Introduction
Liver cancer is one of the most malignant tumors
with increasing incidence and cancer-related mortality worldwide
(1). In recent years, great advances
in the treatment for liver cancer have been achieved, including
surgical technique, radiotherapy and chemotherapy. However, the
long-term survival rate is still low among liver cancer patients
due to the aggressive metastasis and recurrence. Currently, a
variety of studies have revealed that the progression of liver
cancer was closely associated with multiple abnormal molecular
expression and dysfunctional signaling pathways, such as tumor
protein P53, Wnt/β-catenin signaling, NF-κB signaling, and
non-coding RNAs (2–5). However, the precise molecular mechanisms
underlying liver cancer progression still urgently need to be
established, which may contribute to improve therapeutic strategies
for liver cancer patients.
Long non-coding RNAs (lncRNAs) are non-protein
coding RNAs longer than 200 nucleotides. Over the recent years,
studies has shown that lncRNAs have significant functions in a
variety of biological processes, including cell proliferation,
apoptosis, metastasis and inflammation (6,7). It has
been reported that aberrant expression of lncRNAs is involved in
the progression of numerous cancer diseases. Long non-coding RNA
MALAT1 could interact with miR-124 to modulate tongue cancer growth
(8), and MALAT1 also could promote
the tumorigenicity and metastasis of gastric cancer (9). Upregulation of long non-coding RNA
PlncRNA-1 promotes proliferation and induces epithelial-mesenchymal
transition in prostate cancer (10),
and XIST promotes the proliferation of pancreatic cancer by
regulating miR-133a/EGFR (11). These
investigations suggest that targeting lncRNAs may be a new strategy
for diagnosis and therapy in cancers. The lncRNA HOX transcript
antisense RNA (HOTAIR) is expressed form the Homeobox C (HOXC)
locus on chromosome 12, which has been identified to be highly
expressed and involved in poor prognosis in several cancers, such
as breast cancer (12), colorectal
cancer (13), pancreatic cancer
(14), non-small cell lung cancer
(15), and esophageal squamous cell
carcinoma (ESCC) (16). Recently,
several studies have revealed that HOTAIR has an abnormal
expression in liver cancer and is closely associated with the
progression and recurrence of liver cancer (5,17,18). However, the role and underlying
molecular mechanism of HOTAIR in liver cancer is still required
further investigation.
MicroRNAs (miRNAs) are 20–25 nt non-coding RNAs that
regulate the expression of genes by binding to the 3′-untranslated
regions (UTRs) of target mRNAs, and thus, the regulation of miRNAs
in many biological processes including proliferation, cycle arrest,
and apoptosis is of great significance (19,20).
Recently, emerging evidence has demonstrated that the aberrant
expression of miRNAs was involved in the progression of many
cancers, including liver cancer. Several investigations have
reported the regulationship of lncRNAs and miRNAs in controlling
liver cancer progression. One recent study has shown that the
oncogenic function of HOTAIR in liver cancer is partly based on the
negative regulation of miR-1 (21).
Additionally, miR-217 has been shown to function as a potential
tumor suppressor in liver cancer progression (22). However, whether HOTAIR could promote
the growth of liver cancer by regulating miR-217 is still not
clear.
In the present study, we investigated the effects of
HOTAIR on the proliferation and cycle arrest in liver cancer cell
lines and specimens, and explored the regulation of HOTAIR on
miR-217.
Materials and methods
Liver cancer tissues
Twenty-five paired liver cancer tissues and adjacent
non-cancerous liver tissues were obtained from patients who
underwent partial liver resection at Xi'an Honghui Hospital.
Informed consent was obtained from all the patients. The study was
performed in accordance with the Helsinki Declaration and was
approved by the Human Ethics Committee/Institutional Review Board
of Xi'an Honghui Hospital.
Cell culture
The normal human hepatic cell line HL-7702 and liver
cancer cell lines (MHCC 97H, HepG2 and Hep3B) were obtained from
American Type Culture Collection (Manassas, VA, USA). The HepG2
cell line used in this study was reported as misidentified, this
cell line was originally thought to be a hepatocellular carcinoma
cell line but was later shown to derive from a hepatoblastoma
(23). All the cells were maintained
in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10%
fetal bovine serum (FBS) and 1% penicillin/streptomycin, and
cultured in a humidified cell incubator with an atmosphere of 5%
CO2 at 37°C.
Construct and infection of HOTAIR
siRNA lentiviral vector
The oligonucleotides encoding siRNA that directed
against HOTAIR were synthesized by GenePharma Co., Ltd. (Shanghai,
China) and annealed into double strands. Then the siRNA was
inserted into the LV10-CMVRFP-Puro vector (GenePharma) and the
constructed plasmids were confirmed by DNA sequencing. The plasmid
DNAs, along with packaging vectors, were transiently transfected
into HEK293T cells using Lipofectamine 2000 (Invitrogen) according
to the manufacturer's instructions. The virus particles were
collected 72 h after transfection and purified by
ultracentrifugation. For transfection, HepG2 cells were seeded in
the cell plates for 24 h, and then infected with the HOTAIR siRNA
lentiviral vectors or empty lentiviral vectors for control. qRT-PCR
was performed to detect the infection efficiency after 72 h.
RNA extraction, northern blotting and
qRT-PCR
Total RNA from frozen liver cancer and paired
non-cancerous liver tissues or cell lines were extracted with
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) according to the manufacturer's instructions. For
northern blot analysis, RNA samples were resolved in the agarose
gel and then were transferred to nylon membrane, and hybridized
with HOTAIR specific biotin-labeled DNA probes. HRP conjugated
secondary antibodies were used and visualized with a ChemiDoc XRS
imaging system. For qRT-PCR, cDNA was synthesized by reverse
transcribing using a PrimeScript RT reagent kit (Takara, Dalian,
China). The expression levels of HOTAIR and miR-217 were detected
using the SYBR Premix Ex Taq kit (Takara). The relative expression
levels were calculated using the 2−ΔΔCt method. β-actin
was used as an internal control for HOTAIR, and U6 snRNA was used
for miR-217.
CCK-8 assay
Cell proliferation was measured using CCK-8 assay
according to the manufacturer's protocol. Briefly, HepG2 cells were
seeded into 96-well plates and treated according to different
experimental requirements. Each well were incubated with 10 µl
CCK-8 solution at different time points (0, 24, 48, 72 and 96 h)
for 4 h at 37°C. The absorbance was measured at 450 nm using a
spectrophotometer.
Western blotting
Total protein was extracted from liver cancer
tissues and different treated HepG2 cells by using RIPA protein
extraction reagent supplemented with 1 mM phenylmethanesulfonyl
fluoride (PMSF). The concentration of protein was measured by BCA
protein assay (Tiangen, Beijing, China). Then 20 µg of protein was
separated by SDS-PAGE and transferred onto a PVDF membrane (EMD
Millipore, Billerica, MA, USA). The membranes were blocked in 5%
non-fat milk for 1 h at room temperature, and then were probed with
the primary antibodies overnight at 4°C: Anti-Ki67,
anti-proliferating cell nuclear antigen (PCNA), anti-p27,
anti-cyclin D1 and anti-GAPDH (Abcam, Cambridge, UK). Then the
membranes were incubated with the corresponding HRP-conjugated
secondary antibodies for 1 h at room temperature, followed by
detection and visualization using a ChemiDoc XRS imaging system and
analysis software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Flow cytometry
HepG2 cells were seeded on 6-well plates for 24 h,
followed by treatment according to different experimental
requirements. The cells were harvested and washed with PBS twice
and suspended in cold phosphate-buffered saline (PBS). Cells were
then stained with PI/RNase staining solution in dark for 30 min at
37°C, and analyzed using flow cytometry.
Cell transfection
The miR-217 mimic or inhibitor and corresponding
negative control were purchased from GenePharma. Cells transfection
was carried out using Lipofectamine 2000 according to the
manufacturer's instructions. Cells were harvested 48 h after
transfection for further experiments.
Dual luciferase reporter gene
assays
The fragment from HOTAIR containing the predicted
miR-217 binding site was amplified by PCR and cloned into a pGL3
luciferase reporter vector and this vector was named HATAIR WT. To
test the binding specificity, the corresponding mutant was created
by mutating the miR-217 seed region binding site and this vector
was named HOTAIR MUT. HepG2 cells were seeded in 96-well plates,
and after 24 h, the cells were co-transfected with miR-217 mimics
and pGL3 vectors containing HOTAIR WT or HOTAIR MUT. The luciferase
activities was measured using Dual Luciferase reporter assay system
(Promega Corporation, Madison, WI, USA) 48 h after transfection
according to the manufacturer's instructions.
Xenograft transplantation in vivo
Four-week-old BALB/c male nude mice were purchased
from The Experimental Animal Center of Xi'an Jiaotong University
and maintained under specific pathogen-free conditions. The HepG2
cells stably transfected with HOTAIR siRNA lentiviral vector or
empty lentiviral vector were injected into nude mice. Cell
suspension (100 µl) containing 1×107 cells was injected
subcutaneously into the back of each nude mouse. The tumor size was
measured every 6 days and on the 30th day following injection, all
mice were sacrificed to recover the tumors. A portion of each tumor
was fixed in 4% paraformaldehyde and embedded in paraffin for
immunohistochemistry assay, and another portion was used for
qRT-PCR and northern blotting. All the animal experiments were
performed according to relevant national and international
guidelines and were approved by the Animal Experimental Ethical
Committee.
Immunohistochemistry assay
Tumor tissues were fixed with 4% paraformaldehyde,
dehydrated, embedded in paraffin and cut into 4 µm sections. The
specimens were deparaffinized in xylene and rehydrated by using a
series of graded alcohols. The tissue sections were
immunohistochemically stained using the primary antibody anti-Ki67
overnight at 4°C, and then incubated with horseradish
peroxidase-conjugated secondary antibody 1 h at room temperature.
Then the sections were incubated with the Cell and Tissue Staining
Kit HRP-DAB system (R&D Systems, MN, USA) according to the
manufacturer's instructions.
Statistical analysis
Statistical analysis was carried out with GraphPad
Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). The
statistical significance between 2 groups was evaluated using
Student's t-test. Each value was presented as mean ± standard error
of the mean (SEM) of at least three independent experiments.
P<0.05 was considered statistically significant.
Results
Expression of HOTAIR was upregulated
in liver cancer tissues and liver cancer cell lines
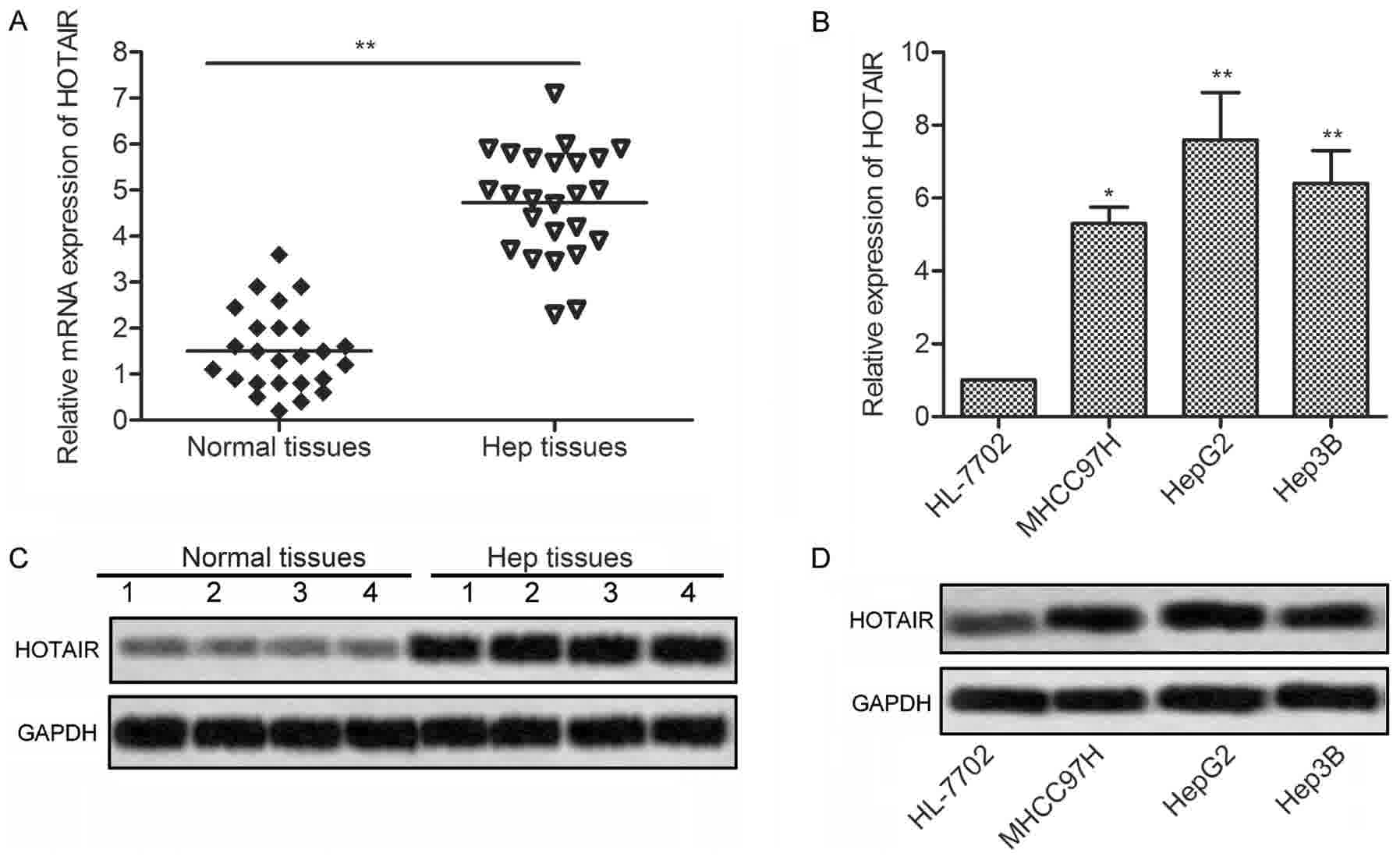
To investigate the effects of HOTAIR in liver
cancer, expression of HOTAIR was detected in 25 liver cancer
tissues and 3 liver cancer cell lines by qRT-PCR and northern
blotting, respectively. As shown in Fig.
1A and C, the expression of HOTAIR was significantly
upregulated in the liver cancer tissues as compared with the normal
tissues. Consistent with the result, the expression of HOTAIR were
markedly increased in 3 liver cancer cell lines (MHCC97H, HepG2 and
Hep3B) compared with normal human hepatic cell line HL-7702
(Fig. 1B and D). These results
suggest that HOTAIR may be involved in the progression of liver
cancer.
HOTAIR inhibition suppressed the
proliferation of HepG2 cells
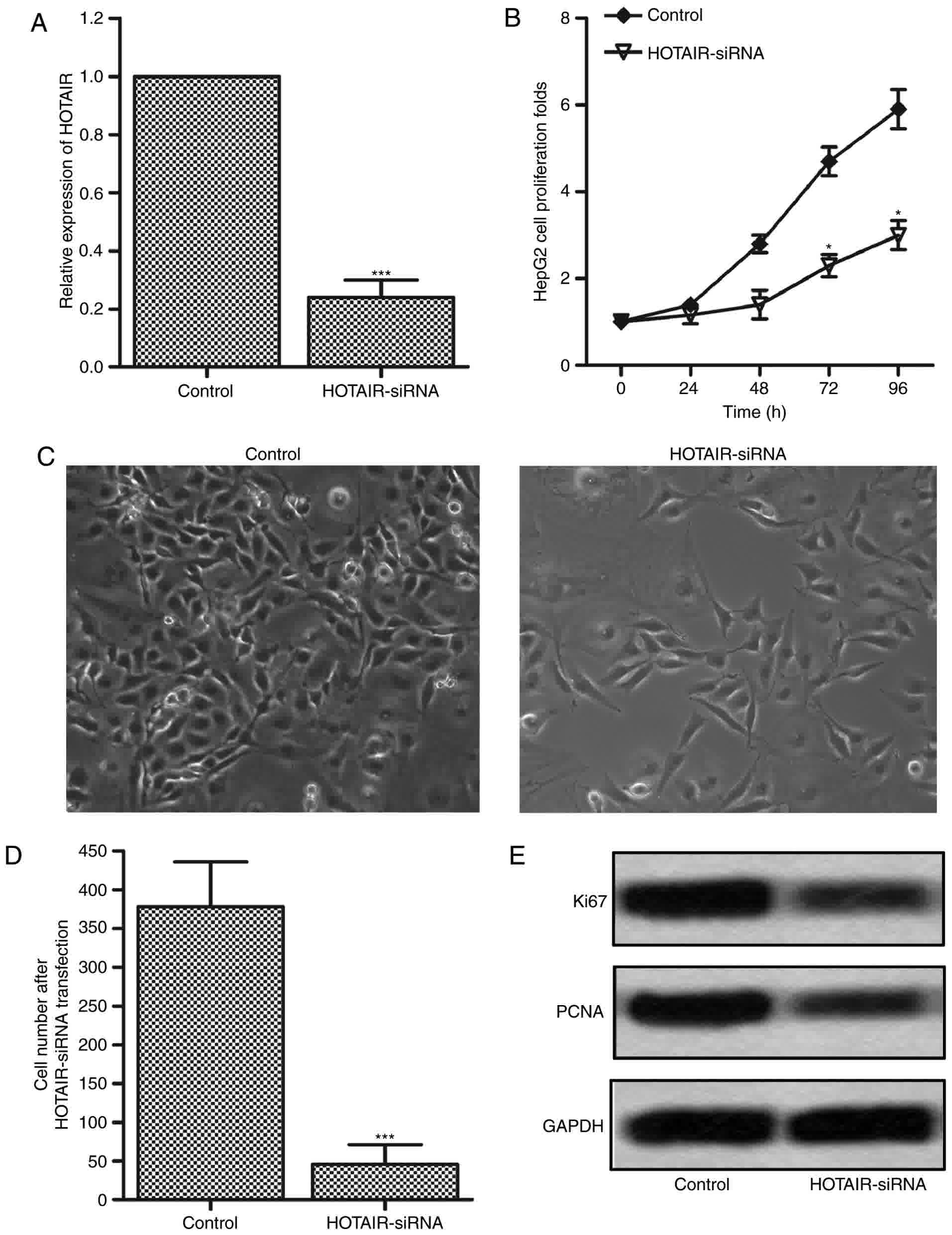
To investigate the effects of HOTAIR on the
proliferation in liver cancer cells, the expression of HOTAIR was
interfered by transfecting HOTAIR siRNA lentiviral vectors into
HepG2 cells (24,25). The interference efficiency was
measured by RT-PCR after 72 h, and the result was shown in Fig. 2A. The expression level of HOTAIR was
significantly downregulated by 80% in HOTAIR-siRNA group as
compared with the control group. The cell proliferation was
measured by CCK-8 assay at 24, 48, 72 and 96 h. The results showed
that cell proliferation in HOTAIR-siRNA group had an obvious
suppression at 72 and 96 h compared with control group (Fig. 2B). These results suggest that
inhibition of HOTAIR plays an important role in the proliferation
suppression in HepG2 cells. The results were confirmed by cell
counting using optical microscope. As shown in Fig. 2C and D, the cell number had an obvious
reduction after HOTAIR-siRNA lentiviral vectors infection.
Furthermore, we detected the protein expression levels of two
proliferation-associated markers, Ki67 and PCAN, by western
blotting. As shown in Fig. 2E, the
expression levels of Ki67 and PCAN were significantly inhibited in
HOTAIR-siRNA group as compared with the control group. These
results indicate that inhibition of HOTAIR suppresses the
proliferation of HepG2 cells.
HOTAIR inhibition induced G0/G1 cell
cycle arrest
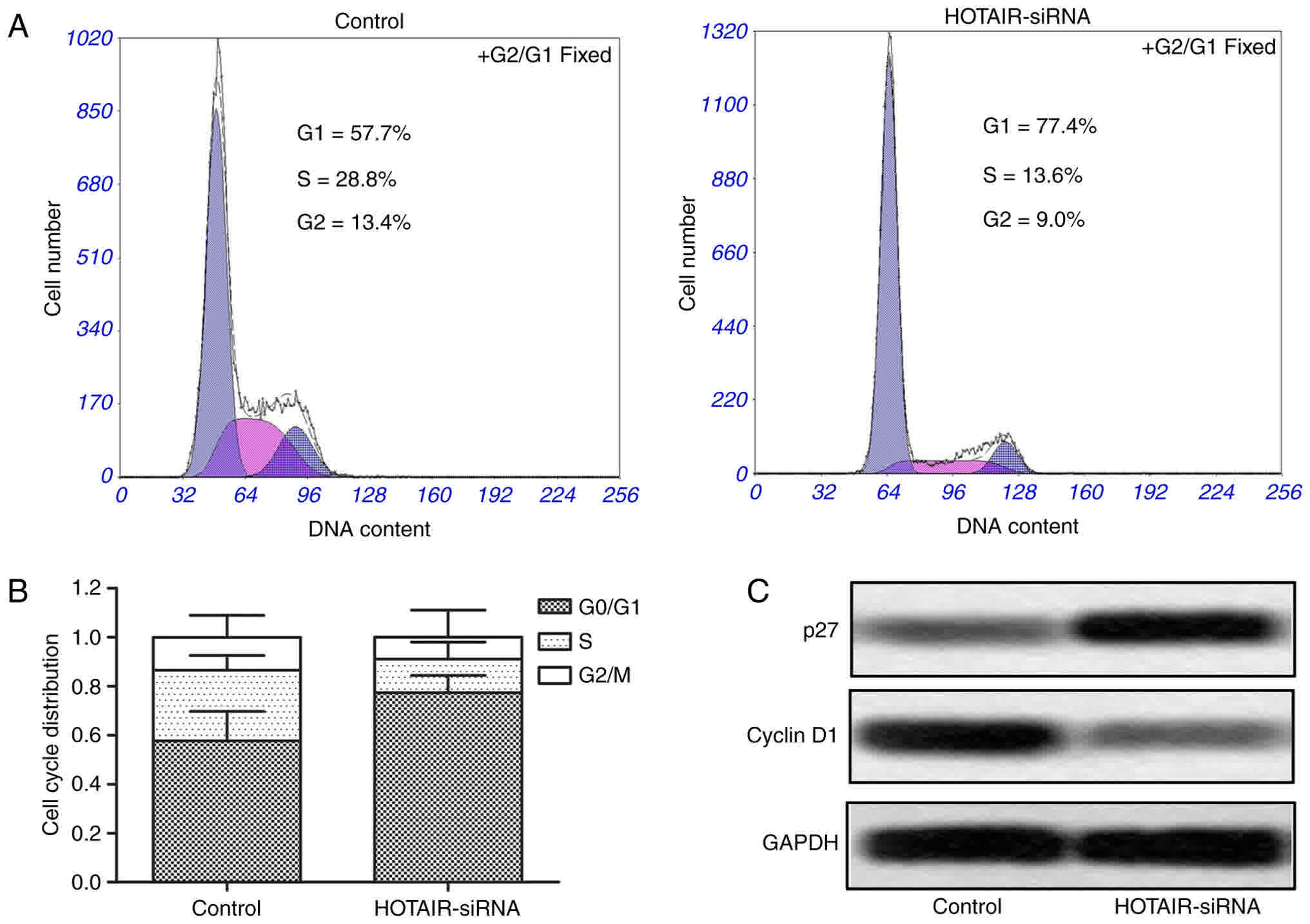
Cell cycle arrest was measured by flow cytometry to
further convince the suppressing effect of HOTAIR siRNA on the
proliferation in HepG2 cells. As shown in Fig. 3A and B, the G0/G1 cell cycle arrest
was significantly increased in HOTAIR-siRNA group compared with
control group. Moreover, we measured the protein expression levels
of two cell cycle markers p27 and cyclin D1, and the results showed
that the expression of p27 had an obvious upregulation, while the
level of cyclin D1 had a significant downregulation in HOTAIR-siRNA
group as compared with control group (Fig. 3C). These results suggest that
inhibition of HOTAIR induces G0/G1 cell cycle arrest in HepG2
cells.
Expression of miR-217 was
downregulated in liver cancer tissues and liver cancer cell
lines
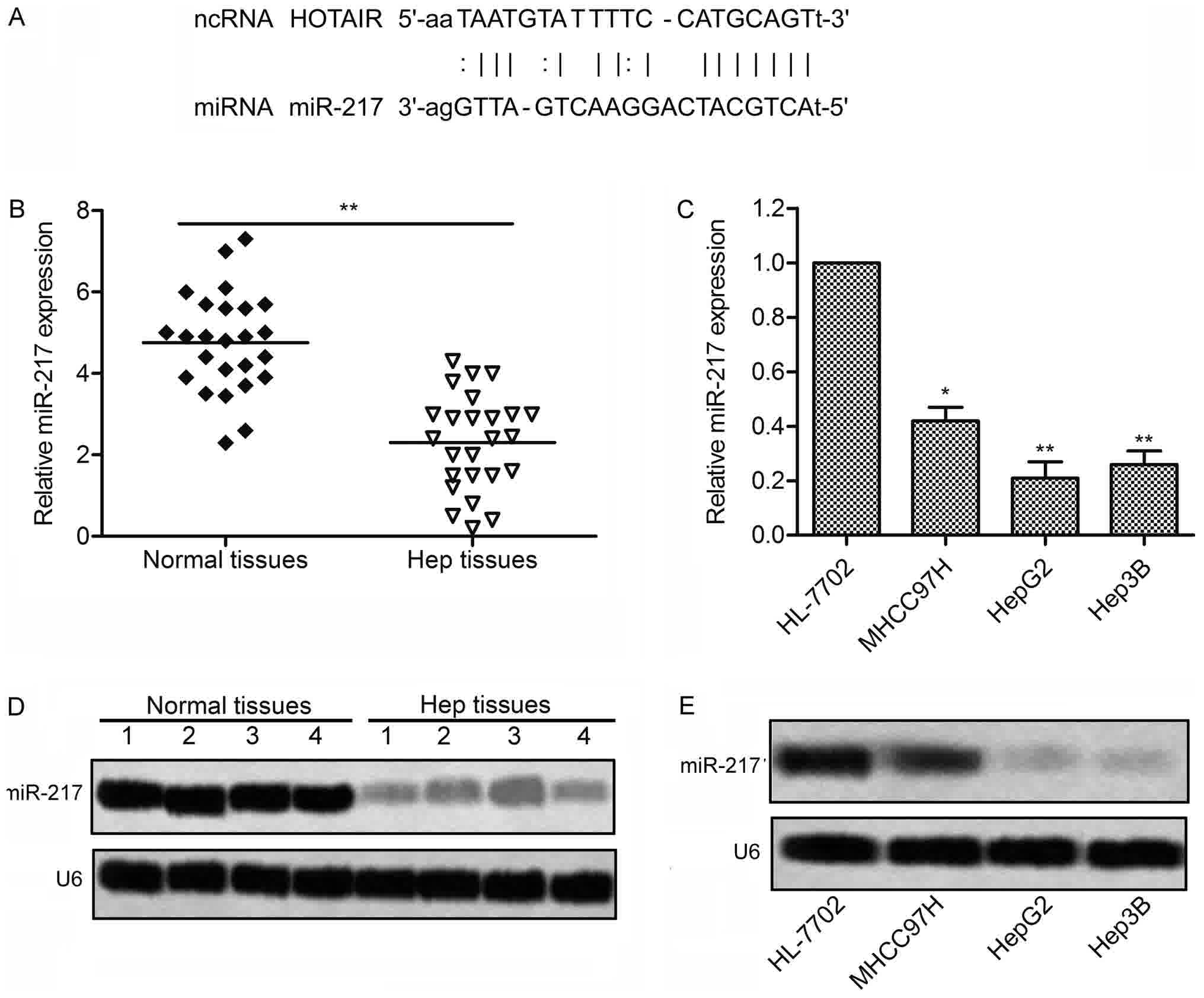
The targeting relationship between HOTAIR and
miR-217 was predicted by StarBase v2.0 (http://starbase.sysu.edu.cn/) (Fig. 4A). We then detected the expression of
miR-217 in 25 liver cancer tissues and 3 liver cancer cell lines by
RT-PCR and northern blotting, respectively. As shown in Fig. 4B and D, the expression of miR-217 had
a significantly downregulation in the liver cancer tissues as
compared with the normal tissues. Consistent with the result,
Expression of miR-217 were markedly downregulated in 3 liver cancer
cell lines compared with normal human hepatic cell line HL-7702
(Fig. 4C and E). These results
suggest that miR-217 plays a crucial role in the progression of
liver cancer, and the expression between HOTAIR and miR-217 has a
close correlation.
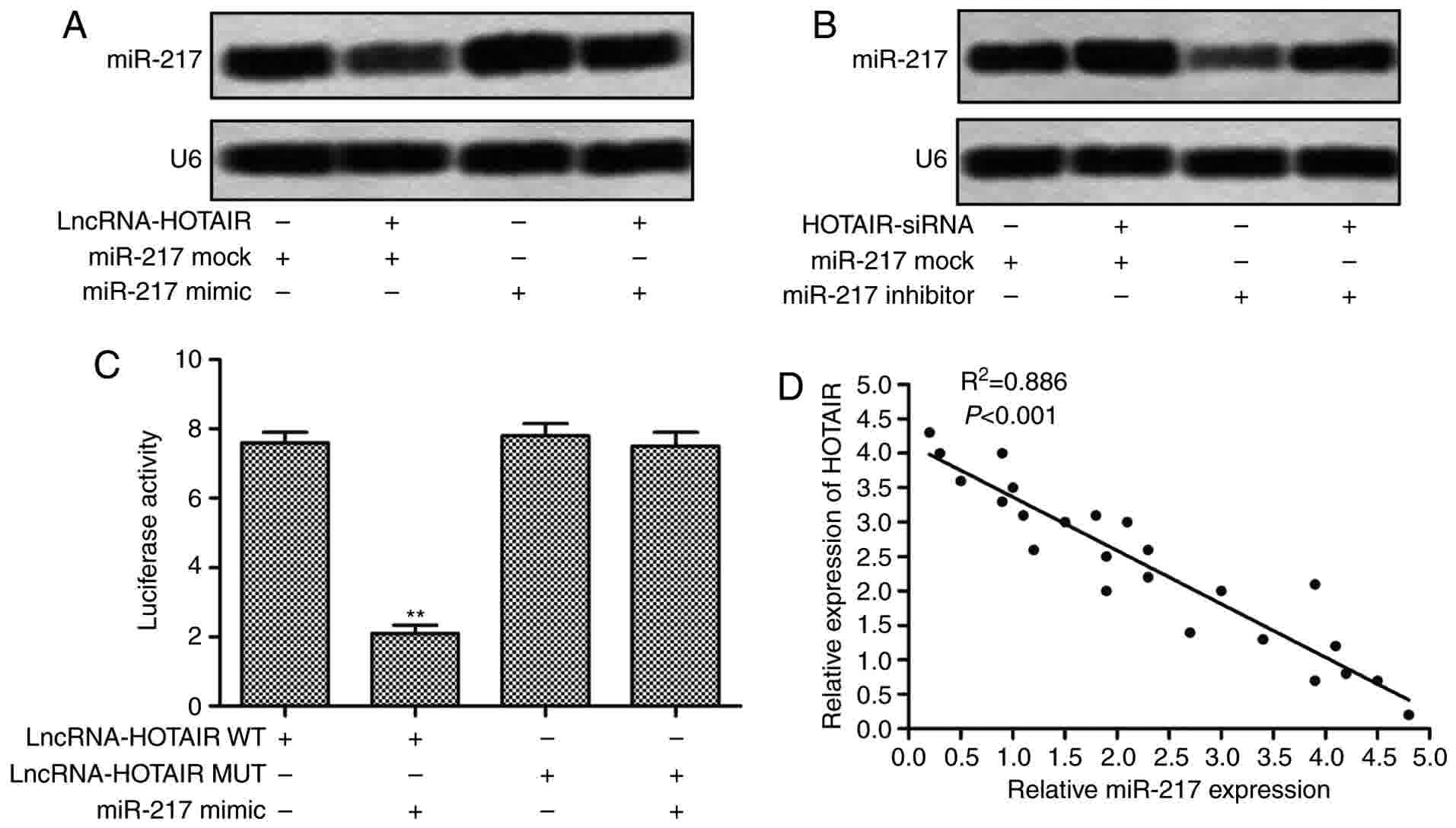
miR-217 is a direct target of
HOTAIR
To further confirm the regulationship of HOTAIR and
miR-217, we co-transfected HepG2 cells with HOTAIR siRNA lentiviral
vectors and miR-217 mimics or inhibitors. As shown in Fig. 5A, compared with miR-217 mock group,
the expression of miR-217 in HepG2 cells was significantly
downregulated when co-transfected with HOTAIR lentvirus vectors and
miR-217 mock. The elevated level of miR-217 was also decreased
adding HOTAIR lentvirus vectors in HepG2 cells transfected with
miR-217 mimic. As shown in Fig. 5B,
when HepG2 cells were co-transfected with HOTAIR siRNA lentvirus
vectors and miR-217 inhibitors, the inhibitor-induced suppression
of miR-217 was significantly reduced, and the expression of miR-217
was markedly increased as compared with that in cells transfected
with miR-217 inhibitors alone. These results imply that HOTAIR
regulate the expression of miR-217 in HepG2 cells. In addition, we
performed the Dual luciferase reporter assay to identify the
targeting relationship between HOTAIR and miR-217, the results
showed that miR-217 mimics transfection markedly decreased the
relative luciferase activity of the plasmid containing HOTAIR
sequences (WT) as compared with that containing mutating sequences
(Fig. 5C). We further found that
there was a negative regulation between the expression of HOTAIR
and miR-217 in liver cancer tissues (Fig.
5D). Taken together, the above results illustrate that HOTAIR
directly targets miR-217 to regulate its expression.
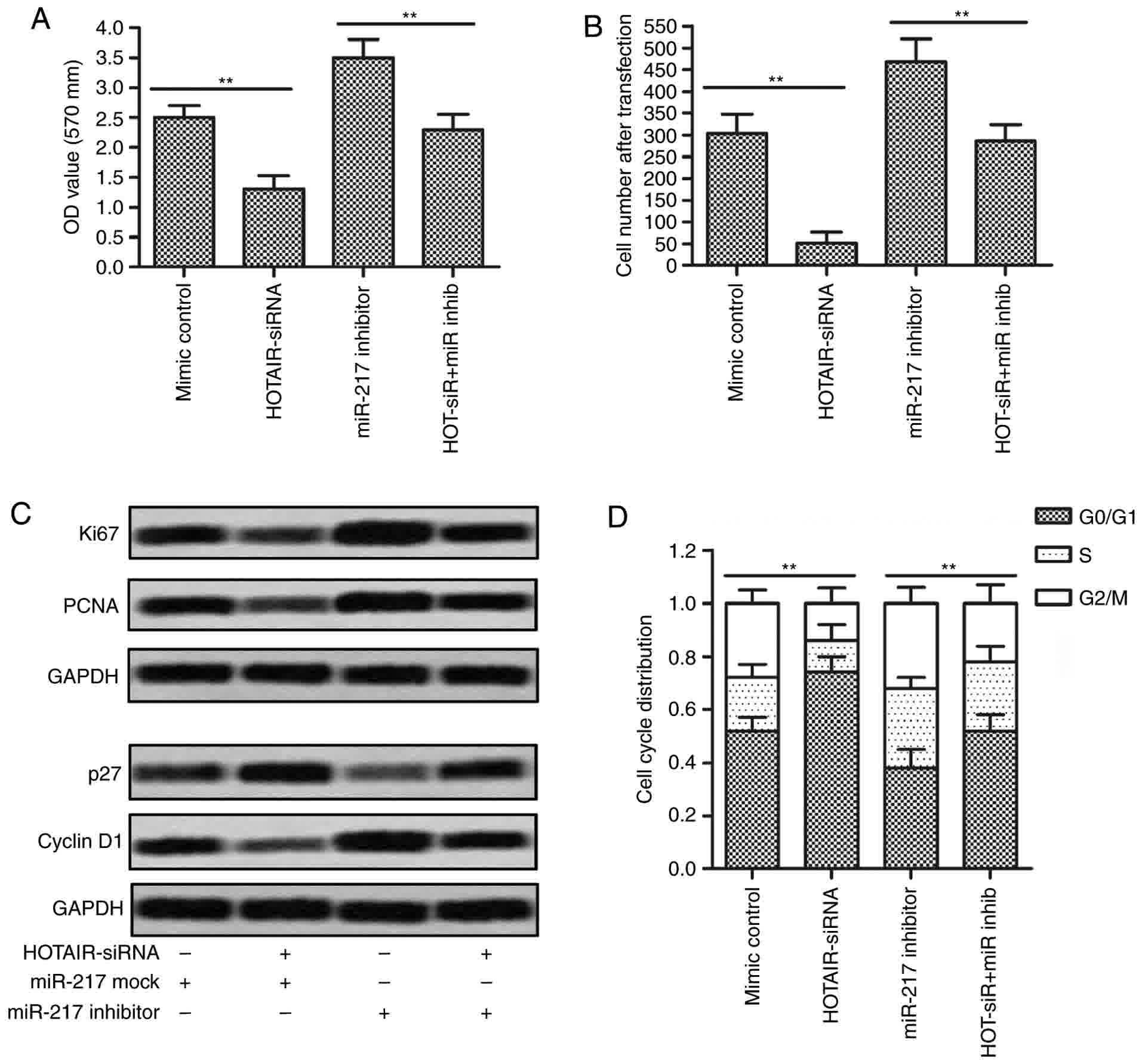
miR-217 was involved in the regulation
of HOTAIR on cell proliferation and cycle arrest in HepG2
cells
To investigate whether the effects of HOTAIR on cell
proliferation and cycle arrest in HepG2 cells were mediated by
miR-217, we transfected HepG2 cells with HOTAIR siRNA lentiviral
vectors, miR-217 inhibitors, or the combination of lentiviral
vectors and inhibitors. CCK-8 assay and cell counting showed that
inhibition of HOTAIR suppressed the proliferation and reduced cell
numbers of HepG2 cells, while inhibition of miR-217 promoted the
proliferation and increased cell numbers compared with control
group. However, the proliferation and cell numbers in HOTAIR siRNA
plus miR-217 inhibitor group and control group showed no
significantly differences (Fig. 6A and
B). As shown in Fig. 6C, western
blotting results revealed that the protein expression levels of
Ki67 and PCNA were downregulated in HOTAIR siRNA group and
upregulated in miR-217 inhibitor group, while the levels did not
significantly altered in HOTAIR siRNA plus miR-217 inhibitor group
as compared with control group. Furthermore, the expression of p27
showed an obvious downregulation and the level of cyclin D1 showed
an obvious upregulation in miR-217 inhibitor group, which were
significantly reversed when co-transfection with HOTAIR siRNA
lentiviral vectors and miR-217 inhibitors. Moreover, miR-217
inhibition-induced reduction of G0/G1 cycle arrest was partly
rescued by inhibition of HOTAIR (Fig.
6D). Taken together, these observations suggest that the
effects of HOTAIR on cell proliferation and cycle arrest in HepG2
cells were mediated by miR-217.
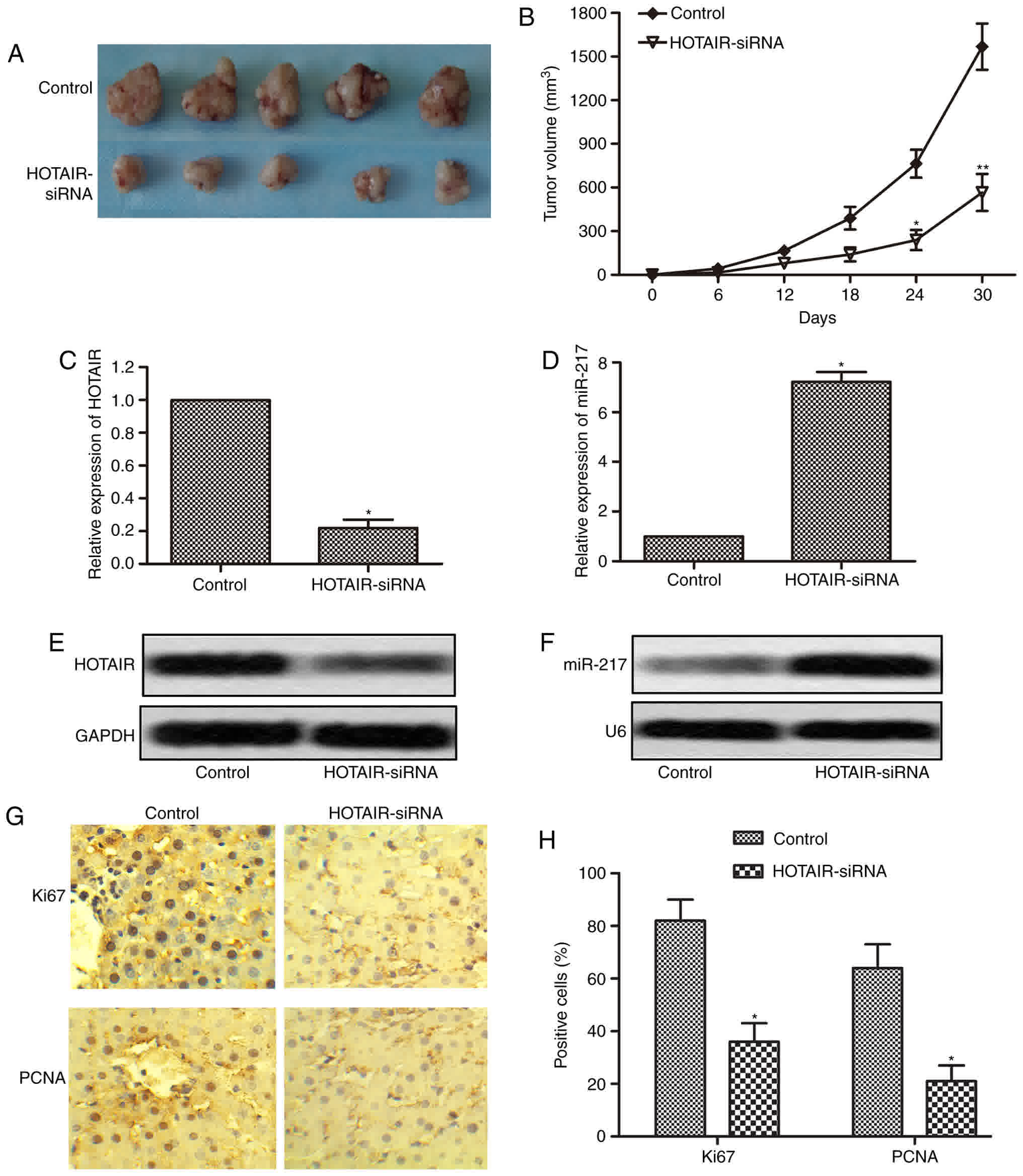
HOTAIR inhibition suppresses tumor
growth in vivo
To further determine whether inhibition of HOTAIR
could suppress the progression of liver cancer, an in vivo
tumor model injected with HepG2 cells or HOTAIR-siRNA cells was
used. Tumor volumes were measured every 6 days until the 30th day
following cells injection. Five representative tumors from each
group at 30th day were photographed and shown in Fig. 7A. The tumors volumes in HOTAIR-siRNA
group were significantly smaller than that in control group
(Fig. 7B). We then measured the
expression of HOTAIR and miR-217 in the tumor tissues, and the
results showed that the expression of HOTAIR was markedly reduced,
while the expression of miR-217 was significantly increased in
HOTAIR-siRNA tumor tissues as compared with control tumor tissues
(Fig. 7C-F). In addition, we further
performed immunohistochemistry assay to check the expression of
Ki67 and PCNA in the tumor tissues. As shown in Fig. 7G and H, compared with control tumor
tissues, Ki67 and PCNA positive cells had an obvious reduction in
HOTAIR-siRNA tumor tissues. These in vivo results together
with in vitro results suggest that inhibition of HOTAIR
suppresses tumor growth by regulating the expression of
miR-217.
Discussion
In recent years, a variety of investigations have
reported that lncRNAs have significant functions in cancer
pathogenesis. HOTAIR functions as an oncogenic lncRNA and it has
been studied in many cancers including liver cancer. However, its
biological roles and underlying molecular mechanisms in liver
cancer are still largely unknown. Previous studies have shown that
the expression of HOTAIR was upregulated in many cancer types
including liver cancer. In the present study, we found that the
expression of HOTAIR had an significantly upregulation in the liver
cancer tissues and liver cancer cell lines, MHCC 97H, HepG2 and
Hep3B, which was consistent with the previous results. These data
confirm that high level of HOTAIR is closely associated with the
progression of liver cancer, suggesting that HOTAIR inhibition may
be an effective therapeutic strategy for liver cancer.
Previous reports have demonstrated that HOTAIR plays
an important role in cancers by regulating cellular events, such as
cell proliferation, cell invasion and tumor metastasis. HOTAIR
promotes human liver cancer stem cell malignant growth through
downregulation of SETD2 (26). HOTAIR
inhibition in liver cancer cells leads to the suppression of cell
proliferation and invasion in vitro, and significantly
inhibited the rate of growth of liver cancer cells in vivo,
partly through the regulation of the Wnt/β-catenin signaling
pathway (27). HOTAIR could promote
migration and invasion of liver cancer cells by inhibiting RBM38.
These studies suggest that HOTAIR regulates the development and
progression of liver cancer through many different signal molecules
or pathways. In the present study, to further explore the
biological effects of HOTAIR on the proliferation and cycle arrest
in liver cancer, HOTAIR siRNA lentiviral vectors were used to
infect HepG2 cells to interfere the expression of HOTAIR. CCK-8
assay and cell counting revealed that the cell proliferation had an
obvious suppression, and the cell number had an obvious reduction
in HOTAIR-siRNA group as compared with control group. Western blot
assay showed that compared with the control group, the protein
expression levels of Ki67 and PCAN, two proliferation-associated
markers, were significantly decreased in HOTAIR-siRNA group.
Moreover, flow cytometry assay showed that the G0/G1 cell cycle
arrest was significantly increased in HOTAIR-siRNA group. The
protein expression level of p27 had an obvious upregulation, while
the level of cyclin D1 had a significant downregulation in
HOTAIR-siRNA group as compared with control group. These results
suggest that HOTAIR inhibition suppresses cell proliferation and
induces G0/G1 cell cycle arrest in HepG2 cells.
LncRNAs have been reported to exert various
biological functions by targeting miRNAs. HOTAIR participates in
inhibiting the expression of miR-205, which has a negative
correlation with the degree of bladder cancer malignancy by
regulating proliferation, migration and invasion of bladder cancer
cells (28). HOTAIR promotes the
progression of glioma by inhibiting miR-326, and further
upregulated the expression of firoblast growth factor 1 (FGF1)
(29). Also, the oncogenic activity
of HOTAIR in gallbladder cancer is partly through repressing
miRNA-130a (30). These reports
demonstrate that the biological activities of HOTAIR in different
cancer types can be mediated miRNAs. Recently, miR-217 was shown to
be involved in the development of liver cancer. Moreover, miR-217
has been reported to be downregulated in many cancers, such as lung
cancer, colorectal cancer, epithelial ovarian cancer, and
pancreatic cancer and function as a tumor suppressor gene (31–34). In
this study, we explored the interaction between HOTAIR and miR-217
by bioinformatics prediction and experiments, and found that HOTAIR
directly targeted miR-217 to regulate its expression. The
expression of miR-217 had an significantly downregulation in the
liver cancer tissues and liver cancer cell lines, and the
expression levels of HOTAIR and miR-217 were inversely correlated
in liver cancer tissues, which further supported the regulation of
miR-217 by HOTAIR. We further investigated whether the effects of
HOTAIR on cell proliferation and cycle arrest in HepG2 cells were
mediated by miR-217. The results showed that the effects of HOTAIR
inhibition on proliferation suppression and G0/G1 cycle arrest
induction in HepG2 cells were in part rescued by miR-217
inhibition. Taken together, these observations suggest that the
effects of HOTAIR on cell proliferation and cycle arrest in HepG2
cells were mediated by miR-217.
To further determine whether inhibition of HOTAIR
could suppress the progression of liver cancer, an in vivo
tumor model was used. And in vivo experiments had shown that
HOTAIR inhibition significantly decreased the tumors volumes, and
the expression of miR-217 was increased, the expression of Ki67 and
PCNA was reduced in HOTAIR-siRNA tumor tissues. These in
vivo results are consistent with the data in vitro
experiments, and suggest that inhibition of HOTAIR suppresses liver
cancer progression and growth by regulating the expression of
miR-217.
In conclusion, the present study demonstrates that
HOTAIR expression is upregulated in liver cancer tissues, and
functions as an oncogene in liver cancer. HOTAIR inhibition
significantly suppresses the progression of liver cancer by
inhibiting cell proliferation and inducing G0/G1 cell cycle arrest
of liver cancer cells, which was partly mediated by upregulating
miR-217. Thus, targeting HOTAIR-miR-217 axis may provide novel
insight into therapeutic targets for the treatment of liver
cancer.
Acknowledgements
The authors thank the Xi'an No. 4 Hospital for
providing technical support.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
LW and JW performed the examinations and analyzed
the data. XW contributed to analysis of the data, and was a major
contributor in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all the patients.
The study was performed in accordance with the Helsinki Declaration
and was approved by the Human Ethics Committee/Institutional Review
Board of Xi'an Honghui Hospital.
Consent for publication
Informed consent was obtained from all the
patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HOTAIR
|
HOX transcript antisense RNA
|
|
miR-217
|
microRNA-217
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
ESCC
|
esophageal squamous cell carcinoma
|
|
3′-UTRs
|
3′-untranslated regions
|
References
|
1
|
Bosch FX, Ribes J, Díaz M and Cléries R:
Primary liver cancer: Worldwide incidence and trends.
Gastroenterology. 127:S5–S16. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dong C, Zhao B, Long F, Liu Y, Liu Z, Li
S, Yang X, Sun D, Wang H, Liu Q, et al: Nogo-B receptor promotes
the chemoresistance of human liver cancer via the ubiquitination of
p53 protein. Oncotarget. 7:8850–8865. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng Z, Liu J, Yang Z, Wu L, Xie H, Jiang
C, Lin B, Chen T, Xing C, Liu Z, et al: MicroRNA-452 promotes
stem-like cells of liver cancer by inhibiting Sox7 involving
Wnt/β-catenin signaling pathway. Oncotarget. 7:28000–28012. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qin Y, Zhao D, Zhou HG, Wang XH, Zhong WL,
Chen S, Gu WG, Wang W, Zhang CH, Liu YR, et al: Apigenin inhibits
NF-κB and snail signaling, EMT and metastasis in human liver
cancer. Oncotarget. 7:41421–41431. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang Z, Zhou L, Wu LM, Lai MC, Xie HY,
Zhang F and Zheng SS: Overexpression of long non-coding RNA HOTAIR
predicts tumor recurrence in liver cancer patients following liver
transplantation. Ann Surg Oncol. 18:1243–1250. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mercer TR, Dinger ME and Mattick JS: Long
non-coding RNAs: Insights into functions. Nat Rev Genet.
10:155–159. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gibb EA, Brown CJ and Lam WL: The
functional role of long non-coding RNA in human carcinomas. Mol
Cancer. 10:382011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang TH, Liang LZ, Liu XL, Wu JN, Su K,
Chen JY, Zheng QY, Huang HZ and Liao GQ: Long non-coding RNA MALAT1
interacts with miR-124 and modulates tongue cancer growth by
targeting JAG1. Oncol Rep. 37:2087–2094. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li Y, Wu Z, Yuan J, Sun L, Lin L, Huang N,
Bin J, Liao Y and Liao W: Long non-coding RNA MALAT1 promotes
gastric cancer tumorigenicity and metastasis by regulating
vasculogenic mimicry and angiogenesis. Cancer Lett. 395:31–44.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jin Y, Cui Z, Li X, Jin X and Peng J:
Upregulation of long non-coding RNA PlncRNA-1 promotes
proliferation and induces epithelial-mesenchymal transition in
prostate cancer. Oncotarget. 8:26090–26099. 2017.PubMed/NCBI
|
|
11
|
Wei W, Liu Y, Lu Y, Yang B and Tang L:
LncRNA XIST promotes pancreatic cancer proliferation through
miR-133a/EGFR. J Cell Biochem. 118:3349–3358. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xue X, Yang YA, Zhang A, Fong KW, Kim J,
Song B, Li S, Zhao JC and Yu J: LncRNA HOTAIR enhances ER signaling
and confers tamoxifen resistance in breast cancer. Oncogene.
35:2746–2755. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kogo R, Shimamura T, Mimori K, Kawahara K,
Imoto S, Sudo T, Tanaka F, Shibata K, Suzuki A, Komune S, et al:
Long noncoding RNA HOTAIR regulates polycomb-dependent chromatin
modification and is associated with poor prognosis in colorectal
cancers. Cancer Res. 71:6320–6326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim K, Jutooru I, Chadalapaka G, Johnson
G, Frank J, Burghardt R, Kim S and Safe S: HOTAIR is a negative
prognostic factor and exhibits pro-oncogenic activity in pancreatic
cancer. Oncogene. 32:1616–1625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Liu Z, Sun M, Liu J, Wang Z and Wei
D: The long non-coding RNA HOTAIR indicates a poor prognosis and
promotes metastasis in non-small cell lung cancer. BMC Cancer.
13:4642013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen FJ, Sun M, Li SQ, Wu QQ, Ji L, Liu
ZL, Zhou GZ, Cao G, Jin L, Xie HW, et al: Upregulation of the long
non-coding RNA HOTAIR promotes esophageal squamous cell carcinoma
metastasis and poor prognosis. Mol Carcinog. 52:908–915. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao J, Jia LI, Jingli DU and Xiaolei LI:
Long non-coding RNA HOTAIR is a marker for liver cancer progression
and tumor recurrence. Oncol Lett. 11:1791–1798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Geng YJ, Xie SL, Li Q, Ma J and Wang GY:
Large Intervening non-coding RNA HOTAIR is associated with liver
cancer progression. J Int Med Res. 39:2119–2128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bartel DP: MicroRNA target recognition and
regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Su DN, Wu SP, Chen HT and He JH: HOTAIR, a
long non-coding RNA driver of malignancy whose expression is
activated by FOXC1, negatively regulates miRNA-1 in liver cancer.
Oncol Lett. 12:4061–4067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Su J, Wang Q, Liu Y and Zhong M: miR-217
inhibits invasion of liver cancer cells through direct suppression
of E2F3. Mol Cell Biochem. 392:289–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
López-Terrada D, Cheung SW, Finegold MJ
and Knowles BB: HepG2 is a hepatoblastoma-derived cell line. Hum
Pathol. 40:1512–1515. 2009. View Article : Google Scholar
|
|
24
|
Zhou Y, Fukuda T, Hang Q, Hou S, Isaji T,
Kameyama A and Gu J: Inhibition of fucosylation by 2-fluorofucose
suppresses human liver cancer HepG2 cell proliferation and
migration as well as tumor formation. Sci Rep. 7:115632017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shao LW, Huang LH, Yan S, Jin JD and Ren
SY: Cordycepin induces apoptosis in human liver cancer HepG2 cells
through extrinsic and intrinsic signaling pathways. Oncol Lett.
12:995–1000. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, An J, Wu M, Zheng Q, Gui X, Li T, Pu
H and Lu D: LncRNA HOTAIR promotes human liver cancer stem cell
malignant growth through downregulation of SETD2. Oncotarget.
6:27847–27864. 2015.PubMed/NCBI
|
|
27
|
Gao JZ, Li J, Jl DU and Li XL: Long
non-coding RNA HOTAIR is a marker for liver cancer progression and
tumor recurrence. Oncol Lett. 11:1791–1798. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun X, Du P, Yuan W, Du Z, Yu M, Yu X and
Hu T: Long non-coding RNA HOTAIR regulates cyclin J via inhibition
of microRNA-205 expression in bladder cancer. Cell Death Dis.
6:e19072015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ke J, Yao Y, Zheng J, Wang P, Liu YH, Ma
J, Li Z, Liu XB, Li ZQ, Wang ZH and Xue YX: Knockdown of long
non-coding RNA HOTAIR inhibits malignant biological behaviors of
human glioma cells via modulation of miR-326. Oncotarget.
6:21934–21949. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ma MZ, Li CX, Zhang Y, Weng MZ, Zhang MD,
Qin YY, Gong W and Quan ZW: Long non-coding RNA HOTAIR, a c-Myc
activated driver of malignancy, negatively regulates miRNA-130a in
gallbladder cancer. Mol Cancer. 13:1562014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo J, Feng Z, Huang Z, Wang H and Lu W:
MicroRNA-217 functions as a tumour suppressor gene and correlates
with cell resistance to cisplatin in lung cancer. Mol Cells.
37:664–671. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang B, Shen ZL, Jiang KW, Zhao G, Wang
CY, Yan YC, Yang Y, Zhang JZ, Shen C, Gao ZD, et al: MicroRNA-217
functions as a prognosis predictor and inhibits colorectal cancer
cell proliferation and invasion via an AEG-1 dependent mechanism.
BMC Cancer. 15:4372015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Li D and Zhang W: Tumor suppressor
role of miR-217 in human epithelial ovarian cancer by targeting
IGF1R. Oncol Rep. 35:1671–1679. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng S, Zhu S, Wang B, Li X, Liu Y, Qin Q,
Gong Q, Niu Y, Xiang C, Chen J, et al: Chronic pancreatitis and
pancreatic cancer demonstrate active epithelial-mesenchymal
transition profile, regulated by miR-217-SIRT1 pathway. Cancer
Lett. 355:184–191. 2014. View Article : Google Scholar : PubMed/NCBI
|