Introduction
Renal cancer types are the most aggressive common
malignancies of all urological cancer types, amongst which clear
cell renal cell carcinoma (ccRCC) is the the most prevalent
(1). The incidence of ccRCC has
increased during past 3 decades, particularly in high income
countries (2). To date, several
pathological and clinical features have been used to predict
patient prognosis, including tumor size, pathological stage, tumor
stage, performance status, localized symptoms and cachexia
(3). However, few molecular
biomarkers exist that may predict patient prognosis and serve as
therapeutic targets, although numerous molecular biomarkers have
been investigated in ccRCC (4–5). Previous
studies have highlighted the mutations of BRCA1 associated
protein-1 and SET domain containing 2, which are inversely
correlated with the outcome of patients with ccRCC (6–8).
Unfortunately, their use is so limited in clinical practice that
further studies are required in order to identify the mechanism of
ccRCC.
Long non-coding RNAs (lncRNAs) are a notable subtype
of non-coding RNAs that are >200 nt in length, but are unable to
translate proteins (9). Previous
years have witnessed the importance of lncRNAs in cancer research
and revealed their notable impact on cancer cell biology (10). lncRNAs serve crucial regulatory
functions in the biological processes of cancer, including
chromatin modification, transcription, post-transcriptional
processing and translation. Emerging evidence has indicated that
lncRNAs may be potential biomarkers and therapeutic targets
(11,12). lncRNA Pvt1 oncogene (non-protein
coding) (PVT1), located at 8q24.21, was revealed to be upregulated
and regulated in the biological processes of various cancer types,
including gastric, lung, pancreatic and breast cancer (13–15).
However, the potential functions of PVT1 and its underlying
mechanism in ccRCC remain unclear.
The present study aimed to investigate the potential
biological functions of PVT1 in ccRCC and determine whether PVT1
may serve as a reliable prognostic marker for the disease. PVT1
expression in ccRCC tissues was investigated and validated using
The Cancer Genome Atlas (TCGA) database. The association between
PVT1 expression and clinical parameters and prognosis of ccRCC
patients was also assessed. Furthermore, the function and
underlying molecular mechanism of PVT1 in apoptosis and the cell
cycle of ccRCC cells was evaluated.
Materials and methods
Patient and tissue samples
A total of 40 paired ccRCC tissues and normal
adjacent tissues, which were obtained from patients (30 males, 10
females; age range 31–66 years; mean age, 51 years) who underwent
radical nephrectomy at Sun Yat-sen Memorial Hospital, Sun Yat-sen
University (Guangzhou, China), were collected. Patients who had
received any chemotherapy or targeted treatments were excluded. The
pathological features of the samples were confirmed by pathologists
at Sun Yat-sen Memorial Hospital. All tissues were stored at −80°C
immediately after surgery for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis. All tissue samples
were collected with written informed consent from all patients and
the study was ethically approved by the Ethic Review Committee of
Sun Yat-sen Memorial Hospital.
ccRCC data from TCGA database
The clinical and pathological information of 517
patients with ccRCC were downloaded from TCGA database (http://cancergenome.nih.gov/) (16). TCGA gene expression profile was
measured using the Illumina HiSeq 2000 RNA Sequencing platform
(Illumina, Inc., San Diego, CA, USA). RNA-Seq by
Expectation-Maximization (RSEM software v1.3.0; provided by the
University of Wisconsin, Madison, WI, USA) normalized count data
was used in this study. Copy number alterations were estimated by
Genomics Identification of Significant Targets in Cancer 2.0
(GISTIC 2.0; provided by the Broad Institute of MIT and Harvard,
Cambridge, MA, USA) (17), which
defined the copy-number alteration of each gene as −2, −1, 0, 1 or
2, representing homozygous deletion, heterozygous deletion, diploid
normal copy number, low-level amplification (gain) and high-level
amplification, respectively. For analysis, the amplification and
gain groups were combined and the homozygous and heterozygous
groups were also combined. The RNA sequences of 448 ccRCC tissues
were downloaded from the Atlas of Noncoding RNAs in Cancer (TANRIC)
database (http://ibl.mdanderson.org/tanric/_design/basic/index.html).
The patients were divided into high and low PVT1 expression groups.
The median value of PVT1 expression (0.803) was used as the cut-off
value.
Cell culture
RCC cell lines, including 786-O, Caki-1, ACHN and
769-P, and the human normal kidney cell line HK-2 were obtained
from the American Tissue Culture Collection (ATCC; Manassas, VA,
USA). 786-O and 769-P cells were cultured in RPMI-1640 medium
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences, Logan, UT, USA). Caki-1 cells
were cultured in ATCC-formulated McCoy's 5a Medium Modified medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% heat-inactivated FBS. ACHN cells were
cultured in Dulbecco's modified eagle's medium (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% heat-inactivated
FBS. HK-2 cells were cultured in keratinocyte-SFM (Gibco; Thermo
Fisher Scientific, Inc.) supplemented with 10% heat-inactivated
FBS. All cells were incubated at 37°C with 5% CO2 once
seeded. The medium was replaced every 2 days.
Small interfering RNA (siRNA)
transfection and RNA extraction
Using Lipofectamine® RNAiMAX (Invitrogen;
Thermo Fisher Scientific, Inc.), ccRCC cells (786-O and Caki-1)
were transfected with siRNA at 37°C for 2 days according to the
manufacturer's protocol. A total of 1×105 ccRCC cells
and 5 µl siRNA per well was used in each transfection in a 6-well
plate. siRNAs were obtained from Shanghai GenePharma Co., Ltd.
(Shanghai, China). The siRNA sequences were as follows: Si-PVT1a,
5′-CAGCCAUCAUGAUGGUACUTT-3′ and si-PVT1b,
5′-GGCACAUUUCAGGAUACUATT−3′. Negative control siRNA for PVT1,
5′-UUCUCCGAACGUGUCACGUTT-3′. Total RNA was extracted at 2 days
post-transfection using TRIzol reagent (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol.
Cell proliferation assay
ccRCC Cells (786-O and Caki-1) transfected with
si-PVT1 and negative control were harvested at 24 h
post-transfection and 1×103 cells were cultured on
96-well plates. Cells were incubated with CellTiter 96®
Aqueous One Solution Reagent kit (MTS; Promega Corporation,
Madison, WI, USA) at 37°C for 3 h and assessed every 24 h for 5
days according to the manufacturer's protocol. Absorbance was
measured with the multifunctional microplate reader SpectraMax M5
(Molecular Devices, LLC, Sunnyvale, CA, USA) at 490 nm.
Flow cytometry analysis
ccRCC Cells (786-O and Caki-1) transfected with
si-PVT1 and negative control were harvested at 48 h
post-transfection. A total of 2×105 cells for each
analysis of apoptosis were stained at room temperature for 15 min
with fluorescein isothiocyanate-Annexin V and propidium iodide
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China) and analyzed
using the FACSVerse flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA). Early apoptotic cells were assessed and compared. A total
of 1×105 cells for each cell cycle analysis were fixed
in 75% ethanol at 4°C overnight and then stained with propidium
iodide at room temperature for 15 min using a cell cycle detection
kit (Nanjing KeyGen Biotech, Co., Ltd.) according to the
manufacturer's protocol. The percentages of cells in the
G0/G1, S and G2/M phases were
assessed within 4 h. The acquired data were analyzed using FlowJo
10.0 (FlowJo LLC, Ashland, OR, USA).
RT-qPCR
Total RNA of ccRCC cells (786-O and Caki-1) was
extracted at 2 days post-transfection using TRIzol reagent (Thermo
Fisher Scientific, Inc.) according to the manufacturer's protocol.
Total RNA was reverse transcribed into cDNA using the PrimeScript™
RT Master mix (Takara Biotechnology Co., Ltd., Dalian, China).
RT-qPCR was performed using SYBR-Green PCR Master mix (Roche
Diagnostics, Basel, Switzerland) with the Roche LightCycler 480
(Roche Diagnostics). GAPDH was used as the internal control. The
RT-qPCR reaction included a preincubation step (95°C for 300 sec),
and 40 cycles of amplification step (95°C for 15 sec, 56°C for 15
sec and 72°C for 15 sec), a melting step (95°C for 10 sec, 65°C for
60 sec, 97°C for 1 sec) and a cooling step (37°C for 30 sec). The
2−ΔΔCq method was used to compare expression (18). The sequences of the primers used are
as follows: GAPDH sense, 5′-GAGCCAAAAGGGTCATCATCTC-3′ and
antisense, 5′-GGTCATGAGTCCTTCCACGATAC-3′; PVT1 sense,
5′-CATCCGGCGCTCAGCT-3′ and antisense, 5′-TCATGATGGCTGTATGTGCCA−3′;
EGFR sense, 5′-CGGGACATAGTCAGCAGTG-3′ and antisense,
5′-GCTGGGCACAGATGATTTTG-3′; MYC proto-oncogene, bHLH transcription
factor (MYC) sense, 5′-CTTCTCTCCGTCCTCGGATTCT-3′ and antisense,
5′-GAAGGTGATCCAG-3′; protein kinase B (AKT) sense,
5′-ACGGGCACATTAAGATCACA-3′ and antisense,
5′-TGCCGCAAAAGGTCTTCATG-3′; cyclin D1 sense, 5′-GAAGGTGATCCAG-3′
and antisense, 5′-GGCGGATTGGAAATGAACTT-3′; and p21 sense,
5′-CGATGCCAACCTCCTCAACGA-3′ and anti-sense,
5′-CATCCGGCGCTCAGCT-3′.
Western blot analysis
A total of 1×106 ccRCC cells (786-O and
Caki-1) were harvested at 48 h post-transfection and lysed with
radioimmunoprecipitation assay protein extraction reagent (Beyotime
Institute of Biotechnology, Haimen, China) supplemented with a
protease inhibitor cocktail (Roche Diagnostics). The total protein
concentration was measured using a Bicinchoninic Acid Protein Assay
kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with
Multiskan MK3 (Thermo Fisher Scientific, Inc., Waltham, MA, USA). A
total of 50 µg protein was added to each panel of 10 (for cyclin
D1, Bax, p21, Myc, AKT and p-AKT) or 7.5% (for PARP and EGFR)
sodium dodecyl sulfate-polyacrylamide gel according to the
molecular weight of the targeted protein. Protein was transferred
onto 0.22-µm nitrocellulose membranes (EMD Millipore, Billerica,
MA, USA) following electrophoresis. The membranes were blocked with
5% skimmed milk at room temperature for 1 h, followed by incubation
with the primary antibodies at 4°C overnight. The membranes were
then incubated with corresponding horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (sc-2004; 1:5,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at room temperature for 1 h.
Primary antibodies (1:1,000 dilution) were against PARP (cat. no.
9532), EGFR (cat. no. 2085), MYC (cat. no. 13987), p21 (cat. no.
2947), cyclin D1 (cat. no. 2922), Bax (cat. no. 5023), AKT (cat.
no. 4685) and p-AKT (cat. no. 4060; Cell Signaling Technology,
Inc., Danvers, MA, USA). GAPDH was used as the reference gene for
EGFR, AKT, MYC and p-AKT, and β-tubulin was used as the reference
gene for PARP, cyclin D1, Bax and p21.
Statistical analysis
χ2 or Kruskal-Wallis tests were used to
analyze the association between the expression of PVT1 and the
clinicopathological parameters. The Kaplan-Meier method and
log-rank test were used for survival analyses. P<0.05 was
considered to indicate a statistically significant difference.
Statistical analyses were performed using SPSS software 17.0 (SPSS
Inc., Chicago, IL, USA). All these experiments were performed three
times.
Results
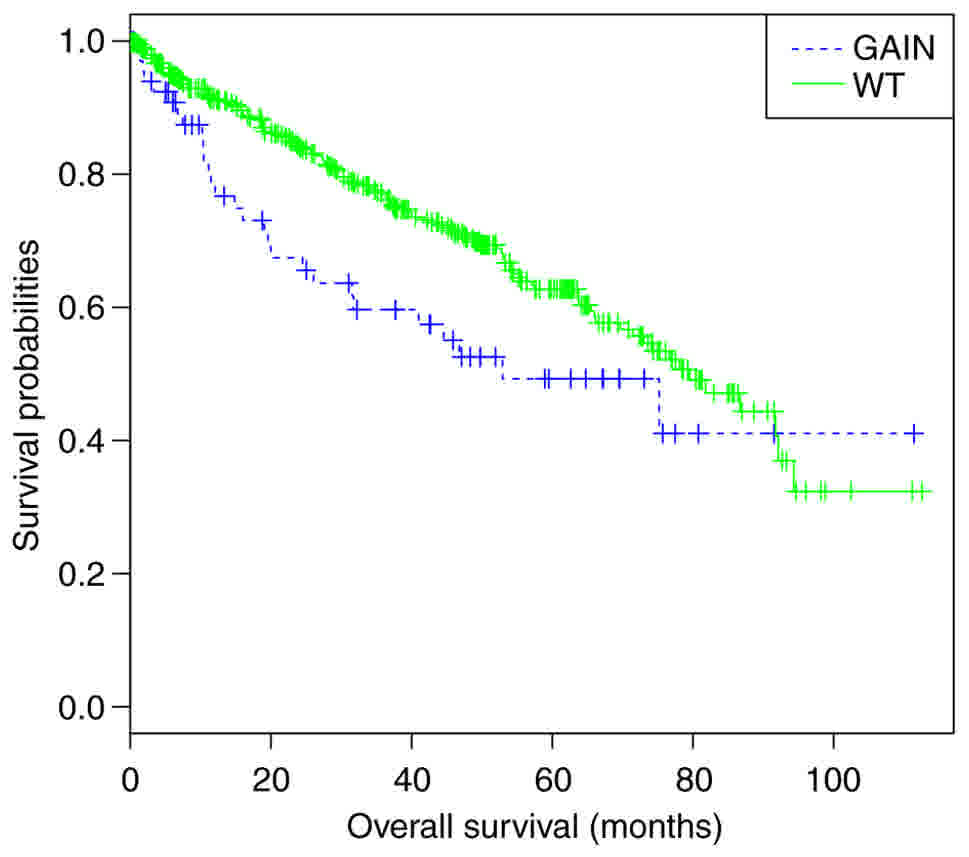
PVT1 copy number variant in ccRCC
affects overall survival
Of the 517 ccRCC cases selected from TCGA, 70 cases
were identified with PVT1 gain/amplification. Next, the association
between PVT1 copy number and patient prognosis was analyzed.
Kaplan-Meier analysis revealed that patients with PVT1 gain
experienced significantly worse overall survival compared with
patients without PVT1 gain (log-rank, P<0.01; Fig. 1).
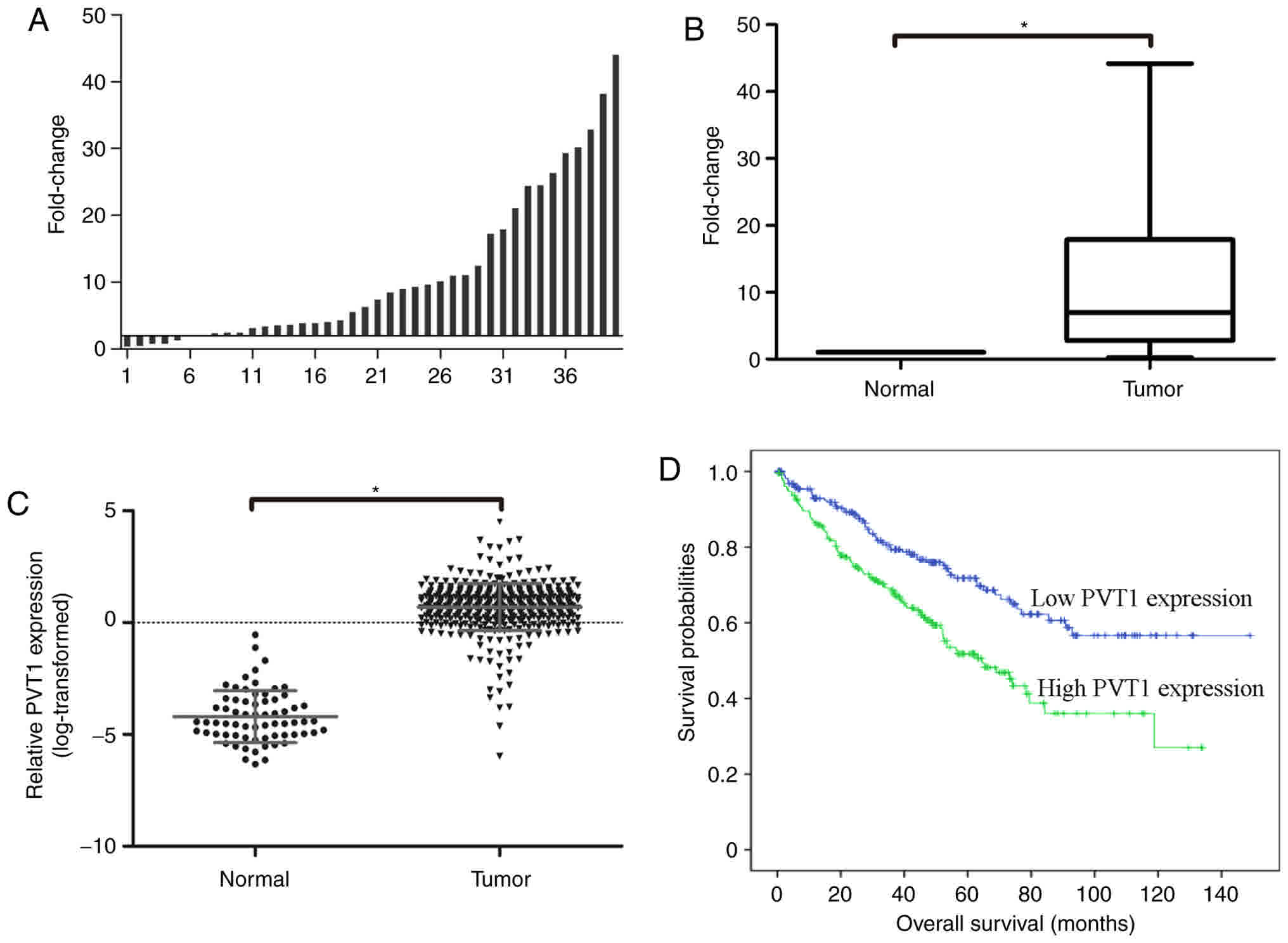
PVT1 is upregulated in ccRCC
tissues
In order to investigate the role of PVT1 in ccRCC,
the expression of PVT1 in 40 paired ccRCC tissues and normal
adjacent tissues was examined using RT-qPCR. A fold change of
>1.5 was designated as upregulated, and the results revealed
that PVT1 was upregulated in 33 (82.5%) of ccRCC tissue cases
compared with normal adjacent tissues (Fig. 2A). As presented in Fig. 2B, the mean expression of PVT1 was
significantly higher in tumor tissues compared with that in
adjacent normal tissues (P<0.001).
To further validate the expression of PVT1 in ccRCC,
PVT1 RNA-seq expression values from the TCGA database were
analyzed. It was identified that PVT1 was significantly upregulated
in ccRCC tissues (n=448) compared with that in normal kidney
tissues (n=67) (Fig. 2C).
PVT1 expression is associated with the
poor prognosis of patients with ccRCC
Subsequently, the association between PVT1
expression and clinical characteristics was analyzed. The results
revealed that PVT1 expression was significantly associated with T
stage (P<0.001), M stage (P=0.024), AJCC stage (P<0.001) and
patient age (P=0.018) (Table I). No
significant association was identified between PVT1 expression and
patient sex, N stage and tumor grade. Next, the association between
PVT1 expression and the prognosis of patients with ccRCC was
assessed. The patients with ccRCC were separated into the high PVT1
expression group (n=224) and the low PVT1 expression group (n=224)
according to PVT1 expression, and Kaplan-Meier analysis was
performed. The result revealed that the overall survival time was
significantly shorter in the high PVT1 expression group compared
with that in the low PVT1 expression group (log-rank, P<0.01;
Fig. 2D).
 | Table I.Association between Pvt1 oncogene
(non-protein coding) expression and clinical characteristics of
patients with clear cell renal cell carcinoma. |
Table I.
Association between Pvt1 oncogene
(non-protein coding) expression and clinical characteristics of
patients with clear cell renal cell carcinoma.
|
Characteristics | n | Expression | F-value | P-value |
|---|
| Sex |
|
| 0.982 | 0.322 |
|
Male | 288 | 0.656 |
|
|
|
Female | 160 | 0.759 |
|
|
| Age, years |
|
| 5.633 | 0.018a |
|
<60 | 205 | 0.566 |
|
|
|
≥60 | 243 | 0.800 |
|
|
| T stage |
|
| 17.90 | <0.001a |
|
T1-2 | 276 | 0.530 |
|
|
|
T3-4 | 172 | 0.953 |
|
|
| M stage |
|
| 5.102 | 0.024a |
| M0 | 376 | 0.644 |
|
|
| M1 | 72 | 0.947 |
|
|
| N stage |
|
| 2.934 | 0.088 |
| N0 | 218 | 0.594 |
|
|
| N1 | 16 | 1.084 |
|
|
| NX | 214 | 0.766 |
|
|
| Tumor grade |
|
| 0.017 | 0.895 |
|
G1-2 | 208 | 0.706 |
|
|
|
G3-4 | 235 | 0.693 |
|
|
| GX | 5 | 0.162 |
|
|
| AJCC stage |
|
| 14.78 | <0.001a |
| I | 215 | 0.477 |
|
|
| II | 46 | 0.626 |
|
|
|
III | 112 | 0.966 |
|
|
| IV | 75 | 0.942 |
|
|
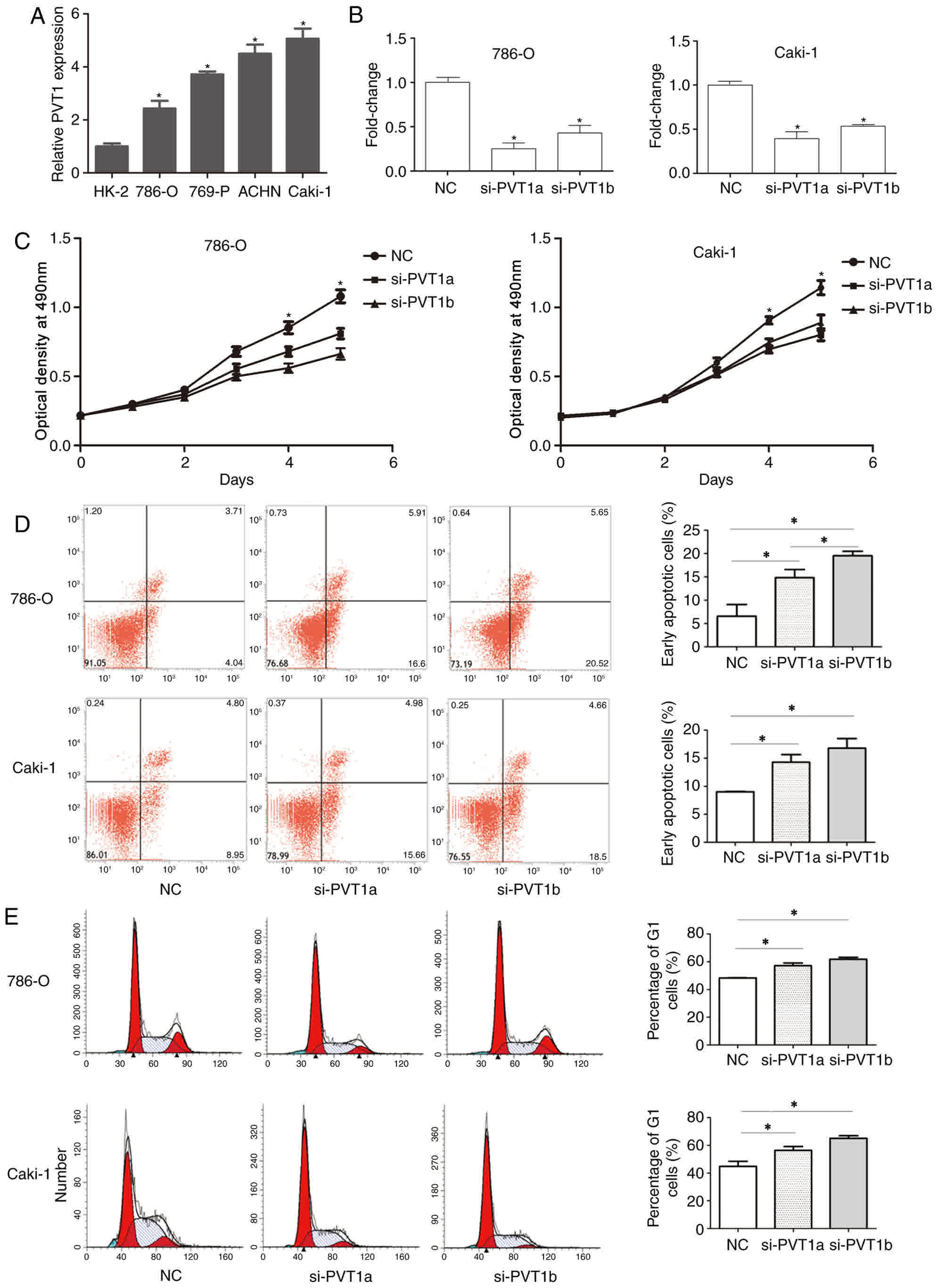
Knockdown of PVT1 significantly
inhibits the proliferation of ccRCC cells
In order to investigate the potential biological
functions of PVT1 in ccRCC, the expression of PVT1 in ccRCC cell
lines (786-O, Caki-1, ACHN and 769-P) and one normal kidney cell
line (HK-2) was examined using RT-qPCR. The results revealed that
PVT1 expression was significantly higher in ccRCC cells compared
with that in HK-2 cells (Fig. 3A).
786-O and Caki-1 cells were then transfected with si-PVT1s or si-NC
using Lipofectamine RNAiMAX Transfection reagent, and the
transfection efficiency was tested using RT-qPCR. The results
revealed that the expression of PVT1 was significantly reduced in
cells transfected with si-PVT1a and si-PVT1b compared with that in
cells transfected with si-NC (Fig.
3B). Next, an MTS assay was performed to examine the effect of
PVT1 on the proliferation of the ccRCC cells. The MTS assay
revealed that the knockdown of PVT1 significantly inhibited the
proliferation of 786-O and Caki-1 cells in a time-dependent manner
compared with cells in the NC group (Fig.
3C).
Knockdown of PVT1 significantly
induces apoptosis and promotes cell cycle arrest in ccRCC
cells
To further investigate the effect of PVT1 on the
growth of ccRCC cells, cell apoptosis analysis and cell cycle
analysis were performed using flow cytometry. Cell apoptosis
analysis indicated that the knockdown of PVT1 significantly
increased the apoptosis rate in 786-O and Caki-1 cells, most
notably in 786-O si-PVT1a group compared with the NC group
(P<0.05; Fig. 3D). Cell cycle
analysis revealed that the knockdown of PVT1 also significantly
promoted cell cycle arrest at the G0/G1 phase compared with the
normal control (P<0.05; Fig. 3E).
No significant difference in G1 phase was found between si-PVT1a
and si-PVT1b.
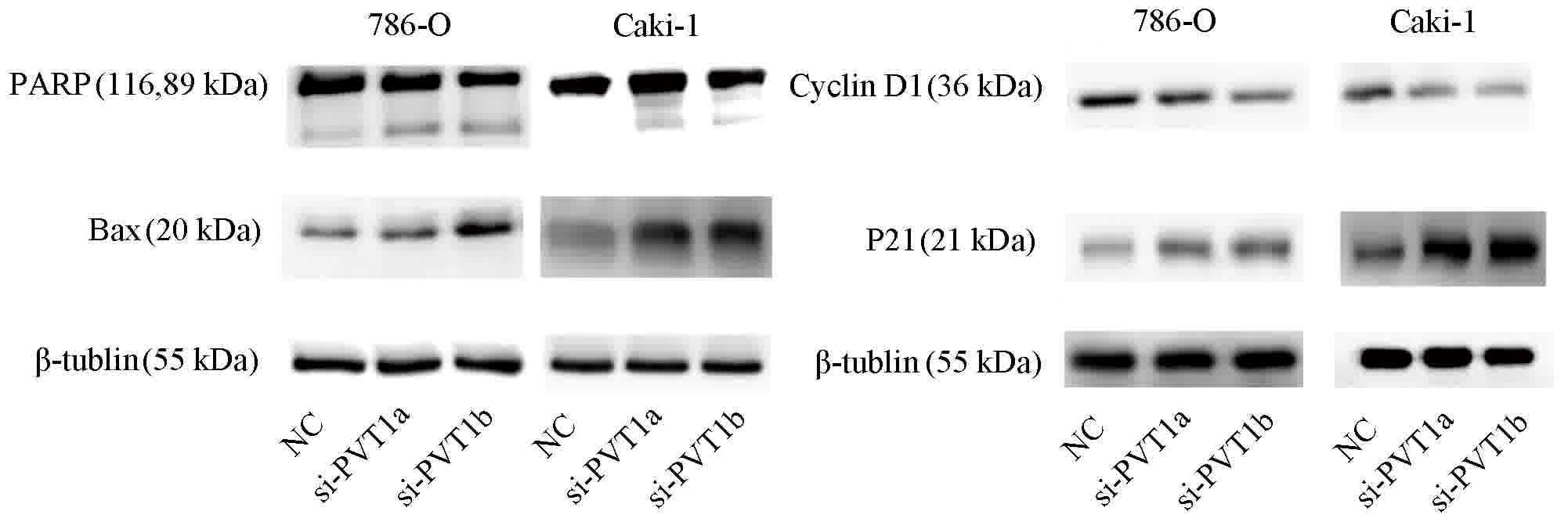
Next, western blotting was performed to support the
results of flow cytometry. The results demonstrated that the
knockdown of PVT1 increased the level of Bax and the cleavage of
PARP, which serve notable functions in the activation of cell
apoptosis. Meanwhile, the knockdown of PVT1 decreased the level of
cyclin D1, a major mediator of the cell cycle, and increased the
level of p21 (Fig. 4).
PVT1 regulates the activation of the
EGFR pathway in ccRCC cells
To investigate the underlying molecular mechanisms
of PVT1 function, a potential pathway involved in cell
proliferation was investigated. As EGFR pathways serve a notable
role in the cell proliferation, apoptosis and cell cycle of ccRCC
(19,20), the mRNA and protein expression levels
of EGFR following the transfection of si-PVT1a and b and si-NC were
investigated. The results demonstrated that the mRNA expression
level of EGFR was significantly downregulated following the
knockdown of PVT1 compared with that of the NC (Fig. 5A), and the protein expression was also
notably downregulated (Fig. 5B).
Furthermore, AKT and MYC, the downstream proteins of EGFR, were
detected at the mRNA and protein level following the knockdown of
PVT1. It was revealed that the mRNA expression levels of AKT and
MYC were significantly downregulated in si-PVT1 transfected groups
compared with that in the NC groups (Fig.
5A), and the protein expression of AKT, phosphorylated (p-)AKT
and MYC in cells transfected with si-PTV1 were notably
downregulated compared with that in the NC groups (Fig. 5B). The results indicated that PVT1 may
potentially regulate cell proliferation, apoptosis and the cell
cycle of ccRCC cells through the activation of the EGFR
pathway.
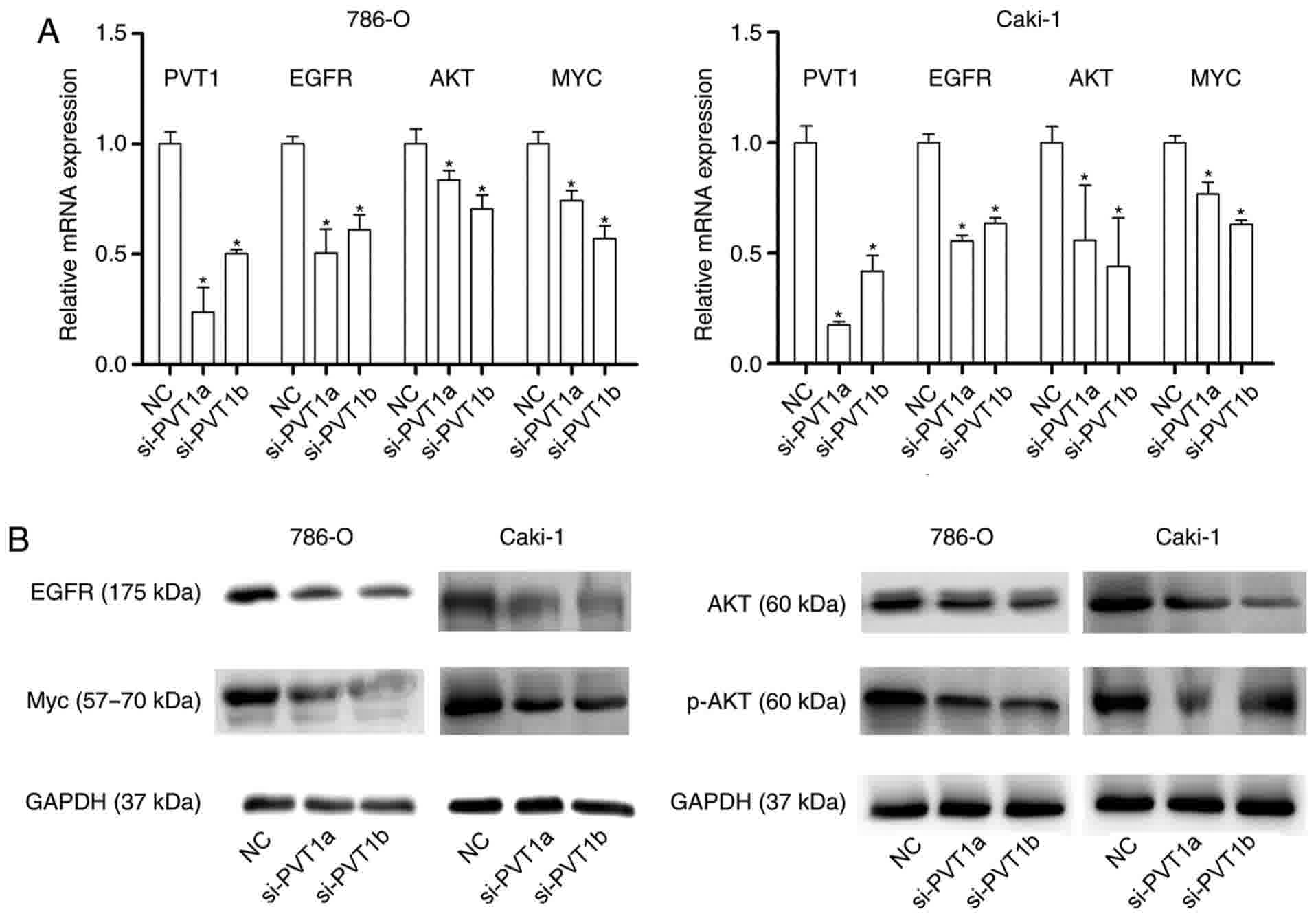 | Figure 5.PVT1 regulates the activation of the
EGFR pathway in clear cell renal cell carcinoma cells. (A) Reverse
transcription-quantitative polymerase chain reaction examined the
mRNA expression of EGFR, AKT and MYC following PVT1-knockdown
(*P<0.05 vs. NC). (B) Western blotting examined the protein
expression of EGFR, AKT, p-AKT and MYC following PVT1-knockdown.
GADPH was used as the reference gene. PVT1, Pvt1 oncogene
(non-protein coding); EGFR, epidermal growth factor receptor; AKT,
protein kinase B; MYC, MYC proto-oncogene, BHLH transcription
factor; p-, phosphorylated; NC, negative control. |
Discussion
Emerging evidence has revealed that lncRNAs are of
great importance in cell development and human diseases (21–23).
lncRNAs have different expression patterns in different diseases,
and their aberrant expression may be involved in the
pathophysiology of various diseases (24). Studies have demonstrated that lncRNAs
may be involved in the progression of various cancer types,
including breast and lung cancer (13,18). To
date, only a few lncRNAs have been studied in renal cancer. Zhang
et al (25) identified that
the overexpression of metastasis associated lung adenocarcinoma
transcript 1 (non-protein coding) (MALAT1) may predict the poor
prognosis of patients with renal cancer, and Hirata et al
(26) demonstrated that MALAT1
promoted tumorigenesis through enhancer of zeste homolog 2. The
functions of growth arrest-specific 5 (27), neuroblastoma-associated transcript 1
(28) and HOX transcript antisense
RNA (29) in the progression of ccRCC
were also investigated.
PVT1, an lncRNA mapped at 8q24, was demonstrated to
be involved in the tumorigenesis of various cancer types. A
previous study suggested that PVT1 expression was significantly
higher in colorectal cancer tissues compared with that in normal
tissues, and that the knockdown of PVT1 may inhibit cell
proliferation (30). Wan et al
(13) additionally revealed that PVT1
promoted lung cancer cell proliferation through the regulation of
large tumor suppressor kinase 2. Various studies additionally
revealed that PVT1 influenced the expression of MYC (14,31). All
these reports indicated that PVT1 may be involved in cancer
pathophysiology (32). However, to
the best of our knowledge, no previous study has reported the
expression pattern and biological function of PVT1 in ccRCC.
In the present study, the association between PVT1
expression and ccRCC was investigated. PVT1 was revealed to be
significantly upregulated in ccRCC tissues compared with that in
adjacent normal tissues (P<0.001). Furthermore, high PVT1
expression was associated with advanced ccRCC stage. The results
demonstrated that PVT1 expression was significantly associated with
age (P=0.018). PVT1 expression in patients ≥60 years old was
significantly higher compared with that in patients <60 years
old. Survival analysis revealed that the overall survival time was
significantly shorter in the high PVT1 expression group compared
with that in the low PVT1 expression group (P<0.01). It was
hypothesized that the association between PVT1 expression and age
affected the survival analysis to a certain extent. Although PVT1
expression is associated with age, PVT1 expression may be an
independent prognostic factor for patients with ccRCC. Furthermore,
the findings were confirmed by TCGA database, as its cohort size
was large enough and the follow-up time was long enough to draw a
conclusion without substantial bias, and it demonstrated that the
survival time of patients with high PVT1 expression was shorter
compared with that of patients with low PVT1 expression. The
results revealed that PVT1 expression was significantly associated
with T stage (P<0.001) and M stage (P=0.024), but not with tumor
grade. Tumor grading is a measure of cell differentiation and is
based on the resemblance of the tumor to the tissue of origin.
Tumor grading is distinguished from staging, which is a measure of
the extent to which the cancer has spread (33). This finding may be due to the fact
that PVT1 serves an important role in the cell cycle and cell
proliferation, which are associated with advanced T stage and M
stages. Additionally, the differentiation of RCC cells was not
markedly affected by PVT1, resulting in non-significant differences
between PVT1 expression and tumor grade. All these results
suggested that PVT1 may promote the tumorigenesis of ccRCC.
To elucidate how PVT1 affects the biological
behavior of ccRCC cells, a series of experiments was designed to
evaluate the biological function of PVT1. It was revealed that the
knockdown of PVT1 induced cell apoptosis and promoted cell cycle
arrest at the G1 phase. The mitochondria-mediated intrinsic pathway
and the death receptor-induced extrinsic pathway are the major
pathways for the regulation of cell apoptosis (34,35). Bax
is a key component for cellular induced apoptosis, which increases
the permeability of the mitochondrial membrane and results in the
activation of the caspase pathway for apoptosis (36). Western blotting in the present study
revealed that the knockdown of PVT1 increased the level of Bax and
the cleavage of PARP, a marker of cells undergoing apoptosis
(37). Therefore, the results of the
present study indicated that the knockdown of PVT1 induced cell
apoptosis through the mitochondria-dependent pathway. In addition,
the knockdown of PVT1 induced cell cycle arrest at the G1 phase by
regulating the level of cyclin D1, which is an important protein
for controlling cell transition through the G1 phase into the DNA
synthesis S phase (38).
EGFR family members are involved in the mechanisms
of cell proliferation and apoptosis, and the cell cycle (39–41). EGFR
signaling may regulate the biological behavior of cancer cells
through AKT, MAPK and numerous other pathways (42). In the present study, it was revealed
that silencing PVT1 decreased the expression of EGFR and its
downstream protein AKT, and p-AKT. Research has also indicated that
MYC expression is regulated by the EGFR family (43). The expression of MYC was confirmed and
it was revealed that the expression of MYC was decreased subsequent
to the silencing of PVT1. Therefore, it was concluded that PVT1
activated the EGFR pathway and participated in the progression of
ccRCC.
In conclusion, the present study demonstrated that
PVT1 expression was significantly upregulated in ccRCC compared
with that in normal tissues, and that the high expression of PVT1
was associated with advanced stage and a poor prognosis. Results
also indicated that the knockdown of PVT1 induced the apoptosis and
cell cycle arrest of ccRCC cells through activation of the EGFR
pathway. These findings indicate that PVT1 may serve as a novel
therapeutic target in ccRCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 81672534 and
81472388), the Key Laboratory of Malignant Tumor Molecular
Mechanism and Translational Medicine of Guangzhou Bureau of Science
and Information Technology [grant no. (2013) 163], the Key
Laboratory of Malignant Tumor Gene Regulation and Target Therapy of
Guangdong Higher Education Institutes (grant no. KLB09001) and the
Guangdong Science and Technology Department (grant no.
2015B050501004).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
WX, WL and ZZ designed the experiments and wrote the
article. WL and ZZ performed the experiments. HC and YC performed
the statistical analysis. All authors have read and approved the
final manuscript for publication.
Ethics approval and consent to
participate
All tissue samples were collected with written
informed consent from all patients and the study was ethically
approved by the Ethic Review Committee of Sun Yat-sen Memorial
Hospital.
Consent for publication
The patients included in the present study gave
their consent for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lipworth L, Tarone RE and McLaughlin JK:
The epidemiology of renal cell carcinoma. J Urol. 176:2353–2358.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paner GP, Stadler WM, Hansel DE, Montironi
R, Lin DW and Amin MB: Updates in the eighth edition of the
tumor-node-metastasis staging classification for urologic cancers.
Eur Urol. Jan 8–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dasgupta P, Kulkarni P, Majid S, Varahram
S, Hashimoto Y, Bhat NS, Shiina M, Deng G, Saini S, Tabatabai ZL,
et al: MicroRNA-203 inhibits long noncoding RNA HOTAIR and
regulates tumorigenesis through epithelial-to-mesenchymal
transition pathway in renal cell carcinoma. Mol Cancer Ther. Feb
13–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu B, Wang J and Jin X: MicroRNA-138
suppresses cell proliferation and invasion of renal cell carcinoma
by directly targeting SOX9. Oncol Lett. 14:7583–7588.
2017.PubMed/NCBI
|
|
6
|
Duns G, van den Berg E, van Duivenbode I,
Osinga J, Hollema H, Hofstra RM and Kok K: Histone
methyltransferase gene SETD2 is a novel tumor suppressor gene in
clear cell renal cell carcinoma. Cancer Res. 70:4287–4291. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kapur P, Peña-Llopis S, Christie A,
Zhrebker L, Pavía-Jiménez A, Rathmell WK, Xie XJ and Brugarolas J:
Effects on survival of BAP1 and PBRM1 mutations in sporadic
clear-cell renal-cell carcinoma: A retrospective analysis with
independent validation. Lancet Oncol. 14:159–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gossage L, Murtaza M, Slatter AF,
Lichtenstein CP, Warren A, Haynes B, Marass F, Roberts I, Shanahan
SJ, Claas A, et al: Clinical and pathological impact of VHL, PBRM1,
BAP1, SETD2, KDM6A, and JARID1c in clear cell renal cell carcinoma.
Genes Chromosomes Cancer. 53:38–51. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huarte M: The emerging role of lncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang S, Xu J and Zeng X: A six-long
non-coding RNA signature predicts prognosis in melanoma patients.
Int J Oncol. Feb 7–2018.(Epub ahead of print). View Article : Google Scholar
|
|
12
|
Chen X, Chen Z, Yu S, Nie F, Yan S, Ma P,
Chen Q, Wei C, Fu H, Xu T, et al: Long noncoding RNA LINC01234
functions as a competing endogenous RNA to regulate CBFB expression
by sponging miR-204-5p in gastric cancer. Clin Cancer Res. Jan
31–2018.(Epub ahead of print). View Article : Google Scholar
|
|
13
|
Wan L, Sun M, Liu GJ, Wei CC, Zhang EB,
Kong R, Xu TP, Huang MD and Wang ZX: Long noncoding RNA PVT1
promotes non-small cell lung cancer cell proliferation through
epigenetically regulating LATS2 expression. Mol Cancer Ther.
15:1082–1094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sarver AL, Murray CD, Temiz NA, Tseng YY
and Bagchi A: MYC and PVT1 synergize to regulate RSPO1 levels in
breast cancer. Cell Cycle. 15:881–885. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang XW, Bu P, Liu L, Zhang XZ and Li J:
Overexpression of long non-coding RNA PVT1 in gastric cancer cells
promotes the development of multidrug resistance. Biochem Biophys
Res Commun. 462:227–232. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cancer Genome Atlas Research Network:
Comprehensive molecular characterization of clear cell renal cell
carcinoma. Nature. 499:43–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mermel CH, Schumacher SE, Hill B, Meyerson
ML, Beroukhim R and Getz G: GISTIC2.0 facilitates sensitive and
confident localization of the targets of focal somatic copy-number
alteration in human cancers. Genome Biol. 12:R412011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song W, Dang Q, Xu D, Chen Y, Zhu G, Wu K,
Zeng J, Long Q, Wang X, He D and Li L: Kaempferol induces cell
cycle arrest and apoptosis in renal cell carcinoma through EGFR/p38
signaling. Oncol Rep. 31:1350–1356. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liang L, Li L, Zeng J, Gao Y, Chen YL,
Wang ZQ, Wang XY, Chang LS and He D: Inhibitory effect of silibinin
on EGFR signal-induced renal cell carcinoma progression via
suppression of the EGFR/MMP-9 signaling pathway. Oncol Rep.
28:999–1005. 2012.PubMed/NCBI
|
|
21
|
Clark BS and Blackshaw S: Long non-coding
RNA-dependent transcriptional regulation in neuronal development
and disease. Front Genet. 5:1642014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li P, Ruan X, Yang L, Kiesewetter K, Zhao
Y, Luo H, Chen Y, Gucek M, Zhu J and Cao H: A liver-enriched long
non-coding RNA, lncLSTR, regulates systemic lipid metabolism in
mice. Cell Metab. 21:455–467. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carrieri C, Forrest AR, Santoro C,
Persichetti F, Carninci P, Zucchelli S and Gustincich S: Expression
analysis of the long non-coding RNA antisense to Uchl1 (AS Uchl1)
during dopaminergic cells' differentiation in vitro and in
neurochemical models of Parkinson's disease. Front Cell Neurosci.
9:1142015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsoi LC, Iyer MK, Stuart PE, Swindell WR,
Gudjonsson JE, Tejasvi T, Sarkar MK, Li B, Ding J, Voorhees JJ, et
al: Analysis of long non-coding RNAs highlights tissue-specific
expression patterns and epigenetic profiles in normal and psoriatic
skin. Genome Biol. 16:242015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang HM, Yang FQ, Chen SJ, Che J and
Zheng JH: Upregulation of long non-coding RNA MALAT1 correlates
with tumor progression and poor prognosis in clear cell renal cell
carcinoma. Tumour Biol. 36:2947–2955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hirata H, Hinoda Y, Shahryari V, Deng G,
Nakajima K, Tabatabai ZL, Ishii N and Dahiya R: Long noncoding RNA
MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and
interacts with miR-205. Cancer Res. 75:1322–1331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qiao HP, Gao WS, Huo JX and Yang ZS: Long
non-coding RNA GAS5 functions as a tumor suppressor in renal cell
carcinoma. Asian Pac J Cancer Prev. 14:1077–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xue S, Li QW, Che JP, Guo Y, Yang FQ and
Zheng JH: Decreased expression of long non-coding RNA NBAT-1 is
associated with poor prognosis in patients with clear cell renal
cell carcinoma. Int J Clin Exp Pathol. 8:3765–3774. 2015.PubMed/NCBI
|
|
29
|
Wu Y, Liu J, Zheng Y, You L, Kuang D and
Liu T: Suppressed expression of long non-coding RNA HOTAIR inhibits
proliferation and tumourigenicity of renal carcinoma cells. Tumour
Biol. 35:11887–11894. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Takahashi Y, Sawada G, Kurashige J, Uchi
R, Matsumura T, Ueo H, Takano Y, Eguchi H, Sudo T, Sugimachi K, et
al: Amplification of PVT-1 is involved in poor prognosis via
apoptosis inhibition in colorectal cancers. Br J Cancer.
110:164–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tseng YY, Moriarity BS, Gong W, Akiyama R,
Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell
TC, et al: PVT1 dependence in cancer with MYC copy-number increase.
Nature. 512:82–86. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Surendran K, Selassie M, Liapis H, Krigman
H and Kopan R: Reduced Notch signaling leads to renal cysts and
papillary microadenomas. J Am Soc Nephrol. 21:819–832. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Martinou JC and Youle RJ: Mitochondria in
apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev
Cell. 21:92–101. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kaufmann T, Strasser A and Jost PJ: Fas
death receptor signalling: Roles of Bid and XIAP. Cell Death
Differ. 19:42–50. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wei MC, Zong WX, Cheng EH, Lindsten T,
Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB and
Korsmeyer SJ: Proapoptotic BAX and BAK: A requisite gateway to
mitochondrial dysfunction and death. Science. 292:727–730. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tewari M, Quan LT, O'Rourke K, Desnoyers
S, Zeng Z, Beidler DR, Poirier GG, Salvesen GS and Dixit VM:
Yama/CPP32 beta, a mammalian homolog of CED-3, is a
CrmA-inhibitable protease that cleaves the death substrate
poly(ADP-ribose) polymerase. Cell. 81:801–809. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sherr CJ: Cancer cell cycles. Science.
274:1672–1677. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chandra A, Lan S, Zhu J, Siclari VA and
Qin L: Epidermal growth factor receptor (EGFR) signaling promotes
proliferation and survival in osteoprogenitors by increasing early
growth response 2 (EGR2) expression. J Biol Chem. 288:20488–20498.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhan Y, Chen Y, Liu R, Zhang H and Zhang
Y: Potentiation of paclitaxel activity by curcumin in human breast
cancer cell by modulating apoptosis and inhibiting EGFR signaling.
Arch Pharm Res. 37:1086–1095. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen W, Zhong X, Wei Y, Liu Y, Yi Q, Zhang
G, He L, Chen F, Liu Y and Luo J: TGF-β regulates survivin to
affect cell cycle and the expression of EGFR and MMP9 in
glioblastoma. Mol Neurobiol. 53:1648–1653. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sigismund S, Avanzato D and Lanzetti L:
Emerging functions of the EGFR in cancer. Mol Oncol. 12:3–20. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chou YT, Lin HH, Lien YC, Wang YH, Hong
CF, Kao YR, Lin SC, Chang YC, Lin SY, Chen SJ, et al: EGFR promotes
lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc
pathway that targets the Ets2 transcriptional repressor ERF. Cancer
Res. 70:8822–8831. 2010. View Article : Google Scholar : PubMed/NCBI
|