Introduction
Osteosarcoma (OS) is the most common primary
malignant bone tumor in children and young adults, affecting three
to five people per million annually (1,2). OS
typically presents in the bones around the knee (60% of cases),
accounting for ~5% of the pediatric malignancies (1,2). The
second most common site for OS to present is the pelvis; other
initial presentation sites have also been reported, including the
end of the humerus, skull and clavicle (3–5). OS
usually occurs in teenagers (60%): The majority of OS cases are
diagnosed before 20 years of age, a total of 75% (6,7).
The treatment outcome of OS has improved markedly
over the course of several years. The current standard treatment
for OS is a combination of surgery and chemotherapy: Preoperative
(neoadjuvant) chemotherapy, limb sparing surgery and postoperative
(adjuvant) chemotherapy. In the late 1970s, the 5-year survival
rate was 10–20% due to apparent lung metastases post-surgery;
however, 5-year survival has improved to the current rate of 50–80%
(8–12). In addition to improvements in surgical
and diagnostic techniques, the introduction of intensive
chemotherapy has reduced the rate of lung metastases. The majority
of patients today receive the same drugs as 25 years ago, including
doxorubicin, cisplatin, high-dose methotrexate and ifosfamide in
varying combinations (13,14). In order to further improve the
survival rate, the development of novel anticancer agents is also
necessary. Genetic studies have been performed to identify novel
therapeutic targets for OS; however, the underlying molecular
mechanisms have not yet been completely established (15–17).
Candidate gene studies and a recent genome-wide association study
have identified several common single nucleotide polymorphisms
associated with OS (18,19).
The recently-discovered epithelial nicotinamide
adenine dinucleotide phosphate (NADPH) oxidase (NOX) family
of enzymes mediate critical physiological and pathological
processes, including cell signaling, inflammation and mitogenesis
through the generation of reactive oxygen species (ROS) (20). The role of the ROS produced by these
enzymes in specific tissue types and distinct cellular compartments
has been the subject of several recent investigations (21,22).
Cancer cells, like non-malignant tissues, produce ROS; in tumors,
reactive oxygen metabolites may act as signaling molecules to
promote cell survival over apoptosis (23,24).
NOX enzymes and the mitochondria are a major source of
cellular ROS (25). There are
currently seven identified enzymes in the NADPH family, including
five NOX enxymes (NOX1-5) (26,27).
NOX enzymes have a fundamental role in numerous cell
functions, including signal transduction, differentiation,
proliferation and cell death (25,26).
However, current understanding of the roles of the NOX
family members in the development and growth of human cancers
remains limited (27–31).
In the present study, it was hypothesized that
NOX-mediated ROS generation conferred anti-apoptotic
activity, and, thus, a growth advantage to OS cells. It was
demonstrated that treatment with a flavoenzyme inhibitor,
diphenylene iodonium (DPI), and the knockdown of NOX2
significantly suppressed ROS generation in OS cells, which induced
apoptosis, indicating that NOX2-mediated ROS may transmit
cell survival signals and provide a potential clinical approach for
OS treatment.
Materials and methods
Cell culture and materials
Five OS cell lines (HOS, MOS, MG-63, NOS-1 and HuO
9N2) were used in this study. The MOS and NOS-1 cell lines were
kindly provided by Dr Masahiko Kanamori (School of Medicine,
University of Toyama, Toyama, Japan) (32–36). Three
cell lines (HOS, MG-63, and HuO 9N2) were obtained from the
Japanese Collection of Research Bioresources cell bank (Ibaraki,
Osaka, Japan). Cells were maintained at 37°C containing 5%
CO2 atmospheric air in Dulbecco's modified Eagle's
medium (DMEM; Sigma-Aldrich, St. Louis, MO, USA) supplemented with
10% heat-inactivated fetal bovine serum (FBS; Sigma-Aldrich), 2 mM
L-glutamine, 200 U/ml penicillin and 100 µg/ml streptomycin. DPI
was purchased from Calbiochem (EMD Millipore, Billerica, MA,
USA).
Ethical approval for the present study was obtained
from the ethical committee of Aichi Medical University (approval
no. 11–039), and informed consent was obtained prior to the start
of the study. Heparinized peripheral blood was collected from
healthy individuals (n=3). Peripheral blood mononuclear cells
(PBMCs) were separated by Ficoll-Hypaque density centrifugation at
400 × g for 30 min at room temperature.
Based on human NOX2 and NOX4 cDNA
sequences, small interfering RNAs (siRNAs) were designed as follows
(Integrated DNA Technologies, Coralville, IA, USA):
5′-UCAGGGUUCUUUAUUCUCUTT-3′ and 5′-AGAGAAUAAAGAACCCUGATT-3′ for
NOX2 siRNA-1; 5′-GUACAAUUCGUUCAGCUCCTT-3′ and
5′-GGAGCUGAACGAAUUGUACTT-3′ for NOX2 siRNA-2;
5′-GCUGAAGUAUCAAACUAAUUUAGAT-3′ and
5′-UCUAAAUUAGUUUGAUACUUCAGCAG-3′ for NOX4 siRNA-1;
5′-GAAUUACAGUGAAGACUUUGUUGAA-3′
and5′-UUCAACAAAGUCUUCACUGUAAUUCAC-3′ for NOX4 siRNA-2.
Universal scrambled siRNA sequences (Invitrogen; Thermo-Fisher
Scientific, Inc., Waltham, MA, USA), which have no significant
homology to mouse, rat or human genome databases, were used as
controls.
Quantification of NOX2 and NOX4 mRNAs
by reverse transcription-quantitative polymerase chain reaction
(RT-qPCR)
The total RNA was purified with a TRIzol reagent
(Thermo-Fisher Scientific, Inc.). The HOS, MOS, MG-63, NOS-1 and
HuO 9N2 OS cell lines (1×105 density) were seeded into
24-well plates and cultured in the presence or absence of
NOX2 and/or siRNAs for 48 h. Cell lysates were prepared in 1
ml TRIzol reagent with adequate mixing. Chloroform (200 µl) was
added and the solution was mixed well and centrifuged for 15 min at
12,000 × g at 4°C. The chloroform and centrifugation steps were
repeated. Then, RNA pellet was precipitated with 2-propanol and
rinsed with 70% ethanol. Purified RNA was dissolved in 20 µl
distilled water. RT was conducted as follows: A total of 8 µl
water, containing 1 µg total RNA, was added to 50 ng random primers
(Thermo-Fisher Scientific, Inc.) and incubated at 65°C for 5 min.
The samples were chilled on ice and cDNA was prepared with
SuperScript III First-Strand Synthesis Supermix (Invitrogen;
Thermo-Fisher Scientific, Inc.) according to the manufacturer's
instructions. The PCR products created with the NOXs 2 and 4
primers were identified by direct DNA sequence analysis.
RT-qPCR was performed with SYBR Premix Ex Taq II
(Takara Bio, Inc., Otsu, Shiga, Japan) in an ABI PRISM 7500
Sequence Detection system (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Briefly, a solution of SYBR Premix Ex Taq II (10
µl) containing sense and antisense primers (10 µM each) was
prepared and aliquoted into individual wells of a MicroAmp Optical
Plate (ABI-PE; Applied Biosystems; Thermo-Fisher Scientific, Inc.):
2 µl cDNA was added to give a final volume of 20 µl. The cycling
conditions for the PCR were 42°C for 5 min, 95°C for 10 sec and 40
cycles of 95°C for 5 sec (denaturation) and 60°C for 34 sec
(annealing/extension). The data were analyzed with Sequence
Detector software (version 1.6; ABI-PE; Applied Biosystems;
Thermo-Fisher Scientific, Inc.). The quantitative cycle (Cq) during
the exponential phase of amplification was determined by real-time
monitoring of fluorescent emission by the nuclease activity of
Taq polymerase. β-actin was used as an internal
control gene for mRNA expression. Relative transcripts were
determined by the formula: 1/2(Cq target - Cq control)
(36). NOX1, NOX2, NOX3, NOX4,
NOX5 and β-actin genes were amplified with specific
primer sequences (Star Oligo, Rikaken, Nagoya, Japan) according to
the NCBI reference sequences (http://www.ensembl.org/Homo_sapiens/index.html). The
PCR primer pairs and probes used, were as follows: NOX1,
5′-AGCGTCTGCTCTCTGCTTGAA-3′ and 5′-GGCTGCAAAATGAGCAGGT-3′ (junction
between exons 3 and 4); NOX2, 5′-TGCCTTTGAGTGGTTTGCAGAT-3′
and 5′-ATTGGCCTGAGACTCATCCCA-3′ (junction between exons 11 and 12);
NOX3, 5′-GAACCCTCGGCTTGGAAAT-3′ (junction between exons 7
and 8) and 5′-TGGCTTACCACCTTGGTAATGA-3′ (junction between exons 8
and 9); NOX4, 5′-CCCTCACAATGTGTCCAACTGA-3′ (junction between
exons 11 and 12) and 5′-GGCAGAATTTCGGAGTCTTGAC-3′; NOX5,
5′-AAGAGTCAAAGGTCGTCCAAGG-3′ and 5′-GCTTTCTTTTCTGGTGCCTGT-3′
(junction between exons 13 and 14); β-actin,
5′-GATGACCCAGATCATGTTTGAGACC-3′ (junction between exons 2 and 3)
and 5′-CGGTGAGGATCTTCATGAGGTAGT-3′. Semi-quantitative RT-PCR was
performed with 30 cycles (94°C for 1 min, 55°C for 1 min, 72°C for
1 min).
Cell transfection
The cells were transfected with NOX2- and/or
NOX4-specific siRNAs, or scramble RNAs, using
Lipofectamine® 2000 (Invitrogen; Thermo-Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Assessment of intracellular ROS
production
HOS, MOS, MG-63, NOS-1 and HuO 9N2 OS cells
(2×105) were seeded in 6-well plates and treated with 10
µM DPI for 48 h. Following this, the cells were transfected with
NOX2- and NOX4-specific siRNAs and cultured for 48 h.
The cells were then incubated with 2.5 µM dihydroethidium (DCFH-DA;
Molecular Probes, Thermo-Fisher Scientific, Inc.) for 30 min at
37°C in the dark. Subsequently, the cells were washed with Hank's
buffer and fixed in 1% paraformaldehyde. Fluorescence-activated
cell sorting (FACS) was used to measure the fluorescence emission
intensities at 488 nm for excitation and at 580 nm for detection.
The histograms were analyzed with the BD FACStation System Data
Management system (BD Biosciences; Franklin Lakes, NJ, USA).
Background fluorescence from a blank sample was subtracted from
each reading to normalize the results.
In vitro 3-(4, 5-dimethyl
thiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay
An MTT assay was used to evaluate cell viability
after 48 h incubation at 37°C. OS cells were incubated (in
triplicate) in 96-well culture plates at 37°C in humidified air
with 5% CO2. Three wells that contained OS cells in
drug-free DMEM were included to determine the control cell survival
rate; another three wells contained only DMEM to calibrate the
spectrophotometer. After two days, 10 µl (5 mg/ml) MTT salt
(Sigma-Aldrich: St Louis, MO, USA) was added to each well in
96-well culture plates for 6 h. The MTT compound was reduced to
colored formazan crystals by the living cells alone. The crystals
were dissolved with 100 µl of acidified isopropanol and the
formazan crystal production was quantified using a
spectrophotometer (562 nm). The optical density (OD) is linearly
related to the cell number. Cell survival (CS) was calculated for
each drug concentration using the following equation:
CS=(ODtreated well/ODcontrol well mean)
×100%.
Measuring apoptosis using flow
cytometry
The externalization of phosphatidylserine was
measured by flow cytometry with fluorescein
isothiocyanate-conjugated Annexin V (BD Pharmingen, San Diego, CA,
USA) (37). Flow cytometry analyses
were performed using a FACSCalibur flow cytometer (BD Biosciences)
and CellQuest Pro Version 4.0.2 (BD Biosciences) software. Cells
(2×105) seeded in 6-well plates were cultured for 48 h
following transfection of NOX siRNAs or scramble RNAs, washed and
resuspended in 100 µl Annexin-binding buffer [10 mM HEPES
(N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid), 140 mM NaCl
and 2.5 mM CaCl2 in PBS], stained with 5 µl Annexin V-Alexa Fluor
488 conjugate for 20 min then analyzed by flow cytometry
(FACSCalibur). The cells that stained positive with Annexin V were
counted as apoptotic populations.
Statistical analysis
The results of MTT assay, apoptosis assay with siRNA
transfection, and RT-qPCR assay were analyzed using one-way
analysis of variance with a Turkey Kramer post hoc test using the
Statview software (version 5; SAS Institute, Inc., Cary, NC, USA).
Results are expressed as the mean ± standard deviation. P<0.05
was considered to indicate a statistically significant
difference.
Results
Inhibition of cell growth and ROS
generation by the flavoenzyme inhibitor DPI
Flavoprotein-dependent ROS play a critical role in
cytokine-mediated signal transduction in normal tissues and tumor
cells. DPI, a flavoenzyme inhibitor, inhibits the membrane-bound,
flavoprotein-containing NOX enzymes (25,30). The
present study examined whether DPI affected cell viability in OS
cell lines using an MTT assay. A total of five OS cell lines were
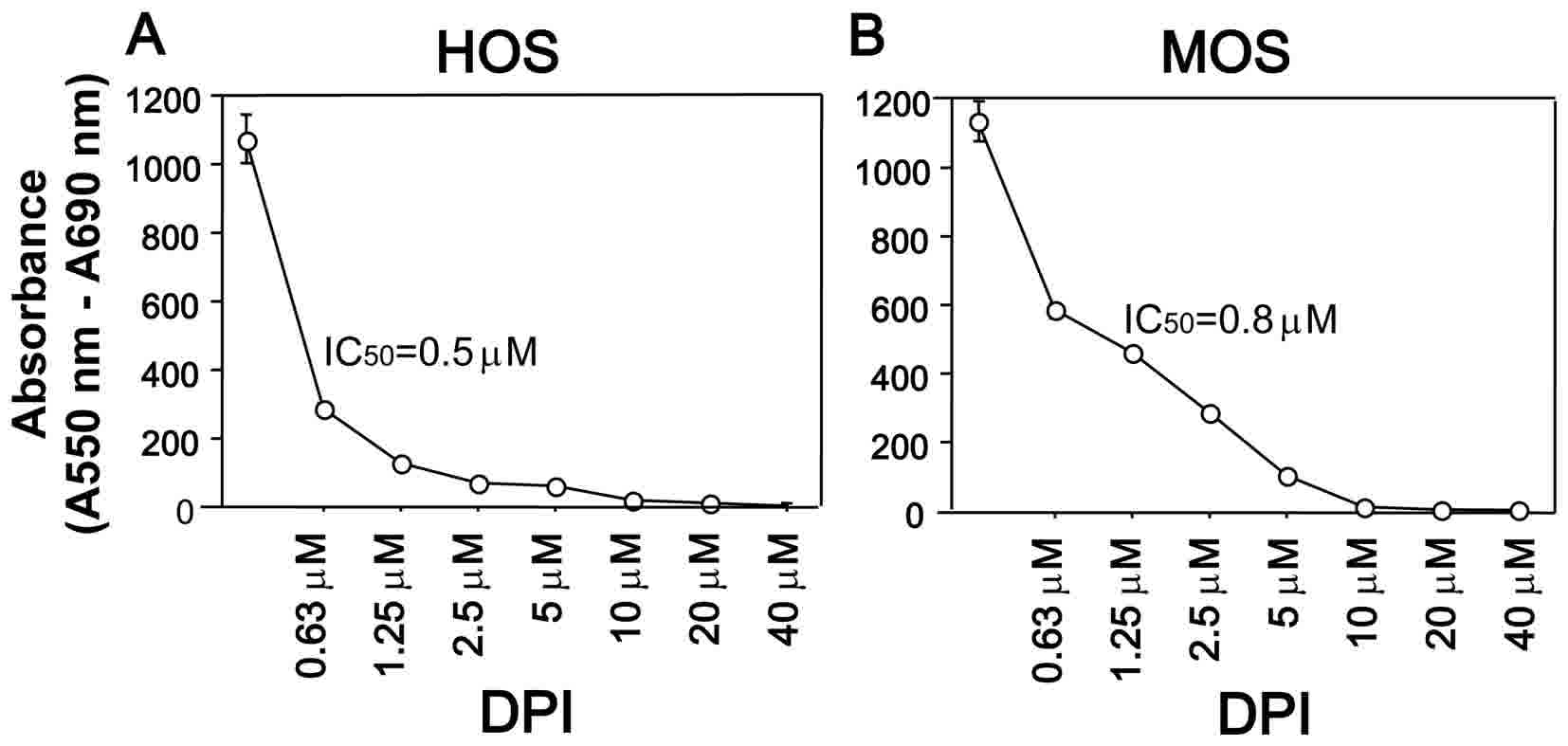
treated with various DPI concentrations for 24 h and the
representative results for HOS and MOS cells are shown in Fig. 1. As hypothesized, DPI treatment
decreased the viability of HOS and MOS cells in a dose-dependent
manner (P<0.0001 for both cell lines); the IC50
values of HOS and MOS cells were 0.5 and 0.8 µM, respectively. The
IC50 values for the other three cell lines were as
follows: 0.9 µM (MG-63), 1.2 µM (NOS-1) and 0.7 µM (HuO 9N2),
respectively.
To determine whether DPI affects ROS generation,
intracellular ROS levels were evaluated by using flow cytometry. It
was observed that untreated HOS cells generated ROS, and DPI
treatment eliminated ROS generation in HOS cells (Fig. 2). Therefore, dichlorofluorescein (DCF)
fluorescence intensity was reduced from 2.8×103 in
untreated cells, to 0.9×103 in DPI-treated cells.
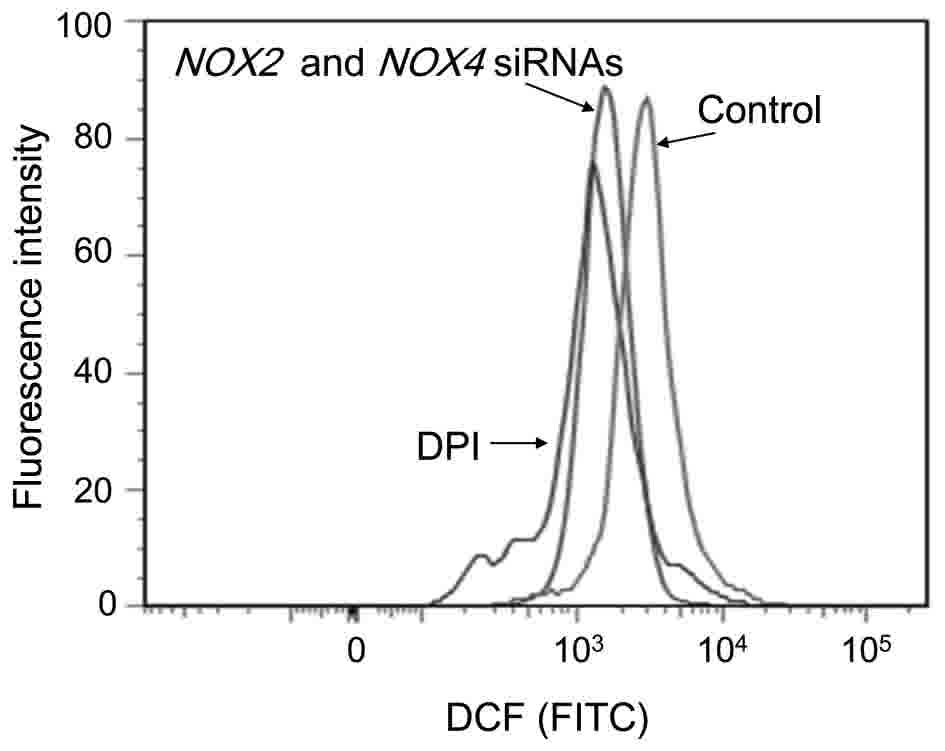 | Figure 2.Inhibition of ROS production by DPI
or NOX2 and NOX4 specific siRNAs. DPI and NOX2 and
NOX4-specific siRNA treatment inhibited ROS production. HOS cells
(1×105) were cultured in a 24-well plate for 48 h and
subsequently treated with, or without (control), DPI (10 µM) for 24
h. HOS cells were also seeded in 6-well plates (2×105),
cultured overnight and transfected with NOX2 and NOX4
specific siRNAs for 48 h. Following cell harvest, the cells were
labeled with DCFH-DA and intracellular ROS levels were measured by
flow cytometry. DCF fluorescence intensities were normalized to
untreated HOS cells. A typical fluorescence profile is exhibited.
The x-axis indicates fluorescence intensity with an excitation
source of 488 nm and emission wavelength of 580 nm. DPI,
diphenylene iodonium; OS, osteosarcoma; ROS, reactive oxygen
species; NOX, NADPH oxidase; DCF, dichlorofluorescein; DCFH-DA, 2′,
7′-dichlorodihydrofluorescein diacetate; siRNA, small interfering
RNA; FITC, fluorescein isothiocyanate. |
Induction of apoptosis by DPI
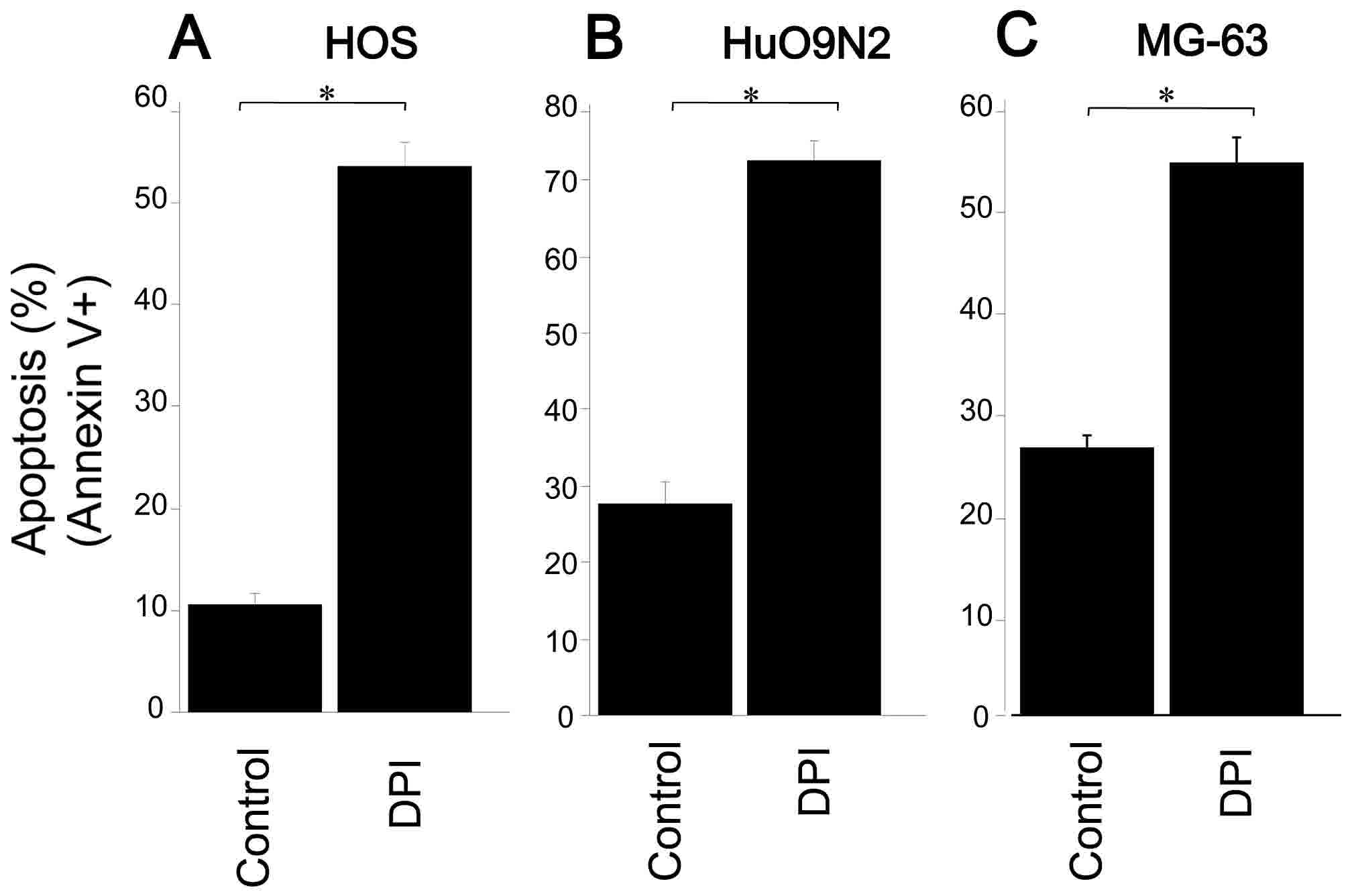
The present study examined the effect of DPI on
apoptosis in OS cells using Annexin V, and observed that DPI
treatment markedly increased apoptosis in HOS (54%), HuO 9N2 (43%)
and MG63 (28%) cells (Fig. 3). The
results suggest that the depletion of ROS, generated by the
NOX-like enzymes, triggered apoptosis in OS cells.
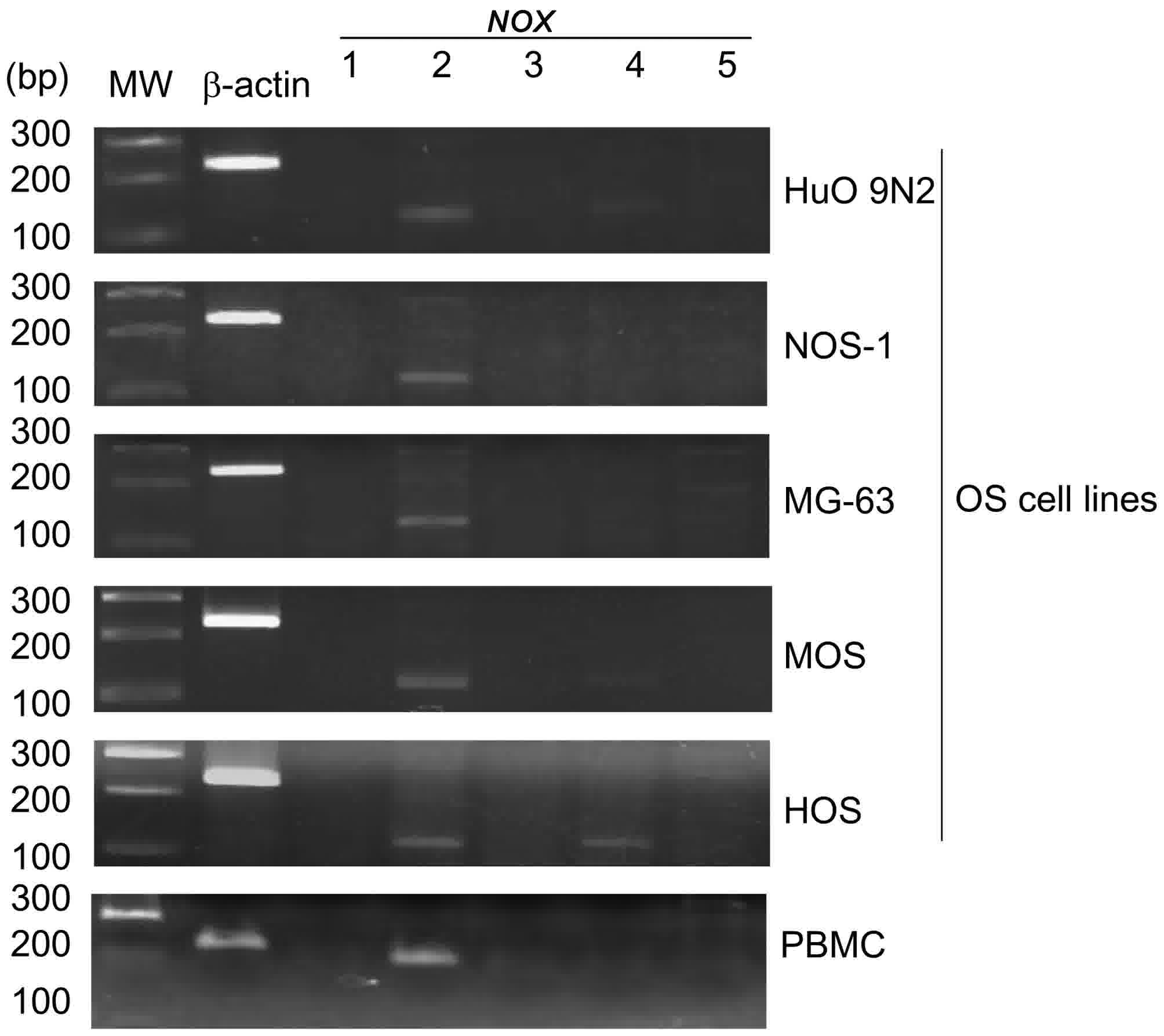
Expression of NOX1-5 mRNAs in human OS
cell lines
NOX family members produce ROS that are
pivotal for cell proliferation. To examine the role of the
NOX family in the proliferation of OS cells, mRNA expression
of the NOX family members was measured in 5 human OS cell
lines by semi-quantitative RT-PCR. NOX2 mRNA was highly
expressed in all of the examined OS cell lines, whereas little or
no NOX1, NOX3 and NOX5 mRNA was detected (Fig. 4). The OS cell lines also expressed
low-moderate levels of NOX4 mRNA (Fig. 4). To provide a comparison, high levels
of NOX2 mRNA were also detected in human PBMCs (Fig. 4).
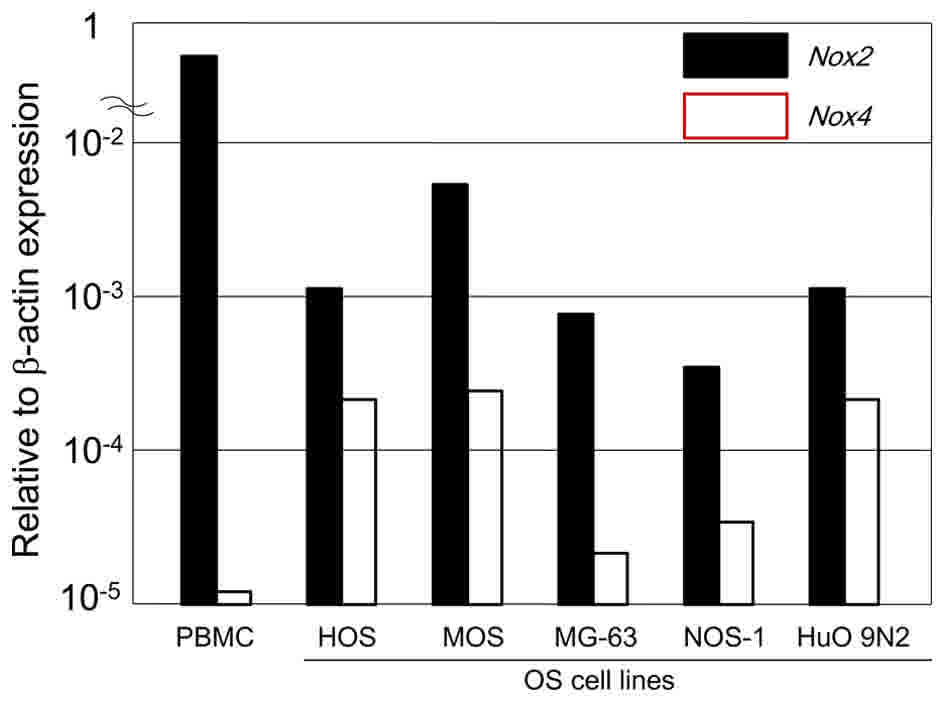
NOX2 and NOX4 expression in human OS
cell lines
The present study measured the expression levels of
NOX family mRNAs relative to β-actin by RT-qPCR (Fig. 5). The expression was graded as low
(NOX/β-actin ratio <2×10−5), moderate (ratio
>2×10−5 and <50×10−5) or high (ratio
>50×10−5). Relative transcripts were determined by
the formula: 1/2(Cqtarget - Cqcontrol) (36). High-level NOX2 mRNA expression
was observed in all of the examined OS cell lines, with the highest
expression detected in MOS cells. Moderate-level NOX4 mRNA
expression was detected in all of the examined OS cell lines.
Therefore, NOX2 mRNA expression was higher than NOX4
mRNA expression in the included OS cell lines. For comparison,
high-level expression of NOX2 and low-level expression of
NOX4 was detected in human peripheral blood mononuclear
cells (Fig. 5).
NOX2 and NOX4 mediate ROS production
in OS cells
The present study utilized RNA interference to
determine whether NOX2/4 mediates ROS generation in OS
cells. Primarily, the effects of NOX2 or
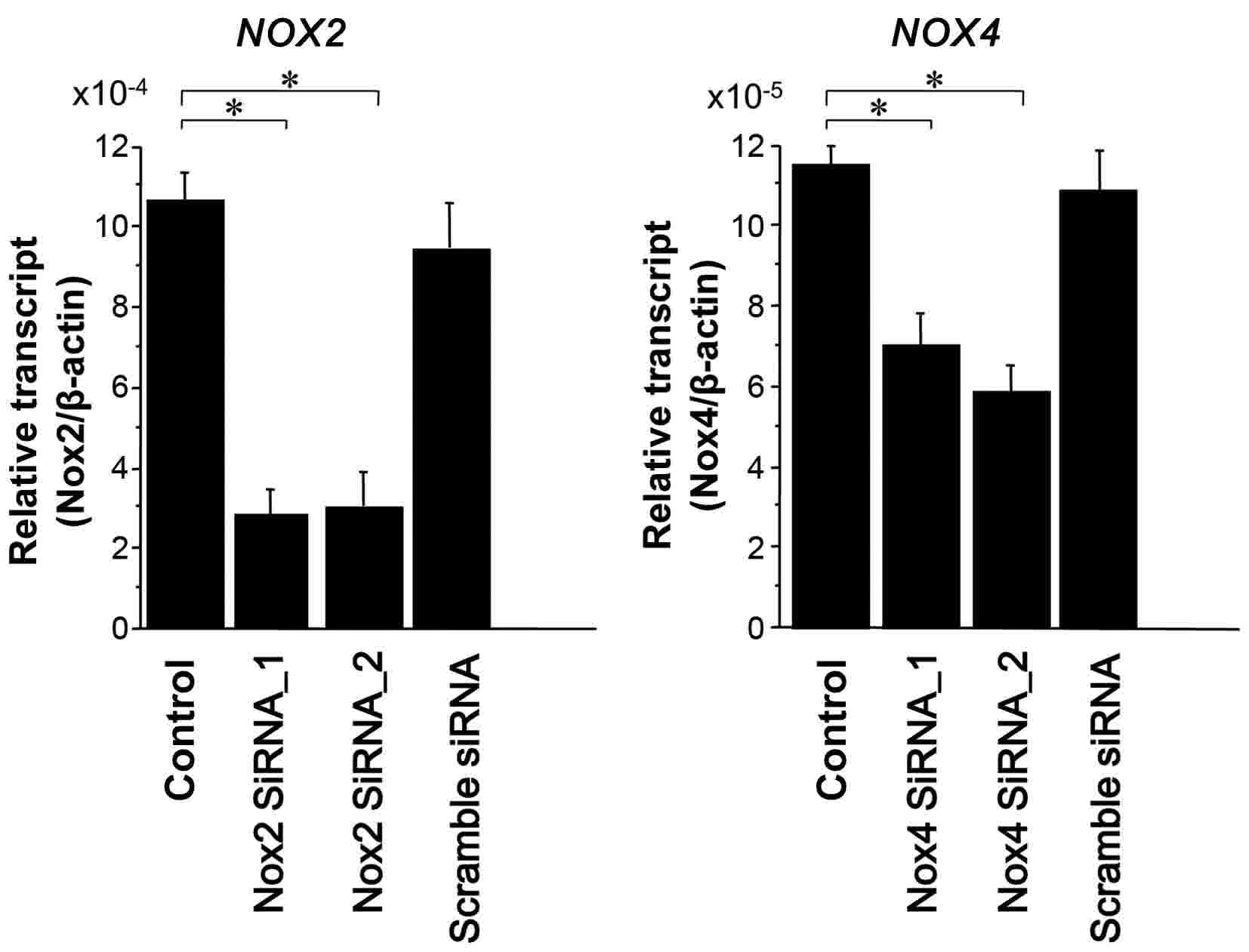
NOX4-specific siRNAs on the endogenous expression of their
mRNAs in HOS cells were evaluated (Fig.
6), revealing that their siRNAs functioned effectively
(Fig. 6). In order to elucidate how
NOX2 and NOX4 mRNA expression affects ROS generation,
siRNAs targeting NOX2 and NOX4 were transiently
transfected into OS cells and intracellular ROS levels were
evaluated using flow cytometry. Double transfection of NOX2
and NOX4 siRNAs reduced DCF fluorescence intensity to
1.1×103 from the untreated control intensity of
2.8×103 (Fig. 2). Thus,
the double transfection of OS cells with NOX2 and
NOX4 siRNAs suppressed intracellular ROS levels (39%)
compared with the controls (Fig. 2).
The results indicate that NOX2 and/or NOX4, at least
in part, are responsible for intracellular ROS generation in OS
cells. However, ROS generation was not completely inhibited by
NOX2 and NOX4 knockdown, suggesting that other
NOX proteins and mitochondrial components may also
contribute to ROS generation.
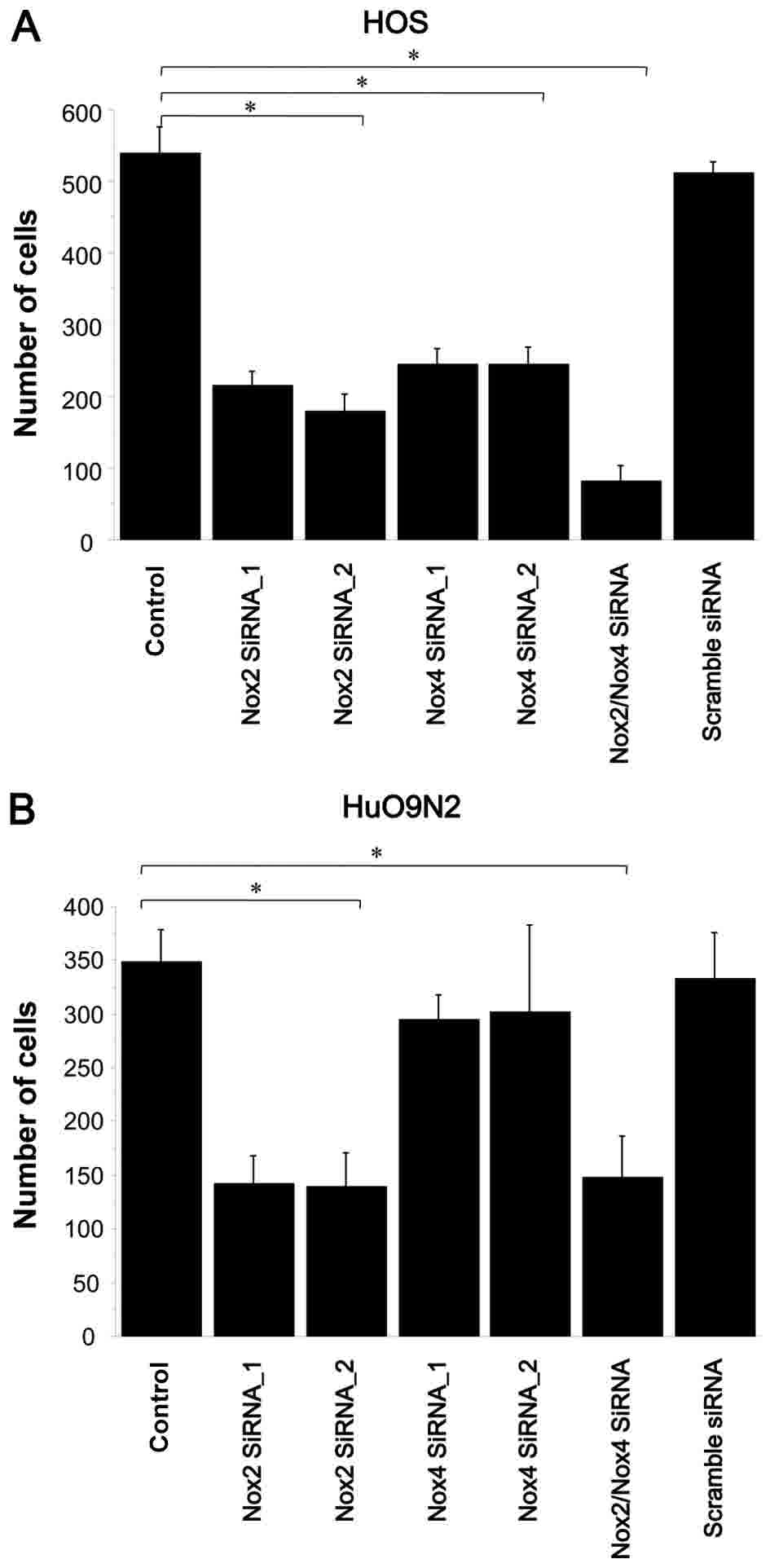
NOX2 and NOX4 siRNAs reduce cell
viability and induce apoptosis
To explore whether NOX2 and
NOX4-mediated ROS affect cell survival, the effect of their
knockdown on cell viability and apoptosis was examined. NOX2
and NOX4 knockdown significantly reduced HOS cell viability,
by 74 and 65%, respectively, relative to that of untreated cells
(P<0.0001 for NOX2 knockdown; P=0.0033 for NOX4
knockdown; Fig. 7A). NOX2
knockdown in HuO 9N2 cells reduced viability by 61% (P=0.0003;
Fig. 7B), while NOX4 knockdown
in the same cell line reduced viability by 11%, which was not
statistically significant. Collectively, these results suggest that
among NOX family members, NOX2 has a major role in
survival of HOS and HuO 9N2 cells.
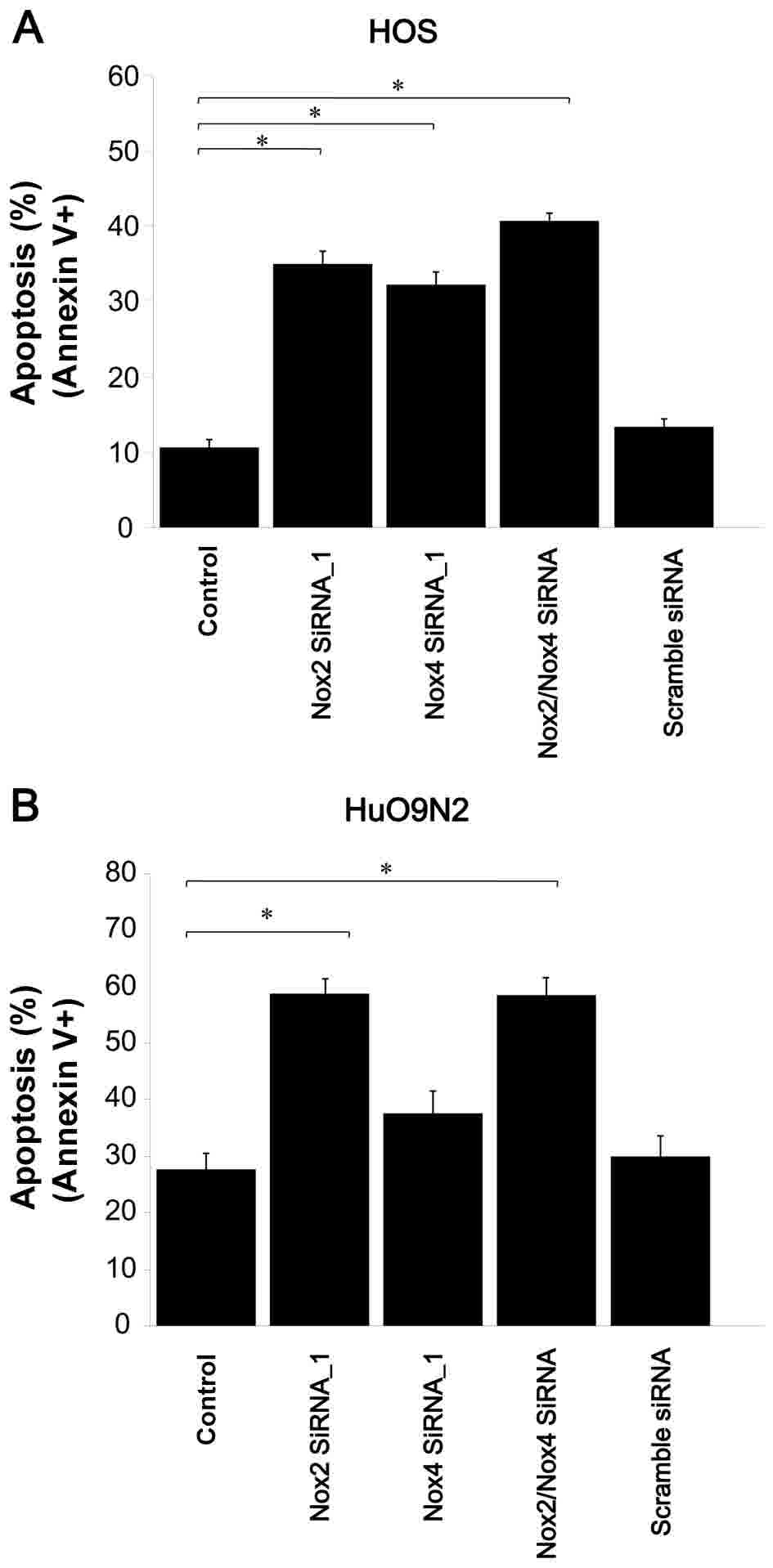
To verify whether this reduced cell viability was
associated with apoptosis, an Annexin V assay was performed and
NOX2 knockdown was observed to markedly induce apoptosis in
HOS and HuO 9N2 cells (P=0.0003 for HOS cells; P<0.0001 for HuO
9N2 cells; Fig. 8A and B). Thus, the
reduction in cell viability observed with siRNA knockdown was
associated with the induction of apoptosis. Therefore, NOX2
and NOX4 siRNAs suppressed ROS generation, and the depletion
of ROS by NOX2 knockdown, and DPI treatment induced
apoptosis in HOS and HuO 9N2 cells.
Discussion
Cancer cells produce ROS that may act as signaling
molecules to promote cell survival and cell growth (23,24,38). It is
possible that chronic inflammation may accelerate the development
and progression of malignant OS, due to cytokine release and ROS
generation.
The present study examined the role of ROS in the
viability and apoptosis of OS cell lines. Although ROS are
considered to cause stress-induced apoptosis, ROS often confer a
survival advantage on cancer cells. The results of the current
study demonstrated that suppressing ROS levels by DPI treatment
reduced the viability of OS cells. Similar results have been
observed in other types of cancer cells, including pancreatic tumor
cells (39). The current study also
investigated whether the NOX2 and 4-mediated ROS generation
conferred anti-apoptotic activity and, thus, a growth advantage to
OS cells. As such, the expression levels of NOX genes in
five human OS cell lines were examined. RT-PCR analysis revealed
that NOX2 and NOX4 mRNAs were expressed in the OS
cell lines; however little or no NOX2, NOX3 and
NOX5 mRNAs were detected. RT-qPCR revealed that NOX2
and NOX4 mRNAs were expressed in OS cells at high and
moderate levels, respectively. In all the examined OS cells,
NOX2 mRNA exhibited the highest expression levels.
NOX2 siRNAs significantly reduced intracellular ROS
generation and OS cell viability. Concordantly, ROS depletion by
DPI treatment or NOX2 knockdown induced apoptosis. The
results of the present study suggested that NOX2-mediated ROS
generation promotes the production of cell survival signals and
that ROS depletion induces apoptosis in OS cells.
NOX4 mRNA overexpression has previously been
reported in primary breast, ovarian, prostate, melanoma and
glioblastoma cancer cell lines (40–42).
NOX4 expression was moderate-high in two of the four tested
ovarian cancer cell lines. Notably, high-level acquired resistance
to cisplatin was associated with a marked decrease in the
NOX4 mRNA levels in A2780/DDP cells (30). Previously, NOX4 was demonstrated to be
an oncoprotein localized in mitochondria (43). In the current study, NOX4
knockdown induced apoptosis in a subset of OS cells. Considering
that NOX2 knockdown significantly reduced cell viability and
induced apoptosis in all the examined OS cell lines, it is possible
that NOX2-mediated ROS generation has a major role in
promoting the survival of OS cells. The present study raises the
possibility that NOX2 may act as an oncoprotein in the pathogenesis
of OS, similar to NOX4 in other types of cancer. Therefore, NOX2
and NOX2-associated signaling molecules may be good candidates for
the targeted therapy of OS. NOX2 has been previously reported to be
involved in a variety of physiological and pathological conditions,
including prion disease (44–47). NOX2 has also been implicated in cancer
biology (48–50) as NOX2 was established to serve a
pro-survival role in human leukemia and be involved to promote
apoptosis in human glioma (48,50).
Considering the possible role of NOX2 in cancer development,
it may be of interest to investigate the correlation between
NOX2 expression and OS prognosis in further studies.
In conclusion, the present study demonstrated that
NOX2-mediated ROS generation promotes the survival of OS
cells and that ROS depletion through NOX2 knockdown and DPI
treatment leads to apoptosis. The current study raises the
possibility of the NOX2-ROS signaling pathway being used as
a novel therapeutic target for OS. For example, antioxidant
treatments targeted to this signaling pathway have the potential to
enhance the therapeutic index of cisplatin-based therapies. Further
studies are required to contribute to the development of targeted
therapies for OS.
Acknowledgements
The authors would like to thank Dr Masahiko Kanamori
and Dr Taketoshi Yasuda (Department of Orthopedic Surgery, School
of Medicine, University of Toyama) for providing the OS cell
lines.
Funding
The present study was supported by the Aichi Cancer
Center to Yuji Miura and a grant of Strategic Research Foundation
Grant-Aided Project for Private Universities from the Ministry of
Education, Culture, Sports, Science and Technology, Japan to
Yoshitaka Hosokawa (grant no. AI 52213).
Availability of data and materials
All data generated or analyzed during this study are
available from the corresponding author on reasonable request.
Authors' contributions
Conception and design, KK, YM, YH and KS;
development of methodology, KK, YM, SK, AO and YH; acquisition of
data (such as provided cells, provided facilities), KK, YM, HK, YH
and SK; analysis and interpretation of data, KK, YM, AO, HK, YH and
SK; writing, review, and/or revision, KK, YM, YH and SK;
administrative, technical or material support (including reporting
or organizing data, preparing vectors), KK, YM, SK, AO, HK, YH and
KS; study supervision, AO, HK, YH and SK.
Ethics approval and consent to
participate
Ethical approval for the present study was obtained
from the ethical committee of Aichi Medical University (approval
no. 11-039), and informed consent was obtained prior to the start
of the present study.
Consent for publication
Informed consent for publication was obtained prior
to the start of the present study.
Competing interests
The authors have no competing interests to
declare.
Glossary
Abbreviations
Abbreviations:
|
DCF
|
dichlorofluorescin
|
|
Duox
|
dual oxidase
|
|
DCFH-DA
|
2′, 7′-dichlorodihydrofluorescein
diacetate
|
|
DPI
|
diphenylene iodonium
|
|
NADPH
|
nicotinamide adenine dinucleotide
phosphate
|
|
NOX
|
NADPH oxidase
|
|
OS
|
osteosarcoma
|
|
PBMC
|
peripheral blood mononuclear cell
|
|
PCR
|
polymerase chain reaction
|
|
ROS
|
reactive oxygen species
|
|
RT-PCR
|
reverse transcription
quantitative-polymerase chain reaction
|
References
|
1
|
Raymond AK, Ayala AG and Knuutila K:
Conventional osteosarcomaFletcher CDM, Unni KK and Mertens F: World
Health Organization Classification of Tumours. Pathology and
Genetics of Tumours of Soft Tissue and Bone. Lyon: IARC Press; pp.
264–270. 2002
|
|
2
|
Arndt CA and Crist WM: Common
musculoskeletal tumors of childhood and adolescence. N Engl J Med.
341:342–352. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mirabello L, Troisi RJ and Savage SA:
International osteosarcoma incidence patterns in children and
adolescents, middle ages and elderly persons. Int J Cancer.
125:229–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mirabello L, Troisi RJ and Savage SA:
Osteosarcoma incidence and survival rates from 1973 to 2004: Data
from the surveillance, epidemiology, and end results program.
Cancer. 115:1531–1543. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anninga JK, Gelderblom H, Fiocco M, Kroep
JR, Taminiau AH, Hogendoorn PC and Egeler RM: Chemotherapeutic
adjuvant treatment for osteosarcoma: Where do we stand? Eur J
Cancer. 47:2431–2445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Uribe-Botero G, Russell WO, Sutow WW and
Martin RG: Primary osteosarcoma of bone. Clinicopathologic
investigation of 243 cases, with necropsy studies in 54. Am J Clin
Pathol. 67:427–435. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: an analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Link MP, Goorin AM, Miser AW, Green AA,
Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick
JA, et al: The effect of adjuvant chemotherapy on relapse-free
survival in patients with osteosarcoma of the extremity. N Engl J
Med. 314:1600–1606. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Anderson P: Chemotherapy for osteosarcoma
with high-dose methotrexate is effective and outpatient therapy is
now possible. Nat Clin Pract Oncol. 4:624–625. 2007.PubMed/NCBI
|
|
10
|
Ritter J and Bielack SS: Osteosarcoma. Ann
Oncol. 21 Suppl 7:vii320–vii325. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ellegast J, Barth TF, Schulte M, Bielack
SS, Schmid M and Mayer-Steinacker R: Metastasis of osteosarcoma
after 16 years. J Clin Oncol. 29:e62–e66. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Andreou D, Bielack SS, Carrle D, Kevric M,
Kotz R, Winkelmann W, Jundt G, Werner M, Fehlberg S, Kager L, et
al: The influence of tumor- and treatment-related factors on the
development of local recurrence in osteosarcoma after adequate
surgery. An analysis of 1355 patients treated on neoadjuvant
cooperative osteosarcoma study group protocols. Ann Oncol.
22:1228–1235. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bielack SS: Osteosarcoma: Time to move on?
Eur J Cancer. 46:1942–1945. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Federman N, Bernthal N, Eilber FC and Tap
WD: The multidisciplinary management of osteosarcoma. Curr Treat
Options Oncol. 10:82–93. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ito M, Barys L, O'Reilly T, Young S,
Gorbatcheva B, Monahan J, Zumstein-Mecker S, Choong PF, Dickinson
I, Crowe P, et al: Comprehensive mapping of p53 pathway alterations
reveals an apparent role for both SNP309 and MDM2 amplification in
sarcomagenesis. Clin Cancer Res. 17:416–426. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Savage SA, Stewart BJ, Liao JS, Helman LJ
and Chanock SJ: Telomere stability genes are not mutated in
osteosarcoma cell lines. Cancer Genet Cytogenet. 160:79–81. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kansara M and Thomas DM: Molecular
pathogenesis of osteosarcoma. DNA Cell Biol. 26:1–18. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Savage SA, Mirabello L, Wang Z,
Gastier-Foster JM, Gorlick R, Khanna C, Flanagan AM, Tirabosco R,
Andrulis IL, Wunder JS, et al: Genome-wide association study
identifies two susceptibility loci for osteosarcoma. Nat Genet.
45:799–803. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mirabello L, Koster R, Moriarity BS,
Spector LG, Meltzer PS, Gary J, Machiela MJ, Pankratz N, Panagiotou
OA, Largaespada D, et al: A genome-wide scan identifies variants in
NFIB associated with metastasis in patients with osteosarcoma.
Cancer Discov. 5:920–931. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Brar SS, Kennedy TP, Sturrock AB,
Huecksteadt TP, Quinn MT, Whorton AR and Hoidal JR: An NAD(P)H
oxidase regulates growth and transcription in melanoma cells. Am J
Physiol Cell Physiol. 282:C1212–C1224. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rhee SG: Cell signaling.
H2O2, a necessary evil for cell signaling.
Science. 312:1882–1883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Storz P: Reactive oxygen species in tumor
progression. Front Biosci. 10:1881–1896. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Szatrowski TP and Nathan CF: Production of
large amounts of hydrogen peroxide by human tumor cells. Cancer
Res. 51:794–798. 1991.PubMed/NCBI
|
|
25
|
Lambeth JD: NOX enzymes and the biology of
reactive oxygen. Nat Rev Immunol. 4:181–189. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bedard K and Krause KH: The NOX family of
ROS-generating NADPH oxidases: physiology and pathophysiology.
Physiol Rev. 87:245–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cheng G, Cao Z, Xu X, Meir EG and Lambeth
JD: Homologs of gp91phox: Cloning and tissue expression of Nox3,
Nox4, and Nox5. Gene. 269:131–140. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Donkó A, Péterfi Z, Sum A, Leto T and
Geiszt M: Dual oxidases. Philos Trans R Soc Lond B Biol Sci.
360:2301–2308. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Geiszt M, Witta J, Baffi J, Lekstrom K and
Leto TL: Dual oxidases represent novel hydrogen peroxide sources
supporting mucosal surface host defense. FASEB J. 17:1502–1504.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Juhasz A, Ge Y, Markel S, Chiu A,
Matsumoto L, van Balgooy J, Roy K and Doroshow JH: Expression of
NADPH oxidase homologues and accessory genes in human cancer cell
lines, tumours and adjacent normal tissues. Free Radic Res.
43:523–532. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu Y, Antony S, Juhasz A, Lu J, Ge Y,
Jiang G, Roy K and Doroshow JH: Up-regulation and sustained
activation of Stat1 are essential for interferon-gamma
(IFN-gamma)-induced dual oxidase 2 (Duox2) and dual oxidase A2
(DuoxA2) expression in human pancreatic cancer cell lines. J Biol
Chem. 286:12245–12256. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kanamori M, Ohmori K, Yasuda T and Yudoh
K: Effects of hyperthermia and differentiation on cultured Dunn
osteosarcoma cells. Cancer Detect Prev. 27:76–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yasuda T, Kanamori M, Nogami S, Hori T,
Oya T, Suzuki K and Kimura T: Establishment of a new human
osteosarcoma cell line, UTOS-1: Cytogenetic characterization by
array comparative genomic hybridization. J Exp Clin Cancer Res.
28:262009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hori T, Kondo T, Kanamori M, Tabuchi Y,
Ogawa R, Zhao QL, Ahmed K, Yasuda T, Seki S, Suzuki K and Kimura T:
Ionizing radiation enhances tumor necrosis factor-related
apoptosis-inducing ligand (TRAIL)-induced apoptosis through
up-regulations of death receptor 4 (DR4) and death receptor 5 (DR5)
in human osteosarcoma cells. J Orthop Res. 28:739–745.
2010.PubMed/NCBI
|
|
35
|
Kanamori M, Sano A, Yasuda T, Hori T and
Suzuki K: Array-based comparative genomic hybridization for
genomic-wide screening of DNA copy number alterations in aggressive
bone tumors. J Exp Clin Cancer Res. 31:1002012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miura Y, Thoburn CJ, Bright EC, Phelps ML,
Shin T, Matsui EC, Matsui WH, Arai S, Fuchs EJ, Vogelsang GB, et
al: Association of Foxp3 regulatory gene expression with
graft-versus-host disease. Blood. 104:2187–2193. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kumar S, Kain V and Sitasawad SL:
Cardiotoxicity of calmidazolium chloride is attributed to calcium
aggravation, oxidative and nitrosative stress, and apoptosis. Free
Radic Biol Med. 47:699–709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong M, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mochizuki T, Furuta S, Mitsushita J, Shang
WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A,
et al: Inhibition of NADPH oxidase 4 activates apoptosis via the
AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic
cancer PANC-1 cells. Oncogene. 25:3699–3707. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ushio-Fukai M and Nakamura Y: Reactive
oxygen species and angiogenesis: NADPH oxidase as target for cancer
therapy. Cancer Lett. 266:37–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Shono T, Yokoyama N, Uesaka T, Kuroda J,
Takeya R, Yamasaki T, Amano T, Mizoguchi M, Suzuki SO, Niiro H, et
al: Enhanced expression of NADPH oxidase Nox4 in human gliomas and
its roles in cell proliferation and survival. Int J Cancer.
123:787–792. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yamaura M, Mitsushita J, Furuta S, Kiniwa
Y, Ashida A, Goto Y, Shang WH, Kubodera M, Kato M, Takata M, et al:
NADPH oxidase 4 contributes to transformation phenotype of melanoma
cells by regulating G2-M cell cycle progression. Cancer Res.
69:2647–2654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Graham KA, Kulawiec M, Owens KM, Li X,
Desouki MM, Chandra D and Singh KK: NADPH oxidase 4 is an
oncoprotein localized to mitochondria. Cancer Biol Ther.
10:223–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
McCann SK, Dusting GJ and Roulston CL:
Nox2 knockout delays infarct progression and increases vascular
recovery through angiogenesis in mice following ischaemic stroke
with reperfusion. PLoS One. 9:e1106022014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li ZY, Jiang WY and Cui ZJ: An essential
role of NAD(P)H oxidase 2 in UVA-induced calcium oscillations in
mast cells. Photochem Photobiol Sci. 14:414–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Sorce S, Nuvolone M, Keller A, Falsig J,
Varol A, Schwarz P, Bieri M, Budka H and Aguzzi A: The role of the
NADPH oxidase NOX2 in prion pathogenesis. PLoS Pathog.
10:e10045312014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Menden H, Welak S, Cossette S, Ramchandran
R and Sampath V: Lipopolysaccharide (LPS)-mediated
angiopoietin-2-dependent autocrine angiogenesis is regulated by
NADPH oxidase 2 (Nox2) in human pulmonary microvascular endothelial
cells. J Biol Chem. 290:5449–5461. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Maraldi T, Prata C, Dalla Sega Vieceli F,
Caliceti C, Zambonin L, Fiorentini D and Hakim G: NAD(P)H oxidase
isoform Nox2 plays a prosurvival role in human leukaemia cells.
Free Radic Res. 43:1111–1121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jones KJ, Chetram MA, Bethea DA, Bryant
LK, Odero-Marah V and Hinton CV: Cysteine (C)-X-C Receptor 4
Regulates NADPH Oxidase-2 during oxidative stress in prostate
cancer cells. Cancer Microenviron. Sep 28–2013.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Li SZ, Hu YY, Zhao J, Zhao YB, Sun JD,
Yang YF, Ji CC, Liu ZB, Cao WD, Qu Y, et al: MicroRNA-34a induces
apoptosis in the human glioma cell line, A172, through enhanced ROS
production and NOX2 expression. Biochem Biophys Res Commun.
444:6–12. 2014. View Article : Google Scholar : PubMed/NCBI
|