Introduction
Colorectal cancer (CRC) is considered to be one of
the major causes of cancer-associated mortality worldwide (1). This disease accounts for 9.7% of all
cancer mortalities and is the most commonly diagnosed cancer
subsequent to lung and female breast cancer (2). Contemporary treatment for CRC primarily
relies on surgical measures and chemotherapy (3). The use of 5-fluorouracil (5-FU) has been
the cornerstone of treating CRC for >50 years. 5-FU, a
pyrimidine analog, is converted to fluorodeoxyuridine monophosphate
(FdUMP) to exert its anti-tumor effect (4). FdUMP is incorporated into DNA and RNA,
resulting in defective synthesis and subsequent cell apoptosis.
However, toxicity that develops at high doses and drug resistance
remain obstacles in the application of 5-FU (4). Over previous years, numerous efforts
have been made to identify the mechanism of resistance to 5-FU. A
number of studies indicated that evading of apoptosis serves an
important role in the resistance to 5-FU (5–7).
Apoptosis is programmed cell death required for
maintaining the balance between cell death and proliferation. There
are two primary pathways that lead to the activation of apoptosis:
The extrinsic pathway and the intrinsic pathway, which is also
termed the mitochondrial pathway (6).
The extrinsic pathway is triggered by several ligands of various
death receptors. Stimuli such as cellular stress, radiation and
chemotherapeutic agents may activate the intrinsic/mitochondrial
pathway (8). The intrinsic pathway
involves the release of mitochondrial intermembrane space proteins
including cytochrome c and Direct IAP-binding protein with
low pI (Smac/Diablo) from the mitochondria to the cytosol (9). Cytochrome c forms a multi-protein
complex with apoptotic protease activating factor 1 (Apaf-1) and
deoxyadenosine triphosphate termed the apoptosome, which in turn
promotes the caspase cascade through cleavage of procaspase-9 into
caspase-9 and subsequently caspase-3 activation (10). Caspase-3 functions as the executioner
caspase, by cleaving various substrates including poly adenosine
5′-diphosphate-ribose polymerase (PARP) and ultimately leading to
morphological and biochemical changes in apoptotic cells (8). The intrinsic pathway is primarily
regulated by the B-cell lymphoma 2 (Bcl-2) family proteins.
According to their roles in the process of apoptosis, the Bcl-2
members may be additionally classified into pro-apoptotic members
and anti-apoptotic members. Overexpression of anti-apoptotic Bcl-2
proteins may often induce insensitivity in cancer cells to various
chemotherapeutic agents; therefore, these proteins are vital
targets for the development of novel cancer therapeutics (11).
Combined therapy, in which another agent is used
simultaneously with 5-FU, may improve outcomes. 5-Fluorouracil
(5-FU) used in combination with other agents such as curcumin,
resveratrol and oxaliplatin may enhance the response rate and
reduce the unfavorable side effects of these agents (12–14). These
data suggest that 5-FU, when administered in combination with other
anti-tumor agents, may result in improved treatment response when
compared with 5-FU treatment alone. Bufalin, a traditional Chinese
medicine also termed Huachansu, is one type of steroid that may be
purified from the skin and parotid venom glands of toads (Bufo
gargarizans or B. melanostictus) (15). Bufalin exhibits a significant
anti-tumor effect in a variety of tumors, including hepatocellular
and colorectal cancer, leukemia and gastric cancer (15–17).
However, additional investigation concerning the
chemo-sensitization effect of bufalin is required.
In the present study, the combined anti-tumor effect
of 5-FU with bufalin on HCT116 human colorectal cancer cells was
investigated. It was identified that bufalin, with 5-FU, may
synergistically induce apoptosis through a mechanism involving
mitochondrial apoptotic pathway activation, which depends on
Bcl-2-associated X protein (Bax). The results of the present study
provide the rationale for the additional evaluation of the
combination of 5-FU/bufalin in human colorectal carcinoma
treatment.
Materials and methods
Reagents and antibodies
Bufalin was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany) and initially dissolved in anhydrous
alcohol at a stock concentration of 20 mg/ml and stored at −20°C.
The bufalin stock solution was freshly diluted to 10, 20 and 30 µM
in the medium prior to use. 5-FU was purchased from Shanghai Xudong
Haipu Pharmaceutical Co. (Shanghai, China). The propidium iodide
(PI)/Annexin V staining assay kit was obtained from BD Biosciences
(San Jose, CA, USA). All other chemicals used were of analytical
grade and were purchased from Sigma-Aldrich (Merck KGaA).
Antibodies against Bax (cat. no., sc-23959) were obtained from
Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Antibodies
against poly adenosine 5′-diphosphate-ribose polymerase (PARP)
(cat. no., 9532), induced myeloid leukemia cell differentiation
protein Mcl-1 (Mcl-1; cat. no., 94296), X-linked inhibitor of
apoptosis protein (XIAP; cat. no., 2045), B-cell lymphoma 2 (Bcl-2;
cat. no., 4223), survivin, Bcl-2-like protein 4 (Bax; cat. no.,
5023), Bcl-2-associated death promotor (Bad; cat. no., cat:9239),
BCl-2 homologous antagonist/killer (Bak; cat. no., 6947) and
β-actin (cat. no., 3700) were purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). RPMI-1640 medium and 10% fetal
bovine serum (FBS) were purchased from HyClone (GE Healthcare Life
Sciences, Logan, UT, USA). Transfection Reagent
Lipofectamine® 2,000 was purchased from Thermo Fisher
Scientific, Inc., (Waltham, MA, USA).
Cell culture
The HCT116 human colorectal cancer cell line used
was purchased from Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China), and cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum, penicillin (100
U/ml), and streptomycin (100 g/ml) (all from Hyclone; GE Healthcare
Life Sciences) at 37°C in a 5% CO2 humidified
atmosphere. Cells were maintained as a monolayer and sub-cultured
every 3 days. Cells were used when the monolayer reached 70%
confluence for all experiments.
MTT viability assay
HCT116 cells were seeded in 96-well plates at
1×104 cells/well for 12 h, and then incubated at 37°C in
the presence various concentrations of 5-FU (5, 10 or 15 µM) with
or without different concentrations of bufalin (10, 20 or 30 nM)
for 24 h. Following this, 20 µl MTT solution (5 mg/ml in PBS, pH
7.2) was added to each well and the plates were incubated at 37°C
for 4 h. Subsequent to removing the medium containing MTT, 150 µl
dimethyl sulfoxide was added to each well. The plates were
incubated at room temperature on a plate shaker for 10 min, and
absorbance at 570 nm was measured using a Bio-Rad 680 microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Each
experiment was conducted in triplicate.
Apoptosis analysis
To detect apoptosis, cells were incubated with 5-FU
(15 µM) with or without bufalin (30 nM) for 24 h at 37°C. The cells
were harvested, washed twice with cold 1X PBS, and re-suspended in
200 µl binding buffer at density of 1×105 cells/ml. The
cells were then stained with 5 µl Annexin-V and PI (BD Biosciences)
for 20 min in the dark at room temperature, and subjected to
analysis by flow cytometry (FACScan, BD Biosciences, Franklin
Lakes, NJ, USA). Apoptosis was evaluated based on the percentage of
cells with Annexin V+/PI+ staining. The results were presented as
mean values from three independent experiments. The results were
analyzed by FlowJo 10.0.5 (Tree Star, Inc., Ashland, OR, USA)
software.
RNA interference
On-target Bax and scramble small interfering (si)RNA
were purchased from Guangzhou RiboBio Co., Ltd (Guangzhou, China).
Bax and scramble small interfering (si)RNA were transfected to the
cells with Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
protocol. Downregulation of the protein was confirmed by
immunoblotting 24 h following transfection.
Immunoblotting and
immunoprecipitation
Cells were separately washed, collected and
homogenized in a lysis buffer (10 mM Tris-HCl, pH 8, 0.32 mM
sucrose, 5 mM EDTA, 2 mM dithiothreitol, 1 mM phenylmethyl
sulfonylfluoride, and 1 % Triton X-100), and centrifuged (13,000 ×
g for 10 min at 4°C). Equal amounts of proteins (50 µg) were
subjected to electrophoresis in a 10% SDS-PAGE gel. The
gel-separated proteins were transferred to Nitropure™
nitrocellulose membranes (Santa Cruz Biotechnology, Inc.) and the
membranes were blocked with 10% fat-free milk in TBST overnight at
4°C and probed with primary antibodies (1:1,000) at 37°C for 2 h.
Each of the targeted proteins was immunostained by specific
antibodies. The membranes were washed three times with TBST and
then incubated for 1 h at room temperature with alkaline
phosphatase-conjugated bovine anti-rabbit (cat no., sc-2379) or
goat anti-mouse (cat no., sc-2039) secondary antibodies (both
1:5,000; Santa Cruz Biotechnology, Inc.). Finally, the protein
bands were visualized by enhanced chemiluminescent reagents (Thermo
Fisher Scientific., Inc.). Equal amounts of protein from cell
lysates (600 µg) were used for Bax immunoprecipitation. All samples
were brought to a final volume of 450 µl with cellular lysis
buffer. Samples were then rotated for 5 h at 4°C with 5 µl of
monoclonal antibodies (BAX 6A7) and 150 µl anti-rabbit IgG magnetic
beads (ready-to-use dilution; cat. no., 11203D; Thermo Fisher
Scientific., Inc.). Then, the supernatants from the beads were
precipitated by a magnetic field. The beads were then washed five
times with the cellular lysis buffer. Finally, the last supernatant
was removed and 25 µl of 5X SDS-PAGE loading buffer (cat. no.,
P0015; Beyotime Institute of Biotechnology, Haimen, China) was
added. The beads were incubated in the loading buffer at 95–100°C
for 5 min, and then centrifuged at 13,400 × g for 5 min at 4°C. The
supernatants were subjected to immunoblotting analysis as
aforementioned. Proteins were detected by the Pierce™
ECL Western Blotting Substrate (Thermo Fisher Scientific, Inc.) and
visualized by the Image Lab™ software, version 6.0 on
the Molecular Imager Gel Doc XR+ System (Bio-Rad Laboratories,
Inc., Hercules, CA, USA).
Preparation of subcellular
fractions
In order to separate the cytosolic and mitochondria
fractions, cells were washed three times in ice-cold PBS. The cells
were then lysed using Cell Lysis and Mitochondria Intact buffer
(cat. no., P3102; Beyotime Institute of Biotechnology) on ice for 5
min and the cell suspensions were centrifuged at 401 × g for 5 min
at 4°C. The supernatant was removed and stored at −20°C as the
cytosolic fraction.
Statistical analysis
Data are presented as the mean ± standard deviation.
Differences between groups were compared using one-way analysis of
variance followed by the Fisher's Least Significant Difference
test. Statistical analysis was performed using SPSS, version 17.0
(SPSS, Inc., Chicago, IL, USA), and P<0.05 was considered to
indicate a statistically significant difference. The half maximal
inhibitory concentration (IC50) and combined effect of
5-FU and bufalin was evaluated using CompuSyn 2.0 software
(ComboSyn Inc., New York, NY, USA). This method of analysis
generally defines the combination as positive (synergistic) when
the combination index (CI) is <0.9, negative (antagonistic) when
the CI is >1.1, or additive when the CI is from 0.9 to 1.1.
Results
Effects of 5-FU, bufalin and
combinations on viability of HCT116 cells
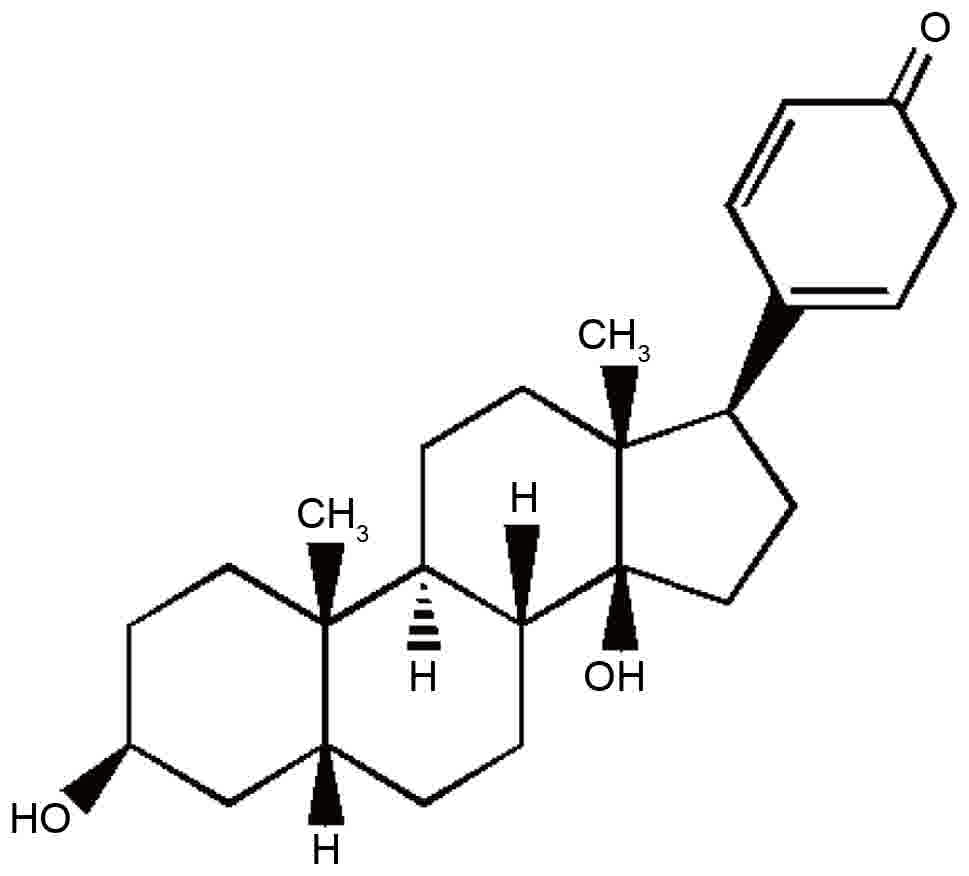
The chemical structure of bufalin is presented in
Fig. 1. The cytotoxic effects of
5-FU, bufalin and their combination treatment effect on the HCT116
human colorectal cancer cell line were evaluated. Firstly, cells
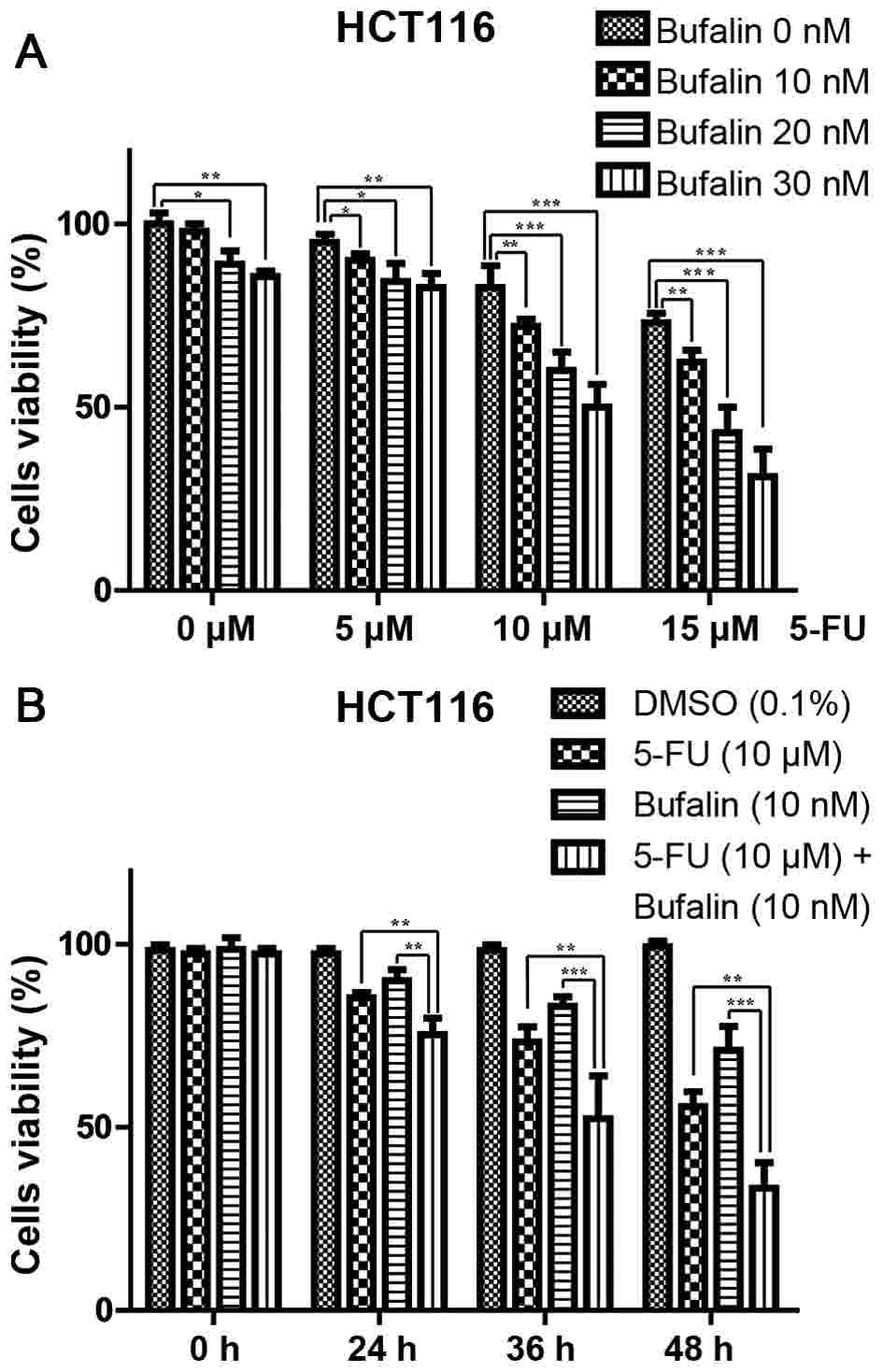
were treated for 24 h with various concentrations of 5-FU (5, 10 or
15 µM) and/or bufalin (0.01, 0.02, 0.03 µM) (Fig. 2A). The cell viability was evaluated
using an MTT assay. The IC50 levels of 5-FU and bufalin
were 28.01±0.91 µM and 58.03±3 nM, respectively. Cells were
subsequently treated with a constant concentration of 5-FU (10 µM)
and/or bufalin (0.01 µM) for 24, 36 and 48 h (Fig. 2B). It was determined that 5-FU and
bufalin inhibited the viability of HCT116 cells in dose- and
time-dependent manners, and their combination demonstrated a more
marked inhibitory effect compared with the single agent (Fig. 2). Then, the CompuSyn software was used
to calculate the CI values, as summarized in Table I. The present study indicated that
5-FU's ability to affect the viability of HCT116 cells was
additionally enhanced when the drug was combined with bufalin.
Based on CI values, the present study selected the combination of
15 µM 5-FU and 0.03 µM bufalin for subsequent experiments
(CI=0.54867).
 | Table I.CI analysis of 5-FU with bufalin in
HCT116 cells. |
Table I.
CI analysis of 5-FU with bufalin in
HCT116 cells.
| 5-FU (µM) | Bufalin (µM) | CI |
|---|
| 5.0 | 0.01 | 0.82295 |
|
| 0.02 | 0.81846 |
|
| 0.03 | 0.78597 |
| 10.0 | 0.01 | 0.76462 |
|
| 0.02 | 0.75372 |
|
| 0.03 | 0.75132 |
| 15.0 | 0.01 | 0.86689 |
|
| 0.02 | 0.77369 |
|
| 0.03 | 0.54867 |
Bufalin sensitizes 5-FU-induced
apoptosis in HCT116 cells
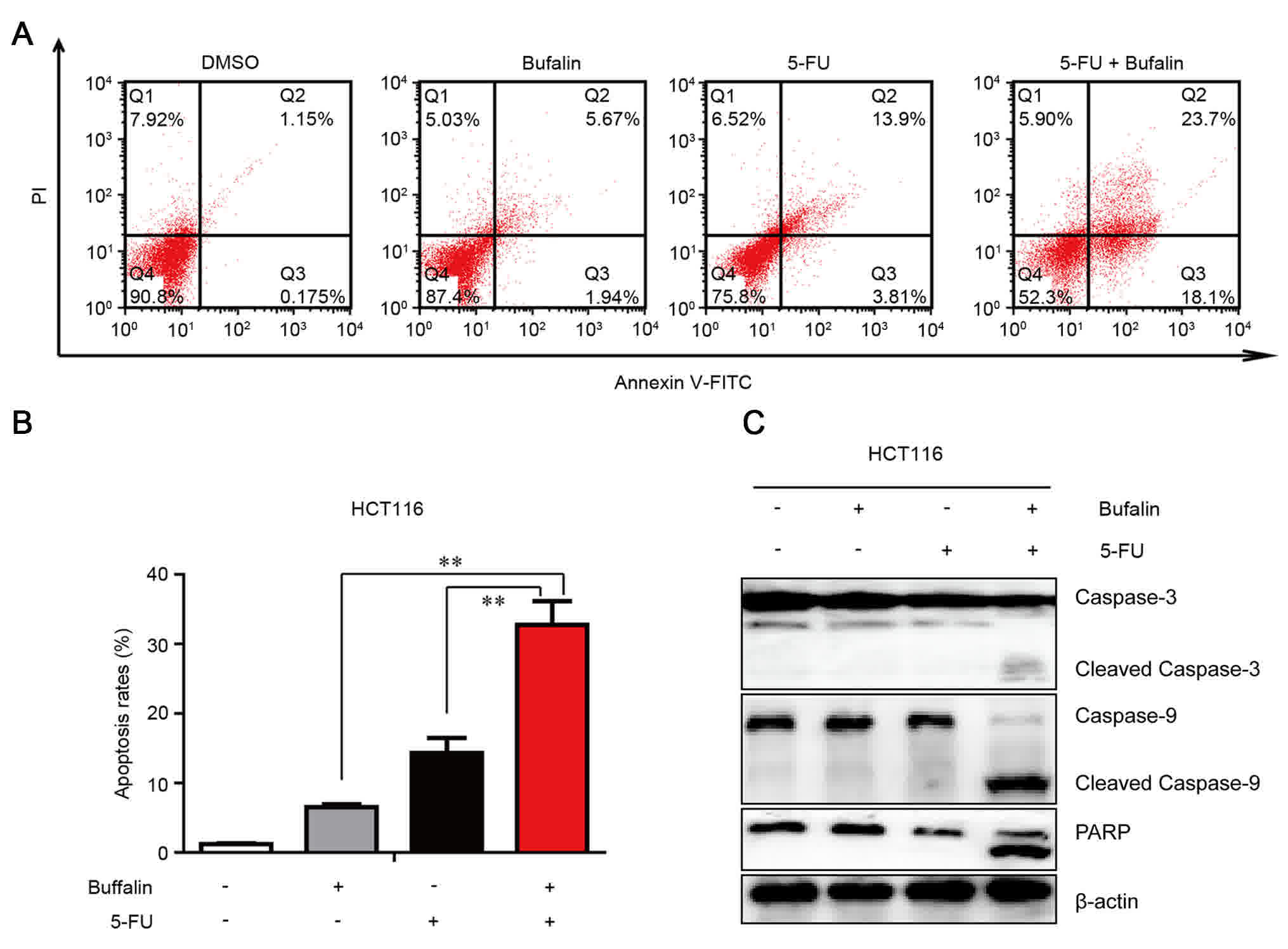
To elucidate how bufalin and 5-FU exert their
synergistic anti-tumor effects on HCT 116 cells, specifically
whether the combined therapy induced apoptosis. The level of
apoptosis was measured by Annexin V/PI staining and flow cytometry.
The results demonstrated that combined treatment of 5-FU with
bufalin significantly increased the percentage of apoptotic cells
compared with 5-FU or bufalin alone for 24 h (Fig. 3A and B). To additionally investigate
the mechanism behind apoptosis, the activation of caspases, namely
caspase-9 and caspase-3, was quantified using western blotting. As
demonstrated in Fig. 3C, the levels
of the activated caspase-3 and caspase-9 fragments were higher in
the cells treated with the combined treatment compared with the
single agent-treated cells. The cleaved PARP was also increased in
the cells with co-treatment. These data indicate that
5-FU/bufalin-induced apoptosis may occur via the mitochondrial
pathway in HCT116 cells.
Bufalin and 5-FU synergistically
induce apoptosis in HCT116 cells via mitochondrial pathway
The members of the Bcl-2 family have important roles
in regulating the process of apoptosis. Therefore, whether the
combined treatment with bufalin and 5-FU induced apoptosis via
altering the levels of the apoptosis-associated members of this
family was examined. Compared with the control and single
agent-treated group, co-treatment with 5-FU and bufalin decreased
the expression of Mcl-1, Bcl-2, XIAP, while it upregulated the
expression of Bax and Bad (Fig. 4A).
Concurrently, the expression of Bak was not affected (Fig. 4A). Additionally, a specific Bax
antibody (6A7) was used to detect the status of Bax. As
demonstrated in Fig. 4B, Bax was
activated following the combined treatment of 5-FU/bufalin. In
addition, the release of mitochondrial proteins cytochrome c
and Smac/Diablo into the cytosol was significantly increased
following the combined treatment of 5-FU and bufalin (Fig. 4C). Therefore, this implies that
5-FU/bufalin-induced apoptosis occurs primarily through the
mitochondrial apoptotic pathway.
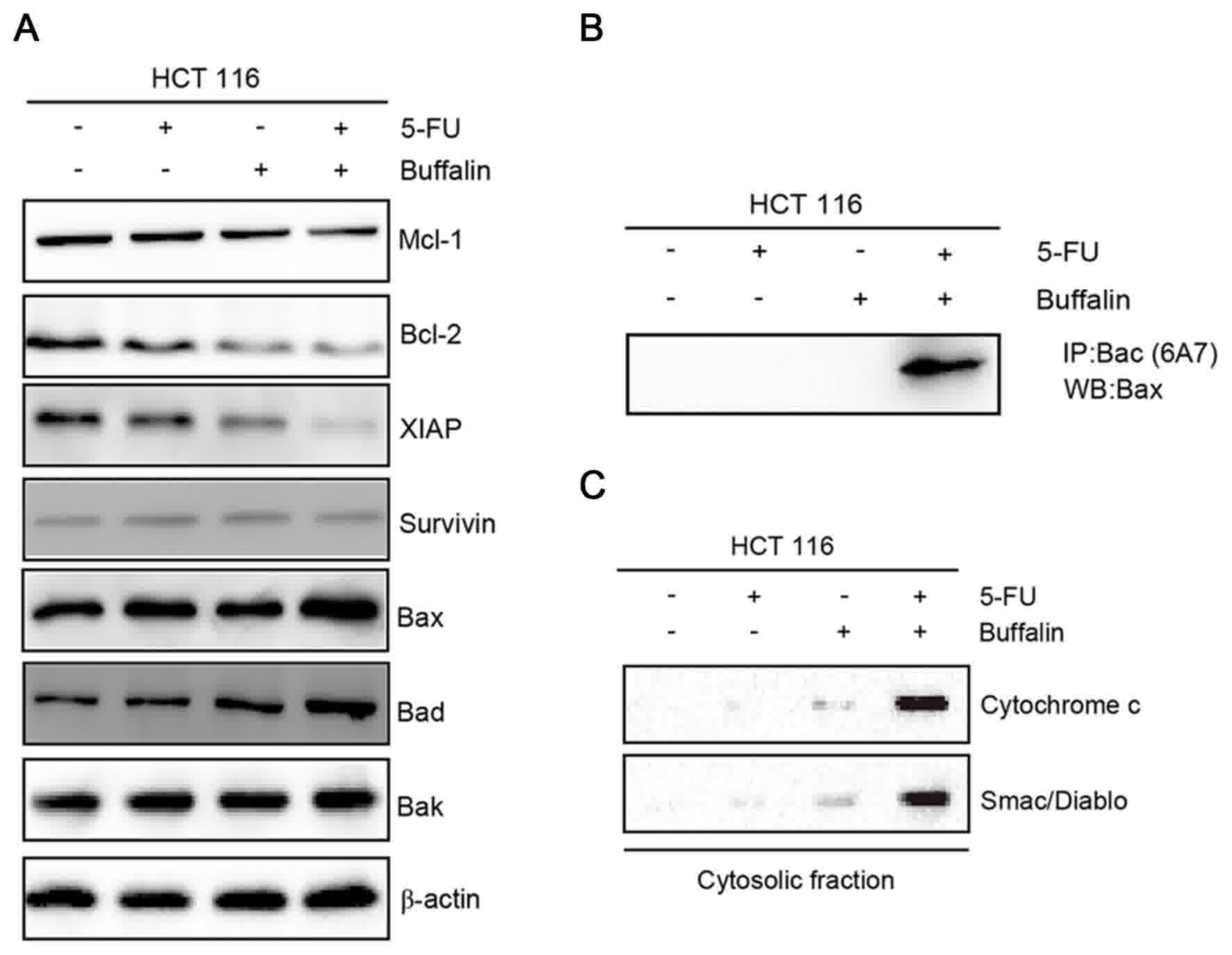 | Figure 4.Effects of 5-FU and bufalin on
apoptosis-associated proteins in HCT116 cells. HCT116 cells were
treated with 5-FU or bufalin alone, or in a combination for 24 h.
(A) Protein levels of Mcl-1, XIAP, Bcl-2, Survivin, Bax, Bad and
Bak were determined by WB, β-actin was used as the loading control.
(B) Bax activation was determined by IP using active
conformation-specific antibody (6A7). (C) Release of cytochrome
c and Smac/Diablo were detected in the cytosolic fraction of
cells. Data are presented as one of triplicate experiments. WB,
western blotting; IP, immunoprecipitation; 5-FU, 5-fluorouracil;
Bcl-2, B-cell lymphoma 2; Mcl-1, induced myeloid leukemia cell
differentiation protein Mcl-1; XIAP, X-linked inhibitor of
apoptosis protein; Bax, Bcl-2-like protein 4; Bad, Bcl-2-associated
death promotor; Bak, Bcl-2 homologous antagonist killer. |
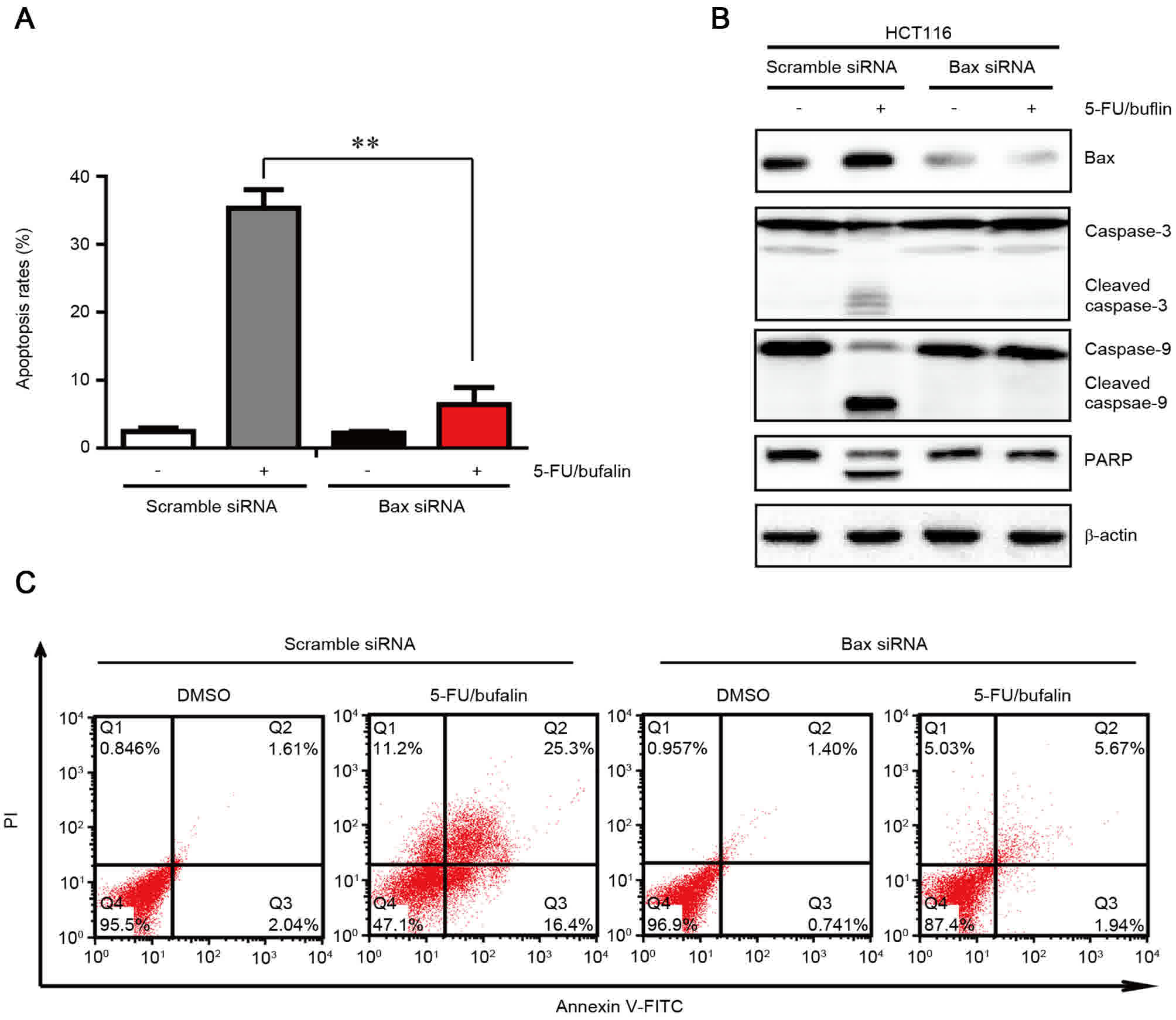
Upregulation of Bax is required for
the induction of apoptosis by co-treatment of 5-FU and bufalin
As it was observed that Bax was activated by the
combinational treatment of 5-FU and bufalin (Fig. 4A), the present study aimed to
determine whether the apoptosis induced by 5-FU/bufalin was
dependent on Bax. In order to examine this, Bax or scramble siRNA
were transfected into cells, followed by treatment with
5-FU/bufalin (Fig. 5). The level of
apoptosis induced by 5-FU/bufalin was significantly attenuated in
cells with silencing of Bax compared with the cells transfected
with scramble siRNA (Fig. 5A and C).
In addition, the western blotting assay indicated that following
treatment with 5-FU/bufalin, cleaved caspase-3, caspase-9 and PARP
were also attenuated by knockdown of Bax (Fig. 5B). These findings indicated that
5-FU/bufalin-triggered apoptosis relied on the activation of Bax in
HCT116 cells.
Discussion
Resistance to 5-FU treatment is one of the major
causes for the failure of chemotherapy in treating advanced
colorectal cancer (4). Therefore, it
is vital to develop novel strategies to increase the effectiveness
of 5-FU for therapeutic purposes. Combination therapy via the
simultaneous administration of various therapeutic agents has
emerged as a crucial strategy for achieving enhanced anti-tumor
activity through synergistic effects. In the present study, it was
observed that combined 5-FU and bufalin treatment had a synergistic
anti-tumor effect against human colorectal cancer cells (CI<1;
Table I).
5-FU exerts its anti-tumor effect by blocking cell
cycle progression, inducing DNA damage, which leads to cellular
apoptosis (4). Previous studies have
indicated that extracts from Chinese traditional medicine may
increase the cytotoxicity of 5-FU against colorectal cancer cells
such as resveratrol, curcumin and ginsenoside (12,18,19).
Bufalin, a major immunoreactive component of the skin and parotid
venom gland of toads, exhibits a variety of effects, including
cardiotonic, anesthetic, blood pressure stimulation, respiration
and antineoplastic (15). Previous
studies have suggested that bufalin may induce growth inhibition,
cell cycle arrest and cellular apoptosis in various tumor cells
(20–22). In addition, bufalin may interfere with
the differentiation and proliferation of cancer stem cells derived
from primary osteosarcoma cells (23). Bufalin also inhibits
epithelial-to-mesenchymal transition and migration by
downregulating TGF-β receptors in human lung cancer cells (24).
In the present study, it was observed that combined
treatment with 5-FU and bufalin was more effective compared with
monotherapy with 5-FU or bufalin alone in the inhibition of HCT116
cell proliferation (Fig. 2). Based on
these findings, the present study investigated whether the enhanced
anti-tumor effects of combined treatment were caused by their
effects on cell apoptosis. Apoptosis is primarily mediated through
the extrinsic and/or intrinsic pathway: In previous studies,
chemotherapeutic agents such as 5-FU induced apoptosis primarily
through the intrinsic pathway (4,25).
Conversely, bufalin was able to trigger apoptosis through either
extrinsic and/or intrinsic pathways (26). In order to clarify through which
pathway 5-FU/bufalin induces apoptosis, the activation of caspases
was investigated and it was identified that caspase-9 activation
was significantly increased following the combined treatment of
5-FU and bufalin (Fig. 3C).
Activation of caspase-3 and cleaving of PARP are hallmarks of
apoptosis that lead to DNA fragmentation and subsequently cell
death. Therefore, 5-FU/bufalin induced apoptosis via the
caspase-9-caspase-3 axis in HCT116 cells.
The present study revealed the downregulation of
anti-apoptotic protein Bcl-2 with a concomitant upregulation of
Bax, Bad and activation of Bax (Fig. 4A
and B). In previous studies, downregulation of Bax contributed
to colorectal carcinogenesis and resistance to 5-FU and increase in
the expression of Bax enhanced the susceptibility to 5-FU (23,24).
Subsequent to silencing of Bax, it was also observed that the
apoptosis induced by 5-FU/bufalin and the cleavage of caspase-3,
−9, PARP was reduced (Fig. 5A and B).
All these findings highlight the critical role of Bax in regulating
the cellular response to 5-FU. An additional pro-apoptotic Bcl-2
member Bad promoted apoptosis through antagonizing the
anti-apoptotic role of Bcl-2 (27).
The upregulation of Bax and Bad, and the downregulation of Bcl-2
may lead to an increase of the Bax/Bcl-2 ratio which will lead to
the permeabilization of outer mitochondrial membrane, resulting in
the release of mitochondrial proteins such as cytochrome c
and/or Smac/Diablo (9). The present
study was also able to detect the release of cytochrome c
and Smac/Diablo in the cytosolic fraction of cells following
co-treatment (Fig. 4C). Cytochrome
c forms a complex termed the apoptosome with Apaf-1 and
procaspase-9. Procaspase-9 may be self-activated within the
apoptosome, resulting in the activation of downstream caspases
including caspase-3. However, this process may be blocked by XIAP
through binding to and inhibiting caspase-3 and caspase-9 (9). The present study observed that XIAP was
also downregulated following the combined treatment of 5-FU and
bufalin (Fig. 4A). The release of
Smac/Diablo in the cytosol also blocked the anti-apoptotic activity
of XIAP by preventing it from binding to caspase-3 (8). XIAP also confers resistance to other
anti-tumor agents such as TRAIL, doxorubicin and cisplatin
(6,28). Additionally, XIAP has also been
implicated in the process of metastasis, making it a viable
potential target for cancer therapy (29). Therefore, the effects of 5-FU/bufalin
on the metastatic properties of colorectal cancer cells should be
investigated further.
In conclusion, 5-FU and bufalin cooperated to
promote apoptosis via the intrinsic pathway, and Bax is required
for the synergistic effect. Although there are multiple studies
concerning 5-FU or bufalin in inhibition of tumors, the anti-tumor
effects of the two agents in combination remain to be elucidated.
Previous studies investigating chemotherapeutics suggested that
traditional chemotherapy agents are not capable of eradicating
cancer stem cells (CSCs) and do not to prevent disease relapse,
indicating that novel strategies should focus on the capability to
target CSCs (7). Bufalin, which has
already been demonstrated to be relatively safe in clinical trials
(30), also possesses the ability to
inhibit the differentiation and proliferation of CSCs (23,31). The
data of the present study may have important clinical implications
for the treatment and prevention of colon cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
Natural Science Foundation of Ningbo (grant no. 2014A610225),
Medical Foundation of Ningbo (grant no. 211B10), Social Development
and Scientific and Technological Projects Foundation of Ningbo
(grant no. 2014C50068), Huamei Foundation of Ningbo No. 2 Hospital
(grant nos. 2015HMKY07, 2015HMKY08 and 2015HMKY36).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
XD was involved in the planning of the article,
provided experimental guidance and cultivated the colon cancer
cells. BZ conducted cell culture and drug treatment experiments. KL
analyzed the data and wrote the paper. JL conducted apoptotic
analysis. YX detected protein expression alterations in the
cells.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mayer RJ: Targeted therapy for advanced
colorectal cancer-more is not always better. N Engl J Med.
360:623–625. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Temraz S, Mukherji D, Alameddine R and
Shamseddine A: Methods of overcoming treatment resistance in
colorectal cancer. Crit Rev Oncol Hematol. 89:217–230. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yu R, Deedigan L, Albarenque SM, Mohr A
and Zwacka RM: Delivery of sTRAIL variants by MSCs in combination
with cytotoxic drug treatment leads to p53-independent enhanced
antitumor effects. Cell Death Dis. 4:e5032013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rich JN and Bao S: Chemotherapy and cancer
stem cells. Cell Stem Cell. 1:353–355. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tummers B and Green DR: Caspase-8:
Regulating life and death. Immunol Rev. 277:76–89. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Susin SA, Lorenzo HK, Zamzami N, Marzo I,
Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler
M, et al: Molecular characterization of mitochondrial
apoptosis-inducing factor. Nature. 397:441–446. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Azmi AS, Wang Z, Philip PA, Mohammad RM
and Sarkar FH: Emerging Bcl-2 inhibitors for the treatment of
cancer. Expert Opin Emerg Drugs. 16:59–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shakibaei M, Mobasheri A, Lueders C, Busch
F, Shayan P and Goel A: Curcumin enhances the effect of
chemotherapy against colorectal cancer cells by inhibition of NF-κB
and Src protein kinase signaling pathways. PLoS One. 8:e572182013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Douillard JY, Sobrero A, Carnaghi C,
Comella P, Díaz-Rubio E, Santoro A and Van Cutsem E: Metastatic
colorectal cancer: Integrating irinotecan into combination and
sequential chemotherapy. Ann Oncol. 14 Suppl 2:ii7–ii12. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giacchetti S, Perpoint B, Zidani R, Le
Bail N, Faggiuolo R, Focan C, Chollet P, Llory JF, Letourneau Y,
Coudert B, et al: Phase III multicenter randomized trial of
oxaliplatin added to chronomodulated fluorouracil-leucovorin as
first-line treatment of metastatic colorectal cancer. J Clin Oncol.
18:136–147. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu CX, Nan KJ and Lei Y: Agents from
amphibians with anticancer properties. Anticancer Drugs.
19:931–939. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miao Q, Bi LL, Li X, Miao S, Zhang J,
Zhang S, Yang Q, Xie YH, Zhang J and Wang SW: Anticancer effects of
bufalin on human hepatocellular carcinoma HepG2 cells: Roles of
apoptosis and autophagy. Int J Mol Sci. 14:1370–1382. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xie CM, Chan WY, Yu S, Zhao J and Cheng
CH: Bufalin induces autophagy-mediated cell death in human colon
cancer cells through reactive oxygen species generation and JNK
activation. Free Radic Biol Med. 51:1365–1375. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Santandreu FM, Valle A, Oliver J and Roca
P: Resveratrol potentiates the cytotoxic oxidative stress induced
by chemotherapy in human colon cancer cells. Cell Physiol Biochem.
28:219–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fishbein AB, Wang CZ, Li XL, Mehendale SR,
Sun S, Aung HH and Yuan CS: Asian ginseng enhances the
anti-proliferative effect of 5-fluorouracil on human colorectal
cancer: Comparison between white and red ginseng. Arch Pharm Res.
32:505–513. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jiang L, Zhao MN, Liu TY, Wu XS, Weng H,
Ding Q, Shu YJ, Bao RF, Li ML, Mu JS, et al: Bufalin induces cell
cycle arrest and apoptosis in gallbladder carcinoma cells. Tumour
Biol. 35:10931–10941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qiu DZ, Zhang ZJ, Wu WZ and Yang YK:
Bufalin, a component in Chansu, inhibits proliferation and invasion
of hepatocellular carcinoma cells. BMC Complement Altern Med.
13:1852013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takai N, Ueda T, Nishida M, Nasu K and
Narahara H: Bufalin induces growth inhibition, cell cycle arrest
and apoptosis in human endometrial and ovarian cancer cells. Int J
Mol Med. 21:637–643. 2008.PubMed/NCBI
|
|
23
|
Chang Y, Zhao Y, Gu W, Cao Y, Wang S, Pang
J and Shi Y: Bufalin inhibits the differentiation and proliferation
of cancer stem cells derived from primary osteosarcoma cells
through Mir-148a. Cell Physiol Biochem. 36:1186–1196. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhao L, Liu S, Che X, Hou K, Ma Y, Li C,
Wen T, Fan Y, Hu X, Liu Y and Qu X: Bufalin inhibits TGF-β-induced
epithelial-to-mesenchymal transition and migration in human lung
cancer A549 cells by downregulating TGF-β receptors. Int J Mol Med.
36:645–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sampath D, Rao VA and Plunkett W:
Mechanisms of apoptosis induction by nucleoside analogs. Oncogene.
22:9063–9074. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hong SH and Choi YH: Bufalin induces
apoptosis through activation of both the intrinsic and extrinsic
pathways in human bladder cancer cells. Oncol Rep. 27:114–120.
2012.PubMed/NCBI
|
|
27
|
Datta SR, Katsov A, Hu L, Petros A, Fesik
SW, Yaffe MB and Greenberg ME: 14-3-3 proteins and survival kinases
cooperate to inactivate BAD by BH3 domain phosphorylation. Mol
Cell. 6:41–51. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Qu Y, Xia P, Zhang S, Pan S and Zhao J:
Silencing XIAP suppresses osteosarcoma cell growth, and enhances
the sensitivity of osteosarcoma cells to doxorubicin and cisplatin.
Oncol Rep. 33:1177–1184. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mehrotra S, Languino LR, Raskett CM,
Mercurio AM, Dohi T and Altieri DC: IAP regulation of metastasis.
Cancer Cell. 17:53–64. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Qi F, Li A, Inagaki Y, Kokudo N, Tamura S,
Nakata M and Tang W: Antitumor activity of extracts and compounds
from the skin of the toad Bufo bufo gargarizans Cantor. Int
Immunopharmacol. 11:342–349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meng Z, Yang P, Shen Y, Bei W, Zhang Y, Ge
Y, Newman RA, Cohen L, Liu L, Thornton B, et al: Pilot study of
huachansu in patients with hepatocellular carcinoma, nonsmall-cell
lung cancer, or pancreatic cancer. Cancer. 115:5309–5318. 2009.
View Article : Google Scholar : PubMed/NCBI
|