Introduction
Hepatocellular carcinoma (HCC) is one of the most
common types of cancer, causing ~662,000 cancer-associated
mortalities worldwide per year (1).
According to an epidemiological survey, HCC has a particularly high
incidence in males, and is most common between the ages of 30 and
50 years (2,3). In the majority of cases, the patients
were either infected by viral hepatitis (type B or C) or exposed to
metabolic toxins such as alcohol or aflatoxin (4). At present, the treatment options for
patients with HCC include surgical resection, liver
transplantation, chemoembolization and molecularly targeted therapy
(5). However, the prognosis for
patients with HCC remains poor (5).
Therefore, it is important to investigate and explore the potential
prognostic factors for HCC.
Post-translational modifications of histones,
particularly the methylation of histones H3 and H4, regulate
chromatin structure and function by promoting the assembly of
protein complexes at specific genomic loci (6). The methylation of histone H3 lysine 4
(H3K4) is a highly conserved modification in the epigenetic
regulation of eukaryotic transcription (6). The aberrant methylation of H3K4 by H3K4
methyltransferases (H3K4MTs) has been demonstrated to be associated
with different types of cancer and developmental disorders
(7,8).
In humans, the MLL/SET1 complexes, which are primarily responsible
for H3K4 methylation, are composed of mixed lineage leukemia (MLL)
proteins 1–4 and the SET domain containing 1A/B catalytic subunit.
Additionally, the full enzyme activity of H3K4MTs requires the
interaction of the catalytic subunit with the core regulatory
subunits, including WD repeat domain 5 (WDR5),
retinoblastoma-binding protein-5 (RbBP5), absent small
homeotic-2-like (ASH2L) and dumpy-30 (DPY-30) (9,10).
Although the precise structure of the assembled catalytic subunit
and core regulatory subunits remains unclear, WDR5 is considered to
be the key for the association of RbBP5, ASH2L and DPY-30 with
MLL1, since RbBP5 and ASH2L do not stably interact with MLL1 in the
absence of WDR5 (11). WDR5 is a
highly conserved protein with a molecular weight of ~36 kDa that is
composed of a short, unstructured N-terminus followed by seven WD40
repeats, which can adopt a seven-bladed β-propeller fold (12). Previous studies revealed that WDR5 can
interact with HDAC3 to activate mesenchymal gene expression, and
may promote epithelial-mesenchymal transition by H3K4 methylation
(11,12). Furthermore, it has been demonstrated
that WDR5 is overexpressed in prostate cancer (13), bladder cancer (14), leukemia (15) and breast cancer (16). It has also been demonstrated that the
overexpression of WDR5 may promote cell proliferation (13–16).
Despite the increased understanding of WDR5, its expression pattern
and association with phenotypic parameters in HCC remains
unclear.
An important mechanism of HCC development is the
accumulation of genetic and epigenetic alterations. Therefore, the
aims of the present study were to measure the expression of WDR5 in
HCC tumor and adjacent tissues, and investigate the association
between its expression and clinicopathological prognostic
variables. Furthermore, the association of WDR5 expression with the
overall survival time of patients with HCC was evaluated.
Materials and methods
Patients and specimens
A total of 113 patients diagnosed with HCC and
treated at the People's Hospital of Rizhao (Rizhao, China) between
August 2004 and September 2011 were enrolled in the present study.
The study was approved and monitored by the Research Ethics
Committee of the People's Hospital of Rizhao. Informed written
consent was obtained from all the participating patients, whose
specimens were anonymized according to the ethical and legal
standards of the Research Ethics Committee. All patients had no
history of chemotherapy or radiotherapy prior to surgery. HCC
tumors were surgically removed from the patients, snap-frozen in
liquid nitrogen and stored at −80°C until further usage. Adjacent
noncancerous tissues were obtained from the distal edge of the
resection, ≥2 cm from the tumor, and were also immediately frozen
in liquid nitrogen and stored at −80°C. The tumor stage of each
patient was reviewed and classified according to the 6th edition of
the tumor-node-metastasis (TNM) classification of International
Union Against Cancer (17).
Histological grade was evaluated independently by pathologists
according to the World Health Organization classification system
(18). All patients completed
follow-up for 5 years after surgery. The survival period was
calculated using the date of the curative resection of the tumor as
the first day, and the date of mortality as the final day. The
clinical and pathological variables of the patients were collected
and are presented in Table I.
 | Table I.Associations between WDR5 tumor
expression and clinicopathological parameters. |
Table I.
Associations between WDR5 tumor
expression and clinicopathological parameters.
|
|
| WDR5 expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Cases, n | High | Low | P-value |
|---|
| Sex |
|
|
| NS |
| Male | 62 | 40 | 22 |
|
|
Female | 51 | 39 | 12 |
|
| Age (years) |
|
|
| NS |
| ≥50 | 65 | 44 | 21 |
|
|
<50 | 48 | 35 | 13 |
|
| AFP (ng/ml) |
|
|
| NS |
|
>400 | 52 | 38 | 14 |
|
| ≤400 | 61 | 41 | 20 |
|
| HBsAg |
|
|
| NS |
|
Negative | 54 | 37 | 17 |
|
|
Positive | 59 | 42 | 17 |
|
| Liver cirrhosis |
|
|
| NS |
| No | 45 | 34 | 11 |
|
| Yes | 68 | 45 | 23 |
|
| Histological
grade |
|
|
| 0.038 |
|
Well/moderate | 53 | 32 | 21 |
|
|
Poor | 60 | 47 | 13 |
|
| Tumor size |
|
|
| 0.023 |
| ≥5 | 58 | 35 | 23 |
|
|
<5 | 55 | 44 | 11 |
|
| TNM stage |
|
|
| 0.035 |
|
I–II | 56 | 34 | 22 |
|
|
III–IV | 57 | 45 | 12 |
|
Immunohistochemical (IHC)
analysis
The paraffin-embedded HCC tumor and normal liver
tissues were cut into 5-µm sections. These sections were
deparaffinized in 100% xylene (Beyotime Institute of Biotechnology,
Shanghai, China) and rehydrated using a series of graded alcohols
(Sinopharm Chemical Reagent Co. Ltd., Shanghai, China). Antigen
retrieval was performed by boiling the sections in pH 6.0 citrate
buffer (Beyotime Institute of Biotechnology) for 5 min.
Subsequently, these sections were blocked with 10% goat serum
(OriGene Technologies, Inc, Beijing, China) for 1 h prior to
incubation with the previously described primary antibody against
WDR5 at 4°C for 2 h, followed by a horseradish
peroxidase-conjugated goat anti-rabbit IgG (cat. no., PI-1000;
dilution, 1:200; Vector Laboratories, Burlingame, CA, USA) at room
temperature for 2 h. The reaction was visualized using
diaminobenzidine substrate chromogen solution (Gene Technology
Biotechnology Co., Ltd., Shanghai, China). These sections were then
counterstained with hematoxylin, and examined under an optical
microscope. The scoring method was performed as previously
described (19).
Cell culture
The SNU-449 and MHCC97H human HCC cell lines were
purchased from American Type Culture Collection (Manassas, VA, USA)
and cultured in RPMI-1640 medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.). The HL-7702 normal
liver cell line obtained from Cell Bank of Chinese Academy of
Sciences (Shanghai, China) was cultured in Dulbecco's modified
Eagle's medium (Thermo Fisher Scientific, Inc.) supplemented with
10% FBS. All cell lines were cultured at 37°C in a 5%
CO2 atmosphere.
Stable WDR5 knockdown in HCC
cells
The short hairpin RNA (shRNA) targeting WDR5 and a
non-targeting shRNA were designed and synthesized according to the
study by Dai et al (16). The
shRNA sequences were as follows: shRNA against WDR5,
5′-GCTCAGAGGATAACCTTGTTT-3′; non-targeting shRNA,
5′-CCTAAGGTTAAGTCGCCCTCG-3′. These shRNAs were cloned into the
pSuper.retro.puro vector (Oligoengine, Seattle, WA, USA) and the
generation of retrovirus and infection were conducted according to
the manufacture's protocol. Stably transfected cell lines were
established by selection with G418 (600 µg/ml). The knockdown of
WDR5 expression in HCC cell lines was confirmed by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analysis. The cells were harvested for cell
proliferation and migration assays.
Cell proliferation assay
The cell proliferation rate was analyzed using an
MTT assay. The wild-type and transfected cell lines were seeded in
96-well plates at a density of ~3×103 cells/well. MTT
solution (20 µl of 5 mg/ml MTT, Invitrogen; Thermo Fisher
Scientific, Inc.) was added to each well, and the plates were
incubated for 4 h at 37°C. Subsequently, 150 µl dimethyl sulfoxide
was added to each well and incubated for 10 min at 37°C to dissolve
the formazan crystals. The optical absorbance was measured at 490
nm using a microplate reader (Thermo Fisher Scientific, Inc,) at
the time points of 12, 24, 48 and 72 h. Assays were repeated >3
times.
RT-qPCR
Total RNA was prepared from the 113 tumor tissues,
113 adjacent non-cancerous tissues and the cell lines using an
RNAiso Plus kit (Takara Bio, Inc., Otsu, Japan) according to the
manufacturer's protocol. The cDNA synthesis was conducted using the
PrimerScript RT Master mix (Takara Bio, Inc.) using the ABI ProFlex
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
RT-qPCR was performed with qSTAR SYBR Master mix (OriGene
Technologies, Inc., Rockville, MD, USA) and a StepOne Plus
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). Each experiment was performed in triplicate. The relative
expression of WDR5 was calculated using the 2−∆∆Cq
method (20). The thermocycling
conditions were as follows: Initial denaturation at 95°C for 10
min, followed by 40 cycles of denaturation at 95°C for 1 min and
annealing/extension at 56°C for 1 min. The transcription level of
GAPDH was used as an internal control. The primers were designed
using Primer 3.0 software and are listed as follows: WDR5, forward,
5′-AATATCCGATGTAGCCTGGTC-3′, and reverse,
5′-TTGGACTGGGGATTGAAGTTG-3′; GAPDH forward,
5′-CAAGGCTGAGAACGGGAAG-3′, and reverse,
5′-TGAAGACGCCAGTGGACTC-3′.
Western blot analysis
The total protein was extracted from the collected
cell lines, and the tumor and corresponding noncancerous tissue
samples using the Whole Protein Extraction kit (Fermentas; Thermo
Fisher Scientific, Inc., Pittsburgh, PA, USA) following the
manufacturer's protocol. The protein concentration was quantified
using the Bradford method. Protein samples (20 µg) were mixed with
6X SDS sample buffer containing 10% β-mercaptoethanol and separated
by 10% SDS-PAGE and then transferred onto polyvinylidene fluoride
membranes (GE Healthcare Life Sciences, Chicago, IL, USA). The
membranes were incubated in 5% fat-free milk for 1 h at room
temperature prior to the addition of antibodies. Primary antibodies
against WDR5 (cat. no., ab178410; dilution, 1:1,000; Abcam,
Cambridge, MA, USA) and GAPDH (cat. no., 2118; dilution, 1:1,000;
Cell Signaling Technology, Inc., Danvers, MA, USA) were incubated
with the membrane for 1 h at room temperature. Subsequent to
washing with PBS with 0.5% Tween, the membranes were incubated with
a horseradish peroxidase-conjugated goat anti-rabbit antibody (cat.
no., 7074; dilution, 1:1,000; Cell Signaling Technology, Inc.) at
room temperature for 1 h. Bands were visualized with an Luminata™
Western HRP Substrates (EMD Millipore, Billerica, MA, USA).
Densitometry was performed using Image J software (version 1.37;
National Institutes of Health, Bethesda, MA, USA). GAPDH protein
level was used as a loading control.
Statistical analysis
SPSS version 20.0 software (IBM Corporation, Armonk,
NY, USA) was used to conduct all statistical analyses. The
χ2 test was used to analyze the association between WDR5
expression and clinicopathological variables. The survival rate was
calculated using the Kaplan-Meier method, and analyzed with the
log-rank test. The univariate and multivariate Cox proportional
hazards regression model analyses were conducted to identify the
factors that were significant for the prognosis of HCC patients.
Student's t test was used to analyze the statistical difference
between two groups. One-way analysis of variance with a Tukey
post-hoc test was used to analyze the statistical difference among
multiple groups. Results are expressed as the mean ± standard error
of the mean. P<0.05 was considered to indicate a statistically
significant difference.
Results
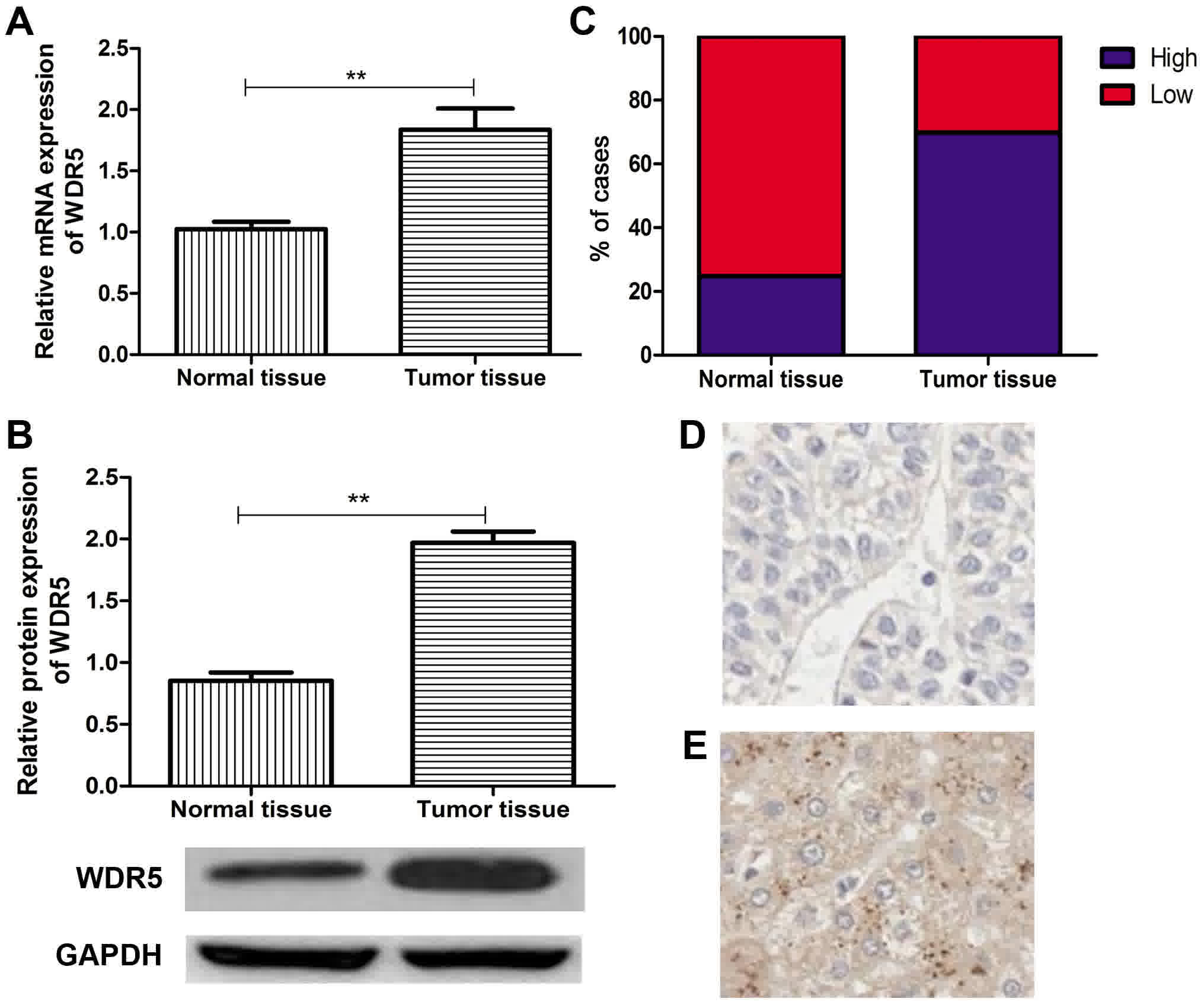
WDR5 is overexpressed in HCC
To investigate whether WDR5 is associated with the
development and progression of HCC, WDR5 mRNA and protein
expression levels were measured in 113 HCC tissues and 113 matched
normal adjacent tissues by RT-qPCR and western blot analyses.
Compared with the normal adjacent liver tissues, the HCC tissues
exhibited a significant increase in WDR5 expression (P<0.05;
Fig. 1A and B). The majority of the
HCC cancer tissues (69.9%, 79/113 cases) exhibited high WDR5
expression, whereas the majority of the normal tissues (75.2%,
85/113 cases) exhibited low WDR5 expression (Fig. 1C). These results demonstrated that
WDR5 was upregulated in HCC tissues.
Furthermore, the WDR5 expression in HCC tissues and
matched normal adjacent tissues was measured by IHC. The level of
WDR5 expression in HCC tissues was markedly higher than in the
normal adjacent liver tissues (Fig. 1D
and E). Based on the WDR5 expression analysis results obtained
from RT-qPCR, western blot analysis and IHC, the patients were
divided into two groups: Low- and high-WDR5 expression groups
(cut-off value: 1.39).
Association between WDR5 expression
and clinicopathological variables
The association between WDR5 expression and the
clinicopathological features of 113 patients with HCC was studied
(Table I). High WDR5 expression in
HCC was significantly associated with histological grade (P=0.038),
tumor size (P=0.023) and TNM stage (P=0.035), but not with sex,
age, α-fetoprotein (AFP) level, hepatitis B surface antigen (HBsAg)
or liver cirrhosis (all P>0.05). These findings suggested that
WDR5 overexpression may contribute to HCC progression.
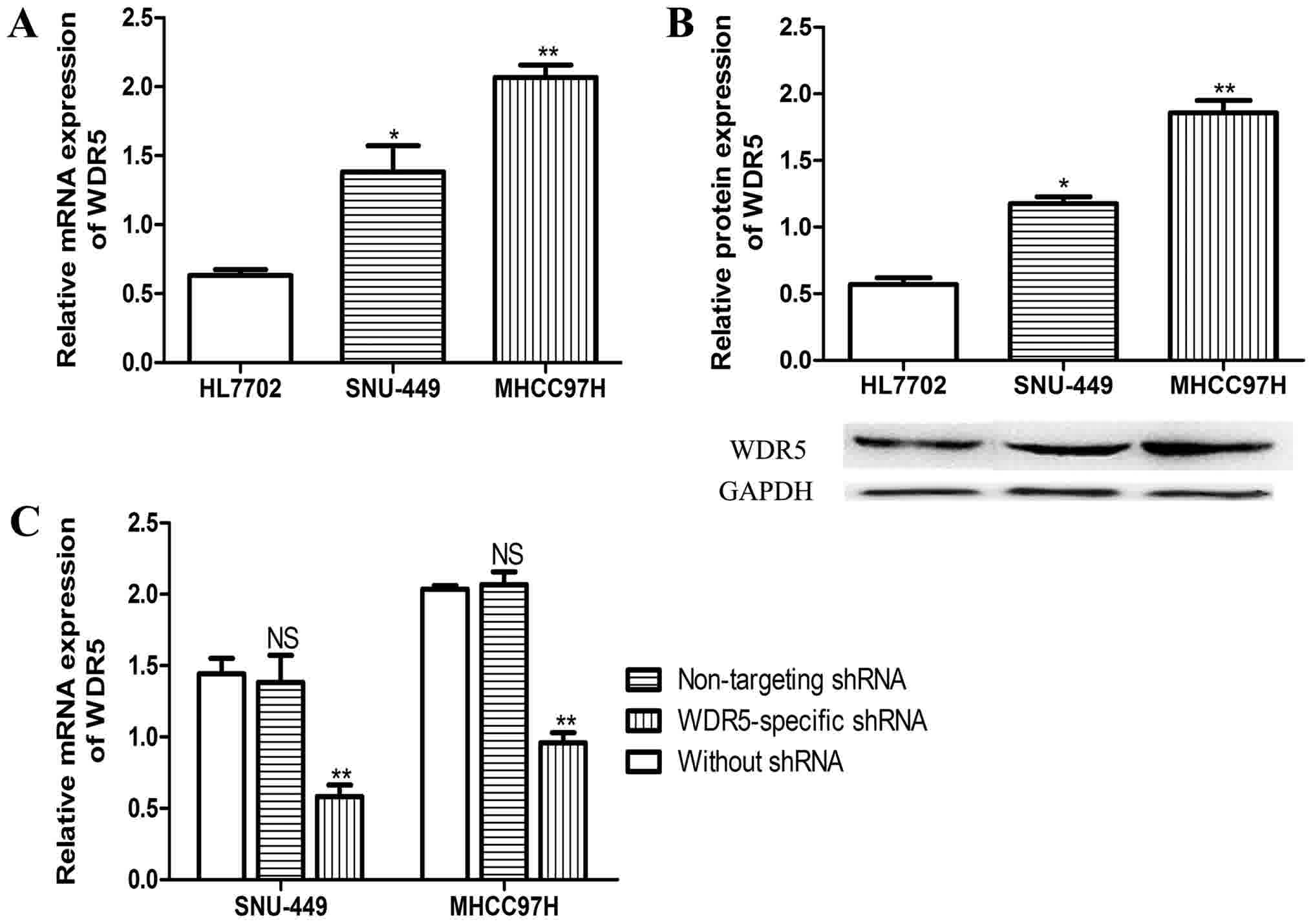
WDR5 overexpression promotes cell
proliferation
In order to examine the role of WDR5 in HCC, the
expression of WDR5 in HCC cell lines and normal liver cell lines
was examined. As presented in Fig. 2A and
B, the mRNA and protein expression levels of WDR5 were markedly
higher in HCC cells than in normal liver cells. WDR5-knockdown HCC
cell lines were created with WDR5-shRNA. To verify the efficacy of
WDR5-specific shRNA, a non-targeting shRNA was transfected into the
HCC cell lines, and the mRNA expression level was evaluated with
RT-qPCR. As displayed in Fig. 2C, it
was demonstrated that the WDR5-shRNA effectively downregulated the
expression of WDR5 in the HCC cell lines in vitro, whereas
the non-targeting shRNA had no significant effect on WDR5 compared
with untransfected cells.
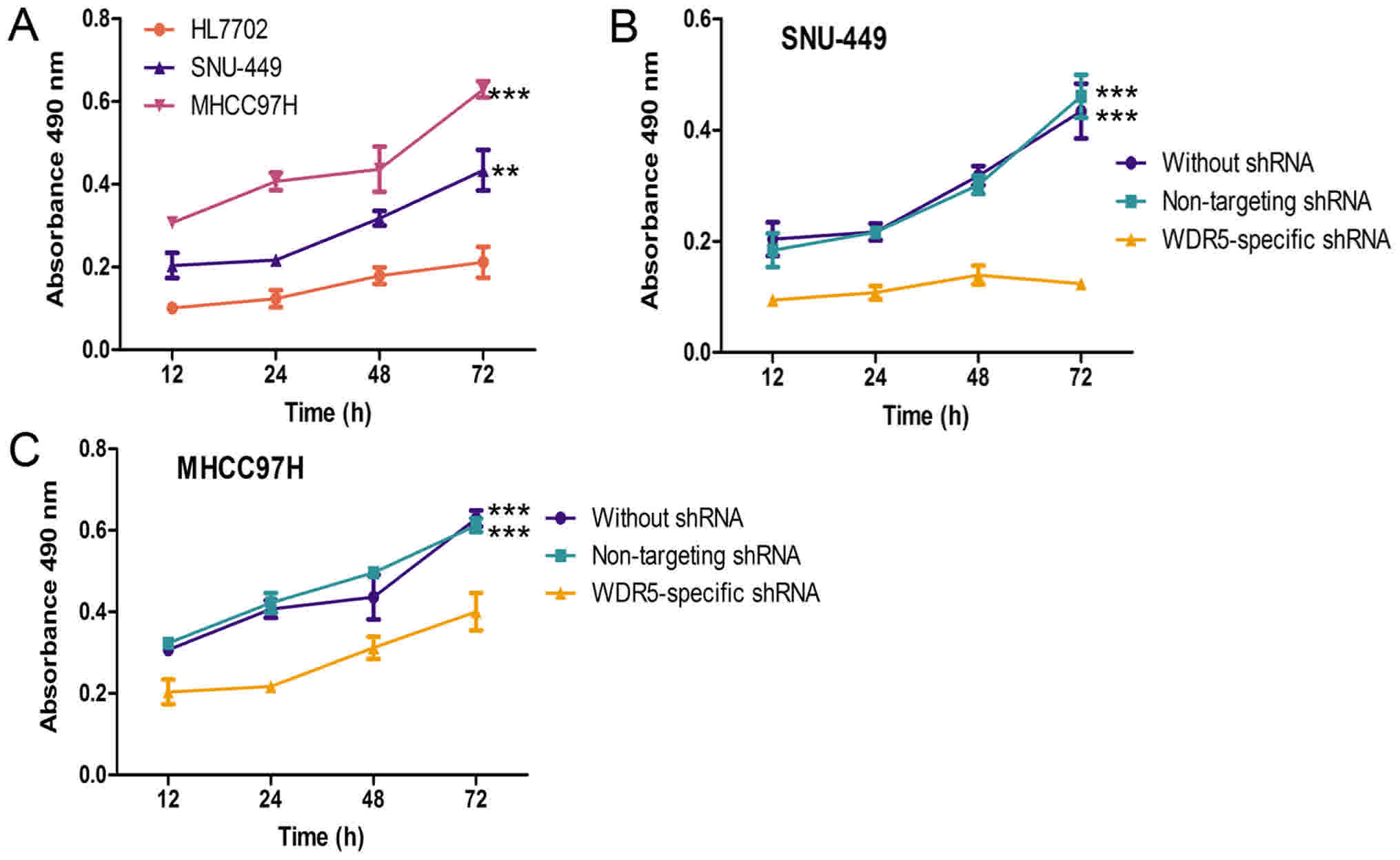
Furthermore, the effect of WDR5 overexpression on
HCC cell lines was analyzed with an MTT assay. As shown in Fig. 3A, the proliferation rates of HCC cell
lines were evidently higher than the normal liver cell line. The
HCC cells transfected with non-targeting shRNA and untransfected
HCC cells had similar proliferation rates, as shown in Fig. 3B-D. Conversely, the HCC cell lines
transfected with WDR5-specific shRNA exhibited a significantly
lower proliferation rate than untransfected HCC cells or HCC cell
lines transfected with non-targeting shRNA. Taken together, these
results suggest that the expression of WDR5 was enhanced in HCC
cell lines, and that WDR5 expression may promote the proliferation
of HCC cells in vitro.
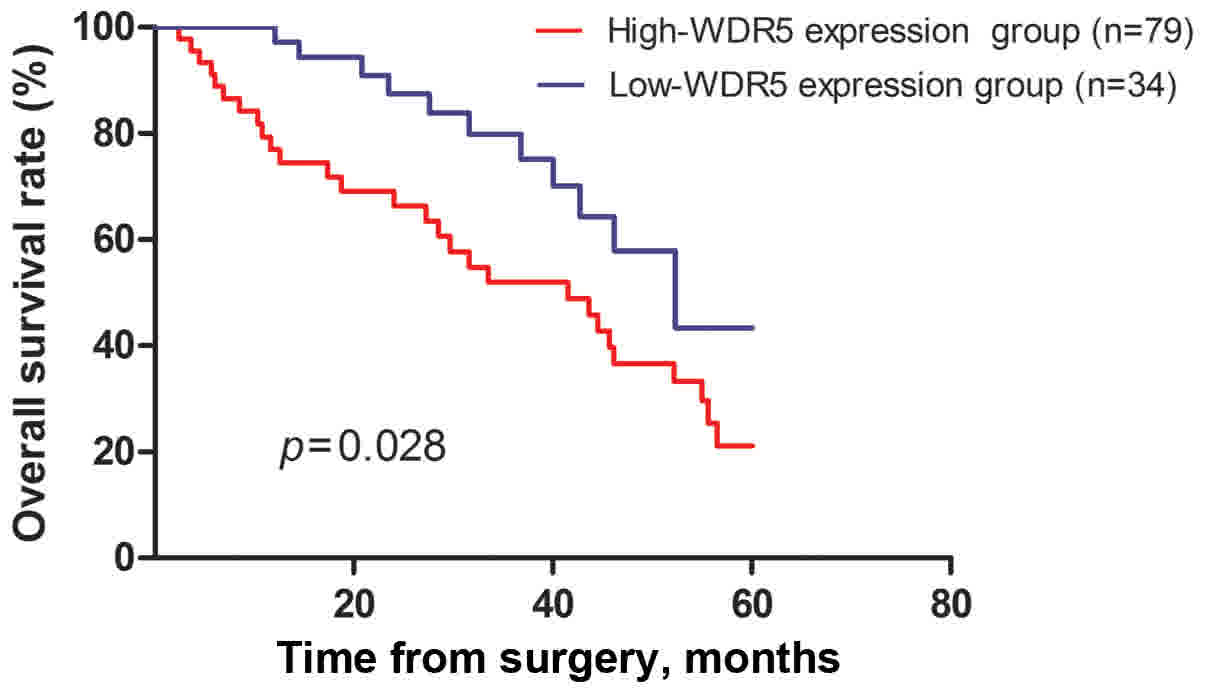
Prognostic value of high WDR5
expression in HCC
Overall survival rates were analyzed to evaluate the
prognostic role of WDR5 expression using Kaplan-Meier survival
curves. The results demonstrated that the 5-year overall survival
rate of patients in the high WDR5 expression group had a
significantly lower survival expectation time than those with low
WDR5 expression (P=0.028, Fig. 4). In
addition, to assess a potential role of WDR5 expression in
predicting the postoperative prognosis for patients with HCC,
univariate and multivariate analysis was conducted using the Cox
proportional hazards model. The results of these analyses are
summarized in Table II. The
univariate analyses demonstrated that WDR5 expression, histological
grade, tumor size and TNM stage were significantly associated with
the outcome of HCC. Multivariate analysis indicated that WDR5
expression was an independent prognostic factor, alongside
histological grade, tumor size and TNM stage.
 | Table II.Univariate and multivariate analyses
of factors associated with overall survival. |
Table II.
Univariate and multivariate analyses
of factors associated with overall survival.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Variables | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| WDR5 (high vs.
low) | 2.151 | 1.141–4.058 | 0.018 | 2.239 | 1.167–4.296 | 0.015 |
| Sex (male vs.
female) | 1.663 | 0.795–3.479 | 0.177 | – | – | – |
| Age (≥50 vs.
<50) | 1.755 | 0.850–3.625 | 0.129 | – | – | – |
| AFP (ng/ml; ≥400
vs. <400) | 1.948 | 0.977–3.887 | 0.058 | – | – | – |
| HBsAg (negative vs.
positive) | 1.846 | 0.904–3.766 | 0.092 | – | – | – |
| Liver cirrhosis (no
vs. yes) | 1.882 | 0.933–3.795 | 0.077 | – | – | – |
| Histological grade
(well/moderate vs. poor) | 1.921 | 1.014–3.640 | 0.045 | 1.946 | 1.032–3.671 | 0.040 |
| Tumor size (≥5 vs.
<5) | 2.066 | 1.081–3.948 | 0.028 | 2.081 | 1.094–3.959 | 0.026 |
| TNM stage (I–II vs.
III–IV) | 2.053 | 1.070–3.942 | 0.031 | 2.007 | 1.046–3.851 | 0.036 |
Discussion
The importance of chromatin modification in
carcinogenesis has become increasingly evident in recent years
(6). Histone modification represents
a large proportion of chromatin modifications (6); therefore, the proteins responsible for
histone modifications, including methylation, acetylation,
phosphorylation, ubiquitylation and ADP ribosylation, have
attracted significant attention. Previous studies have demonstrated
that the high expression of trimethylated histone H3K4 is
associated with the prognosis in HCC (21), and that its expression can be
regulated by enhancer of zeste homolog 2 (EZH2) (22) or WDR5 (14). EZH2 has been identified as an oncogene
in bladder (23), breast (24) and prostate cancer (23). WDR5, the core subunit of the MLL/SET1
complex, is reported as the essential component for complex
assembly and methyltransferase activity. WDR5 is also reported to
perform a critical role in embryonic stem cell self-renewal and
epithelial-mesenchymal transition (7). Furthermore, WDR5 was reported to be
overexpressed in prostate (13),
bladder (14) and breast cancer
(16), and leukemia (15), and was identified as a prognostic
indicator in these types of cancer. Additionally, the knockdown of
WDR5 expression may result in cell proliferation arrest. However,
the WDR5 expression pattern and clinical significance in HCC are
incompletely characterized. Therefore, in the present study, the
focus was to investigate the WDR5 expression in HCC tumors, and the
association between WDR5 expression and clinicopathological
characteristics.
In the present study, the results of RT-qPCR and
western blot analysis demonstrated that WDR5 mRNA and protein
expression levels were significantly upregulated in HCC tissue
compared with the adjacent normal liver tissues. In order to
confirm this, IHC analysis was performed to measure the location
and expression of WDR5 in HCC tissue, which demonstrated that WDR5
expression was indeed upregulated in HCC tissue compared with
normal adjacent tissue. These findings indicated that WDR5 may
serve an important role in HCC.
WDR5 silencing has been reported to reduce the
proliferation breast (16) and
prostate cancer cells (13).
Therefore, to investigate the role of WDR5 expression in the
progression of HCC, shRNA-based knockdown was performed to
downregulate the expression of WDR5 in HCC cell lines in
vitro. These results demonstrated that the HCC cell lines
transfected with WDR5-specific shRNA exhibited lower WDR5
expression compared with the wild-type HCC cell lines, which proved
the efficacy of WDR5-specific shRNA. An MTT assay indicated that
the proliferation rate of HCC cell lines transfected with
WDR5-specific shRNA was significantly lower than the wild-type and
non-targeting shRNA-transfected HCC cell lines. These findings
suggest that WDR5 overexpression promoted cell proliferation.
In addition, the association of WDR5 expression with
clinicopathological features and prognosis was investigated. WDR5
expression was associated with tumor size, tumor stage and
histological grade based on statistical analysis, indicating the
importance of WDR5 in tumor cell growth. The patients were divided
into two groups based on the median expression level of WDR5 to
determine whether WDR5 expression had prognostic value. The
association between WDR5 expression and survival time was assessed
by using a Kaplan-Meier curve. Log-rank test analysis demonstrated
that the patients with high WDR5 expression had a significantly
shorter overall survival time than those with low WDR5 expression.
The effects of WDR5 expression and other clinicopathological
features on the prognosis of HCC were analyzed using univariate and
multivariate analyses. The results indicated that WDR5 expression,
histological grade, tumor size and TNM stage were significant
predictors for a relatively poor outcome. Conversely, sex, age,
serum AFP level, HBsAg, and liver cirrhosis did not significantly
affect the outcome of HCC. Notably, multivariate analysis indicated
that, in addition to the histological grade, tumor size and TNM
stage, WDR5 expression was also an independent predictor for the
overall survival of patients with HCC.
In conclusion, WDR5 overexpression is frequently
observed in HCC tissues. Furthermore, it was demonstrated WDR5
expression promoted cell proliferation. However, the upstream and
downstream genes require further study to be identified. In
addition, the present study provided clinical evidence that WDR5
tumor expression is positively associated with the poor overall
survival of patients with HCC, and may also serve as an independent
prognostic factor to determine the outcome of HCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZJC and HBL participated in experimental design,
interpreted the results and wrote the manuscript; FL, CLM, YHM, XGZ
and JDL performed experiments and analyzed the results; FL, CLM and
XGZ coordinated the experimental work, interpreted the results and
contributed to the critical revision; ZJC and HBL designed the
research plan, interpreted the results and wrote the
manuscript.
Ethics approval and consent to
participate
The study was approved and monitored by the Research
Ethics Committee of the People's Hospital of Rizhao (Rizhao,
China). Informed written consent was obtained from all the
participating patients.
Consent for publication
The study participants provided consent for the
publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alejandro F, Josep ML and Jordi B:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen WQ, Zheng RS, Baade PD, Zhang SW,
Zeng HM, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in
China, 2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ogunwobi OO and Liu C: Therapeutic and
prognostic importance of epithelial-mesenchymal transition in liver
cancers: Insights from experimental models. Crit Rev Oncol Hematol.
83:319–328. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Inokawa Y, Nomoto S, Hishida M, Hayashi M,
Kanda M, Nishikawa Y, Takeda S, Sugimoto H, Fujii T, Yamada S,
Hayashi M, Kanda M, Nishikawa Y, Takeda S, Sugimoto H, Fujii T,
Yamada S, Kodera Y, et al: Detection of doublecortin
domain-containing 2 (DCDC2), a new candidate tumor suppressor gene
of hepatocellular carcinoma by triple combination array analysis. J
Exp Clin Cancer Res. 32:652013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bannister AJ and Kouzarides T: Regulation
of chromatin by histone modifications. Cell Res. 21:381–395. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ang YS, Tsai SY, Lee DF, Monk J, Su J,
Ratnakumar K, Ding J, Ge Y, Darr H, Chang B, et al: Wdr5 mediates
self-renewal and reprogramming via the embryonic stem cell core
transcriptional network. Cell. 145:183–197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wysocka J, Swigut T, Milne TA, Dou Y,
Zhang X, Burlingame AL, Roeder RG, Brivanlou AH and Allis CD: WDR5
associates with histone H3 methylated at K4 and is essential for H3
K4 methylation and vertebrate development. Cell. 121:859–872. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Trievel RC and Shilatifard A: WDR5, a
complexed protein. Nat Struct Mol Biol. 16:678–680. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dou Y, Milne TA, Ruthenburg AJ, Lee S, Lee
JW, Verdine GL, Allis CD and Roeder RG: Regulation of MLL1 H3K4
methyltransferase activity by its core components. Nat Struct Mol
Biol. 13:713–719. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shinsky SA, Hu M, Vought VE, Ng SB,
Bamshad MJ, Shendure J and Cosgrove MS: A non-active-site SET
domain surface crucial for the interaction of MLL1 and the
RbBP5/Ash2L heterodimer within MLL family core complexes. J Mol
Biol. 426:2283–2299. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schuetz A, Allali-Hassani A, Martín F,
Loppnau P, Vedadi M, Bochkarev A, Plotnikov AN, Arrowsmith CH and
Min J: Structural basis for molecular recognition and presentation
of histone H3 by WDR5. EMBO J. 25:4245–4252. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JY, Banerjee T, Vinckevicius A, Luo Q,
Parker JB, Baker MR, Radhakrishnan I, Wei JJ, Barish GD and
Chakravarti D: A role for WDR5 in integrating threonine 11
phosphorylation to lysine 4 methylation on histone H3 during
androgen signaling and in prostate cancer. Mol Cell. 54:613–625.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen X, Xie WB, Gu P, Cai QQ, Wang B, Xie
Y, Dong W, He W, Zhong G, Lin T and Huang J: UpregulatedWDR5
promotes proliferation, self-renewal and chemoresistance in bladder
cancer via mediating H3K4 trimethylation. Sci Rep. 5:82932015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ge Z, Song EJ, Kawasawa YI, Li J, Dovat S
and Song C: WDR5 high expression and its effect on tumorigenesis in
leukemia. Oncotarget. 7:37740–37754. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dai X, Guo W, Zhan C, Liu X, Bai Z and
Yang Y: WDR5 expression is prognostic of breast cancer outcome.
PLoS One. 10:e01249642015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wittekind C, Compton CC, Greene FL and
Sobin LH: TNM residual tumor classifcation revisited. Cancer.
94:2511–2516. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou L, Rui JA, Ye DX, Wang SB, Chen SG
and Qu Q: Edmondson-steiner grading increases the predictive
efficiency of TNM staging for long-term survival of patients with
hepatocellular carcinoma after curative resection. World J Surg.
32:1748–1756. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen Z, Yang P, Li W, He F, Wei J, Zhang
T, Zhong J, Chen H and Cao J: Expression of EZH2 is associated with
poor outcome in colorectal cancer. Oncol Lett. 15:2953–2961.
2018.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
He C, Xu J, Zhang J, Xie D, Ye H, Xiao Z,
Cai M, Xu K, Zeng Y, Li H and Wang J: High expression of
trimethylated histone H3 lysine 4 is associated with poor prognosis
in hepatocellular carcinoma. Hum Pathol. 43:1425–1435. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Margueron R and Reinberg D: The Polycomb
complex PRC2 and its mark in life. Nature. 469:343–349. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weikert S, Christoph F, Kollermann J,
Müller M, Schrader M, Miller K and Krause H: Expression levels of
the EZH2 polycomb transcriptional repressor correlate with
aggressiveness and invasive potential of bladder carcinomas. Int J
Mol Med. 16:349–353. 2005.PubMed/NCBI
|
|
24
|
Bachmann IM, Halvorsen OJ, Collett K,
Stefansson IM, Straume O, Haukaas SA, Salvesen HB, Otte AP and
Akslen LA: EZH2 expression is associated with high proliferation
rate and aggressive tumor subgroups in cutaneous melanoma and
cancers of the endometrium, prostate, and breast. J Clin Oncol.
24:268–273. 2006. View Article : Google Scholar : PubMed/NCBI
|