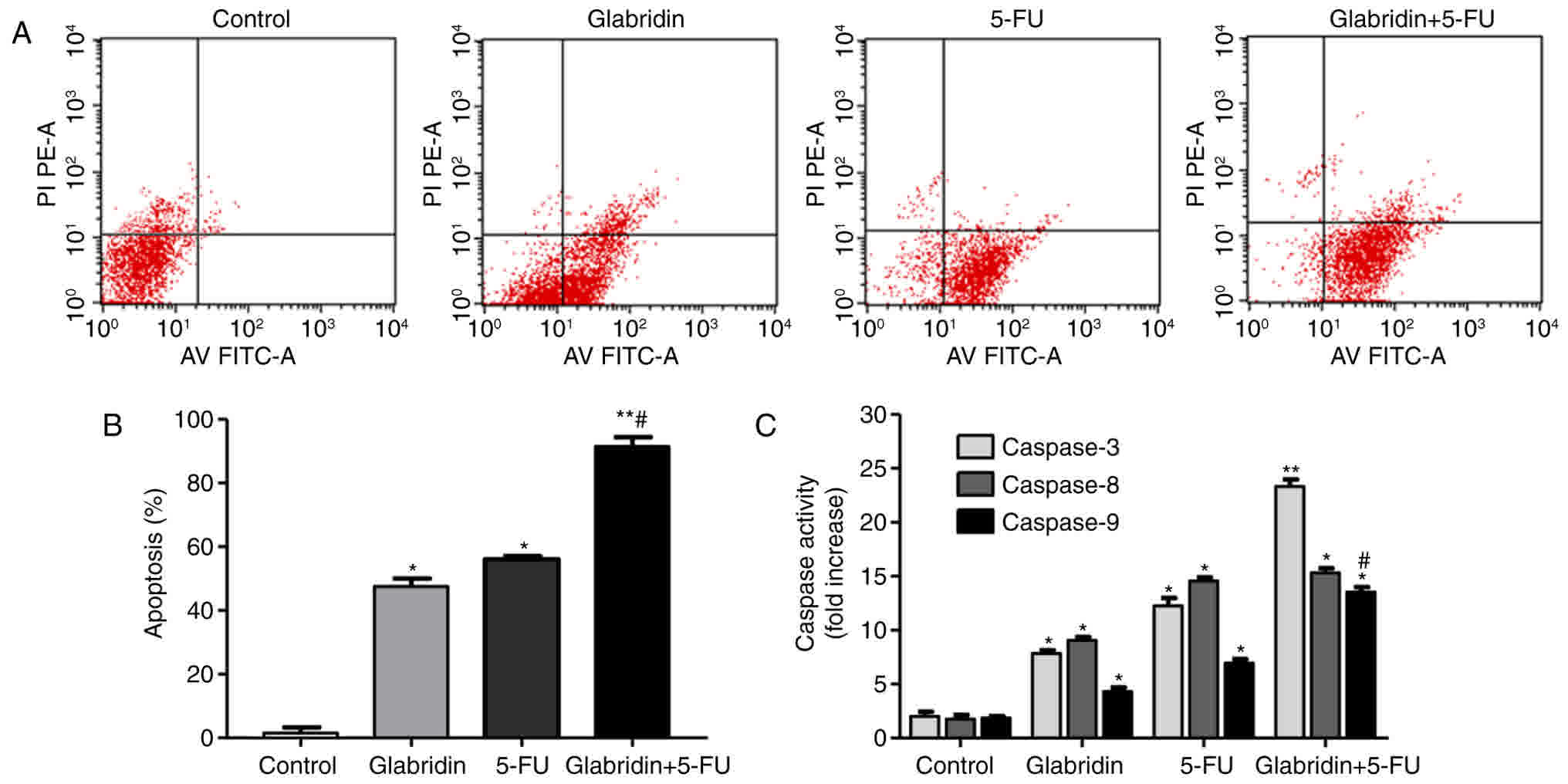
Apoptotic cell death was identified by flow
cytometric analysis of Annexin V-FITC-stained apoptotic cells. The
results of the present study demonstrated that the apoptosis of
MKN-45 cells was observed following treatment with 8 µg/ml
glabridin, 0.1 mM 5-FU and the combined treatment, respectively.
Additionally, glabridin plus 5-FU significantly induced the
apoptosis of the MKN-45 cells compared with glabridin or 5-FU alone
groups (Fig. 3). As shown in Fig. 3B, the apoptotic percentages of the
glabridin, 5-FU, combination and control groups were approximately
55.37±3.13, 56.16±2.08, 93.88±4.05 and 2.35±0.61%, respectively. To
investigate the apoptotic mechanisms in cells treated with
glabridin and 5-FU, the activity of caspases −3, −8 and −9 were
assayed using caspase activity assay kits. As shown in Fig. 3C, caspases −3, −8 and −9 were all
activated following induction with glabridin, 5-FU or glabridin
plus 5-FU. In comparison with the control, caspase-3, −8, and −9
levels were demonstrated to be significantly higher in the
glabridin, 5-FU and combination groups (P<0.01). Compared with
the activity in the combination group, caspase-3 and −9 activities
were identified to be significantly lower (P<0.01) in the
glabridin and 5-FU groups. As a result, it was observed that
glabridin had an enhanced effect on the MKN-45 cells by increasing
the activity of caspase-3, −8, and −9, which are precursors of
apoptosis (Fig. 3C).
Glabridin combined with 5-FU inhibits
the invasion of MKN-45 cells
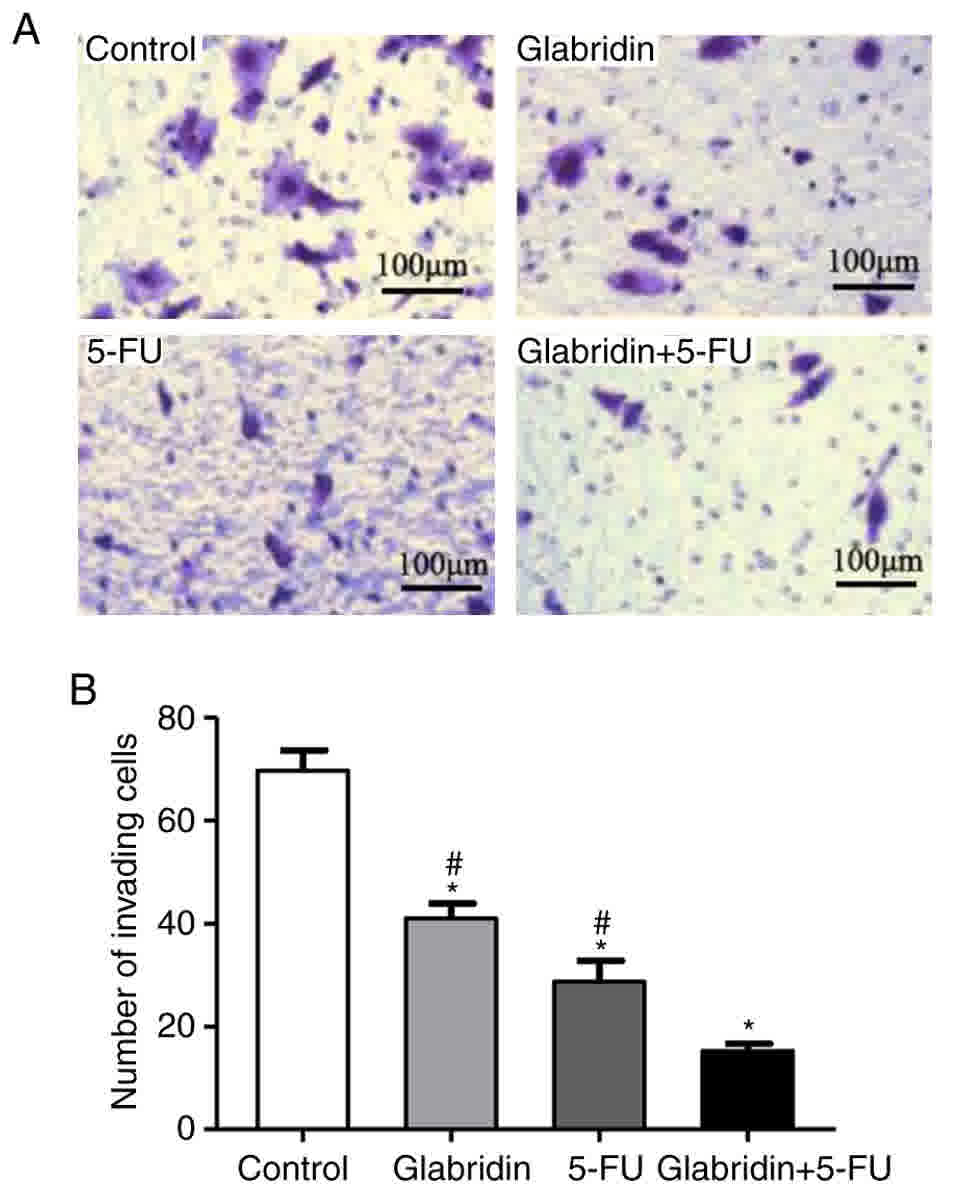
Cell invasion assays were conducted to further
examine the effect of glabridin, 5-FU and the two-drug combination
on cell invasion. In these experiments, it was demonstrated that
glabridin combined with 5-FU significantly decreased the chemotaxis
and invasion of MKN-45 cells compared with that of the controls
(Fig. 4). The quantity of invading
cells was also reduced by glabridin and 5-FU in a Matrigel invasion
assay (Fig. 4A). The number of
invading cells was 69.67% (control), 40.96% (glabridin), 28.70%
(5-FU) and 15.23% (glabridin combined with 5-FU) (Fig. 4B).
Cell apoptosis-, cycle progression-,
invasion- and angiogenesis-related genes are influenced by
glabridin and 5-FU
The results of the present study confirmed that
glabridin combined with 5-FU decreases proliferation and colony
formation, and promotes the apoptosis of MKN-45 cells. These
results influenced the decision to examine the effects of
glabridin, 5-FU and the two-drug combination on the gene expression
of MKN-45 cells. Glabridin combined with 5-FU significantly
weakened the expression of the anti-apoptotic gene Bcl-2 and
increased expression of the pro-apoptotic gene Bax (Fig. 5A). These results suggest that
glabridin combined with 5-FU may promote apoptosis of MKN-45 cells
by upregulating the Bax gene and downregulating the Bcl-2 gene.
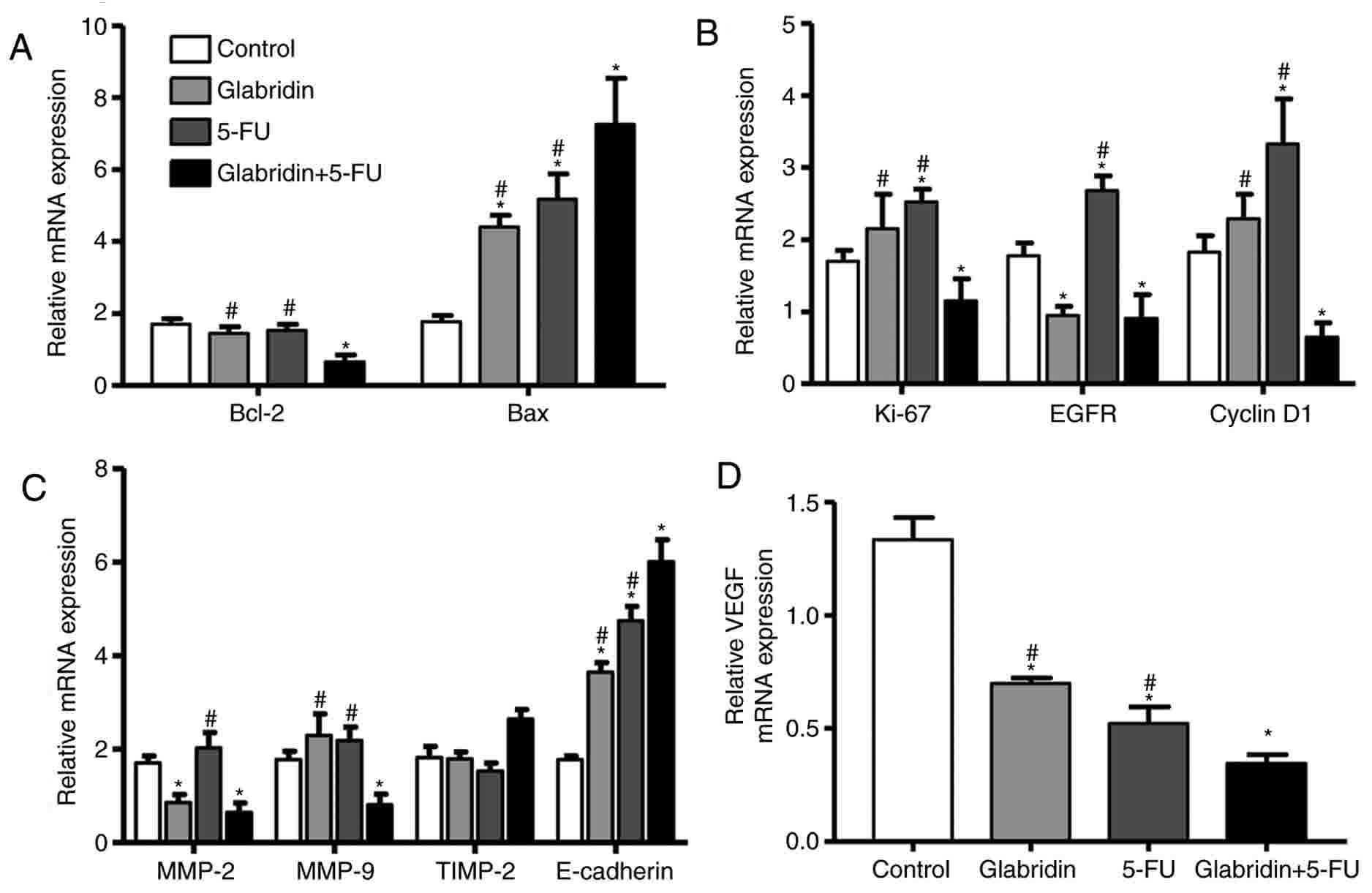 | Figure 5.Determining the amount of mRNA
expression by reverse transcription-quantitative polymerase chain
reaction. (A) Apoptosis-, (B) cell cycle progression-, (C)
invasion- and (D) angiogenesis-related genes are influenced by
glabridin, 5-FU and glabridin plus 5-FU. *P<0.05 (vs. control
group) or #P<0.05 (glabridin+5-FU vs. 5-FU or glabridin) was
considered to indicate a statistically significant difference.
VEGF, vascular endothelial growth factor; 5-FU, 5-fluorouracil;
Bcl-2, apoptosis regulator Bcl-2; Bax, apoptosis regulator BAX;
Ki-67, proliferation marker protein Ki-67; EGFR, epidermal growth
factor receptor; MMP, matrix metalloproteinase; TIMP, tissue
inhibitor of metalloproteinase. |
To understand the regulation of genes by glabridin
with regard to cell proliferation and colony formation, RT-qPCR was
performed to examine the relative mRNA expression levels of EGFR,
cyclin D1 and Ki-67. It was found that glabridin significantly
decreased mRNA expression of EGFR, but had no effect on mRNA
expression of Ki-67 and cyclin D1 compared with the control
(Fig. 5B). Glabridin combined with
5-FU significantly downregulated the expression of Ki-67, EGFR and
cyclin D1 gene compared with the control. This data demonstrates
that glabridin plus 5-FU could induce MKN-45 cell apoptosis and
inhibit cell cycle progression by reducing mRNA expression of
cyclin D1, EGFR and Ki-67.
Additionally, regulation of the MMP-2, TIMP-2, MMP-9
and E-cadherin genes for MKN-45 cell invasion was examined.
Glabridin alone significantly downregulated mRNA expression of
MMP-2, and glabridin and 5-FU significantly upregulated mRNA
expression of E-cadherin compared with the control. Notably,
glabridin in conjunction with 5-FU could significantly alter the
mRNA expression of the MMP-2, MMP-9 and E-cadherin genes (Fig. 5C). The effect of glabridin and 5-FU on
the expression of the VEGF gene was examined, and it was found that
the mRNA expression of VEGF was most significantly decreased by
treatment of glabridin plus 5-FU (Fig.
5D). Taken together, the results of the present study suggested
that glabridin combined with 5-FU may inhibit gastric cancer cell
invasion, and angiogenesis by regulation of MMP-2, MMP-9,
E-cadherin and VEGF genes.
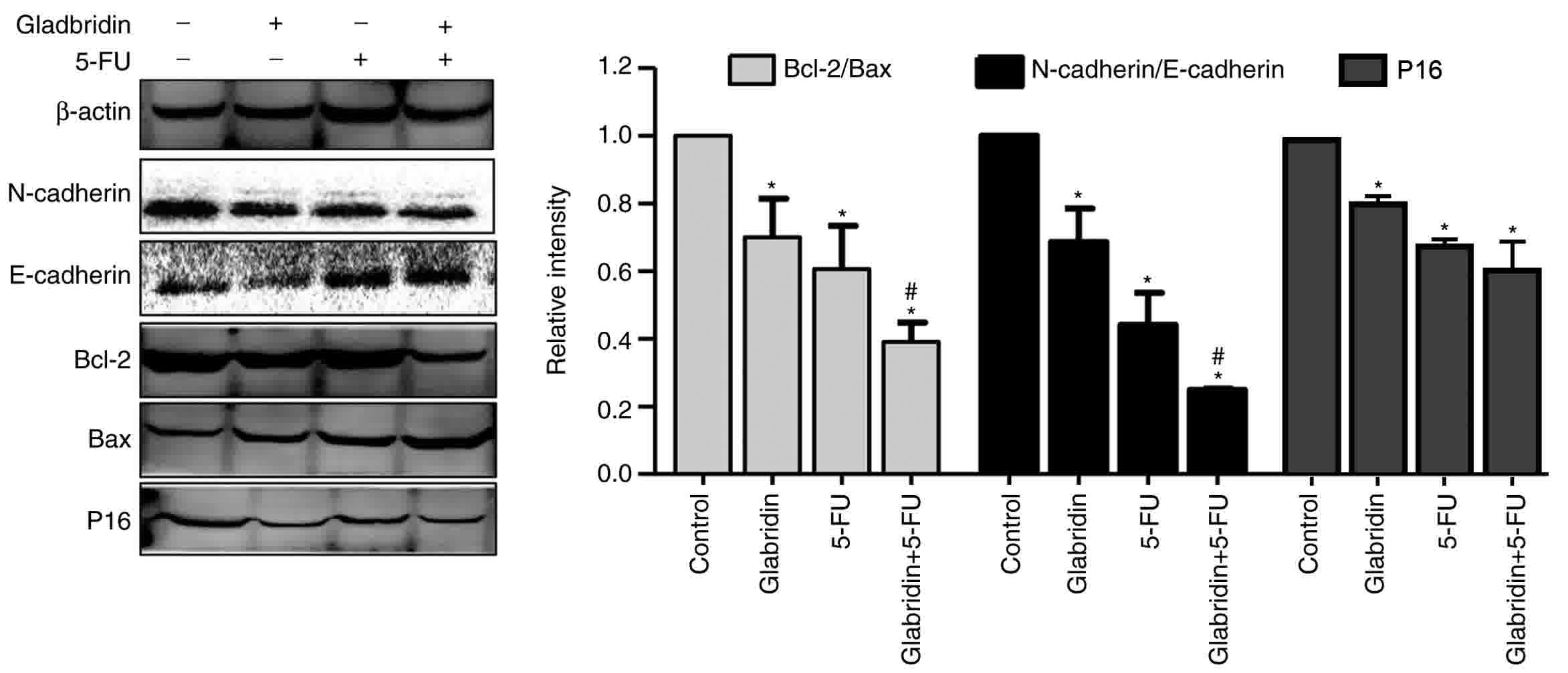
Effects of glabridin combined with
5-FU on the protein expression of MKN-45 cells
RT-qPCR analysis demonstrated that glabridin plus
5-FU can affect the proliferation, invasion and apoptosis
capabilities of MKN-45 cells. p16 is a key regulatory protein for
the cell cycle, with western blot analysis revealing that the
expression of p16 in the glabridin-treated MKN-45 cells was
significantly decreased compared with that in the control group
(Fig. 6). The results demonstrated
that glabridin alone and combined with 5-FU could reduce the
expression of p16 protein. Western blot analysis also demonstrated
that the apoptosis proteins Bcl-2 and Bax were affected in
glabridin-treated MKN-45 cells, and that the glabridin plus 5-FU
combination synergistically decreased the Bcl-2/Bax protein ratio
(Fig. 6). Similarly, compared with
the 5-FU-treated MKN-45 cells group, the expression of
N-cadherin/E-cadherin was significantly decreased in the glabridin
combination with 5-FU-treated MKN-45 cells group. Cadherins are a
class of transmembrane proteins whose primary function is to form
an adherent junction to bind cells within the tissue (23). The results revealed that the glabridin
in conjunction with 5-FU may affect the apoptosis, proliferation
and invasion of cancer cells by altering the expression of Bcl-2,
Bax, E-cadherin, N-cadherin and p16 proteins.
Discussion
Gastric cancer incidence and mortality have
increased worldwide, and according to the latest report by the
World Health Organization, the incidence of gastric cancer in the
global incidence of malignancy ranks fifth mortality rate ranks
third (24). Hu et al reported
that that the annual number of new cases of gastric cancer in China
is 400,000, accounting for 42% of the global gastric cancer cases,
with a mortality rate of ~300,000 (25,26).
Chemotherapy is the principal treatment for patients with gastric
cancer. However, it produces a number of adverse side effects such
as damage to normal, functional cells. This has culminated in the
requirement for the development of drugs with fewer adverse side
effects and increased efficiency. Glabridin is an isoflavane, a
type of isoflavonoid, which is found in the root extract of
licorice (Glycyrrhiza glabra) (27). The effects of glabridin as a
phytoestrogen for the treatment of breast and endometrial cancer
have been investigated (13,17,28).
Furthermore, a number of studies have demonstrated that glabridin
has anti-proliferative, anti-invasion and antitumor angiogenesis
capabilities in breast cancer cells, endometrial cancer and
hepatocarcinoma cells (17–19). The results of the present study
indicate that glabridin either alone or in conjunction with 5-FU
may significantly inhibit colony formation and invasion, and
promote the apoptosis of MKN-45 cells.
The p16 protein is an important factor in cell cycle
regulation and differentiation. Previous studies have identified
that the p16 gene is a tumor suppressor gene that participates in
the development of multiple tumors (29,30). It
has been reported that p16 specifically inhibits the activity of
CDK4, and prevents cells from the G1 phase entering into the S
phase. When the expression of p16 is decreased, CDK4 expression is
upregulated, and p16 combined with cyclin D1 can promote cell
mitosis, thereby promoting cell proliferation, leading to tumor
formation (31–34). Benassi et al (31) investigated the mechanisms regulating
cell-cycle progression in human osteosarcomas and
Retinoblastoma-associated protein (pRb)/p16/CDK4 expression was
analyzed in 39 high-grade osteosarcomas; the data confirmed the
important role of the pRb/p16/CDK4 pathway in osteosarcoma
development (31). Notably, it was
identified in the present study that glabridin reduces the
proliferation and colony formation of MKN-45 cells. This result
validates those of previous studies, which hypothesized an
inhibitory role for glabridin in various types of cancer cells
(35–37). However, compared with 5-FU, the
present study unexpectedly identified that the combination of
glabridin and 5-FU was more effective in significantly inhibiting
the proliferation of MKN-45 cells. According to the experimental
results, we hypothesize that glabridin plus 5-FU may involve a
combination of increased expression of p16 protein and reduced mRNA
expression of the cyclin D1, EGFR and Ki-67 genes, although the
molecular mechanisms responsible for these actions have yet to be
established. Therefore, we hypothesize that glabridin may inhibit
gastric cancer cell proliferation through the p16/CDK4/cyclin D1
pathway.
The results of the present study demonstrated that
glabridin and 5-FU are capable of up or downregulating several key
genes, including Bcl-2, Bax, MMP-9, VEGF, MMP-2 and E-cadherin, all
of which are important regulators of cell invasion, proliferation
and apoptosis. The experimental results confirm that glabridin or
glabridin with 5-FU inhibited MKN-45 cell proliferation and
invasion, and suggest that the combination of glabridin and 5-FU
may inhibit the invasion of cancer cells by downregulating the
MMP-9 and MMP-2 genes. The role of these downstream pathway
mediators in gastric cancer is presently unclear. Caspase and Bcl-2
families serve important roles in apoptosis. It has previously been
reported caspase-3, −8, and −9 activation exhibits a vital role in
early apoptosis, as regulated by a range of factors, including the
Bcl-2 protein family (22). The
mitochondria-mediated apoptotic pathway is also regulated by
members of the Bcl-2 family (38) and
is dependent on the balance of the anti-apoptotic protein Bcl-2 and
the pro-apoptotic protein Bax. The present study indicated that
glabridin shifts the equilibrium of Bcl-2 family members toward
apoptosis and elevates the expression of Bax and procaspases 3, 8
and 9. The results demonstrated that the glabridin plus 5-FU
combination synergistically decreased the Bcl-2/Bax protein ratio,
making the MKN-45 cells more susceptible to apoptosis.
In summary, glabridin alone or in combination with
5-FU inhibits MKN-45 cell proliferation, survival and invasion. The
data suggest that using glabridin alone or in conjunction with 5-FU
may be an effective therapeutic strategy to eliminate gastric
cancer cells via the induction of apoptosis. The present study also
suggests that the p16/CDK4/cyclin D1 pathway may be a novel target
site for gastric cancer therapeutics, and the combined use of
glabridin and 5-FU chemotherapy may pose a useful approach to treat
gastric cancer. However, a limitation of the present study is that
only one gastric cancer cell line was used, although it
successfully demonstrated that glabridin plus 5-FU can effectively
promote apoptosis or inhibit proliferation and invasion of the
MKN-45 cell line by different methods, including assessment of mRNA
and protein levels. Ultimately, the intrinsic mechanism of
glabridin plus 5-FU inhibiting the proliferation and invasion of
MKN-45 cells requires further experimental verification.
Furthermore, due of the high cost of antibodies, a number of the
PCR genes were not further validated at the protein level,
consequently these may be included in the next phase of
research.
Acknowledgements
The authors would like to thank Dr. Sandhya Mana for
providing comments on earlier versions of the manuscript.
Funding
No funding was received.
Availability of data and materials
The authors declare that materials described in the
manuscript, including all relevant raw data, will be freely
available to any scientist wishing to use them for non-commercial
purposes, without breaching participant confidentiality.
Authors' contributions
XL conceived and designed the study. LZ and HC
conducted experiments; MW, XS, JZ and FD analyzed the data. JZ also
drafted the manuscript. XL and LZ designed the experiments and
revised the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kásler M, Ottó S and Kenessey I: The
current situation of cancer morbidity and mortality in the light of
the national cancer registry. Orv Hetil. 158:84–89. 2017.(In
Hungarian). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun X, Liu W, Wu S, Han H, Lin Y and Dai
X: The morbidity and mortality trend and prediction of lung cancer
in residents of Nangang District of Harbin in China during the past
10 years. Zhongguo Fei Ai Za Zhi. 8:514–517. 2005.(In Chinese).
PubMed/NCBI
|
|
3
|
Fukunaga S, Nagami Y, Shiba M, Ominami M,
Tanigawa T, Yamagami H, Tanaka H, Muguruma K, Watanabe T, Tominaga
K, et al: Long-term prognosis of expanded-indication
differentiated-type early gastric cancer treated with endoscopic
submucosal dissection or surgery using propensity score analysis.
Gastrointest Endosc. 85:143–152. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dittmar Y, Schüle S, Koch A, Rauchfuss F,
Scheuerlein H and Settmacher U: Predictive factors for survival and
recurrence rate in patients with node-negative gastric cancer-a
European single-centre experience. Langenbecks Arch Surg.
400:27–35. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Greenhill C: Gastric cancer. Metformin
improves survival and recurrence rate in patients with diabetes and
gastric cancer. Nat Rev Gastroenterol Hepatol. 12:1242015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang Q and Ye M: Chemical analysis of the
Chinese herbal medicine Gan-Cao (licorice). J Chromatogr A.
1216:1954–1969. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang R, Yuan BC, Ma YS, Zhou S and Liu Y:
The anti-inflammatory activity of licorice, a widely used Chinese
herb. Pharm Biol. 55:5–18. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen X, Liu Z, Meng R, Shi C and Guo N:
Antioxidative and anticancer properties of Licochalcone A from
licorice. J Ethnopharmacol. 198:331–337. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang LP and Li JG: Glabridin reduces
lipopolysaccharide-induced lung injury in rats by inhibiting p38
mitogen activated protein kinase/extracellular regulated protein
kinases signaling pathway. Zhonghua Yi Xue Za Zhi. 96:3893–3897.
2016.(In Chinese). PubMed/NCBI
|
|
10
|
Liu C, Sui H, Zhao Q, Zhang X and Wang W:
Enhanced skin permeation of Glabridin using eutectic mixture-based
nanoemulsion. Drug Deliv Transl Res. 7:325–332. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ito C, Oi N, Hashimoto T, Nakabayashi H,
Aoki F, Tominaga Y, Yokota S, Hosoe K and Kanazawa K: Absorption of
dietary licorice isoflavan glabridin to blood circulation in rats.
J Nutr Sci Vitaminol (Tokyo). 53:358–365. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cao J, Chen X, Liang J, Yu XQ, Xu AL, Chan
E, Wei D, Huang M, Wen JY, Yu XY, et al: Role of P-glycoprotein in
the intestinal absorption of glabridin, an active flavonoid from
the root of Glycyrrhiza glabra. Drug Metab Dispos. 35:539–553.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ye X, Jiang F, Li Y, Mu J, Si L, Wang X,
Ning S and Li Z: Glabridin attenuates the migratory and invasive
capacity of breast cancer cells by activating microRNA-200c. Cancer
Sci. 105:875–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tsai YM, Yang CJ, Hsu YL, Wu LY, Tsai YC,
Hung JY, Lien CT, Huang MS and Kuo PL: Glabridin inhibits
migration, invasion, and angiogenesis of human non-small cell lung
cancer A549 cells by inhibiting the FAK/rho signaling pathway.
Integr Cancer Ther. 10:341–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tamir S, Eizenberg M, Somjen D, Stern N,
Shelach R, Kaye A and Vaya J: Estrogenic and antiproliferative
properties of glabridin from licorice in human breast cancer cells.
Cancer Res. 60:5704–5709. 2000.PubMed/NCBI
|
|
16
|
Jiang F, Mu J, Wang X, Ye X, Si L, Ning S,
Li Z and Li Y: The repressive effect of miR-148a on TGF beta-SMADs
signal pathway is involved in the glabridin-induced inhibition of
the cancer stem cells-like properties in hepatocellular carcinoma
cells. PLoS One. 9:e966982014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang F, Li Y, Mu J, Hu C, Zhou M, Wang X,
Si L, Ning S and Li Z: Glabridin inhibits cancer stem cell-like
properties of human breast cancer cells: An epigenetic regulation
of miR-148a/SMAd2 signaling. Mol Carcinog. 55:929–940. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hsieh MJ, Lin CW, Yang SF, Chen MK and
Chiou HL: Glabridin inhibits migration and invasion by
transcriptional inhibition of matrix metalloproteinase 9 through
modulation of NF-κB and AP-1 activity in human liver cancer cells.
Br J Pharmacol. 171:3037–3050. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Retraction statement: ‘Glabridin
attenuates the migratory and invasive capacity of breast cancer
cells by activating microRNA-200c’. by. Xianqing Ye, Fei Jiang,
Yuan Li, Juan Mu, Lu Si, Xingxing Wang, Shilong Ning, Zhong L..
Cancer Sci. 106:1252015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nabekura T, Yamaki T, Ueno K and Kitagawa
S: Inhibition of P-glycoprotein and multidrug resistance protein 1
by dietary phytochemicals. Cancer Chemother Pharmacol. 62:867–873.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khazraei-Moradian S, Ganjalikhani-Hakemi
M, Andalib A, Yazdani R, Arasteh J and Kardar GA: The effect of
licorice protein fractions on proliferation and apoptosis of
gastrointestinal cancer cell lines. Nutr Cancer. 69:330–339. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou X, Wang W, Li P, Zheng Z, Tu Y, Zhang
Y and You T: Curcumin enhances the effects of 5-fluorouracil and
oxaliplatin in inducing gastric cancer cell apoptosis both in vitro
and in vivo. Oncol Res. 23:29–34. 2016. View Article : Google Scholar
|
|
23
|
Alimperti S and Andreadis ST: CDH2 and
CDH11 act as regulators of stem cell fate decisions. Stem Cell Res.
14:270–282. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Saika K and Sobue T: Cancer statistics in
the world. Gan To Kagaku Ryoho. 40:2475–2480. 2013.(In Japanese).
PubMed/NCBI
|
|
25
|
Zeng H, Zheng R, Zhang S, Zuo T, Xia C,
Zou X and Chen W: Esophageal cancer statistics in China, 2011:
Estimates based on 177 cancer registries. Thorac Cancer. 7:232–237.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kinoshita T, Kajiyama K, Hiraga Y,
Takahashi K, Tamura Y and Mizutani K: Isoflavan derivatives from
Glycyrrhiza glabra (licorice). Heterocycles. 43:581–588. 1996.
View Article : Google Scholar
|
|
28
|
Vaya J, Belinky PA and Aviram M:
Antioxidant constituents from licorice roots: Isolation, structure
elucidation and antioxidative capacity toward LDL oxidation. Free
Radic Biol Med. 23:302–313. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fulmer CG, Hoda RS, Pirog EC, Park KJ and
Holcomb K: Cytomorphology of gastric-type cervical adenocarcinoma
on a ThinPrep Pap test: Report of a p16-positive tumor case. Diagn
Cytopathol. 44:710–713. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kosemehmetoglu K, Ardic F, Karslioglu Y,
Kandemir O and Ozcan A: p16 expression predicts neoadjuvant tumor
necrosis in osteosarcomas: Reappraisal with a larger series using
whole sections. Hum Pathol. 50:170–175. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Benassi MS, Molendini L, Gamberi G,
Ragazzini P, Sollazzo MR, Merli M, Asp J, Magagnoli G, Balladelli
A, Bertoni F and Picci P: Alteration of pRb/p16/cdk4 regulation in
human osteosarcoma. Int J Cancer. 84:489–493. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dobashi Y, Goto A, Fukayama M, Abe A and
Ooi A: Overexpression of cdk4/cyclin D1, a possible mediator of
apoptosis and an indicator of prognosis in human primary lung
carcinoma. Int J Cancer. 110:532–541. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maelandsmo GM, Flørenes VA, Hovig E,
Oyjord T, Engebraaten O, Holm R, Børresen AL and Fodstad O:
Involvement of the pRb/p16/cdk4/cyclin D1 pathway in the
tumorigenesis of sporadic malignant melanomas. Br J Cancer.
73:909–916. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rossi KA, Markwalder JA, Seitz SP, Chang
CH, Cox S, Boisclair MD, Brizuela L, Brenner SL and Stouten PF:
Understanding and modulating cyclin-dependent kinase inhibitor
specificity: Molecular modeling and biochemical evaluation of
pyrazolopyrimidinones as CDK2/cyclin A and CDK4/cyclin D1
inhibitors. J Comput Aided Mol Des. 19:111–122. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lee SK, Park KK, Park JH, Lim SS and Chung
WY: The inhibitory effect of roasted licorice extract on human
metastatic breast cancer cell-induced bone destruction. Phytother
Res. 27:1776–1783. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jo EH, Hong HD, Ahn NC, Jung JW, Yang SR,
Park JS, Kim SH, Lee YS and Kang KS: Modulations of the Bcl-2/Bax
family were involved in the chemopreventive effects of licorice
root (Glycyrrhiza uralensis Fisch) in MCF-7 human breast cancer
cell. J Agric Food Chem. 52:1715–1719. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jo EH, Kim SH, Ra JC, Kim SR, Cho SD, Jung
JW, Yang SR, Park JS, Hwang JW, Aruoma OI, et al: Chemopreventive
properties of the ethanol extract of chinese licorice (Glycyrrhiza
uralensis) root: Induction of apoptosis and G1 cell cycle arrest in
MCF-7 human breast cancer cells. Cancer Lett. 230:239–247. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brahmbhatt H, Oppermann S, Osterlund EJ,
Leber B and Andrews DW: Molecular pathways: Leveraging the BCL-2
interactome to kill cancer cells-mitochondrial outer membrane
permeabilization and beyond. Clin Cancer Res. 21:2671–2676. 2015.
View Article : Google Scholar : PubMed/NCBI
|