Introduction
Pancreatic cancer (PC) is one of the most lethal
malignancies of the human digestive system due to its rapid
progression, high recurrence rate and strong chemoresistance
(1,2).
PC is rarely diagnosed at an early stage due to the fact that
patients with localized PC have no recognizable signs or symptoms.
Therefore, the majority of PC patients do not receive a definitive
diagnosis until late, once the PC cells have metastasized to other
organs (3,4). Even in cases where surgical resection is
performed, the local recurrence rate of PC is high and the 5-year
survival rate remains at only 5% following surgery. Despite recent
progress in chemotherapeutics and understanding of the molecular
biological mechanisms of PC, limited progress has been made in
therapeutic methods for metastatic disease. Due to the fact that
the incidence of PC has been markedly increasing over recent years
(5), it is necessary to find novel
therapies for PC (6).
Gemcitabine (2′,2′-difluoro-2′-deoxycytidine, dFdC;
GEM), a pyrimidine analog, which is phosphorylated to diphosphate
and triphosphate forms to inhibit DNA polymerase and ribonucleotide
reductase (7), is now used as the
standard palliative treatment for PC (8–11).
Originally, GEM was approved as the first-line treatment for PC by
the US Food and Drug Administration in 1997 based upon the study by
Burris et al (9). Almost 2
decades later, GEM was approved for the treatment of advanced PC.
GEM has demonstrated marked effects on the survival time of PC
patients when used in various forms of therapy, including GEM
monotherapy, combination treatment with GEM and in a number of
other active cytotoxic agents or regimens (12–15).
However, there are a number of factors that have been reported to
cause GEM resistance (16).
The reversible phosphorylation of proteins serves a
crucial function in regulating numerous biological responses. In
mammalian cells, >99% of this phosphorylation occurs on serine
or threonine (Ser/Thr) residues. This type of protein phosphatase
dephosphorylates a range of proteins involved in a wide range of
cellular processes, including apoptosis, cell differentiation, cell
survival, the response to DNA damage, and the regulation of ion
channels and circadian rhythms (17,18). The
reversible phosphorylation of proteins may also impact on several
signaling pathways, including those controlled by kinases, such as
apoptosis signal-regulating kinase 1/mitogen-activated protein
kinase kinase kinase 5 (ASK1/MAP3K5), protein kinase,
DNA-activated, catalytic polypeptide and RAF1 (19–21). The
targeting protein of Ser/Thr protein phosphatase 5 (PPP5C), a
member of the phosphoprotein phosphatase (PPP) family of Ser/Thr
phosphatases, is an enzyme encoded by the PPP5C gene (22). PPP5C is broadly expressed and has
distinct structural properties compared with other phosphatases in
the PPP family (23,24). PPP5C belongs to the protein
phosphatase-5 subfamily and contains only 1 single polypeptide
chain (25–27). It has been reported that PPP5
functions upstream of p53 and that it phosphorylates p53 to
regulate the induction of p21 (WAF1/Cip1), as well as to mediate
the growth arrest pathway (25,28). PPP5C
has been demonstrated to interact with ASK1 (19), cryptochrome circadian clock 2
(29), guanine nucleotide-binding
protein subunit α-12 (30) and
Ras-related C3 botulinum toxin substrate 1 (31). ASK1 could activate c-Jun N-terminal
kinase (JNK) and p38 mitogen-activated protein kinases (MAPK) in a
Raf-independent manner in response to a number of stresses. ASK1 is
additionally associated with cancer, diabetes, and cardiovascular
and neurodegenerative diseases (32).
The result of PPP5C-knockdown by siRNA or
oligonucleotides has revealed that PPP5C is also associated with
the stress response and inhibition of the proliferation rate of
tumor cells, including ovarian cancer (33), glioma (34) and liver carcinoma (35) cells. Additionally, a previous study
demonstrated that an elevated level of PPP5C protein is directly
associated with Alzheimer's disease (35,36).
However, the function of PPP5C in PC has not been reported prior to
the present study. In the present study, shRNA was constructed to
silence the expression of PPP5C in the PC PANC-1 cell line and the
effects of PPP5C on PANC-1 cells treated with GEM were also
investigated.
Materials and methods
Cell lines and cell culture
The human embryonic kidney 293 cell line (HEK 293)
and the human PC cell line PANC-1 were used in the present study
and were purchased from the Cell Bank of the Chinese Academy of
Sciences (Shanghai, China). The cell lines were cultured in
Dulbecco's modified Eagle's medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA; cat. no. SH30243.01B+), supplemented with
10% fetal bovine serum (Biological Industries, Beit-Haemek, Israel;
cat. no. 04-001-1A) and maintained at 37°C in a humidified
incubator with 5% CO2.
Construction of the lentivirus vector
for PPP5C short hairpin (sh)RNA and virus packaging
Based upon the sequence of PPP5C (NM_001204284.1), a
responsible shRNA sequence
(5′-GAGACAGAGAAGATTACAGTACTCGAGTACTGTAATCTTCTCTGTCTCTTTTT-3′) was
generated to target PPP5C and a control shRNA sequence
(5′-TTCTCCGAACGTGTCACGTCTCGAGACGTGACACGTTCGGAGAA-3′) was designed
and synthesized. The recombinant vectors, PPP5C shRNA (shPPP5C) and
control shRNA (shCon), were designated to carry the corresponding
shRNA. T4 DNA ligase (New England BioLabs, Inc., Ipswich, MA, USA)
was used to construct shRNA fragments (50 ng), which were cloned
into the lentiviral expression vector pGP (Shanghai Hollybio,
Shanghai, China) prior to being digested by EcoRI and
BamHI (New England BioLabs, Inc.). Lentivirus was generated
by co-transfection of HEK293 cells with recombinant vectors and
packaging plasmids (pVSVG-I and pCMV ∆ R8.92; Shanghai Hollybio).
The supernatants were collected 96 h after transfection to extract
the lentivirus that may express PPP5C shRNA or control shRNA. The
lentivirus was then purified via ultracentrifugation in a condition
of 100,000 × g for 30 min at 4°C. PANC-1 cells were infected with
the concentrated virus at a multiplicity of infection of 10 and
mock-infected cells were used as negative controls. Since the
lentivirus carries a green fluorescence protein (GFP) as a reporter
and this GFP is driven by the cytomegalovirus promoter, the titer
of lentivirus was determined by counting the number of cells that
expressed GFP under fluorescence microscopy under ×100
magnification (Olympus Corporation, Tokyo, Japan) following 48 h of
infection. The efficiency of PPP5C-knockdown was subsequently
measured by reverse transcription quantitative polymerase chain
reaction (RT-qPCR) and western blot analysis.
RNA extraction and RT-qPCR
PANC-1 cells were harvested following 72 h of
lentivirus infection and all of the RNA from the cultured cells was
extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA; cat. no. 15596-026) according
to the manufacturer's protocols. The purity and integrity of
extracted RNA was assessed using spectrophotometry and agarose gel
electrophoresis, respectively. The first strand in the
complementary DNA of the extracted RNA was synthesized from the
aforementioned RNA (2 µg) using reverse transcription reagents
(Promega Corporation, Madison, WI, USA; cat. no. M1705). The
primers used in this study were: PPP5C forward,
5′-CCCAACTACTGCGACCAGAT-3′ and reverse, 5′-CCCGTCACCTCACATCATTC-3′;
and β-actin forward, 5′-GTGGACATCCGCAAAGAC-3′ and reverse,
5′-AAAGGGTGTAACGCAACTA-3′. The expression of PPP5C mRNA was
evaluated by RT-qPCR using the SYBR Green Core Reagents kit on
BioRad CFX96 Touch™ Real-Time PCR system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The conditions of the PCR involved
incubating all of the samples at 95°C for 1 min, followed by 40
cycles of denaturation at 95°C for 5 sec, and annealing and
extension at 60°C for 20 sec. β-actin was used as the input
reference. The relative gene expression levels were quantified
using the 2−ΔΔCq method (37).
Western blot analysis
PANC-1 cells were harvested following 6 days of
lentivirus infection. Cells were harvested and washed twice using
ice-cold phosphate-buffered saline (PBS) and then lysed in ice-cold
2X SDS Lysis Buffer [100 mM Tris-HCl (pH 6.8), 10 mM EDTA, 4% SDS
and 10% glycine]. The protein concentration of cell lysate was
determined using the BCA protein assay kit (Pierce; Thermo Fisher
Scientific, Inc.; cat. no. 23235). Extracted proteins (30 µg) were
separated on 10% SDS-PAGE prior to being transferred
electrophoretically onto a polyvinylidene fluoride membrane
(Bio-Rad Laboratories, Inc.; cat. no. 162-0177). The proteins were
blocked in 5% skimmed milk for 1 min at room temperature and probed
with specific antibodies at 4°C overnight. The primary antibodies
used were rabbit anti-PPP5C (Proteintech Group Inc., Chicago, IL,
USA; cat. no. 117515-1-AP; 1:1,000 dilution), and rabbit anti-p-JNK
(cat. no. 4668, 1:1,000 dilution), rabbit anti-JNK (cat. no. 9252;
1:1,000 dilution), rabbit anti-p-p38 (cat. no. 9215; 1:500
dilution), rabbit anti-p38 (cat no. 9212; 1:1,000 dilution), rabbit
anti-caspase3 (cat. no. 9661; 1:500 dilution), rabbit anti-PARP
(cat. no. 9542; 1:1,000 dilution), rabbit anti-p-p53(Ser315) (cat.
no. 2528; 1:500 dilution) and rabbit anti-p53 antibody (Ser15)
(cat. no. 11094; 1:500 dilution) (all from Cell Signaling
Technology, Inc., Danvers, MA, USA). One rabbit anti-GADPH antibody
was used as loading control (cat. no. 11205; 1:1,000 dilution;
Proteintech Group Inc.). Subsequently, the membranes were washed 3
times in Tris-buffered saline (TBST) prior to being incubated with
goat anti-rabbit Immunoglobulin G horseradish peroxidase-conjugated
secondary antibody (cat. no. SC-2054; 1:5,000 dilution; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) for 1 h at room temperature.
In addition, the JNK blot was stripped and reprobed with p-JNK, p38
was stripped and reprobed with p-38, and PARP was stripped and
reprobed with p-38. Stripping buffers consisted of 0.94 g glycine
(cat. no. GB0235; Sangon Biotech Co., Ltd., Shanghai, China) and 5
g sodium dodecyl sulfonate (cat. no. A500228; Sangon Biotech Co.,
Ltd.) in 500 ml water. The blot was placed into the stripping
buffers prior to being agitated in the shaker for 8 min at room
temperature. Subsequently, the blot was washed in 1X TBST 3 times,
for 5 min each time. The blot was then blocked and reacted with all
antibodies indicated as above in the aforementioned manner. The
target bands were visualized using an enhanced chemiluminescence
kit (GE Healthcare Life Sciences, Uppsala, Sweden) according to the
manufacturer's protocols.
MTT assay
Cell proliferation and viability were determined by
MTT assay. Cells were plated at a density of 5×103
cells/well in 96-well culture plates following 72 h of lentivirus
infection and treatment with various concentrations of GEM (1, 5
and 10 µΜ). Next, 20 µl MTT solution (5 mg/ml dissolved in PBS) was
added to each well followed by 4 h of incubation at 37°C. Following
incubation, 100 µl stop buffer acidic isopropanol (0.01 M HCl, 10%
SDS and 5% isopropanol) was added to each well to stop the
reaction. The culture plates with incubated cells were gently
agitated for 10 min and analyzed using an Epoch Microplate
Spectrophotometer version 2 (BioTek Instruments, Inc., Winooski,
VT, USA) at a wavelength of 595 nm.
Cell cycle analysis
Cell cycle analysis was conducted using propidium
iodide (PI) according to the manufacturer's protocols. PANC-1 cells
were seeded onto 6-cm wide dishes at a density of 5×105
cells/well. Following lentivirus infection for 4 days and treatment
with 5 µΜ GEM, PANC-1 cells were collected, washed, fixed with 75%
ethanol at 4°C overnight and then stained with PI. Finally, the
distribution of the cell cycle was assessed using a
fluorescence-associated cell sorting (FACS) assay with FACSCalibur
(Beckman Coulter, Inc., Brea, CA, USA) and the results were
analyzed using ModFit LT 3.1 software (Verity Software House, Inc.,
Topsham, ME, USA).
Annexin V staining apoptosis
analysis
To assess the rate of apoptosis, PANC-1 cells
infected with lentivirus and treated with 1 µΜ GEM were stained
using the Annexin V-APC/7-AAD Apoptosis Detection kit (cat. no.
KGA1026; Nanjing KeyGen Biotech Co., Ltd., Nanjing, China). The
cells were analyzed on a FACSCalibur (Beckman Coulter, Inc.) using
the CellQuest Pro software (version 5.1) (BD Biosciences, San Jose,
CA, USA) and the percentage of each quadrant was calculated using
this software.
Statistical analysis
All results are expressed as the mean ± standard
deviation. Statistical analysis was performed using unpaired
Student's t-test or one-way analysis of variance followed by
Dunnett's multiple comparisons test. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed using SPSS 13.0 statistical software (SPSS
Inc., Chicago, IL, USA).
Results
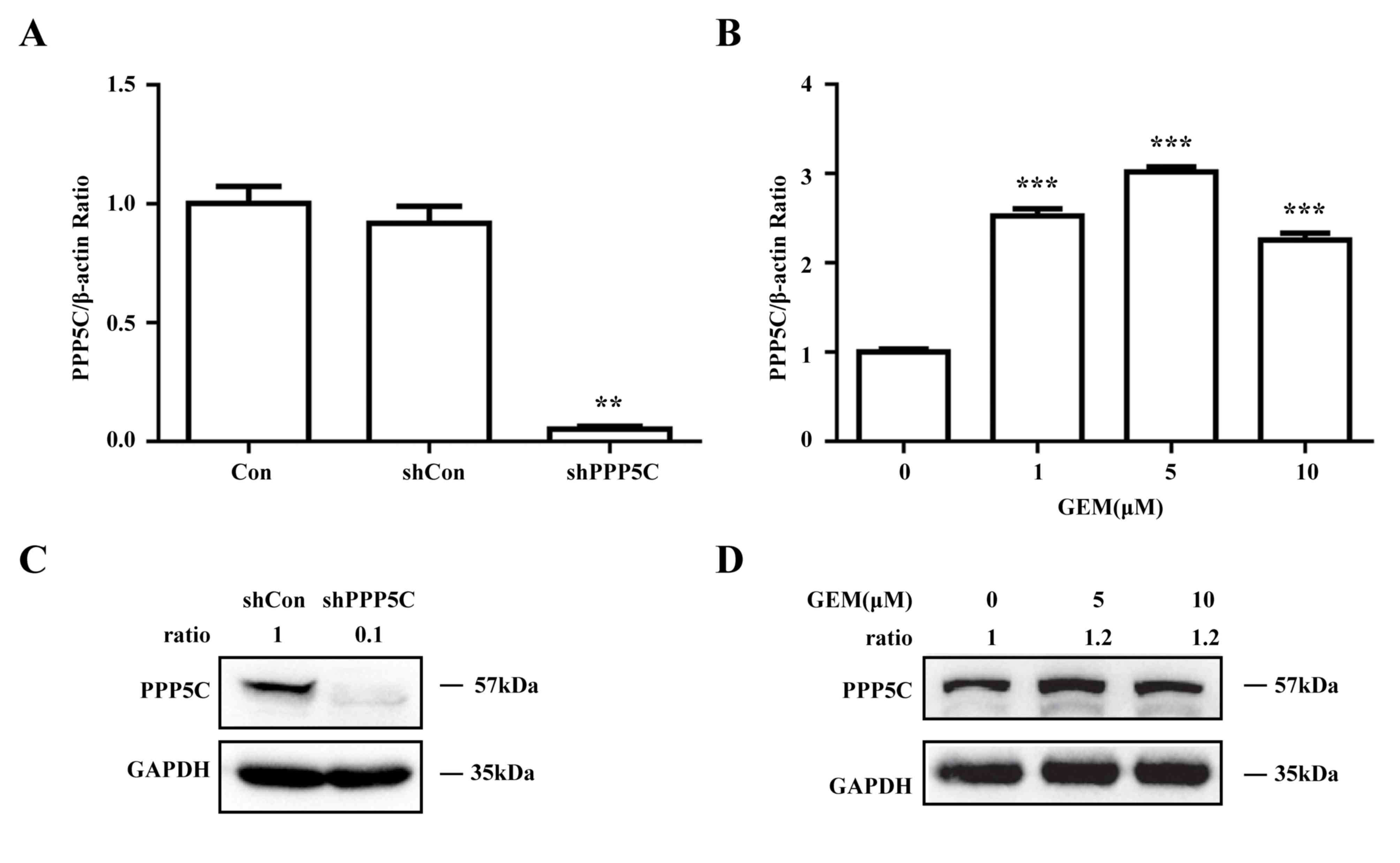
Endogenic PPP5C is inhibited by
shPPP5C and induced by GEM in the PC PANC-1 cell line
To investigate the role served by PPP5C in PC, the
PANC-1 cell line was infected with a lentivirus vector carrying
shRNA targeting PPP5C. PPP5C-knockdown efficiency was verified
using RT-qPCR and western blot analysis. Compared with that in the
negative control cells (shCon), the PPP5C mRNA expression in PPP5C
shRNA-infected PANC-1 cells (shPPP5C) was decreased by 94%
(Fig. 1A). Likewise, PPP5C protein
expression was significantly decreased in the PANC-1 cells infected
with shPPP5C (Fig. 1B). In addition,
the effect of GEM on PPP5C expression in the PANC-1 cells was
detected by qRT-PCR and western blot analysis. The results
indicated that GEM could increase intracellular PPP5C mRNA and
protein expression at concentrations of 1, 5 and 10 µm compared
with the untreated control (Fig. 1C and
D). These results indicate that intracellular PPP5C was
successfully silenced and that it could be enhanced by GEM in
PANC-1 cells.
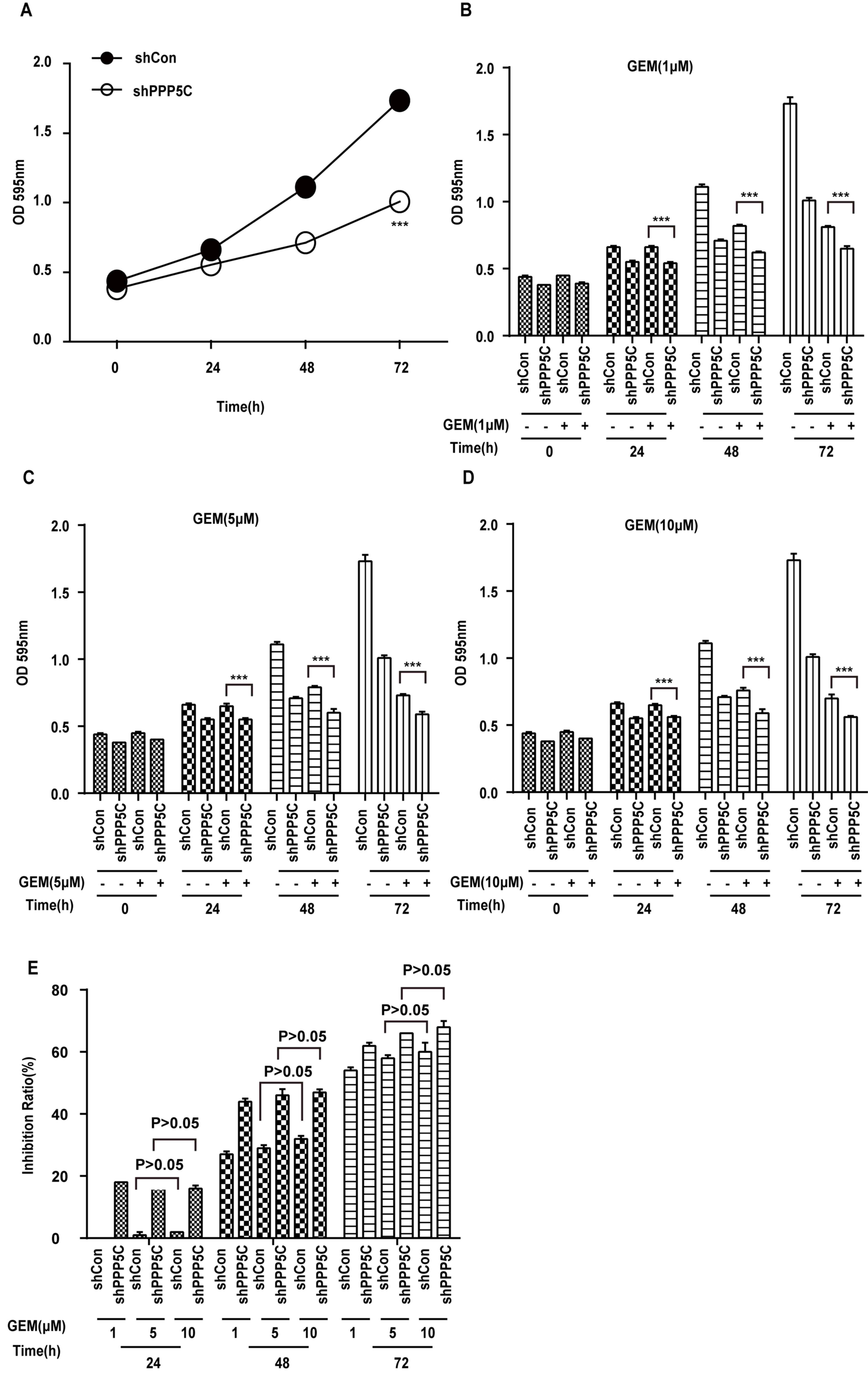
PPP5C silencing enhances the
chemosensitivity of PANC-1 cells to GEM
To determine whether or not PANC-1 cells with
reduced PPP5C expression were more sensitive to GEM, PANC-1 cells
were stably transfected with shPPP5C or shCon using a continuous
72-h MTT assay. As demonstrated in Fig.
2A, shPPP5C markedly inhibited the proliferation of PANC-1
cells compared with shCon. Following addition of GEM at varying
concentrations, the cell growth rates were significantly decreased
(Fig. 2B-D) compared with shCon cells
(Fig. 2A) using a Student's t-test.
As demonstrated in Fig. 2E, the
inhibition ratio was significantly increased in the shPPP5C group
following GEM treatment. In addition, statistical analysis revealed
that regardless of whether or not PPP5C was silenced, the
inhibitory effect of 5 or 10 µM GEM exhibited no marked differences
at 24, 48 and 72 h, indicating that the effect of GEM had reached
saturation (Fig. 2E). Even if the
effect of GEM had reached saturation, the shPPP5C group remained
able to further inhibit PANC-1 cell proliferation compared with the
shCon group (the inhibition ratio at different GEM concentrations
exhibited no dose-response effect; Fig.
2E). These results indicate that PPP5C-knockdown sensitizes
PANC-1 cells to GEM treatment.
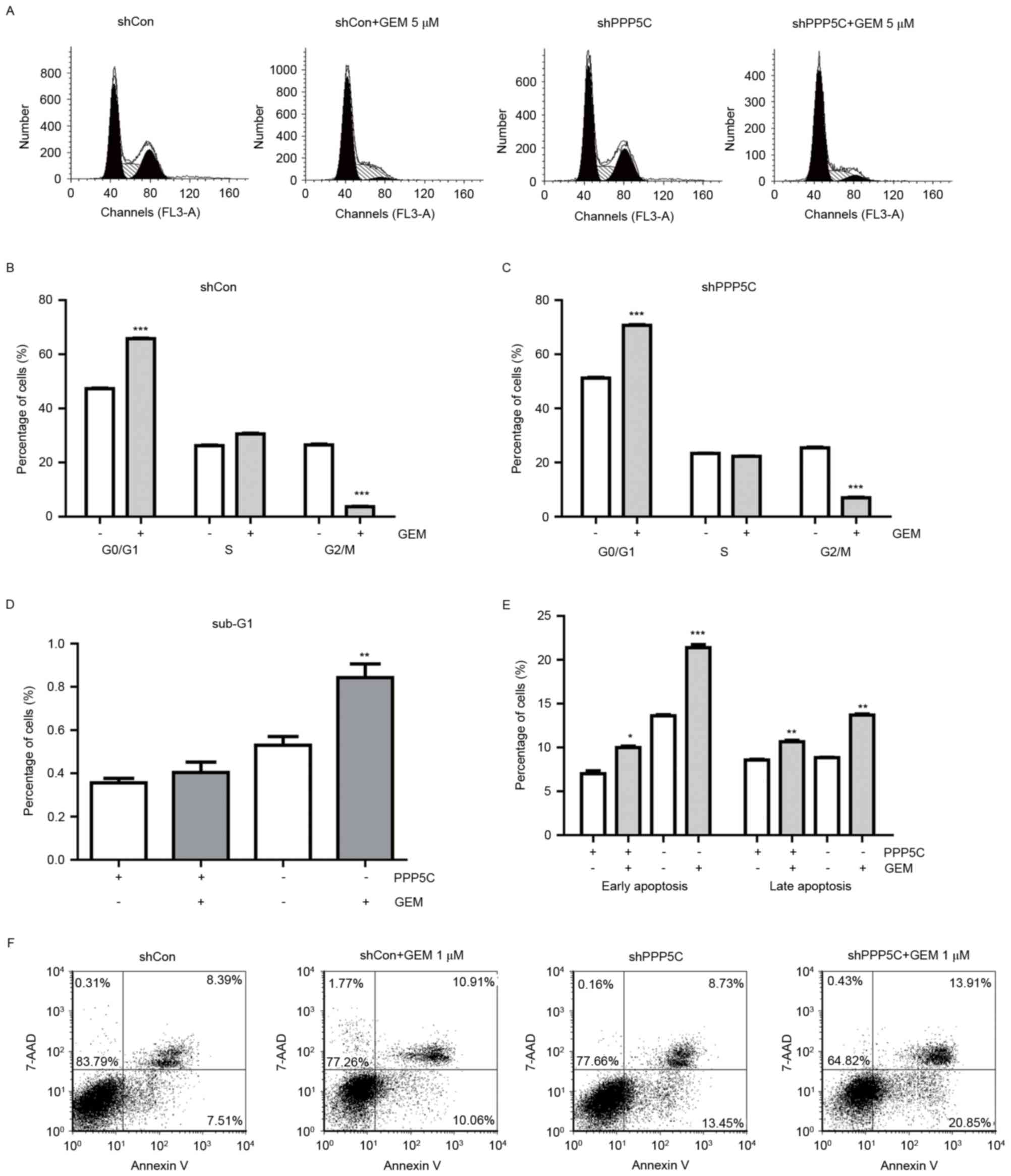
GEM enhances shPPP5C-induced PANC-1
cell cycle arrest and apoptosis
In order to identify the mechanisms underlying the
antiproliferation effect, the distribution of cells in the cell
cycle phases was analyzed using flow cytometry (Fig. 3A). The proportion of G0/G1 phase cells
was markedly increased while the G2/M phase population was
decreased under GEM treatment when compared with PANC-1 cells
treated with the single shCon or shPPP5C alone (Fig. 3B and C). These data suggested that the
combined treatment could further arrest the cell cycle at the
G0/G1 phase in PANC-1 cells. In addition, GEM
treatment plus PPP5C silencing resulted in a significant increase
in the cell population in the sub-G1 phase (Fig. 3D), suggesting the presence of cell
apoptosis.
To further examine the effect of combined treatment
and that of PPP5C silencing alone on cell apoptosis in PANC-1
cells, Annexin V-APC/7-AAD staining was performed. As demonstrated
in (Fig. 3E and F), flow cytometry
analysis revealed that the percentage of early apoptotic cells
(Annexin V+/7-AAD−) was higher in the shPPP5C
group compared with that in the shCon group, while no difference
was observed in the percentage of late apoptotic cells (Annexin
V+/7-AAD+) between the shPPP5C and shCon
groups. The percentages of early and late apoptotic cells were
revealed to be significantly increased in the shPPP5C group when
PPP5C silencing was combined with GEM treatment in comparison with
cells treated with shCon, shPPP5C or GEM alone. More specifically,
the apoptosis rate (for early and late apoptotic cells) was ~35.08%
in the group treated with GEM and shPPP5C, which was significantly
higher than that in the shCon (15.57%), shPPP5C (22.46%) or GEM
plus shCon (20.64%) groups. These results further corroborated the
hypothesis that silencing of PPP5C enhanced the apoptotic effect of
GEM in vitro.
Mechanism of GEM-enhanced
shPPP5C-induced PANC-1 cell apoptosis
To investigate the underlying mechanism of
GEM-enhanced shPPP5C-induced PANC-1 cell apoptosis,
apoptosis-associated proteins were determined by western blot
analysis (Fig. 4). The results
demonstrated that the protein expression of cleaved caspase 3 and
poly (ADP-ribose) polymerase (PARP) was increased in the shPPP5C
group compared with that in the shCon group (Fig. 4A and C). Additionally, the expression
of tumor suppressor p53 was also determined and the protein
expression ratio of p-p53/p53 was also upregulated (Fig. 4B and C). In addition, the association
between MAPK family-associated proteins (JNK, p-JNK, p38 and p-p38)
and shPPP5C-mediated apoptosis was investigated. As demonstrated in
(Fig. 4A and C), the protein
expression ratio of p-JNK/JNK was increased, while almost no change
was observed in that of p-p38/p38. These results indicate that the
effect of silencing PPP5C on cell apoptosis resulted from the
altered expression of the associated anti-apoptotic proteins.
Additionally, the combined treatment of GEM and shPPP5C resulted in
a marked increase in p-JNK/JNK, cleaved caspase 3 and PARP, while
the expression ratio of p-p38/p38 and p-p53/p53 exhibited almost no
change, suggesting that PPP5C silencing combined with GEM may, to a
certain extent, promote further cell apoptosis (Fig. 4C).
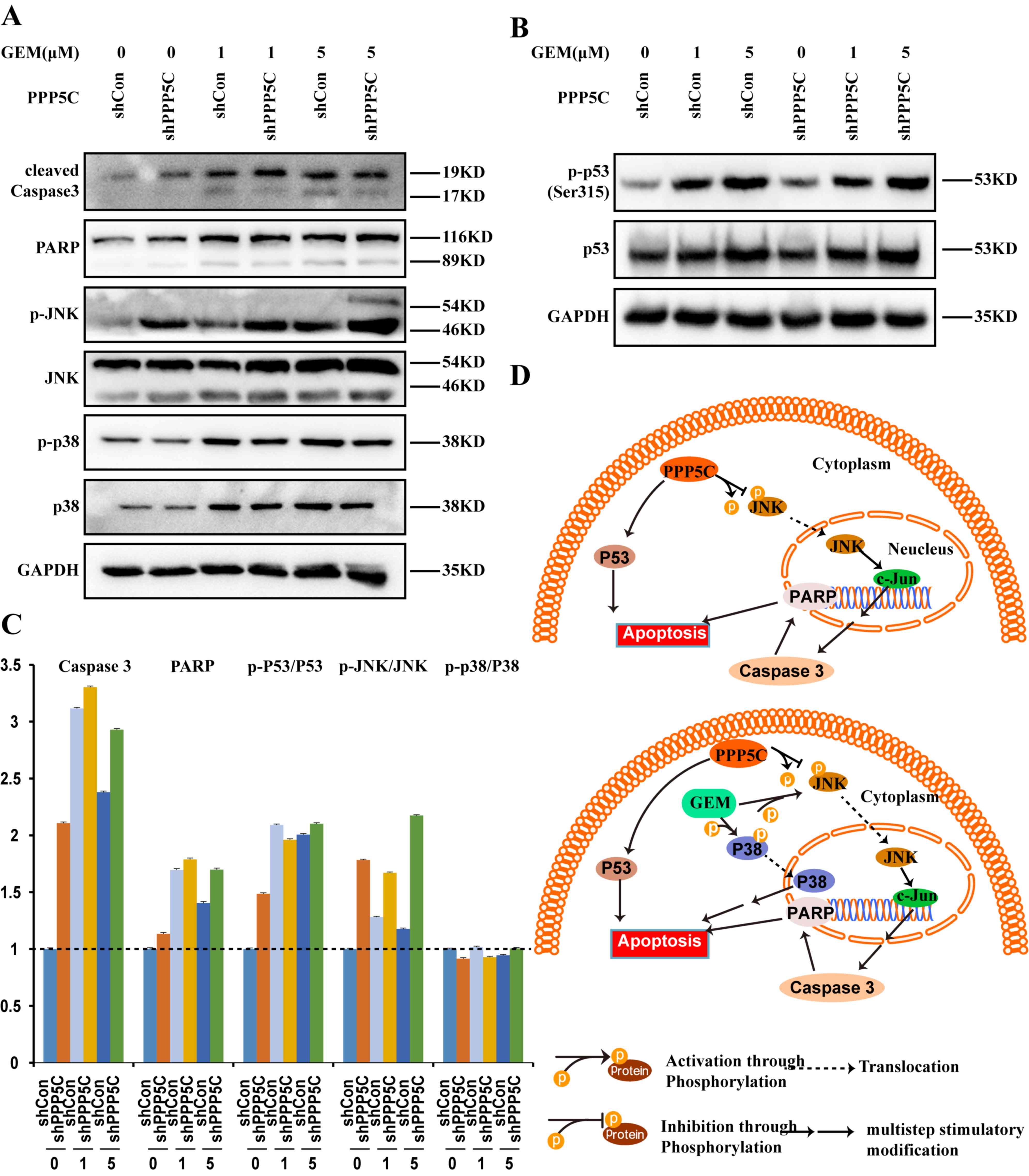 | Figure 4.Effects of PPP5C-knockdown on
apoptosis pathway-associated molecular expression. (A) The levels
of caspase-3, PARP, JNK, p-JNK, p38 and p-p38 protein in PANC-1
cells were detected following PPP5C silencing by western blot
analysis. (B) The levels of p-p53 (ser315) and p53 protein in
PANC-1 cells were analyzed following PPP5C silencing by western
blot analysis. (C) The expression of JNK, p-JNK, p38 and p-p38
proteins in PANC-1 cells transduced with shCon, shPPP5C or the
combined treatment of GEM and shPPP5C or shCon was analyzed by
western blot analysis. (D) Proposed model of the role served by
PPP5C silencing in cell apoptosis. GAPDH was used as an internal
loading control. PPP5C, serine/threonine protein phosphatase 5;
JNK, c-Jun N-terminal kinases; shCon, short hairpin control;
shPPP5C, short hairpin PPP5C; GEM, gemcitabine; p-,
phosphorylated-; t-, total-. |
Discussion
The majority of PC patients exhibit a strong
resistance to chemotherapy. The exact mechanisms underlying this
remain unknown. One underlying mechanism of chemoresistance is the
insensitivity to drug-induced apoptosis (38). GEM is widely used for several cancer
types, not only for PC, but also for lung and bladder cancer types.
GEM monotherapy has been the standard treatment for metastatic PC
for decades and combination therapy of GEM with a number of other
agents has been demonstrated to increase the median survival time
to a certain extent (9). However,
although GEM is still used as a first-line treatment option, the
modest survival benefit of GEM treatment has been revealed to be
unsatisfactory (39). GEM is a unique
antimetabolite that may inhibit the activity of ribonucleotide
reductase and may terminate DNA elongation processes (40). However, the occurrence of drug
resistance is common in cancer treatment with various agents due to
multiple factors, including the attenuation of nucleoside
transporters, the augmentation of efflux transporters, the
acquisition of apoptotic resistance due to overexpression of
anti-apoptotic proteins (e.g., heat shock proteins,
cyclooxygenase-2, nuclear factor erythroid 2-related factor 2 and
B-cell lymphoma 2 family members), and the constitutive activation
of survival signaling pathways (16,41).
Therefore, GEM resistance is a significant obstacle to effective
chemotherapy.
It has been reported that the overexpression of
PPP5C has been observed in several solid cancer types (42–44). In
addition, overexpression of PPP5C has been demonstrated to promote
cell proliferation and tumor progression (35). PPP5C expression is responsive to
hypoxia inducible factor-1 and estrogen, which plays important role
in cancer progression. It has been reported that PPP5C serves an
important role in glioma metastasis, as downregulation of PPP5C
mitigated cell migration in U251 and U373 cell lines (34). In human breast cancer, a strong
association was observed between high levels of PPP5C expression
and the occurrence of invasive ductal carcinoma in patients with
metastatic disease at the time of diagnosis (45). In rats, PPP5C mRNA levels markedly
increased in malignant ascites hepatomas (46). Furthermore, a protein microarray
analysis of mantle-cell lymphoma also revealed elevated PPP5C
expression (47). With regards to PC,
increased PPP5C expression was observed in PANC-1 cells treated
with GEM in the present study. The association between PPP5C levels
and tumor progression requires elucidation in order to find an
effective strategy to overcome GEM resistance.
Based upon previous reports of PPP5C in other cancer
types and the observation of PPP5C overexpression in PANC-1 cells
by GEM treatment, we hypothesized that the suppression of PPP5C
expression may serve an important role in PC growth and may promote
the effect of GEM treatment on PC cells. In the present study, the
effect of PPP5C-knockdown or the combination of this with GEM
treatment on the cellular functions of PANC-1 cell was
investigated. The results of the MTT assay demonstrated that the
proliferation of PANC-1 cells was significantly impaired in the
combination treatment group, which indicated that PPP5C silencing
enhanced the chemosensitivity of PANC-1 cells to GEM. Additionally,
the effects of PPP5C-knockdown alone or the combination treatment
of GEM and PPP5C silencing on cell cycle and apoptosis were also
studied. The results demonstrated that suppressed PPP5C expression
combined with GEM treatment in PANC-1 cells led to cell arrest in
the G0/G1 phase and increased cell apoptosis.
Therefore, these results suggest that PPP5C may serve a central
role in the tumor progression process of PC and in GEM
resistance.
In particular, PPP5 has been revealed to act as a
suppressor of ASK (48,49), p53 and DNA-dependent protein kinase,
catalytic subunit (45). PPP5C
appears to interact with ASK1, which is associated with the c-JNK
and p38 signaling pathways. The present study observed that, in the
shPPP5C silencing group, the protein expression ratio of p-JNK/JNK
was increased, while almost no change was observed in that of
p-P38/P38. Meanwhile, combined treatment with GEM and shPPP5C also
resulted in a marked increase in p-JNK/JNK and no change in
p-p38/p38. These results indicate that the combined treatment could
further induce cell apoptosis via the c-JNK pathway. Furthermore,
western blot analysis demonstrated that PPP5C silencing
significantly increased the protein levels of cleaved caspase-3,
p-p53 and cleaved-PARP, which participate in the cell apoptotic
pathway. As the prime activator of PARP is DNA damage and its
overexpression has been proven to be associated with the
pathogenesis of numerous tumors (50), there may be several links between p53
and PARP. These findings support the hypothesis that the
involvement of MAPK signaling pathways affects the results of
anticancer therapy on PC cells (51).
Combined with the previous findings that the activation of the p38
kinase promotes the activation of p53 and further triggers the
activation of caspase-9 and −3 in PC (52), the results of the present study
revealed that the downstream target of PPP5C is p53 (Fig. 4D). It is widely acknowledged that
MAPKs are responsive to various stress stimuli. Upon activation,
JNK phosphorylates and regulates various cell cycle and apoptotic
mediators. p-p53 may initiate the p53 response and may lead to cell
arrest, as well as apoptosis within cell cycles (53). However, the detailed mechanisms
underlying this remain to be elucidated.
In summary, the present study demonstrated that
PPP5C silencing has a potential novel therapeutic function in PC.
Further investigation is required to elucidate the precise
mechanisms of PPP5C for PC treatment with GEM, such that a novel
gene-targeted therapy for PC may be established.
Acknowledgements
The authors would like to thank the National Natural
Science Foundation of China (grant no. 81201733), the Zhejiang
Provincial Medicine Foundation (grant no. 2012KYB018) and the
Zhejiang Provincial Chinese Medicine Program (grant no. 2013ZQ021)
for providing financial support.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clin. 63:318–348. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hurton S, MacDonald F, Porter G, Walsh M
and Molinari M: The current state of pancreatic cancer in Canada:
Incidence, mortality, and surgical therapy. Pancreas. 43:879–885.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miwa T, Kokuryo T, Yokoyama Y, Yamaguchi J
and Nagino M: Therapeutic potential of targeting protein for Xklp2
silencing for pancreatic cancer. Cancer Med. 4:1091–1100. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zuo J, Zhang C, Ren C, Pang D, Li Y, Xie
X, Tang Z and Jiang X: Secretory leukocyte protease inhibitor is a
proliferation and survival factor for pancreatic cancer cells. Clin
Transl Oncol. 17:314–321. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Funel N, Del Chiaro M, Cahen DL and
Laukkarinen J: Pancreatic cancer. Gastroenterol Res Pract.
2015:8090362015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Thota R, Pauff JM and Berlin JD: Treatment
of metastatic pancreatic adenocarcinoma: A review. Oncology
(Williston Park). 28:70–74. 2014.PubMed/NCBI
|
|
8
|
Oettle H, Post S, Neuhaus P, Gellert K,
Langrehr J, Ridwelski K, Schramm H, Fahlke J, Zuelke C, Burkart C,
et al: Adjuvant chemotherapy with gemcitabine vs observation in
patients undergoing curative-intent resection of pancreatic cancer:
A randomized controlled trial. JAMA. 297:267–277. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burris HA III, Moore MJ, Andersen J, Green
MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo
AM, Tarassoff P, et al: Improvements in survival and clinical
benefit with gemcitabine as first-line therapy for patients with
advanced pancreas cancer: A randomized trial. J Clin Oncol.
15:2403–2413. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tempero MA, Behrman S, Ben-Josef E, Benson
AB III, Cameron JL, Casper ES, Hoffman JP, Karl RC, Kim P, Koh WJ,
et al: Pancreatic adenocarcinoma: Clinical Practice Guidelines in
Oncology. J Natl Compr Canc Netw. 3:598–626. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oettle H and Riess H: Gemcitabine in
combination with 5-fluorouracil with or without folinic acid in the
treatment of pancreatic cancer. Cancer. 95:912–922. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Poplin E, Feng Y, Berlin J, Rothenberg ML,
Hochster H, Mitchell E, Alberts S, O'Dwyer P, Haller D, Catalano P,
et al: Phase III, randomized study of gemcitabine and oxaliplatin
versus gemcitabine (fixed-dose rate infusion) compared with
gemcitabine (30-minute infusion) in patients with pancreatic
carcinoma E6201: A trial of the Eastern Cooperative Oncology Group.
J Clin Oncol. 27:3778–3785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Colucci G, Labianca R, Di Costanzo F,
Gebbia V, Cartenì G, Massidda B, Dapretto E, Manzione L, Piazza E,
Sannicolò M, et al: Randomized phase III trial of gemcitabine plus
cisplatin compared with single-agent gemcitabine as first-line
treatment of patients with advanced pancreatic cancer: The GIP-1
study. J Clin Oncol. 28:1645–1651. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chauffert B, Mornex F, Bonnetain F,
Rougier P, Mariette C, Bouché O, Bosset JF, Aparicio T, Mineur L,
Azzedine A, et al: Phase III trial comparing intensive induction
chemoradiotherapy (60 Gy, infusional 5-FU and intermittent
cisplatin) followed by maintenance gemcitabine with gemcitabine
alone for locally advanced unresectable pancreatic cancer.
Definitive results of the 2000–01 FFCD/SFRO study. Ann Oncol.
19:1592–1599. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Philip PA, Benedetti J, Corless CL, Wong
R, O'Reilly EM, Flynn PJ, Rowland KM, Atkins JN, Mirtsching BC,
Rivkin SE, et al: Phase III study comparing gemcitabine plus
cetuximab versus gemcitabine in patients with advanced pancreatic
adenocarcinoma: Southwest Oncology Group-directed intergroup trial
S0205. J Clin Oncol. 28:3605–3610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Minami K, Shinsato Y, Yamamoto M,
Takahashi H, Zhang S, Nishizawa Y, Tabata S, Ikeda R, Kawahara K,
Tsujikawa K, et al: Ribonucleotide reductase is an effective target
to overcome gemcitabine resistance in gemcitabine-resistant
pancreatic cancer cells with dual resistant factors. J Pharmacol
Sci. 127:319–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cohen PT: Novel protein serine/threonine
phosphatases: Variety is the spice of life. Trends Biochem Sci.
22:245–251. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cohen PT, Brewis ND, Hughes V and Mann DJ:
Protein serine/threonine phosphatases; an expanding family. FEBS
Lett. 268:355–359. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Morita K, Saitoh M, Tobiume K, Matsuura H,
Enomoto S, Nishitoh H and Ichijo H: Negative feedback regulation of
ASK1 by protein phosphatase 5 (PP5) in response to oxidative
stress. EMBO J. 20:6028–6036. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Douglas P, Moorhead GB, Ye R and
Lees-Miller SP: Protein phosphatases regulate DNA-dependent protein
kinase activity. J Biol Chem. 276:18992–18998. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
von Kriegsheim A, Pitt A, Grindlay GJ,
Kolch W and Dhillon AS: Regulation of the Raf-MEK-ERK pathway by
protein phosphatase 5. Nat Cell Biol. 8:1011–1016. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yong WH, Ueki K, Chou D, Reeves SA, von
Deimling A, Gusella JF, Mohrenweiser HW, Buckler AJ and Louis DN:
Cloning of a highly conserved human protein serine-threonine
phosphatase gene from the glioma candidate region on chromosome
19q13.3. Genomics. 29:533–536. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang J, Roe SM, Cliff MJ, Williams MA,
Ladbury JE, Cohen PT and Barford D: Molecular basis for TPR
domain-mediated regulation of protein phosphatase 5. EMBO J.
24:1–10. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hinds TD Jr and Sánchez ER: Protein
phosphatase 5. Int J Biochem Cell Biol. 40:2358–2362. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chinkers M: Protein phosphatase 5 in
signal transduction. Trends Endocrinol Metab. 12:28–32. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Golden T, Swingle M and Honkanen RE: The
role of serine/threonine protein phosphatase type 5 (PP5) in the
regulation of stress-induced signaling networks and cancer. Cancer
Metastasis Rev. 27:169–178. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Swingle MR, Honkanen RE and Ciszak EM:
Structural basis for the catalytic activity of human
serine/threonine protein phosphatase-5. J Biol Chem.
279:33992–33999. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zuo Z, Dean NM and Honkanen RE:
Serine/threonine protein phosphatase type 5 acts upstream of p53 to
regulate the induction of p21(WAF1/Cip1) and mediate growth arrest.
J Biol Chem. 273:12250–12258. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao S and Sancar A: Human blue-light
photoreceptor hCRY2 specifically interacts with protein
serine/threonine phosphatase 5 and modulates its activity.
Photochem Photobiol. 66:727–731. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamaguchi Y, Katoh H, Mori K and Negishi
M: Galpha(12) and Galpha(13) interact with Ser/Thr protein
phosphatase type 5 and stimulate its phosphatase activity. Curr
Biol. 12:1353–1358. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chatterjee A, Wang L, Armstrong DL and
Rossie S: Activated Rac1 GTPase translocates protein phosphatase 5
to the cell membrane and stimulates phosphatase activity in vitro.
J Biol Chem. 285:3872–3882. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hattori K, Naguro I, Runchel C and Ichijo
H: The roles of ASK family proteins in stress responses and
diseases. Cell Commun Signal. 7:92009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng X, Zhang L, Jin B, Zhang F, Zhang D
and Cui L: Knockdown of protein phosphatase 5 inhibits ovarian
cancer growth in vitro. Oncol Lett. 11:168–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhi X, Zhang H, He C, Wei Y, Bian L and Li
G: Serine/threonine protein phosphatase-5 accelerates cell growth
and migration in human glioma. Cell Mol Neurobiol. 35:669–677.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feng L, Sun P, Li Z, Liu M and Sun S:
Knockdown of PPP5C inhibits growth of hepatocellular carcinoma
cells in vitro. Appl Biochem Biotechnol. 175:526–534. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu F, Iqbal K, Grundke-Iqbal I, Rossie S
and Gong CX: Dephosphorylation of tau by protein phosphatase 5:
Impairment in Alzheimer's disease. J Biol Chem. 280:1790–1796.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-(delta delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Moore MJ, Goldstein D, Hamm J, Figer A,
Hecht JR, Gallinger S, Au HJ, Murawa P, Walde D, Wolff RA, et al:
Erlotinib plus gemcitabine compared with gemcitabine alone in
patients with advanced pancreatic cancer: A phase III trial of the
National Cancer Institute of Canada Clinical Trials Group. J Clin
Oncol. 25:1960–1966. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang P, Chubb S, Hertel LW, Grindey GB
and Plunkett W: Action of 2′,2′-difluorodeoxycytidine on DNA
synthesis. Cancer Res. 51:6110–6117. 1991.PubMed/NCBI
|
|
41
|
Li L and Leung PS: Use of herbal medicines
and natural products: An alternative approach to overcoming the
apoptotic resistance of pancreatic cancer. Int J Biochem Cell Biol.
53:224–236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Golden T, Aragon IV, Zhou G, Cooper SR,
Dean NM and Honkanen RE: Constitutive over expression of
serine/threonine protein phosphatase 5 (PP5) augments
estrogen-dependent tumor growth in mice. Cancer Lett. 215:95–100.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Amable L, Grankvist N, Largen JW, Ortsäter
H, Sjöholm Å and Honkanen RE: Disruption of serine/threonine
protein phosphatase 5 (PP5:PPP5c) in mice reveals a novel role for
PP5 in the regulation of ultraviolet light-induced phosphorylation
of serine/threonine protein kinase Chk1 (CHEK1). J Biol Chem.
286:40413–40422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jeong JY, Johns J, Sinclair C, Park JM and
Rossie S: Characterization of Saccharomyces cerevisiae protein
Ser/Thr phosphatase T1 and comparison to its mammalian homolog PP5.
BMC Cell Biol. 4:32003. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Golden T, Aragon IV, Rutland B, Tucker JA,
Shevde LA, Samant RS, Zhou G, Amable L, Skarra D and Honkanen RE:
Elevated levels of Ser/Thr protein phosphatase 5 (PP5) in human
breast cancer. Biochim Biophys Acta. 1782:259–270. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shirato H, Shima H, Nakagama H, Fukuda H,
Watanabe Y, Ogawa K, Matsuda Y and Kikuchi K: Expression in
hepatomas and chromosomal localization of rat protein phosphatase 5
gene. Int J Oncol. 17:909–912. 2000.PubMed/NCBI
|
|
47
|
Ghobrial IM, McCormick DJ, Kaufmann SH,
Leontovich AA, Loegering DA, Dai NT, Krajnik KL, Stenson MJ, Melhem
MF, Novak AJ, et al: Proteomic analysis of mantle-cell lymphoma by
protein microarray. Blood. 105:3722–3730. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhou G, Golden T, Aragon IV and Honkanen
RE: Ser/Thr protein phosphatase 5 inactivates hypoxia-induced
activation of an apoptosis signal-regulating kinase 1/MKK-4/JNK
signaling cascade. J Biol Chem. 279:46595–46605. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Huang S, Shu L, Dilling MB, Easton J,
Harwood FC, Ichijo H and Houghton PJ: Sustained activation of the
JNK cascade and rapamycin-induced apoptosis are suppressed by
p53/p21(Cip1). Mol Cell. 11:1491–1501. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Michels J, Obrist F, Castedo M, Vitale I
and Kroemer G: PARP and other prospective targets for poisoning
cancer cell metabolism. Biochem Pharmacol. 92:164–171. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hong MY, Gao JL, Cui JZ, Wang KJ, Tian YX,
Li R, Wang HT and Wang H: Effect of c-Jun NH2-terminal
kinase-mediated p53 expression on neuron autophagy following
traumatic brain injury in rats. Chin Med J (Engl). 125:2019–2024.
2012.PubMed/NCBI
|
|
52
|
Bu HQ, Liu DL, Wei WT, Chen L, Huang H, Li
Y and Cui JH: Oridonin induces apoptosis in SW1990 pancreatic
cancer cells via p53- and caspase-dependent induction of p38 MAPK.
Oncol Rep. 31:975–982. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Harris SL and Levine AJ: The p53 pathway:
Positive and negative feedback loops. Oncogene. 24:2899–2908. 2005.
View Article : Google Scholar : PubMed/NCBI
|