Introduction
Apoptosis serves an important function in the normal
development and stability of organisms (1). Apoptosis imbalance is associated with
numerous diseases, including developmental defects, autoimmune
diseases, neurodegenerative disorders and, in particular, tumor
occurrence, development and metastasis (2). Research on inducing tumor cell apoptosis
has become a field of interest in cell biology and the biomedical
sciences. The aim of current research on tumor cell apoptosis is to
develop safer and more effective therapeutic agents for treating
tumors. Isoimperatorin (ISOIM) belongs to the 6,7-furan coumarin
family of compounds and is the major effective component in the
umbelliferae family, which includes Angelica dahurica,
Heracleum, coastal glehnia root, Chinese angelica and
Peucedanum ostruthium, and is commonly used in traditional
Chinese medicine (3). ISOIM is a
secondary plant metabolite that possesses multiple pharmacological
properties, including analgesic, antiviral, antitumor,
anti-inflammatory, antibacterial and anti-hypertensive properties
(4–8).
ISOIM may inhibit numerous types of human tumor cell from
proliferating, including lung cancer A549, ovarian cancer SK-OV-3,
skin cancer SK-MEL-2, glioblastoma XF498, HCT-15 colon cancer and
MCF-7 breast cancer cells (4–9). A previous study reported that ISOIM may
inhibit SGC-7901 cells from proliferating and alter the expression
levels of pro-apoptotic and anti-apoptotic proteins (10). However, the present study has certain
limitations, including assessing too few cell lines, not observing
cell morphology or detecting the cell cycle.
Therefore, the present study used the stomach cancer
BGC-823 cell line as an in vitro model to confirm the
effects of ISOIM, assess changes in apoptosis-associated proteins
in the B-cell lymphoma 2 (Bcl-2) and caspase-3 families in
ISOIM-treated cells and to determine the molecular mechanism of
ISOIM-induced BGC-823 cell apoptosis.
Materials and methods
Reagents
ISOIM was obtained from Shanghai Aladdin Bio-Chem
Technology Co., Ltd. (Shanghai, China), maintained in 100 mM stock
solutions in ethanol and stored at −20°C. The stock solutions were
colorless to inhibit them from influencing the results of MTT, flow
cytometry (FCM) and acridine orange (AO)/ethidium bromide (EB)
staining (3). MTT, bisbenzimide
(Hoechst 33258), AO, EB and propidium iodide (PI) were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). RPMI-1640
medium and 100% fetal bovine serum were purchased from Gibco;
Thermo Fisher Scientific, Inc. (Waltham, MA, USA). Mouse monoclonal
antibodies against human caspase-3 (cat. no. sc7272; 1:200),
Bcl-2-associated X (cat. no. sc-4239; 1:200), Bcl-2 (cat. no.
sc509; 1:200), cytochrome c (cat. no. sc13561; 1:200),
cyclin D1 (cat. no. sc4074; 1:500), cyclin dependent kinase 1 (cat.
no. sc-53219; 1:500), cyclin B1 (cat. no. sc-4073; 1:300) and p21
(cat. no. sc-6246; 1:500) were obtained from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). Furthermore, horseradish
peroxidase conjugated rabbit anti-Mouse IgG antibody (A9044, Sigma)
were also used at room temperature for 1 h and detected using an
enhanced chemiluminescence system (Pierce; Thermo Fisher
Scientific, Inc.). All other reagents and solvents used were of
analytical grade.
Cell culture and induction of
ISOIM
BGC823, HGC-27 and MGC-803 human gastric cancer
cells were obtained from the Shanghai Institute of Biochemistry and
Cell Biology (Shanghai, China). The BGC823, HGC-27 and MGC-803
cells were cultured in RPMI-1640 medium with 10% fetal bovine serum
(Gibco; Thermo Fisher Scientific, Inc.) and 5% CO2 at
37°C for 48 h. Cells were treated with RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) containing various concentrations
(0.025, 0.05, 0.10, 0.15 and 0.2 mM) of ISOIM 24 h after
seeding.
MTT assay
BGC-823 human gastric cancer cells were seeded on a
96-well plate (1×105/ml). Following incubation for 24 h,
cells were treated with multiple concentrations of ISOIM (0.025,
0.05, 0.10, 0.15 and 0.2 mM) for 48 h. Subsequently, the medium was
discarded and 20 µl MTT (5 mg/ml) was added to each well. Cells
were incubated for 4 h at 37°C, after which the medium was replaced
with 150 µl dimethyl sulfoxide. The optical density was measured
using a microplate reader (Enspire; PerkinElmer, Inc., Waltham, MA,
USA) at 490 nm.
Hematoxylin and eosin (H&E)
staining of BGC-823
H&E staining was performed as previously
described (8); the treatment group
cells were treated with 0.1 mM ISOIM for 48 h.
Hoechst 33258 and AO/EB staining of
BGC-823
After fixing with 100% methanol for 5 min, cells
were washed with PBS twice. BGC-823 cells seeded onto coverslips
(105/ml) were stained with Hoechst 33258 for 10 min at
room temperature and observed using a fluorescence microscope
(magnification, ×400). The treatment group cells were treated with
0.1 mM ISOIM for 48 h. For AO/EB staining, after washing with PBS
three times, the control and treated groups were stained with AO/EB
staining solution (10 µg/ml) at room temperature for 3 min and
observed using a fluorescence microscope (magnification, ×200).
FCM analysis the cell cycle of
BGC-823
FCM assays were performed as previously described
(11). Treatment group cells were
treated with 0.05, 0.10 or 0.15 mM ISOIM for 48 h.
FCM analysis for the cell apoptosis
rate
BGC-823 cells were incubated in Annexin
V-fluorescein isothiocyanate (Beyotime Institute of Biotechnology,
Haimen, China) in darkness for 10 min at room temperature.
Following centrifugation (800 × g for 5 min at 4°C) and cell
resuspension in Annexin V-FITC binding buffer (Beyotime Institute
of Biotechnology), cells were stained with 10% PI staining solution
(Beyotime Institute of Biotechnology) at room temperature.
Following filtration with a 200-mesh sieve, cells were detected
using flow cytometry. Treatment group cells were treated with 0.1
mM ISOIM for 48 h.
Western blot analysis
Western blot assays were performed as previously
described (12). Treatment group
cells were treated with 0.05, 0.1 and 0.15 mM ISOIM for 48 h at
37°C.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 19.0; SPSS, Inc., Chicago, IL, USA), including
the calculation of half-maximal inhibitory concentration
(IC50). Data were represented as mean ± standard
deviation from at least 3 independent experiments. Student's t-test
or one-way analysis of variance followed by by Bonferroni's test
was used for comparison of 2 or >2 datasets, respectively.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
Anti-proliferative effects of ISOIM on
BGC-823 cells
Since mitochondrial succinate dehydrogenase in
living cells may reduce MTT to a bluish-purple, water-insoluble
crystal, MTT is used to detect the number of viable cells and the
proliferation of cells. In the present study, multiple gastric
cancer cell lines were assessed, including BGC823
(IC50=0.115 mM), HGC-27 (IC50=0.120 mM) and
MGC-803 (IC50=0.146 mM). The BGC-823 cell line was the
most sensitive to ISOIM of these cell lines and was therefore
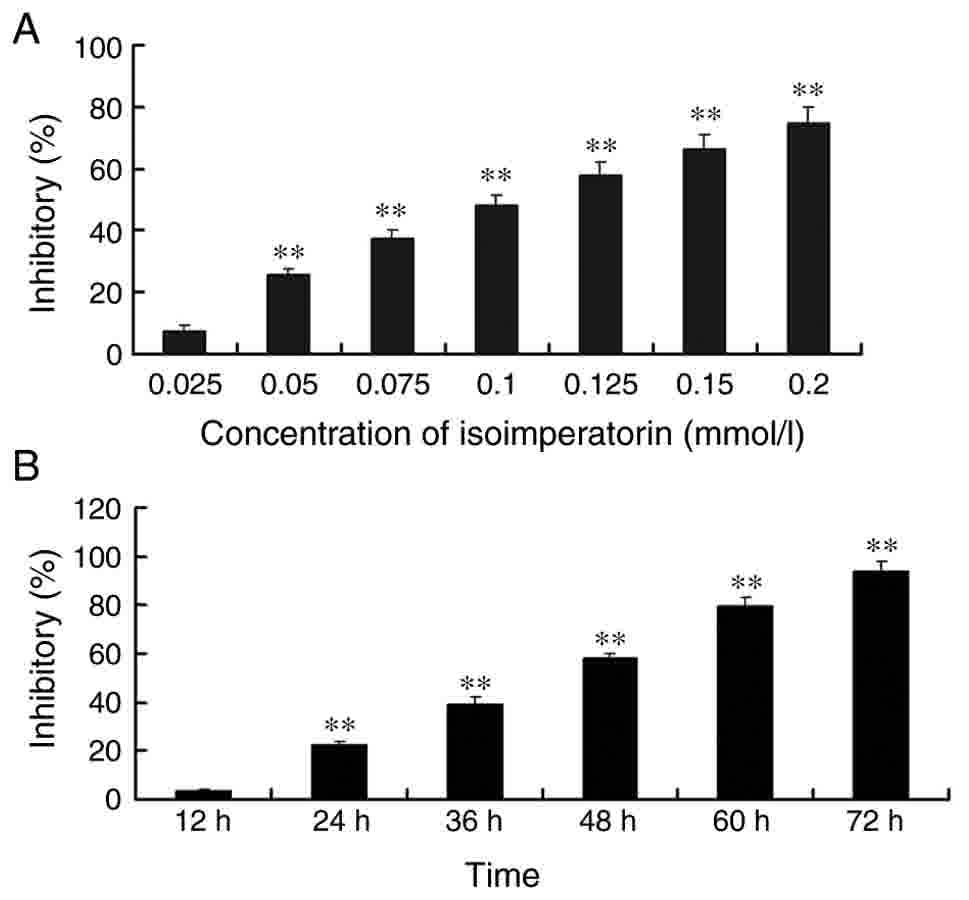
chosen for further study. ISOIM inhibited BGC-823 cell
proliferation in a dose- and time-dependent manner (Fig. 1). The cytotoxic effect was increased
in BGC-823 cells following increasing the concentrations of ISOIM
(0.025, 0.05, 0.10, 0.15 and 0.2 mM). The inhibition rate of cells
treated with ISOIM for 48 h ranged between 7.69 and 74.92%.
Cell shape changes in BGC-823 cells
induced by ISOIM
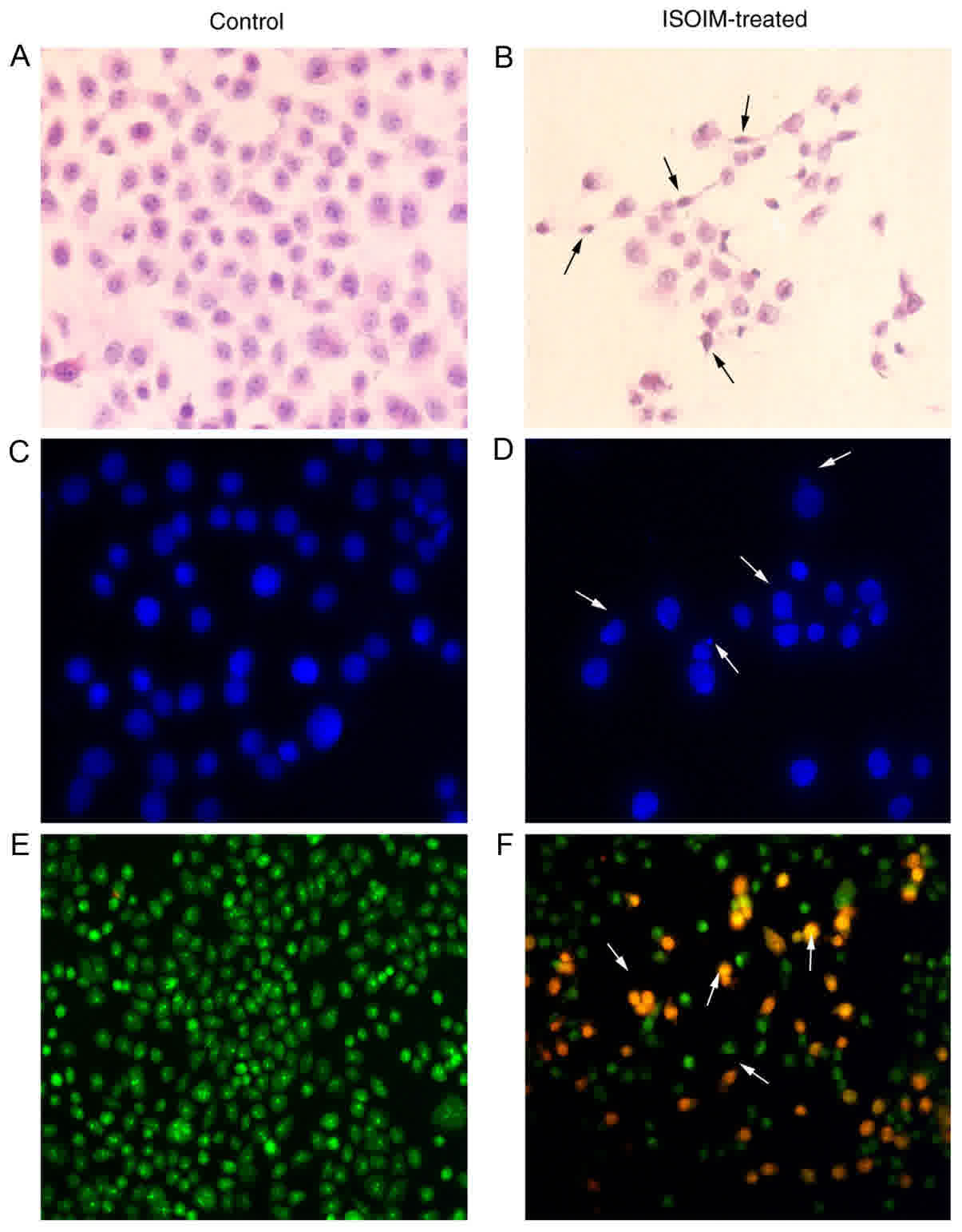
Microscopic observation (Fig. 2) revealed that BGC-823 cells exhibited
morphological changes in apoptotic cells following ISOIM treatment,
including decreased size, cell membrane shrinkage, nuclear
pyknosis, decreased numbers of nucleoli, highly condensed nuclear
chromatin, budding and foaming from the cell membrane (Fig. 2B, D and F).
Following Hoechst 33258 staining, an uneven
distribution, condensation and karyorrhexis of nuclear fluorescence
appeared in BGC-823 cells treated with ISOIM (Fig. 2D). Following AO/EB staining, nuclear
chromatin turned green; pyknotic shaped or round beads represented
early apoptotic cells. Nuclear chromatin turned orange; pyknotic
shaped or round beads represented late apoptotic cells. Numerous
apoptotic cells were observed in BGC-823 cells treated with ISOIM
(Fig. 2F). Control group cells were
shown in Fig. 2A, C and E.
ISOIM-induced cell cycle changes in
BGC-823 cells
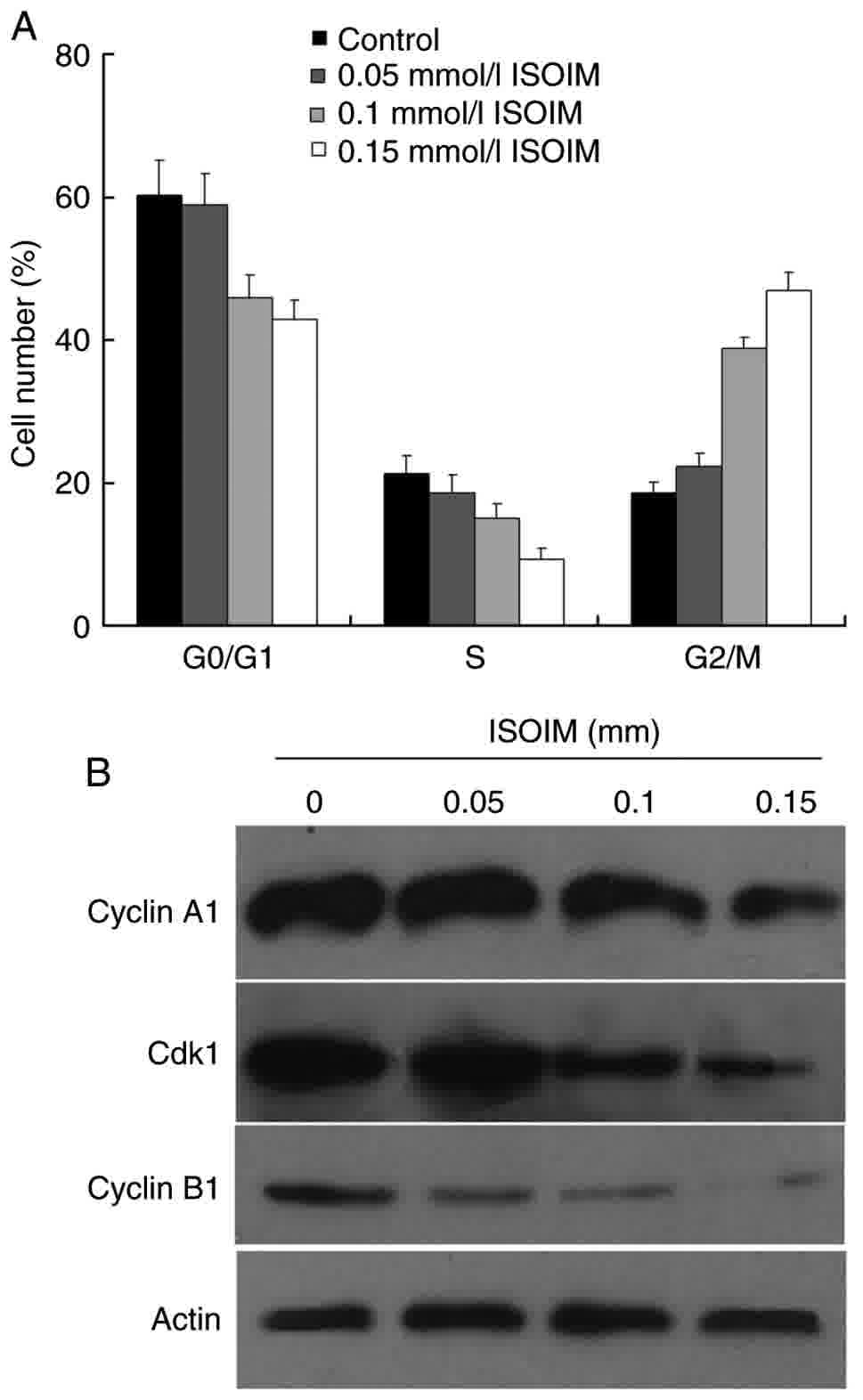
To assess how ISOIM induced apoptosis in BGC-823
cells, FCM analysis was used to evaluate changes to the cell cycle
distribution in BGC-823 cells induced by ISOIM. The results
revealed that the number of ISOIM-treated cells in the
G2/M phase was increased compared with that of the
control group (from 18.49–46.96%; Fig.
3A). The number of cells in the S and
G0/G1 phases decreased following treatment
with ISOIM. The results indicated that ISOIM might be associated
with induction of G2/M cell cycle arrest and subsequent
apoptosis in BGC-823 cells. The expression of G2/M
regulatory proteins, including cyclin A1, cyclin B1 and CDK1, was
detected at different concentrations of ISOIM for 48 h. The results
demonstrated that the expression of mitosis-promoting factors,
including cyclin A1, cyclin B1 and CDK1, was downregulated in
treated cells compared with that in control cells (Fig. 3B).
Effect of cell apoptosis in BGC-823
cells induced by ISOIM
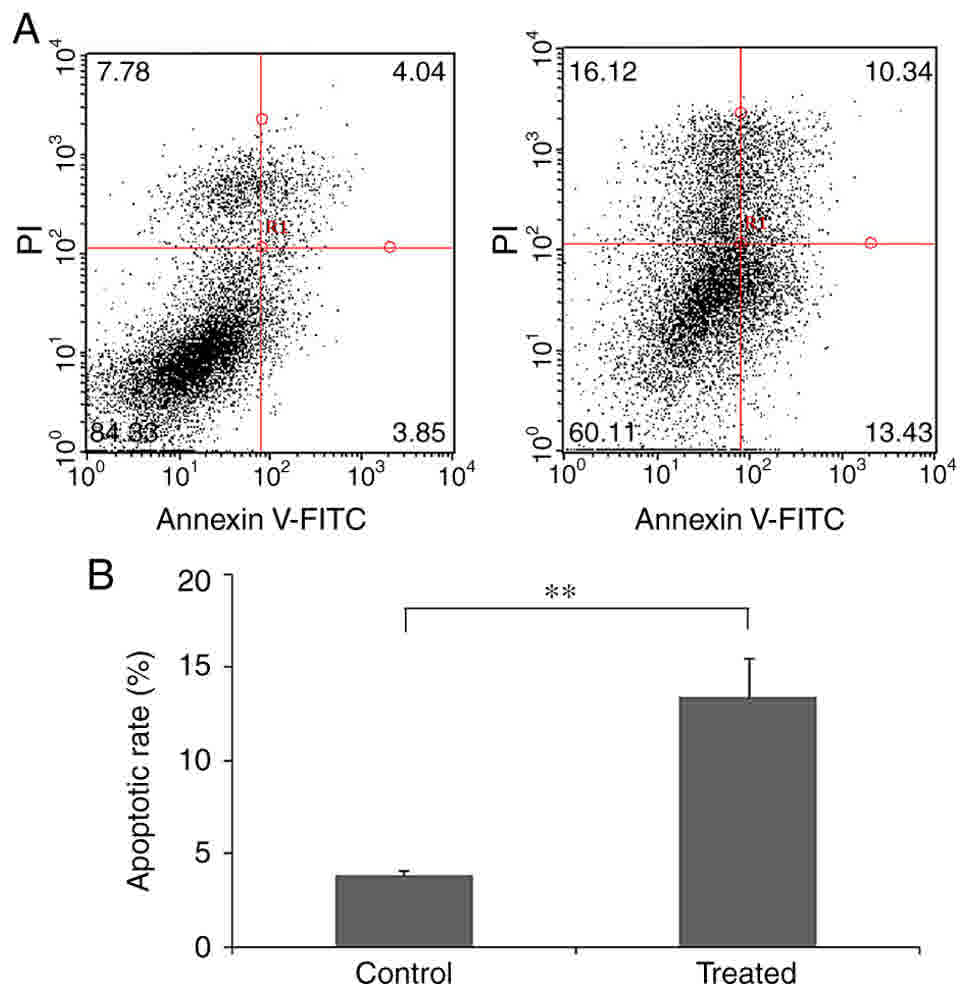
The cell apoptosis rate in cells treated with ISOIM
was also assessed using FCM. The cell apoptosis rate reached 23.77%
while the early apoptosis rate was 13.43% under 0.1 mM ISOIM
treatment (Fig. 4). The results of
the present study confirmed the pro-apoptotic effect of ISOIM on
BGC-823 cells.
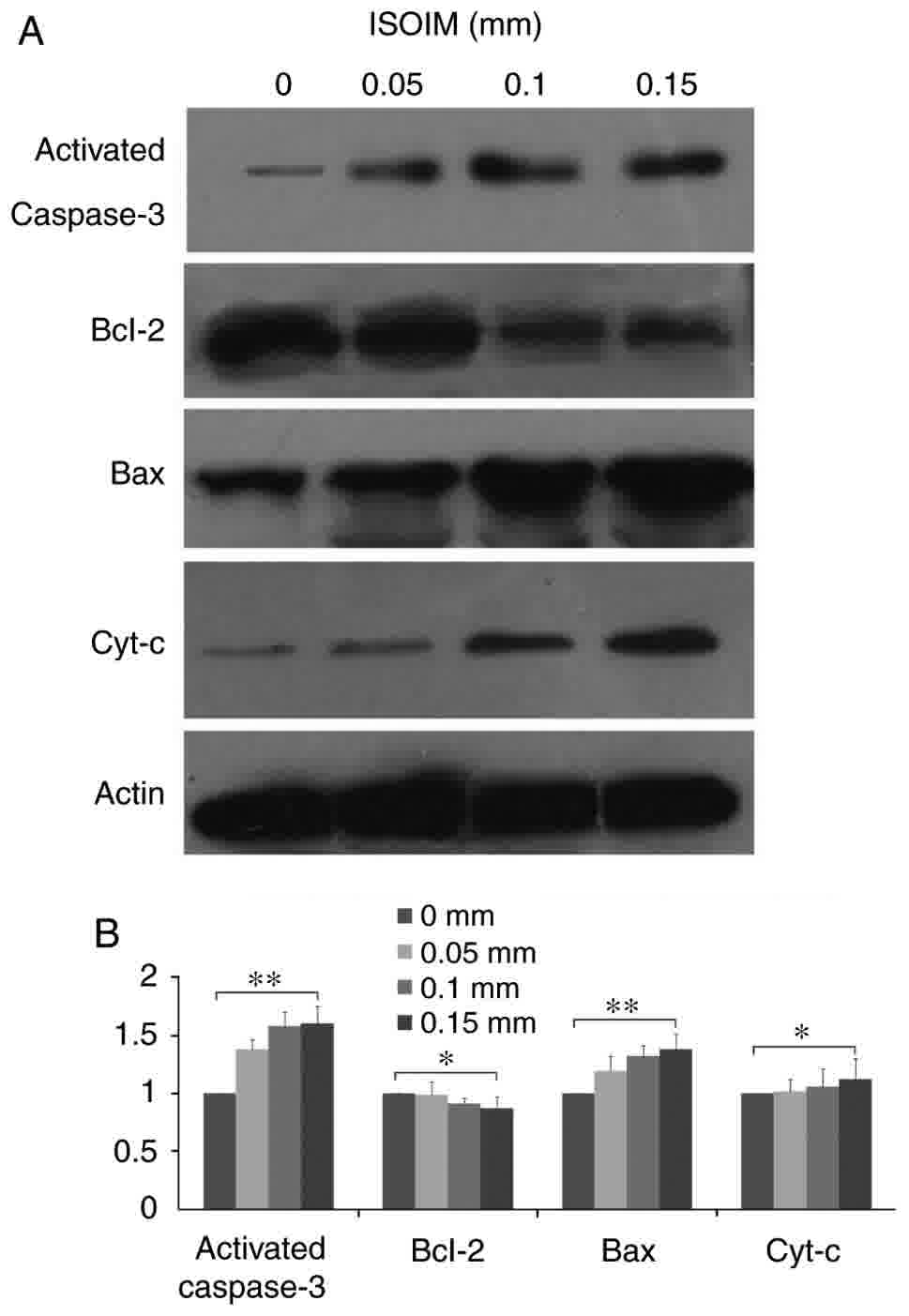
Alterations in apoptosis regulatory
proteins induced by ISOIM in BGC-823 cells
To further evaluate the potential mechanism of
ISOIM-induced apoptosis, the present study analyzed the effect of
ISOIM on the levels of apoptosis regulatory proteins using western
blotting. The levels of cytochrome c and caspase-3 increased
following treatment with ISOIM compared with those of the untreated
controls (Fig. 5), which induced a
cascade of caspase activity (P≤0.01) that resulted in
mitochondria-mediated apoptosis. Furthermore, the levels of Bax
increased (P≤0.01) and the Bcl-2 levels decreased (P≤0.05),
resulting in a decrease in the anti-apoptotic/pro-apoptotic
(Bcl-2/Bax) protein ratio prior to and following ISOIM
treatment.
Discussion
Mitochondria serve a key function in the intrinsic
apoptosis pathway (13–15). Previous studies have indicated that
coumarin compounds induce apoptosis in cells via a
mitochondria-dependent pathway (16–19).
Consequently, the primary aim of the present study was to assess
the potential mechanism by which ISOIM induces human BGC-823
gastric cancer cells to undergo apoptosis.
In the present study, the dose- and time-dependent
proliferation inhibition of BGC-823 cells by ISOIM was
demonstrated. Previous studies have revealed that ISOIM may induce
apoptosis in multiple types of human malignant tumors (9,20,21). The results of the present study
indicated that, following treatment with 0.1 mM ISOIM for 48 h, a
substantial degree of apoptosis in BGC-823 cells was detected. As a
coumarin compound, the effects of ISOIM on BGC-823 cells
corresponded to previous reports of the effects of other coumarin
compounds on human breast cancer MCF7, human monocyte U937, mouse
hepatocellular carcinoma Hepa-1, mouse adipocyte 3T3-L1, ovarian
cancer, and human HL-60 and NALM-6 leukemia cells (14–16,22–25).
The results of cell cycle analysis in the present study confirmed
that ISOIM could significantly arrest the BGC-823 cell cycle at the
G2/M phase and inhibit tumor cells from dividing,
inducing apoptosis. The results of the present study on the cell
cycle were consistent with the results of a prior study pertaining
to the effects of other coumarin compounds on other types of tumor
cell (17).
The Bcl-2 family are major regulators during the
release of mitochondrial apoptotic factor (18). Bcl-2 functions in regulating
mitochondrial permeability transition (pore opening and closing of
apoptotic factors) is considered to be a primary regulator of
apoptosis (26,27). Bax functions by releasing cytochrome
c, and that Bax transfers from the cytoplasm to the
mitochondrial membrane under the stimulus of apoptotic signals and
subsequently activates mitochondrially mediated apoptosis (28). Bcl-2 is an anti-apoptotic protein that
inhibits the promotion of apoptosis proteins on the mitochondrial
membrane by forming oligomers that affect their anti-apoptotic
functions (29). In addition, the
caspase family functions crucially in mediating cell apoptosis
(30). Caspase-3, which is downstream
of the apoptosis cascade, is a crucial effector caspase and
facilitates apoptosis; it serves as the main effector of apoptosis
and the convergence point of apoptotic signaling (31). Activated caspase-3 indicates
irreversible apoptosis (32).
Therefore, the expression of caspase-3 reflects the level of
apoptosis and the existence of an apoptosis promoter (33). To clarify whether ISOIM induces
apoptosis in BGC-823 cells via the mitochondrial pathway, the
present study evaluated the expression of Bcl-2, Bax, caspase-3 and
cytochrome c using western blotting. The results
demonstrated that ISOIM downregulated the levels of Bcl-2 and
upregulated the levels of cytochrome c, caspase-3 and Bax.
The results of the present study revealed that caspase-3 was
activated by ISOIM in a dose-dependent manner, and that ISOIM
induced human gastric cancer cells to apoptosis. In vitro
and in vivo analysis is a useful strategy for assessing
anticancer drugs. The present study used in vitro analysis
and demonstrated that ISOIM may induce apoptosis and its potential
molecular mechanism; however, one deficiency of the present study
is the lack of in vivo data, which should represent a future
research direction in this field.
To conclude, the present study established that
ISOIM, by activating pro- and anti-apoptotic genes, inhibits the
cell cycle of BGC-823 gastric cancer cells at the G2/M
transition, inhibits proliferation and induces the apoptosis of
BGC-823 cells by initiating the activation of the mitochondrial
pathway. Therefore, further assessing the mechanism of ISOIM, a
anticancer compound, in inducing the apoptosis of human BGC-823
gastric cancer cells may be important in preventing this disease
and anticancer research.
Acknowledgements
Not applicable.
Funding
This study was supported by the Henan Planning
Project of Science and Technology (grant no. 132102310118).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
HBY, HRG, YJR and FXF performed the experiments.
HTT, ZJG, WS and SMH analyzed the data. AFZ designed the
experiments and wrote the manuscript.
Ethics and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors confirm that they have no competing
interests.
References
|
1
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei Y and Ito Y: Preparative isolation of
imperatorin, oxypeucedanin and isoimperatorin from traditional
Chinese herb ‘bai zhi’ Angelica dahurica (Fisch. ex Hoffm)
Benth. et Hook using multidimensional high-speed counter-current
chromatography. J Chromatogr A. 1115:112–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim YK, Kim YS and Ryu SY:
Antiproliferative effect of furanocoumarins from the root of
Angelica dahurica on cultured human tumor cell lines.
Phytother Res. 21:288–290. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Moon TC, Jin M, Son JK and Chang HW: The
effects of isoimperatorin isolated from Angelicae dahuricae
on cyclooxygenase-2 and 5-lipoxygenase in mouse bone marrow-derived
mast cells. Arch Pharm Res. 31:210–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim DK, Lim JP, Yang JH, Eom DO, Eun JS
and Leem KH: Acetylcholinesterase inhibitors from the roots of
Angelica dahurica. Arch Pharm Res. 25:856–859. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Park HY, Kwon SB, Heo NK, Chun WJ, Kim MJ
and Kwon YS: Constituents of the stem of Angelica gigas with
rat lens aldose reductase inhibitory activity. J Korean Society
Applied Biol Chem. 54:194–199. 2011. View Article : Google Scholar
|
|
8
|
Baek NI, Ahn EM, Kim HY and Park YD:
Furanocoumarins from the root of Angelica dahurica. Arch
Pharm Res. 23:467–470. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kleiner HE, Reed MJ and DiGiovanni J:
Naturally occurring coumarins inhibit human cytochromes P450 and
block benzo[a]pyrene and 7,12-dimethylbenz[a]anthracene DNA adduct
formation in MCF-7 cells. Chem Res Toxicol. 16:415–422. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tong K, Chang X and Chen W: Isoimperatorin
induces apoptosis of the SGC-7901 human gastric cancer cell line
via the mitochondria-mediated pathway. Oncol Lett. 13:518–524.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yan S, Yuan L, Hong L, Jin L, Xiaohua S
and Wenchang Z: 2,5-Hexanedione induces human ovarian granulosa
cell apoptosis through Bcl-2, BAX, and CASPASE-3 signaling
pathways. Arch Toxicol. 86:205–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao X, Yang W, Shi C, Ma W, Liu J, Wang Y
and Jiang G: The G1 phase arrest and apoptosis by intrinsic pathway
induced by valproic acid inhibit proliferation of BGC-823 gastric
carcinoma cells. Tumour Biol. 32:335–346. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cohen GM: Caspases: The executioners of
apoptosis. Biochem J. 326:1–16. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reed JC: Cytochrome c: Can't live with
it-can't live without it. Cell. 91:559–562. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kroemer G and Reed JC: Mitochondrial
control of cell death. Nat Med. 6:513–519. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang JY, Della-Fera MA and Baile CA:
Esculetin induces mitochondria-mediated apoptosis in 3T3-L1
adipocytes. Apoptosis. 11:1371–1378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Singh RK, Lange TS, Kim KK and Brard L: A
coumarin derivative (RKS262) inhibits cell-cycle progression,
causes pro-apoptotic signaling and cytotoxicity in ovarian cancer
cells. Invest New Drugs. 29:63–72. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Łazarenkow A, Nawrot-Modranka J,
Brzezińska E, Krajewska U and Różalski M: Synthesis, preliminary
cytotoxicity evaluation of new 3-formylchromone hydrazones and
phosphorohydrazone derivatives of coumarin and chromone. Med Chem
Res. 21:1861–1868. 2012. View Article : Google Scholar
|
|
20
|
Marumoto S and Miyazawa M: Beta-secretase
inhibitory effects of furanocoumarins from the root of Angelica
dahurica. Phytother Res. 24:510–513. 2010.PubMed/NCBI
|
|
21
|
Kang SY and Kim YC: Neuroprotective
coumarins from the root of Angelica gigas:
Structure-activity relationships. Arch Pharm Res. 30:1368–1373.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okuyama T, Takata M, Nishino H, Nishino A,
Takayasu J and Iwashima A: Studies on the antitumor-promoting
activity of naturally occurring substances. II. Inhibition of
tumor-promoter-enhanced phospholipid metabolism by umbelliferous
materials. Chem Pharm Bull (Tokyo). 38:1084–1086. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kleiner HE, Vulimiri SV, Reed MJ,
Uberecken A and DiGiovanni J: Role of cytochrome P450 1a1 and 1b1
in the metabolic activation of 7,12-dimethylbenz[a]anthracene and
the effects of naturally occurring furanocoumarins on skin tumor
initiation. Chem Res Toxicol. 15:226–235. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang PY, Rui YC, Li K, Huang XH, Jiang JM
and Yu L: Expression of intercellular adhesion molecule-1 in U937
foam cells and inhibitory effect of imperatorin. Acta Pharmacol
Sin. 23:327–330. 2002.PubMed/NCBI
|
|
25
|
Kleiner HE, Vulimiri SV, Miller L, Johnson
WH Jr, Whitman CP and DiGiovanni J: Oral administration of
naturally occurring coumarins leads to altered phase I and II
enzyme activities and reduced DNA adduct formation by polycyclic
aromatic hydrocarbons in various tissues of SENCAR mice.
Carcinogenesis. 22:73–82. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Green DR and Kroemer G: The
pathophysiology of mitochondrial cell death. Science. 305:626–629.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Q and Lesnefsky EJ: Blockade of
electron transport during ischemia preserves bcl-2 and inhibits
opening of the mitochondrial permeability transition pore. FEBS
Lett. 585:921–926. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jurgensmeier JM, Xie Z, Deveraux Q,
Ellerby L, Bredesen D and Reed JC: Bax directly induces release of
cytochrome c from isolated mitochondria. Proc Natl Acad Sci USA.
95:4997–5002. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Merino D and Bouillet P: The Bcl-2 family
in autoimmune and degenerative disorders. Apoptosis. 14:570–583.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhivotovsky B: Caspases: The enzymes of
death. Essays Biochem. 39:25–40. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lakhani SA, Masud A, Kuida K, Porter GA
Jr, Booth CJ, Mehal WZ, Inayat I and Flavell RA: Caspases 3 and 7:
Key mediators of mitochondrial events of apoptosis. Science.
311:847–851. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nobuhiro M, Keiko N, Hiromi T, Takehiko S
and Yukuto Y: An endoplasmic reticulum stress-specific caspase
cascade in apoptosis. Cytochrome c-independent activation of
caspase-9 by caspase-12. J Biol Chem. 277:34287–34294. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Porter AG and Janicke RU: Emerging roles
of caspase-3 in apoptosis. Cell Death Differ. 6:99–104. 1999.
View Article : Google Scholar : PubMed/NCBI
|