Introduction
Monocyte chemoattractant protein-1 (MCP-1), also
known as chemokine ligand 2, is one of the chemokine family members
(1). MCP-1 mediates neoplasm-induced
osteoclastogenesis in different types of cancer, including myeloma,
breast cancer and prostate cancer, due to its specific function,
which is associated with osteoclast development and maturation
(2). Kim et al (3) reported that MCP-1 was induced by
receptor activator of nuclear factorκB ligand (RANKL), where it
promoted human osteoclast differentiation. Furthermore,
MCP-1-treated human peripheral blood mononuclear cells (PBMCs)
formed tartrate-resistant acid phosphatase (TRAP)-positive
multinucleated cells, suggesting that MCP-1 promotes osteoclast
fusion (4,5).
In contrast to distant bone metastasis, local bone
invasion is a common complication of oral squamous cell carcinoma
(OSCC). Lesions of the gingiva, hard palate and retromolar trigone
may involve the maxillary and/or mandibular bone (6). It is now clear that osteoclasts are
involved in this resorption process (7). These osteoclasts differentiate at the
bone surface and dissolve mineral components. Since osteoclasts
serve important roles in the progression of bone invasion in OSCC,
it is essential to identify an efficient therapeutic osteoclast
target, which may improve clinical approaches.
Our previous study examined whether a decrease in
MCP-1 expression would inhibit OSCC bone invasion (8). To begin with, MCP-1 protein and mRNA
expression in OSCC tissues and several OSCC cell lines were
confirmed. Subsequently, SCC25 cells were transfected with a
dominant-negative variant of MCP-1 with 7-amino acid truncated
(7ND) in the pcDNA vector (SCC25-7ND). Following this, it was
revealed that 10% SCC25-7ND cell conditioned medium efficiently
inhibited human osteoclast formation. By establishing an animal
model of OSCC bone invasion, histological analysis identified
significantly fewer SCC25-7ND osteoclasts within the calvariae
compared with SCC25 cells. These results demonstrated the relevance
of MCP-1 in the study of OSCC bone invasion and indicated that 7ND
may be used as a blocking agent to inhibit MCP-1. Therefore, the
present study aimed to utilize the synthetic 7ND protein in order
to determine whether it inhibits in vitro differentiation of
osteoclasts and whether it reduces SCC25 bone invasion in
vivo.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM),
α-Modified Eagle Medium (α-MEM), fetal bovine serum (FBS),
trypsin-EDTA, antibiotics (100 U/ml penicillin G and 100 mg/ml
streptomycin) and phosphate buffered saline (PBS) were purchased
from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). The primary
monoclonal mouse anti-human MCP-1 antibody was obtained from Abcam
(cat. no. ab9858; Cambridge, MA, USA) and the primary fluorescein
isothiocyanate (FITC) rat anti-mouse cluster of differentiation 14
(CD14) antibody was purchased from BD Biosciences (cat. no. 561710;
San Jose, CA, USA). The horseradish peroxidase (HRP)-conjugated
goat anti-mouse IgG secondary antibody was supplied by Bio-Rad
Laboratories, Inc. (cat. no. STAR137P; Hercules, CA, USA).
Recombinant human cytokines of colony stimulating factor1 (rhCSF1),
RANKL (rhRANKL) and MCP-1 (rhMCP-1) were purchased from PeproTech,
Inc. (Rocky Hill, NJ, USA), A TRAP staining kit was obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Immunohistochemistry
OSCC tissue samples from 10 patients with bone
invasion were examined in order to determine MCP-1 expression.
Adjacent non-cancerous tissues from the same patients served as
control. Following being fixed in 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) for 1 week at room temperature, serial
paraffin-embedded tissue sections (5 µm thick) were dewaxed using
xylene, rehydrated in a descending alcohol series (100, 85 and 75%
ethanol) and treated with 0.3% hydrogen peroxide in PBS. Antigen
retrieval was performed by heating (50°C) sections in a microwave
oven (twice, for 4 min each time) with 0.2% citrate buffer (pH=6).
Non-specific binding was blocked with 5% bovine serum albumin in
PBS for 30 min at room temperature, and sections were subsequently
incubated with the primary MCP-1 antibody (dilution, 1:100)
overnight at 4°C. Sections were then treated with Dako REAL™
EnVision™/HRP, Rabbit/Mouse reagent of the kit(horseradish
peroxidase-conjugated polymer; cat. no. K5007; Envision Detection
System; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) for
30 min at room temperature, followed by 3,3′-diaminobenzidine (DAB)
detection solution for 1 min at room temperature. The primary
antibody was replaced with non-immune serum (Wuhan Boster
Biological Technologies, Ltd., Wuhan, China) as a negative control.
All sections were counterstained with Mayer's hematoxylin for 3 min
at room temperature, dehydrated and mounted with mounting medium.
The final results were visualized with a light microscope
(magnification, ×200) and images were captured using a digital
camera.
Cloning, protein expression and
purification
The full-length 7ND gene was amplified using primers
designed based on the full-length MCP-1 gene (GenBank: S71513.1,
forward, 5′-TCGCGAGCTATAGAAGAATCA-3′ and reverse,
5′-TGTTCAAGTCTTCGGAGTTTG-3′). The 7ND coding region was cloned into
the pMCSG7 vector (9). The
recombinant plasmid was sequenced and the plasmid harboring the 7ND
gene was transformed into BL21 (DE3) E. coli. The cells were
cultured in Luria-Bertani medium (Wuhan Boster Biological
Technologies, Ltd.) containing ampicillin (100 µg/ml) at 37°C until
the OD600 reached 0.8. The culture was induced with 0.2
mmol/l iso-propyl-β-D-thiogalactoside for 20 h at 16°C. Cells were
harvested by centrifugation at 4°C (5,000 × g for 5 min), lysed by
sonication and clarified by centrifugation at 4°C (12,000 × g for
20 min). The supernatant was then applied to a
nickel-nitrilo-triacetic acid (Ni-NTA) resin gravity column
(Qiagen, Inc., Valencia, CA, USA) that had been previously
equilibrated with PBS (137 mmol/l NaCl, 2.7 mmol/l KCl, 50 mmol/l
Na2HPO4 and 10 mmol/l
KH2PO4; pH 7.4). The column was washed with
100 ml PBS, followed by washing with 100 ml 20 mmol/limidazole in
PBS and elution with 300 mmol/l imidazole in PBS. Following buffer
exchange, the His-tag was cleaved by tobacco etch virus treatment.
Uncut protein was separated by a second Ni-affinity chromatography.
Protein-containing fractions were pooled, concentrated and loaded
onto a Superdex G200 size exclusion chromatography column (GE
Healthcare, Chicago, IL, USA), equilibrated with 20 mmol/l Tris-HCl
at pH 8.0, 150 mmol/l NaCl and 2 mmol/l DTT.
Western blot analysis
Total protein was extracted using
radioimmunoprecipitation lysis buffer (Thermo Fisher Scientific,
Inc.) and clarified by centrifugation at 4°C (12,000 × g for 20
min). Protein concentration was determined using a bicinchoninic
acid protein assay kit (Pierce; Thermo Fisher Scientific, Inc.). A
total of 40 µg protein was separated by 10% SDS-PAGE (Bio-Rad
Laboratories, Inc.), prior to being transferred onto polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA) and
subsequently blocked with 5% dry skimmed milk in Tris-buffered
saline for 1 h at room temperature. The membranes were incubated
with an HRP-conjugated human anti-His antibody (cat. no. ab219465;
1:10,000; Abcam) overnight at 4°C, washed twice with PBS and
subsequently incubated with HRP-conjugated goat anti-mouse IgG
secondary antibodies for 1 h (cat. no. STAR137P; 1:3,000; Hercules)
at room temperature. Protein bands were subsequently detected and
visualized using a Super Signal WestPico chemiluminescent substrate
(Thermo Fisher Scientific, Inc.).
Cell lines and culture
The OSCC SCC25 cell line was obtained from American
Type Culture Collection (Manassas, VA, USA). SCC25 cells were
cultured in DMEM, supplemented with 10% FBS and antibiotics (100
U/ml penicillin G and 100 mg/ml streptomycin) at 37°C in an
incubator containing 5% CO2 and 20% O2.
Cell proliferation assay
SCC25 cells were seeded onto 96-well plates
(5×103 cells/well) and were allowed to attach overnight,
prior to being treated with 7ND protein (0, 25, 50, 100 or 200
ng/ml) for 1–3 days. A volume of 20 µl MTT (5 mg/ml; Thermo Fisher
Scientific, Inc.) was added to each well for 4 h at 37°C. Following
removal of solution and addition of dimethyl sulfoxide (150
µl/well; Sigma-Aldrich; Merck KGaA) to dissolve the purple-formazan
the absorbance was read at 590 nm on a BioTek plate reader (Beckman
Coulter, Inc., Brea, CA, USA).
Magnetic activated cell sorting (MACS)
of CD14+ monocytes
Human PBMCs were isolated from the blood of healthy
volunteers using BD vacutainer cell preparation tubes containing
sodium citrate, as previously described (8). Following centrifugation at 1,500 × g for
30 min of room temperature, the cell layer on top of the
Ficoll-Paque was collected, resuspended in 10 ml α-MEM and
centrifuged (225 × g for 10 min) at room temperature.
CD14+ monocytes were purified by incubation with MACS
CD14+ microbeads (Miltenyi Biotec, Inc., Cambridge, MA,
USA) for 15 min at 4°C. Cells were subsequently washed in
CD14+ isolation buffer (0.5% fetal calf serum and 2 mM
EDTA; pH=8) and passed through a MACS magnetic cell separator
(Miltenyi Biotec, Inc.). CD14+ monocytes were collected
and utilized for subsequent experiments.
Migration assay
Transwell inserts (5 µm pore; Corning Incorporated,
Corning, NY, USA) were used as previously described (10). Prior to loading, CD14+
monocytes (1×105 cells/ml) were incubated for 1 h with
various concentrations of 7ND protein (0, 25, 50 or 100 ng/ml), and
were subsequently seeded into the upper chamber [serum-free culture
medium of α-modified-minimum essential medium (α-MEM); Thermo
Fisher Scientific, Inc.] of Transwell inserts, while 600 µl
complete medium of α-MEM with 10% FBS (Thermo Fisher Scientific,
Inc.) containing 10 ng/ml rhMCP-1 was placed into the lower
chamber. Following 3 h of incubation, non-migrating cells were
scraped from the upper chamber, and migrated cells were stained
with Hoechst 33342 (Sigma-Aldrich; Merck KGaA) at room temperature
for 5 min and were observed using a fluorescence microscope
(magnification, ×200). Four fields were randomly selected and
non-overlapping images were captured for each of three triplicate
culture wells. In each image, the total number of stained cells was
counted by two independent assessors.
Osteoclast differentiation assay
CD14+ monocytes were seeded onto 24-well
plates (1×105 cells/well) with 600 µl medium (α-MEM; pH
7.4; containing 10% FBS and 1% penicillin/streptomycin),
supplemented with rhCSF1 (25 ng/ml) and rhRANKL (40 ng/ml) to
induce osteoclast differentiation. Groups were arranged as follows:
Group 1, CD14+ monocytes with rhCSF1 (25 ng/ml) and
rhRANKL (40 ng/ml); and Group 2, CD14+ monocytes with
rhCSF1 (25 ng/ml), rhRANKL (40 ng/ml) and 7ND protein (50 ng/ml).
Medium was changed every 3 days and mature osteoclasts appeared in
one week. Osteoclasts were subsequently fixed in 10% formalin for 5
min at room temperature. Staining of TRAP was used to characterize
osteoclasts (5 min at room temperature). TRAP-positive cells with
three or more nuclei were considered to be multinucleated
osteoclasts. Rhodamine-conjugated phalloidin (Thermo Fisher
Scientific, Inc.) was used to label F-actin and DAPI staining
(Thermo Fisher Scientific, Inc.) was used to visualize nuclei for 5
min at room temperature by using a fluorescence microscope
(magnification, ×200). Four fields were randomly selected and
non-overlapping images were captured for each of three triplicate
culture wells. In each image, the total number of TRAP-positive
multinucleated osteoclasts and the number of their F-actin rings
were counted by two independent assessors.
In vivo animal model of OSCC bone
invasion
A total of 18 BALB/-c nude mice (female, 6–7 weeks,
15–18 g) were obtained from the animal resources center (Sun
Yat-sen University, Guangzhou, China), housed in the animal
facility and cared for by animal housing staff. The housing
conditions included specific pathogen free animal rooms (20–26°C,
20–50 Pa, 12/12 h dark-light cycle, positive atmosphere/clean area
and negative atmosphere/affected area). The holding food and water
were checked and prepared by animal housing staff each day. All
protocols were reviewed and approved by the University Ethics
Committee of Sun Yat-Sen University (2016–334QX). The humane
endpoints were conditions which severely affect the normal diet or
breath of the nude mice, absolute values included the fast growth
of tumors i.e., (if tumor volume ≥3.26 cm3, or weight
loss i.e., the body weight is ≤10 g). OSCC SCC25 cells
(5×106/100 µl) were subcutaneously injected into the
area overlaying the calvaria when the mice were 6–7 weeks old. Mice
were randomly divided into three groups (n=6/group): The negative
control group (Group 1) received PBS; the positive control group
(Group 2) received SCC25 cells; and the experimental group (Group
3) received SCC25 cells plus 7ND protein (30 µg/ml). 7ND protein
was subcutaneously injected into the same location overlaying the
calvaria every other day. All animals were sacrificed after 4
weeks, and tumors and calvariae were fixed in 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) for 1 week at room temperature. The
maximum tumor volume observed in any of the mice was 3.26
cm3.
Micro-computed tomography (µCT)
imaging
All calvariae were surgically removed from
PBS-treated control, SCC25 and SCC25+7ND tumor-bearing nude mice,
were fixed in 70% ethanol for 1 day at room temperature and scanned
using a µCT instrument (SCANCO Medical AG, Brüttisellen,
Switzerland). µCT-analyzer software (version 3.0; Volume Graphics
GmbH, Heidelberg, Germany) was used to analyze the calvarial
structure using the global segmentation method (8). Two-dimensional images were used to
generate three-dimensional reconstruction. The calvarial area was
outlined for analysis and quantification as previously described
(8). The amount of resorbed bone was
defined as the percentage of resorbed bone volume divided by the
total bone volume.
Flow cytometry
Bone marrow cells (BMCs) of each mouse were
extracted from the tibia on week 4, and blocked in 5% bovin serum
albumin (Wuhan Boster Biological Technologies, Ltd.) for 15 min at
room temperature. The CD14+ subpopulation of BMCs was
evaluated by incubating 1×106 cells with FITC rat
anti-mouse CD14 antibody (cat. no. 561710; BD Biosciences, San
Jose, CA, USA) in PBS (dilution, 1:100) at 4°C for 30 min. To wash
off excess antibody following staining, PBS was added and
subsequently centrifuged cell pellet was obtained (225 × g, 5 min,
room temperature). Flow cytometry analysis (FACS) was performed on
a FACScan flow cytometer (BD Biosciences) as previously reported
(8). The unstained cells were gated
out and data acquisition with analysis was performed using FlowJo
LLC software (version 7.6; FlowJo LLC, Ashland, OR, USA).
Histological and immunohistochemical
analysis
Subsequent to being fixed in 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) for 1 week at room temperature, tumor
specimens were embedded in paraffin using a tissue processor.
Serial 5-µm paraffin-embedded sections were cut on a rotary
microtome (Leica Microsystems, Inc., Buffalo Grove, IL, USA) and
were stained with H&E at room temperature for 1 h.
Immunohistochemical staining of sections was performed by
incubating serial sections with the MCP-1 primary antibody
(dilution, 1:100) overnight at 4°C, followed by incubation (1 h)
with Dako REAL™ EnVision™/HRP, Rabbit/Mouse reagent (horseradish
peroxidase-conjugated polymer; cat. no. K5007; Envision Detection
System; Dako; Agilent Technologies, Inc.) at room temperature and
DAB (Dako; Agilent Technologies, Inc.) staining for 3 min at room
temperature. Specimens treated with non-immune serum served as a
negative control.
All tumor-bearing calvaria were decalcified in 10%
EDTA (pH=7.4) for 2 weeks at room temperature, prior to being
processed for paraffin embedding. Serial 5 µm sections were stained
with H&E and TRAP separately, each for 1 h at room temperature.
Analysis of the number of TRAP-positive osteoclasts at the
tumor-bone interface was performed as previously described
(8). For each section, an area of 2
mm2 with the tumor-bone interface was defined for
counting the number of osteoclasts. Four fields of this area were
randomly selected and the number of TRAP-positive osteoclasts was
counted using a light microscope (magnification, ×200).
Statistical analysis
Results are presented as the mean ± standard error
of the mean of ≥3 independent experiments. Data analysis was
performed using SPSS software (version 20.0; IBM Corp., Armonk, NY,
USA). Student's t-test was used to compare two means. One-way
analysis of variance, followed by Student-Newman-Keuls test, was
applied to compare two or more means. P<0.05 was considered to
indicate a statistically significant difference.
Results
MCP-1 protein is expressed in OSCC
tissues with bone invasion
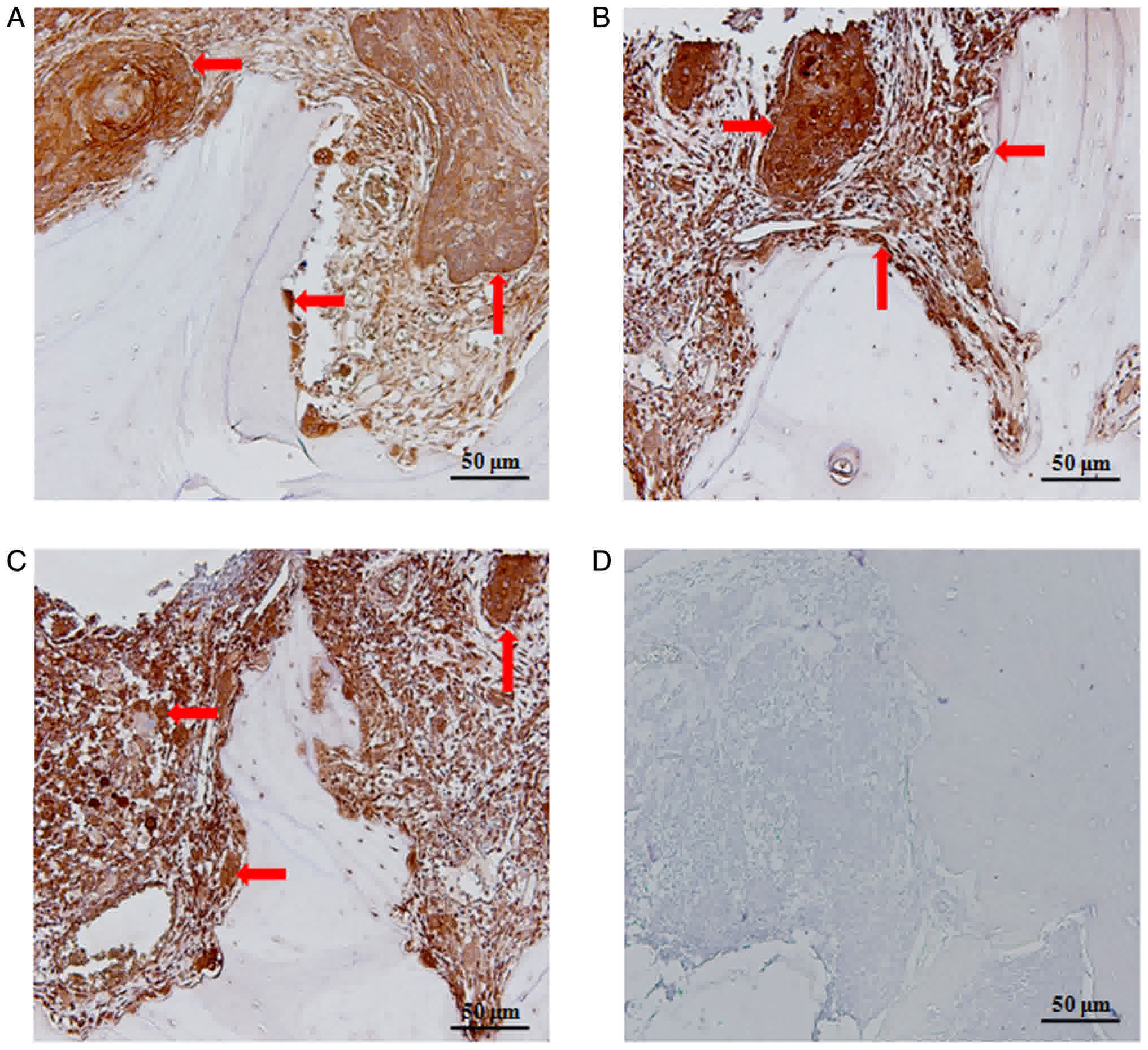
Strong staining of the MCP-1 protein was observed in
10 of the OSCC tissue samples. Immunohistochemistry (IHC)
demonstrated that MCP-1 was localized to osteoclasts and the
cytoplasm of OSCC cells at the tumor-bone interface (Fig. 1A-C). Control sections did not exhibit
any staining (Fig. 1D).
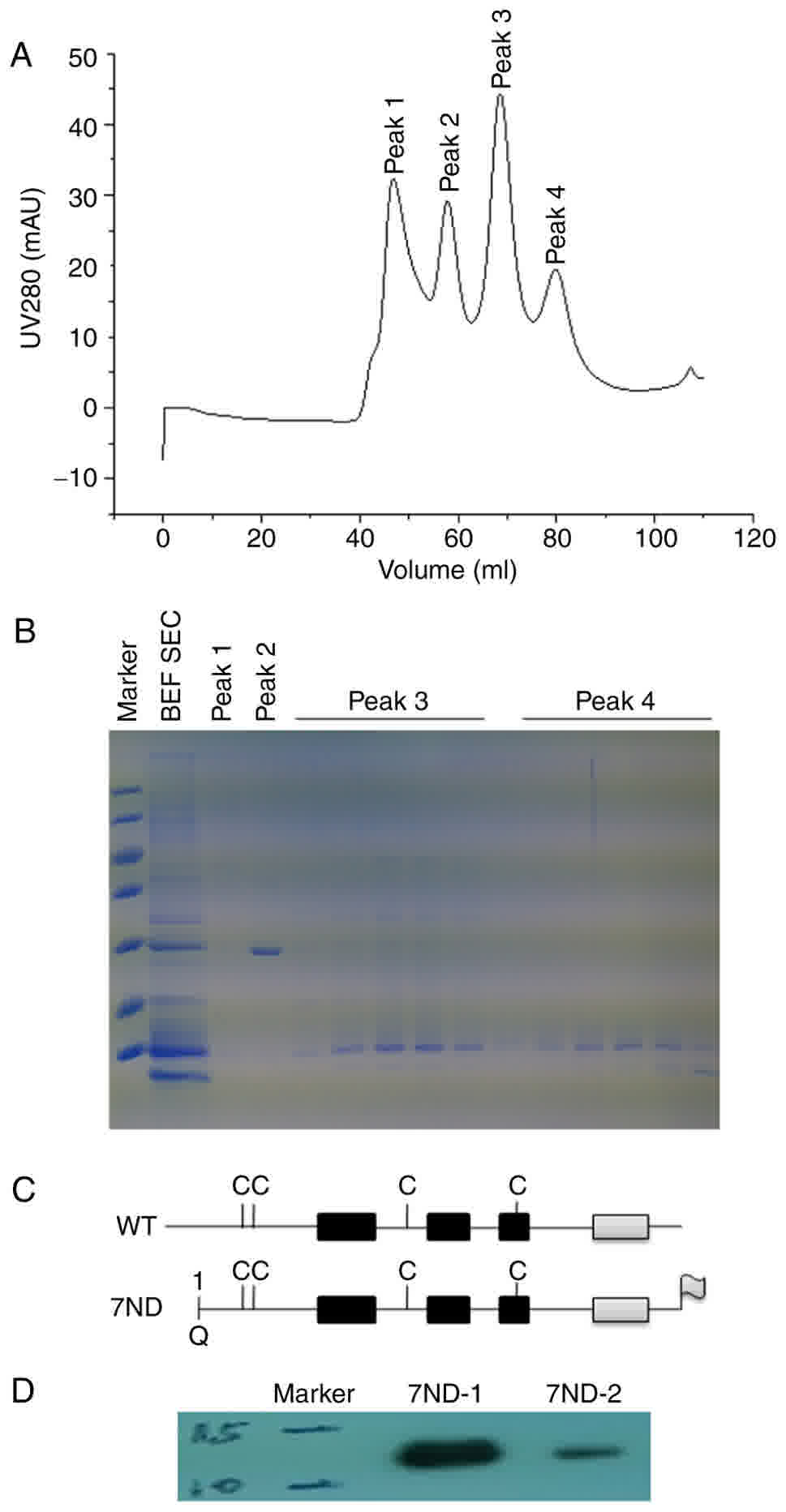
In vitro synthesis and validation of
the 7ND protein
Based on the standard protocol (9), the 7ND protein was cloned, expressed and
purified in vitro. Uncut protein was separated by a second
Ni-affinity chromatography column and SDS-PAGE prior to and
following purification (Fig. 2A-B).
7ND is an N-terminal deletion mutant of MCP-1 and lacks amino acids
2 to 8, and a schematic structural diagram of MCP-1 and 7ND is
presented in Fig. 2C. Western blot
analysis also confirmed the size of the synthetic 7ND protein
(Fig. 2D).
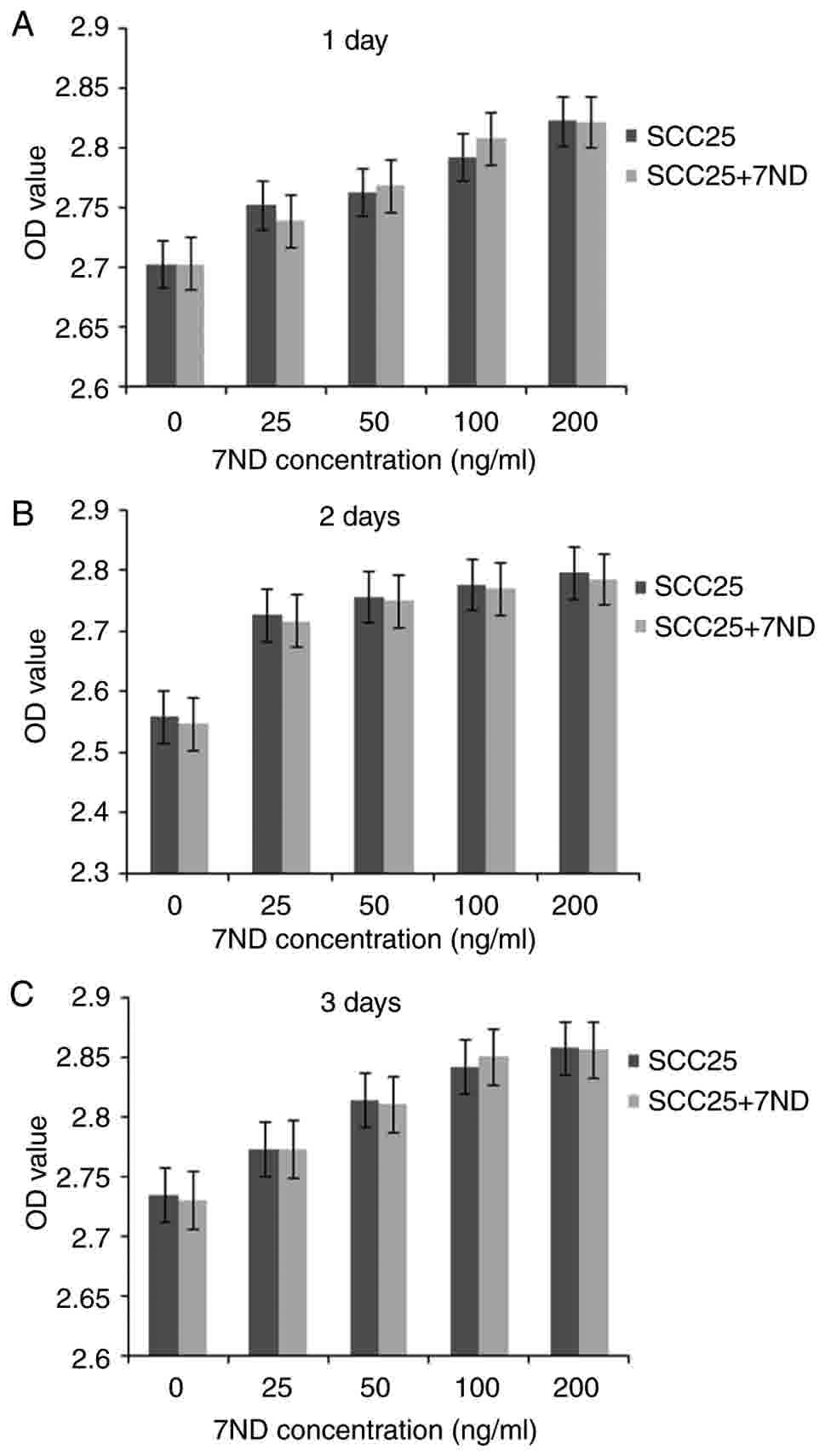
The 7ND protein does not affect SCC25
cell proliferation
Results of the MTT assay indicated that the 7ND
protein did not affect SCC25 cell proliferation following 3 days of
treatment at various concentrations (Fig.
3A-C). No significant differences were observed between pre-
and post-treatment measurements at any concentration.
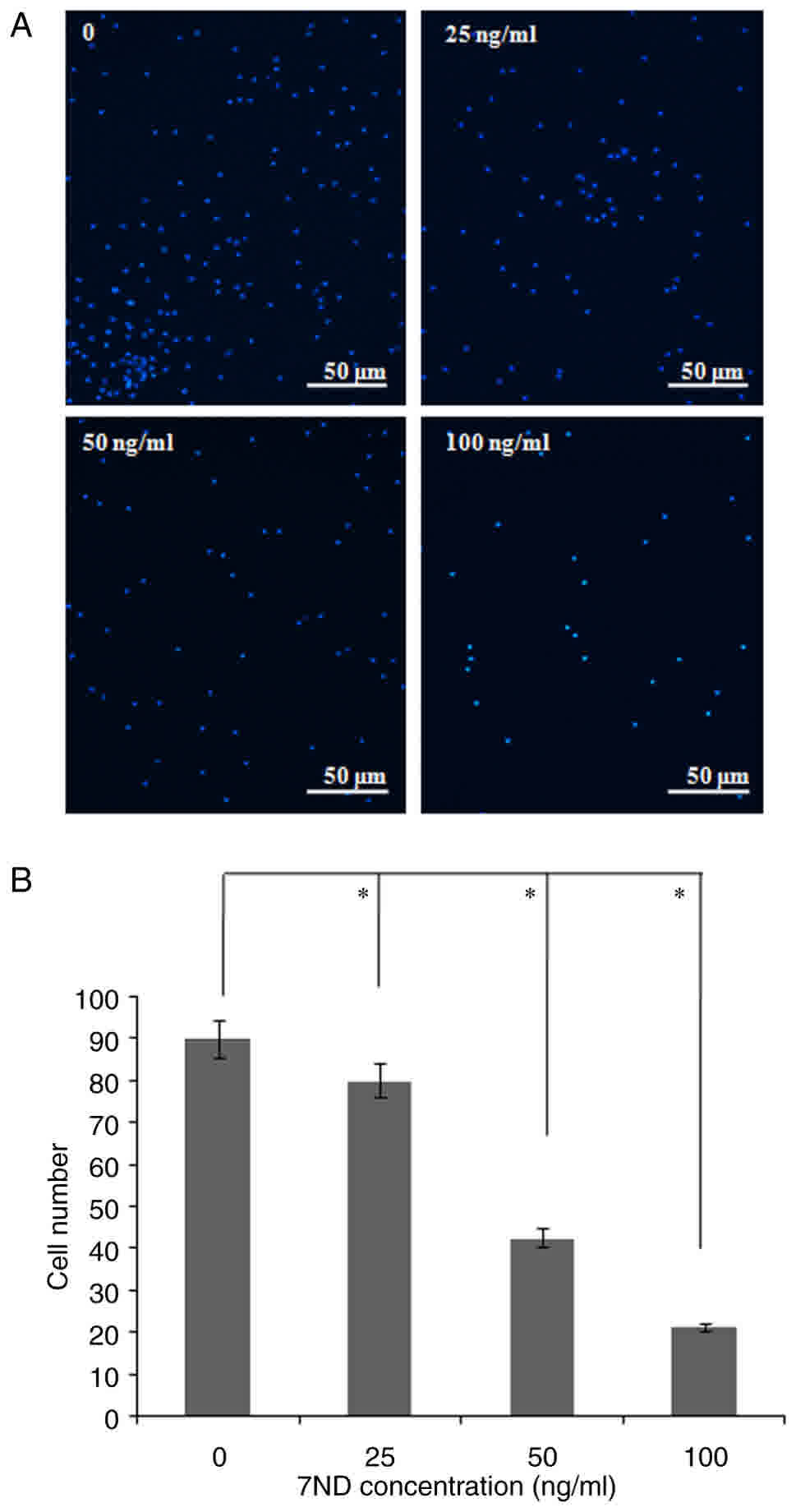
7ND inhibits the migration of
CD14+ monocytes
A migration assay was performed in order to
determine the effects of the 7ND protein on the migration of
CD14+ monocytes. Compared with control CD14+
monocytes, various concentrations of the 7ND protein (25, 50 and
100 ng/ml) efficiently inhibited CD14+ monocyte
migration (Fig. 4A-B).
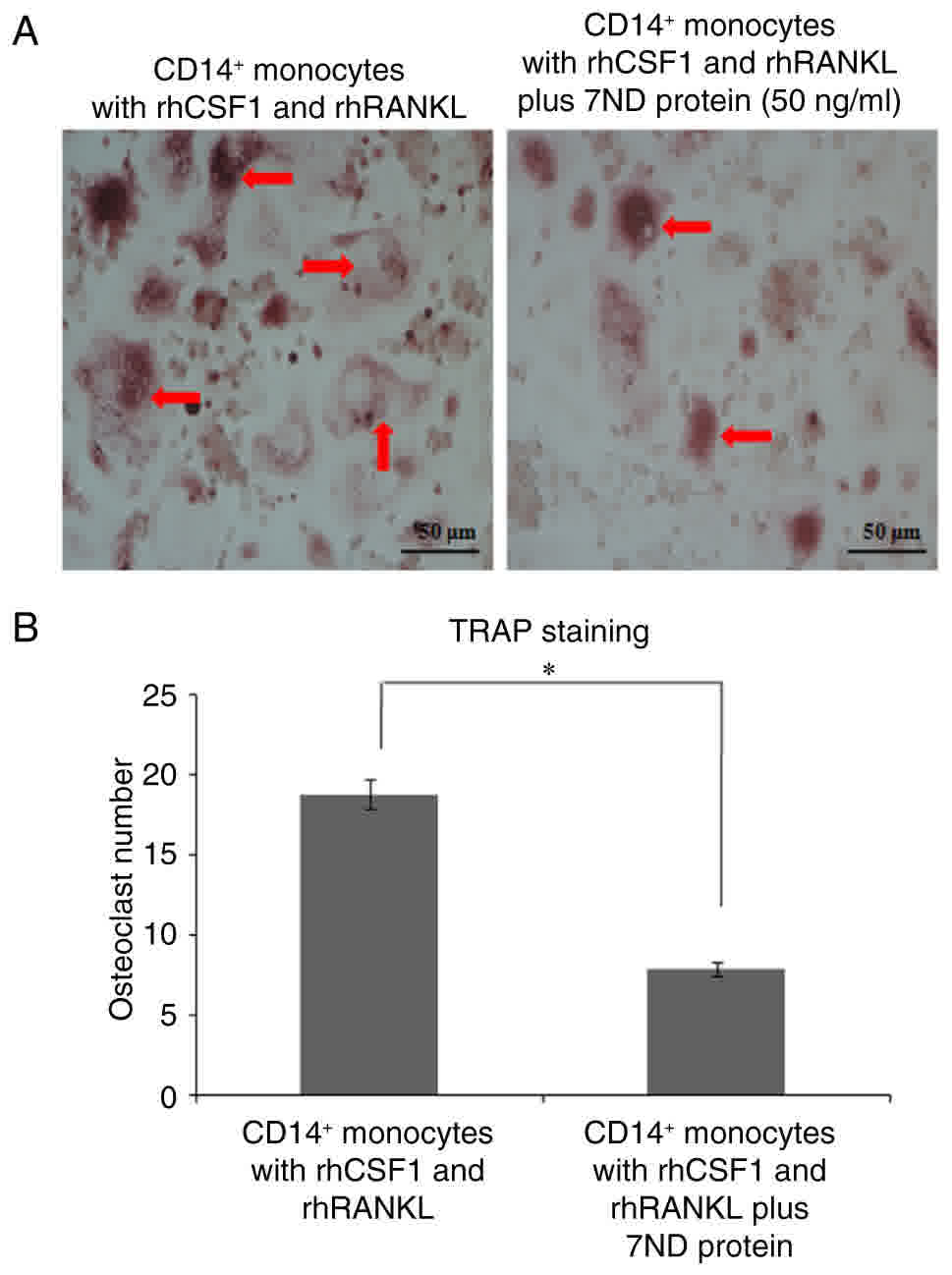
The 7ND protein inhibits the
differentiation of CD14+ monocytes into osteoclasts
The differentiation of osteoclasts from the positive
control group with CD14+ monocytes was induced by the
addition of rhCSF1 and rhRANKL for 3–4 days in culture (Fig. 5A-B). The effect of the 7ND protein (50
ng/ml) on osteoclast differentiation was examined, and TRAP
staining revealed that there were significantly fewer osteoclasts
in the presence of 7ND (Fig. 5A-B).
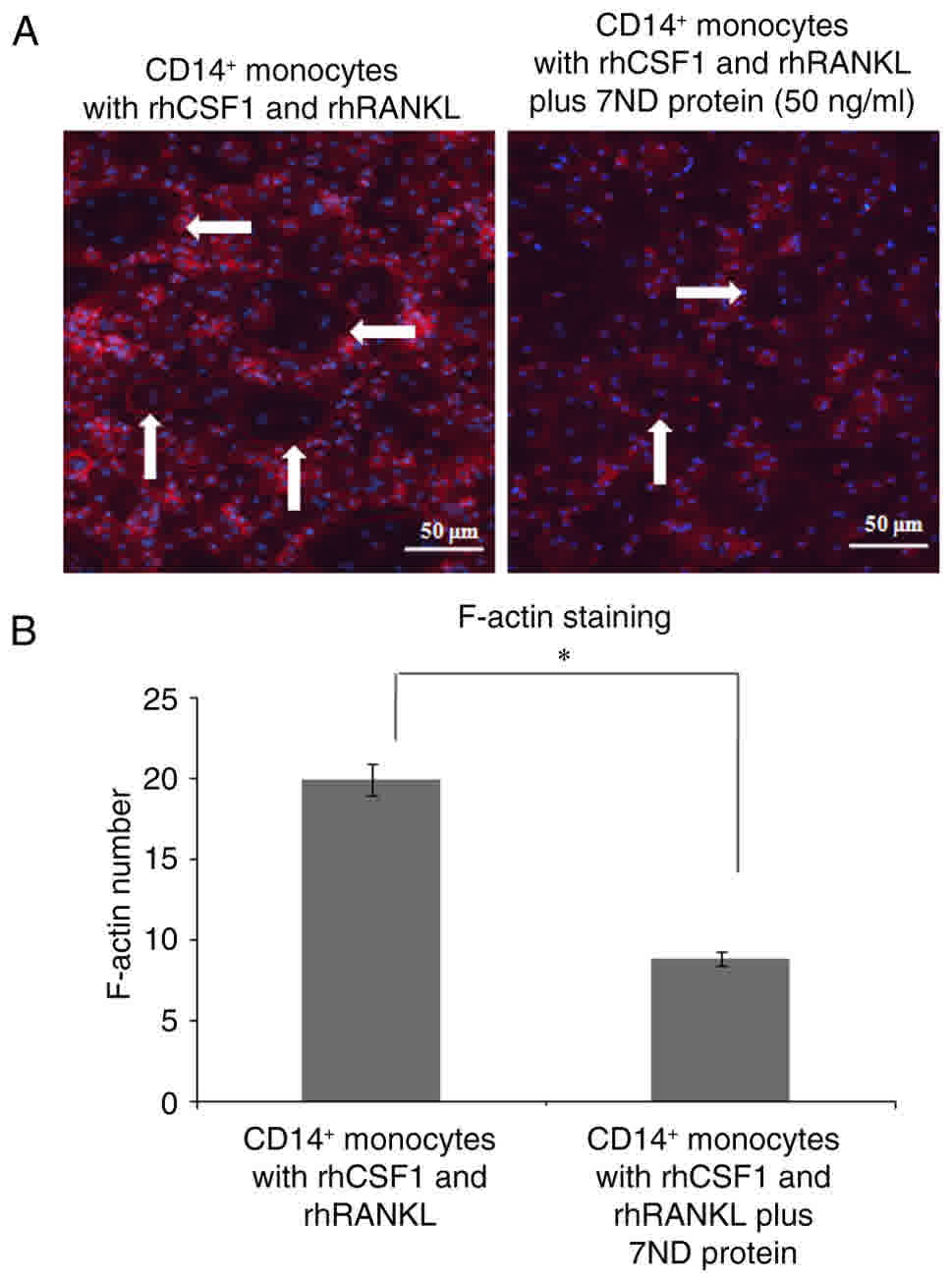
Immunofluorescence also revealed significantly less staining of
F-actin in the presence of 7ND (Fig.
6A-B).
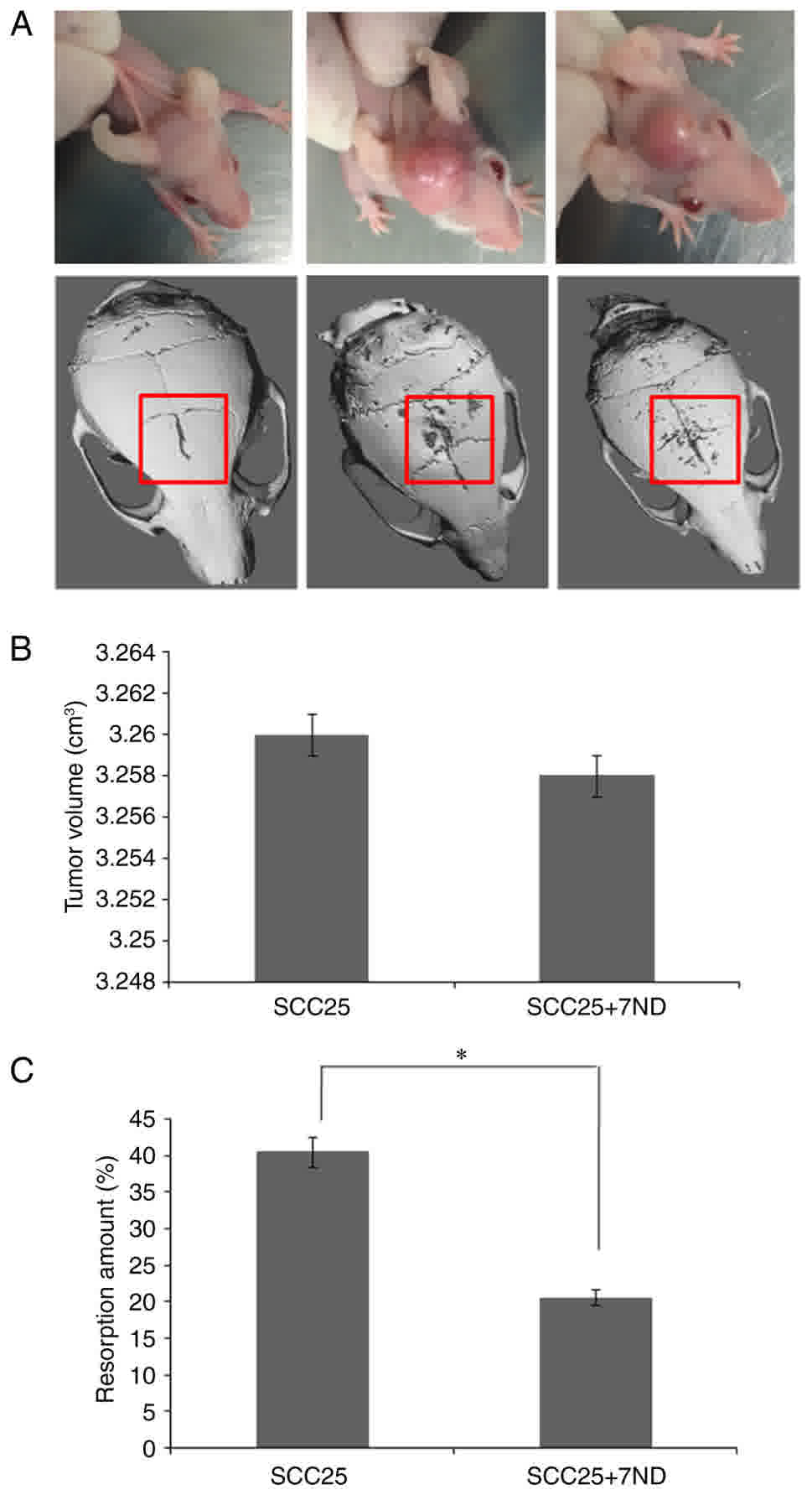
The 7ND protein reduces the SCC25
cell-induced resorption area of calvariae
An animal model of SCC25 cell bone invasion was
utilized as previously described (8),
and tumor cells were injected subcutaneously through the center of
the calvaria. Data regarding the tumor volume (width × length ×
depth) were recorded at week 4, and the results demonstrated that
the average tumor volume was similar in the SCC25+7ND group and the
SCC25 group (Fig. 7A-B). µCT analysis
revealed a significant decrease in the bone resorption area of the
calvaria in the SCC25+7ND group (Fig.
7A-B).
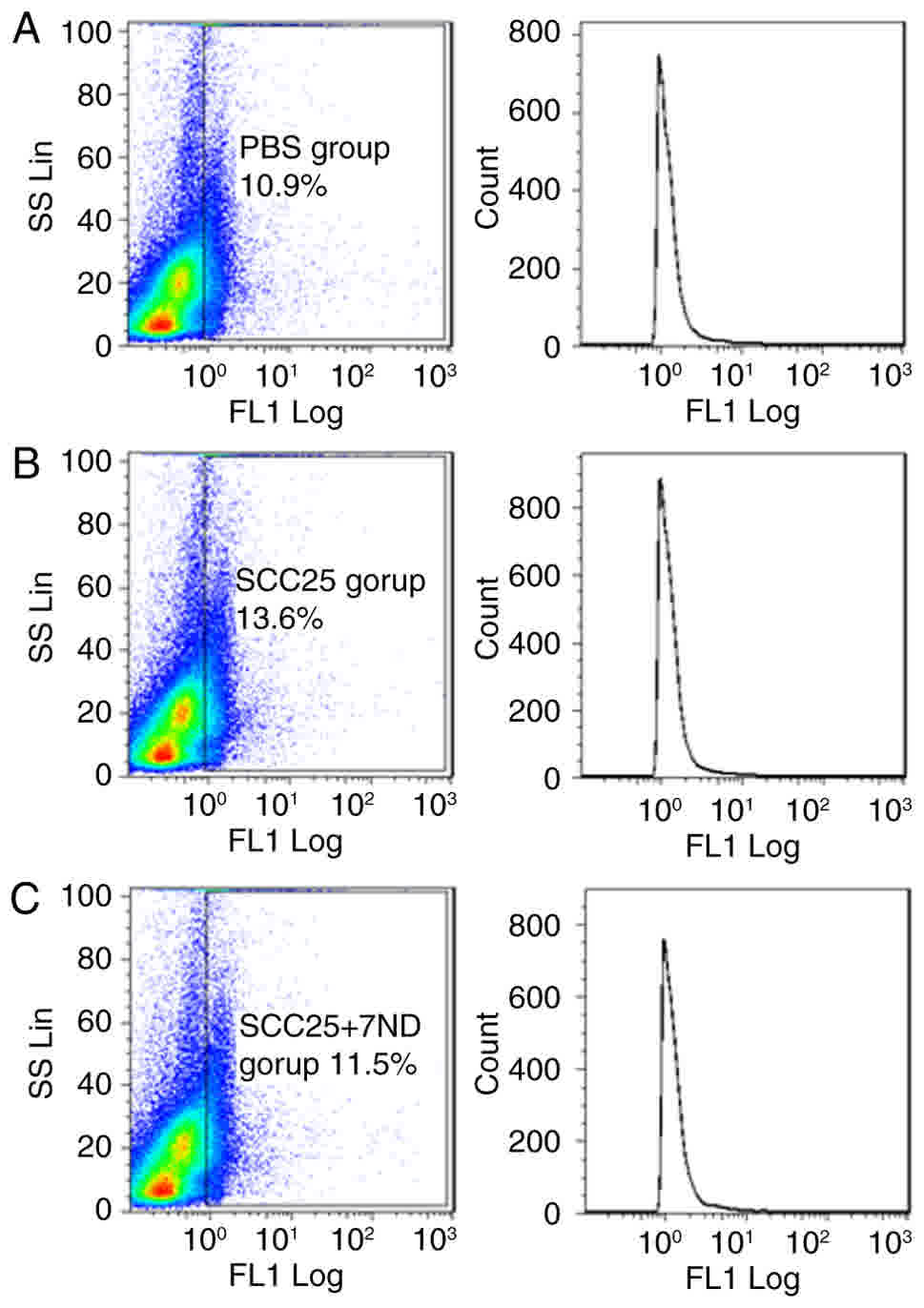
The CD14+ subpopulation of
BMCs is reduced in mice injected with 7ND
FACS analysis revealed that the CD14+
subpopulation of BMCs was reduced in mice of the SCC25+7ND group
(11.5%; Fig. 8C), compared with the
group of mice that received SCC25 cells only (13.6%, Fig. 8B). A significant difference was
observed between these two groups.
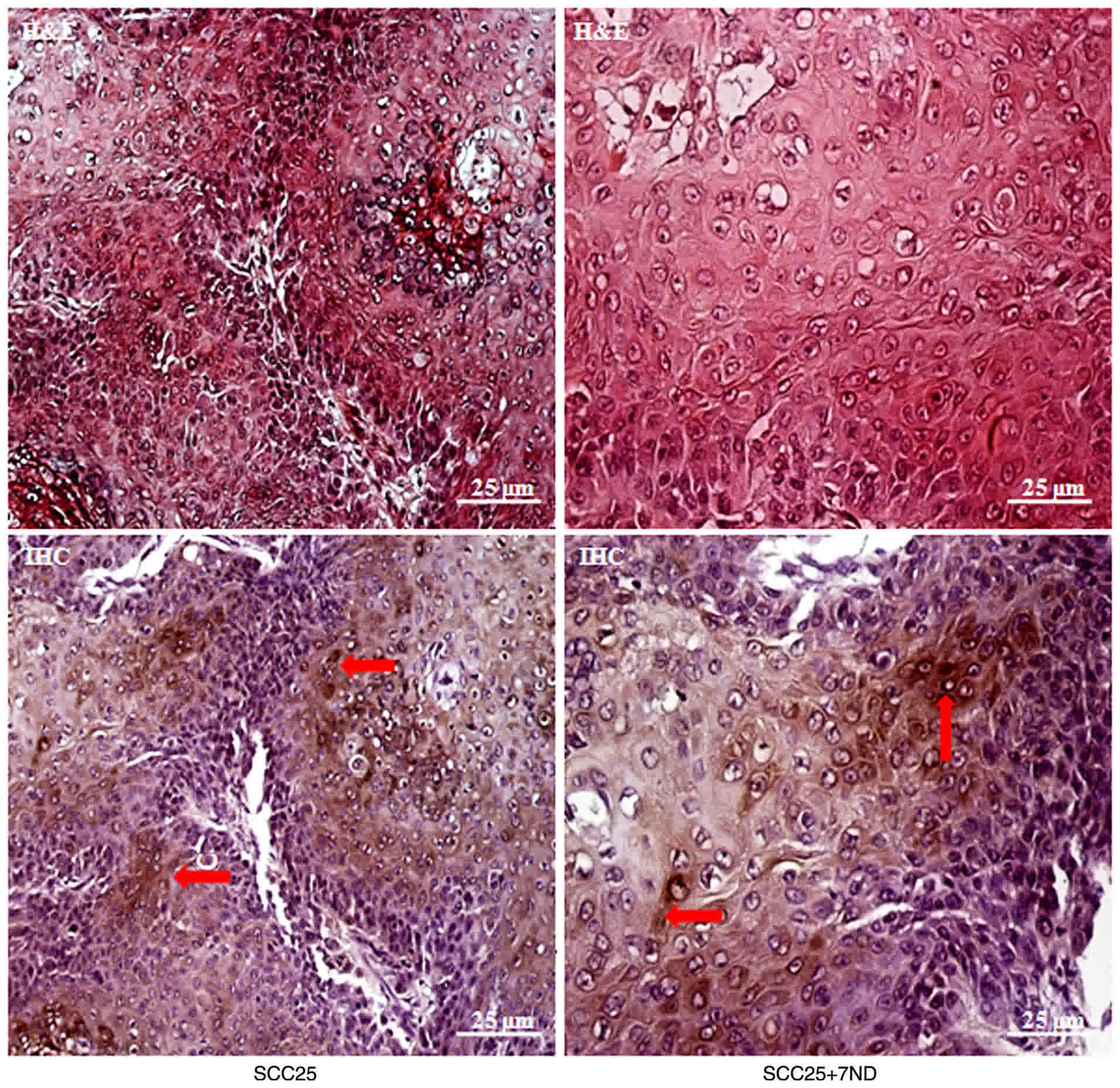
MCP-1 protein is localized to the
cytoplasm of tumor cells in vivo
Well-differentiated OSCC formed in the two groups
following injection with SCC25 cells with or without 7ND protein
(Fig. 9). Immunohistochemistry was
performed in order to examine MCP-1 protein localization, and
expression was revealed to be primarily in the cytoplasm and the
cell membrane of tumor cells (Fig.
9).
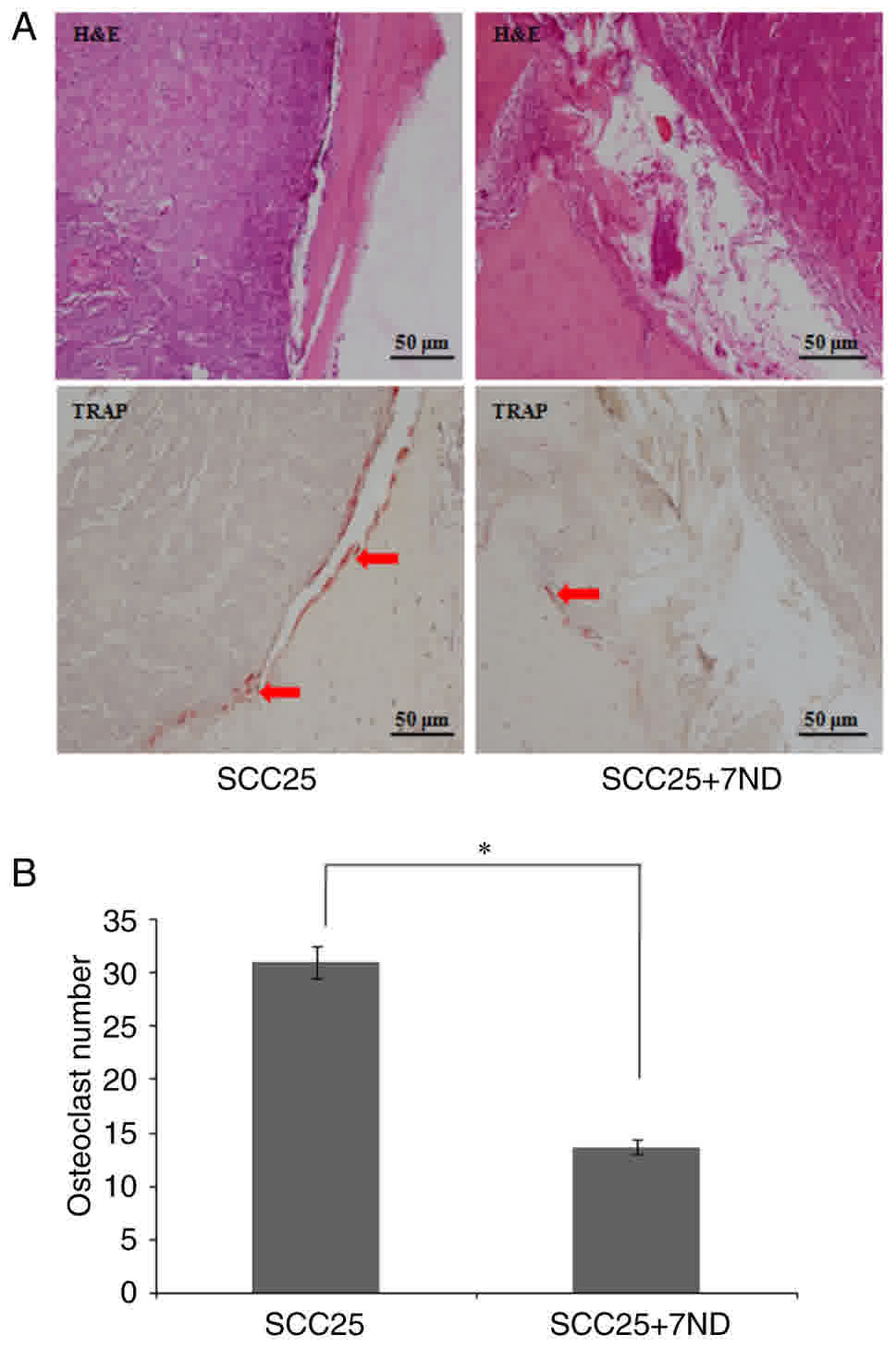
7ND decreases the number of
osteoclasts in bone resorption lacunae
The two groups revealed osteoclast accumulation in
the resorption lacunae (Fig. 10A).
TRAP staining was used to locate and count osteoclasts. Results
revealed significantly fewer osteoclasts localized to the
tumor-bone interface in the SCC25+7ND group, compared with the
SCC25 group (Fig. 10A-B).
Discussion
7ND is an N-terminal deletion mutant of MCP-1
(11). 7ND and wild-type MCP-1 form a
heterodimer and bind to the MCP-1 receptor to inhibit monocyte
chemotaxis (12). Ikeda et al
(13) first suggested using the 7ND
protein for skeletal muscle-directed in vivo electroporation
and, in 2001, reported that transgenic mice expressing 7ND blocked
the C-C motif chemokine receptor 2 pathway in vivo (14). Blockage of endogenous MCP-1 activity
has also been demonstrated to inhibit tumor-associated vessel
formation and early proliferation of melanoma cells in nude mice
(15).
Recent studies on the 7ND protein have revealed
promising results, particularly for total joint replacement in
end-stage arthritis (16–19). Yao et al (16) demonstrated that the 7ND protein
decreased MCP-1-induced migration of THP-1 macrophage cells in a
dose-dependent manner (16). A
therapeutic strategy of local 7ND delivery at the implant site was
further confirmed by the same group (17). By embedding the particles into the
space between the periosteum and the calvaria bone, mice were
treated with local injection of the 7ND protein. Compared with the
PBS-treated control group, 7ND treatment significantly decreased
particle-induced osteolysis, and led to an increase in bone volume
and mineral density. The same group also reported the development
of a biodegradable, layer-by-layer (LBL) coating platform, which
permits efficient loading and controlled release of 7ND from the
surface of orthopedic implants (18,19). The
LBL technique is suitable for an irregularly shaped material
surface, and accomplishes higher drug loading and more controlled
release. This is particularly useful for the rehabilitation of
defective maxilla and/or mandible caused by OSCC. Compared with
systemic treatment, the local delivery system has several
advantages. The most important point is to retain the biological
activity of synthesized protein, which may disturb the progression
of local bone invasion.
In the present study, the 7ND protein was cloned,
expressed and purified, based on the structure of MCP-1 and the 7ND
protein. The results of SDS-PAGE and western blot analysis
confirmed the expression of the 7ND protein. Considering that it
works as a dominant negative inhibitor of MCP-1, various
concentrations of the 7ND protein were examined and the results
revealed that 7ND efficiently inhibited the transmigration of
CD14+ monocytes, with their differentiation into
osteoclasts. These results confirmed the basic function of the
synthetic 7ND protein in vitro, which is the basis for
animal experiments involving 7ND injection in vivo.
Furthermore, the synthetic 7ND protein did not have inhibitory
effects on SCC25 tumor cell proliferation; therefore, it was
reasonable to inject these tumor cells with the 7ND protein in
vivo. Previous studies have attempted to modify the structure
of the 7ND protein in order to improve its efficiency. Severin
et al (20) constructed the
7ND-Fc fusion protein in order to extend its half-life. It was
revealed that 7ND-Fc had antagonistic activity in experimental
autoimmune encephalomyelitis.
Researchers have aimed to identify suitable
biomarkers for OSCC bone invasion. Efficient markers to quickly
identify the presence of bone invasion and effective targets to
successfully treat patients are required. Russmueller et al
(21) demonstrated that the
upregulation of osteoprotegrin expression was associated with bone
invasion, poor tumor regression and decreased long-term survival in
93 patients with OSCC. Tada et al (22) evaluated the effects of an NF-κB
inhibitor (IMD-0560) using a mouse model of jaw bone invasion. It
was demonstrated that IMD-0560 protected against zygoma and
mandible destruction by SCCVII mouse OSCC cells. Furthermore, Hsu
et al (23) reported the
combined use of radiation and a multi-kinase inhibitor of the
extracellular signal-regulated kinase-NF-κB signaling pathway in an
in situ human OSCC-bearing mouse model, and Sorafenib
suppressed radiation-induced NF-κB activity and its downstream
proteins.
Although the animal model used in the present study
was distinct from those used in the afore mentioned studies, the
advantages of calvariain jection animal models have been discussed
previously (8). In the present study,
the progression of OSCC bone invasion was reduced following 7ND
treatment and significantly fewer osteoclasts accumulated at the
tumor-bone interface, compared with the control group of untreated
SCC25 cells. Therefore, the results of the present study suggested
that 7ND has an inhibitory function in vivo, where it may
circulate in the blood to inhibit monocyte migration in order to
efficiently inhibit osteoclast differentiation. This observation
was also confirmed by flow cytometric analysis, since the CD14+
subpopulation of BMCs was significantly reduced in the mice in the
SCC25+7ND group, compared with the group of mice that received
SCC25 cells only.
In conclusion, the results of the present study
demonstrated the potential value of the 7ND protein in reducing the
progression of OSCC bone invasion. The MCP-1 variant, 7ND, not only
works in vitro to inhibit osteoclast differentiation, but
also reduces the progression of osteoclastic bone invasion in
vivo.
Acknowledgements
The present study was supported by the Foundation of
Sun Yat-Sen University (grant no. 13 ykpy 41), the Medical
Scientific Research Foundation of Guangdong Province (grant no.
B2014164) and the National Natural Science Foundation of China
(grant no. 81500839).
Funding
No funding was received.
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
SL contributed to the work of immunohistochemistry
and cell culture; CZ contributed to the work of 7ND cloning,
expression, purification and validation; MC, HL and SZ contributed
to the work of histological staining and data analysis; JZ
contributed to the work of research design and manuscript revising;
JQ contributed to the work of animal model establishment and
research design.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients and healthy donors enrolled in the present study. All
protocols were reviewed and approved by the University Ethics
Committee of Sun Yat-Sen University (2016-334QX).
Consent for publication
All participants of the study provided consent for
the data to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lehmann MH, Torres-Domínguez LE, Price PJ,
Brandmüller C, Kirschning CJ and Sutter G: CCL2 expression is
mediated by type I IFN receptor and recruits NK and T cells to the
lung during MVA infection. J Leukoc Biol. 99:1057–1064. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qidwai T: Chemokine genetic polymorphism
in human health and disease. Immunol Lett. 176:128–138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim MS, Day CJ and Morrison NA: MCP-1 is
induced by receptor activator of nuclear factor-{kappa}B ligand,
promotes human osteoclast fusion, and rescues granulocyte
macrophage colony-stimulating factor suppression of osteoclast
formation. J Biol Chem. 280:16163–16169. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim MS, Day CJ, Selinger CI, Magno CL,
Stephens SR and Morrison NA: MCP-1-induced human osteoclast-like
cells are tartrate-resistant acid phosphatase, NFATc1, and
calcitonin receptor-positive but require receptor activator of
NFkappaB ligand for bone resorption. J Biol Chem. 281:1274–1285.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Morrison NA, Day CJ and Nicholson GC:
Dominant negative MCP-1 blocks human osteoclast differentiation. J
Cell Biochem. 115:303–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Quan J, Johnson NW, Zhou G, Parsons PG,
Boyle GM and Gao J: Potential molecular targets for inhibiting bone
invasion by oral squamous cell carcinoma: A review of mechanisms.
Cancer Metastasis Rev. 31:209–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quan J, Elhousiny M, Johnson NW and Gao J:
Transforming growth factor-β1 treatment of oral cancer induces
epithelial-mesenchymal transition and promotes bone invasion via
enhanced activity of osteoclasts. Clin Exp Metastasis. 30:659–670.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quan J, Morrison NA, Johnson NW and Gao J:
MCP-1 as a potential target to inhibit the bone invasion by oral
squamous cell carcinoma. J Cell Biochem. 115:1787–1798. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gu J, Feng Y, Feng X, Sun C, Lei L, Ding
W, Niu F, Jiao L, Yang M, Li Y, et al: Structural and biochemical
characterization reveals LysGH15 as an unprecedented ‘EF-hand-like’
calcium-binding phage lysine. PLoS Pathog. 10:e10041092014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gong QM, Quan JJ, Jiang HW and Ling JQ:
Regulation of the stromal cell-derived factor-1alpha-CXCR4 axis in
human dental pulp cells. J Endod. 36:1499–1503. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Ernst CA and Rollins BJ: MCP-1:
Structure/activity analysis. Methods. 10:93–103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y and Rollins BJ: A dominant
negative inhibitor indicates that monocyte chemoattractant protein
1 functions as a dimer. Mol Cell Biol. 15:4851–4855. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikeda Y, Yonemitsu Y, Kataoka C, Kitamoto
S, Yamaoka T, Nishida K, Takeshita A, Egashira K and Sueishi K:
Anti-monocyte chemoattractant protein-1 gene therapy attenuates
pulmonary hypertension in rats. Am J Physiol Heart Circ Physiol.
283:H2021–H2028. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ni W, Egashira K, Kitamoto S, Kataoka C,
Koyanagi M, Inoue S, Imaizumi K, Akiyama C, Nishida KI and
Takeshita A: New anti-monocyte chemoattractant protein-1 gene
therapy attenuates atherosclerosis in apolipoprotein E-knockout
mice. Circulation. 103:2096–2101. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Koga M, Kai H, Egami K, Murohara T, Ikeda
A, Yasuoka S, Egashira K, Matsuishi T, Kai M, Kataoka Y, et al:
Mutant MCP-1 therapy inhibits tumor angiogenesis and growth of
malignant melanoma in mice. Biochem Biophys Res Commun.
365:279–284. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yao Z, Keeney M, Lin TH, Pajarinen J,
Barcay K, Waters H, Egashira K, Yang F and Goodman S: Mutant
monocyte chemoattractant protein 1 protein attenuates migration of
and inflammatory cytokine release by macrophages exposed to
orthopedic implant wear particles. J Biomed Mater Res A.
102:3291–3297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang X, Sato T, Yao Z, Keeney M,
Pajarinen J, Lin TH, Loi F, Egashira K, Goodman S and Yang F: Local
delivery of mutant CCL2 protein-reduced orthopaedic implant wear
particle-induced osteolysis and inflammation in vivo. J Orthop Res.
34:58–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keeney M, Waters H, Barcay K, Jiang X, Yao
Z, Pajarinen J, Egashira K, Goodman SB and Yang F: Mutant MCP-1
protein delivery from layer-by-layer coatings on orthopedic
implants to modulate inflammatory response. Biomaterials.
34:10287–10295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nabeshima A, Pajarinen J, Lin TH, Jiang X,
Gibon E, Córdova LA, Loi F, Lu L, Jämsen E, Egashira K, et al:
Mutant CCL2 protein coating mitigates wear particle-induced bone
loss in a murine continuous polyethylene infusion model.
Biomaterials. 117:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Severin IC, Souza AL, Davis JH, Musolino
N, Mack M, Power CA and Proudfoot AE: Properties of 7ND-CCL2 are
modulated upon fusion to Fc. Protein Eng Des Sel. 25:213–222. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Russmueller G, Moser D, Würger T, Wrba F,
Christopoulos P, Kostakis G, Seemann R, Stadler V, Wimmer G, Kornek
G, et al: Upregulation of osteoprotegerin expression correlates
with bone invasion and predicts poor clinical outcome in oral
cancer. Oral Oncol. 51:247–253. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tada Y, Kokabu S, Sugiyama G, Nakatomi C,
Aoki K, Fukushima H, Osawa K, Sugamori Y, Ohya K, Okamoto M, et al:
The novel IκB kinase β inhibitor IMD-0560 prevents bone invasion by
oral squamous cellcarcinoma. Oncotarget. 5:12317–12330. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu FT, Chang B, Chen JC, Chiang IT, Liu
YC, Kwang WK and Hwang JJ: Synergistic effect of sorafenib and
radiation on human oral carcinoma in vivo. Sci Rep. 5:153912015.
View Article : Google Scholar : PubMed/NCBI
|