Introduction
Intrahepatic cholangiocarcinoma (ICC) is the second
most common type of malignant primary hepatic tumor in numerous
areas of the world, including North America, Europe, Australia, and
Japan (1). ICC is a type of malignant
tumor that originates from peripheral intrahepatic biliary
epithelia (2,3). The incidence of ICC and the
ICC-associated mortality rate has increased in several regions
around the world since the 1970s (4–6). Cancer
stem cells (CSCs) are defined as a small subgroup of cancer cells
with the ability of self-renewal that may lead to tumor recurrence.
A number of studies have suggested that CSCs lead to poor prognosis
by promoting tumor recurrence and metastasis (7–9). It has
previously been reported that the maintenance of CSC
characteristics depends on the tumor microenvironment (TME)
(10). The TME consists of tumor
cells and stromal cells, including mesenchymal cells, endothelial
cells and immune cells, and serves an important role in regulating
tumorigenesis, cell invasion and metastasis (11).
Macrophages, a main component of tumor-infiltrating
immunocytes, infiltrate a variety of cytokines, chemokines, growth
factors and matrix metalloproteases, and contribute to tumor
progression and recurrence (12,13).
Macrophages are classified into M1 and M2 subtypes due to their
polarization manners (14). The M1
subtype appears to be tumor suppressive, whereas the M2 subtype is
tumor supportive in tumors (15). M2
subtype macrophages upregulate cluster of differentiation (CD)206,
tumor growth factor-β and interleukin-10 (16). Macrophages that invade the TME are
tumor-associated macrophages (TAMs), expressing similar molecular
and functional characteristic of the M2 subtype (12). Abundant macrophage infiltration is a
histological feature of ICC, and those macrophages in ICC express
similar functional characteristics to the M2 subtype; furthermore,
the increased density of macrophages in ICC was associated with a
poor prognosis (17). Certain studies
have demonstrated that a high density of TAMs was associated with
poor prognosis in numerous other types of cancer (18,19).
Therefore, researching the molecular mechanisms underlying TAM
recruitment may promote the development of therapeutics to
effectively improve ICC treatment.
Periostin (POSTN), also known as OSF-2, is a member
of the fasciclin family and is a disulfide-linked cell adhesion
protein (20). POSTN participates in
the multifarious field of tumorigenic processes via signaling
pathways, including protein kinase B/phosphoinositide-3 kinase,
integrin and Wnt-1 (21,22). Zhou et al (23), revealed that POSTN secreted by stem
cells may serve as a chemoattractant for recruiting M2 TAMs in
clinical specimens and in an animal model of glioblastoma. In
addition, POSTN acts as an important promoter in tumor progression,
including growth, angiogenesis, metastasis and invasion, in certain
types of malignant cancer (22,24,25). The
present study demonstrated that CD44+ ICC stem cells
secrete POSTN, and the density of CD206+ TAMs was
associated with the expression level of POSTN in ICC.
Materials and methods
Patients and specimens
A total of 50 patients (age, 43–75 years; median
age, 59.8 years; 32 males and 18 females) with curative liver
resection and pathology-proven ICC at the Hunan Provincial People's
Hospital (Changsha, China) between May 2001 and February 2007 were
included in the current study. Tumor stage was re-examined
according to the 2009 International Union Against Cancer TNM
Classification system (26). The
present study was approved by the Hunan Provincial People's
Hospital Research Ethics Committee. Written informed consent was
obtained from all patients prior to enrollment in the present
study.
Immunohistochemical examination
For immunohistochemical analysis of POSTN (TA804575;
1:100; OriGene Technologies, Inc., Beijing, China) and CD206
(SC-376232; 1:100; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA), tissue sections (thickness=4 mm) were deparaffinized in 100%
xylene and rehydrated in graded concentrations (100, 95, 70 and
50%) of ethanol. Following incubation with 1% bovine serum albumin
(BSA; Beijing Dingguo Changsheng Biotechnology Co., Ltd., Beijing,
China) in PBS (pH 7.4) at 37°C for 30 min, the tissue sections were
then incubated with primary antibody for 1 h at room temperature,
followed by incubation with the secondary biotinylated mouse
antibody (TA130008; 1:100; OriGene Technologies, Inc., Beijing,
China) at 37°C for 30 min. Following PBS washing, tissue sections
were subsequently treated with streptavidin-peroxidase (S5512;
Sigma Aldrich; Merck KgaA, Darmstadt, Germany) at 37°C for 30 min.
Finally, the results were visualized following a 15-min incubation
with diaminobenzidine (DAB; Beyotime Institute of Biotechnology,
Haimen, China) at room temperature for 5 min. Horseradish
peroxidase was detected using 3,3′-diaminobenzidine (Phoenix
Biotechnologies, San Antonio, TX, USA) substrate for 5 min, washed
with distilled water, and counterstained with Gill's no. 3
hematoxylin (Sigma-Aldrich; Merck KGaA) at room temperature for 15
sec and mounted. The results were observed under a light microscope
(IX51; Olympus Corporation, Tokyo, Japan; magnification, ×100).
Immunofluorescent staining
For immunohistochemical analysis of POSTN (TA500070;
1:100; OriGene Technologies, Inc.), CD44 (ab51037; 1:100; Abcam,
Cambridge, UK) and CD206 (SC-34577; 1:100; Santa Cruz
Biotechnology, Inc.), tissue sections were prepared as
aforementioned. The sections were then incubated with primary
antibody for 1 h at room temperature. The secondary biotinylated
[goat anti-mouse IgG (ab150117; 1:100; Abcam), donkey anti-goat IgG
(ab150079; 1:100; Abcam), donkey anti-mouse IgG (ab150131; 1:100;
Abcam), Goat anti-mouse IgG (ab150129; 1:100; Abcam)] antibody was
applied for identifying primary antibody and incubated at 37°C for
30 min. The nuclei were counterstained at room temperature for 10
min with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich; Merck KGaA).
The results were observed and representative images were captured
using an inverted fluorescent microscope (BX41, Olympus
Corporation, Tokyo, Japan) (magnification, ×100 and ×200).
Cell cultures and cell sorting
HCCC-9810 and THP-1 cells from the Cell Bank of
Shanghai Institute of Biological Sciences (Shanghai, China) were
cultured in RPMI-1640 medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum
(FBS; GE Healthcare, Chicago, IL, USA), 100 U/ml penicillin and 100
µg/ml streptomycin. Cells were incubated in stem cell medium with
B27 (Gibco, Thermo Fisher Scientific, Inc.), 10 ng/ml epidermal
growth factor (Prospec-Tany TechnoGene, Ltd., East Brunswick, NJ,
USA) and basic fibroblast growth factor (Prospec-Tany TechnoGene,
Ltd.) supplement at 37°C for 12 h to expose surface markers, and
samples were sorted using a BD FACSVantage SE (BD Biosciences,
Franklin Lakes, NJ, USA). Phycoerythrin (PE)-conjugated anti-human
CD44 antibody (130-095-194; 1:10; Miltenyi Biotec, Cologne,
Germany) was used to label HCCC-9810 cells according to the
manufacturer's instructions. CD44+ and CD44−
cell subpopulations were sorted by fluorescence-activated cell
sorting with anti-REA (130-104-693; Miltenyi Biotec, Cologne,
Germany). The purity of sorted cells was evaluated using a
FACSCalibur™ flow cytometry system (BD Biosciences) and
analyzed using flow cytometry on the MACSQuant Analyzer 10.
CD44+ cells were enriched with stem cell medium and
sorted as stem cells; similarly, CD44¯ cells were used
as non-stem cells. All sorted cells were cultured in RPMI-1640
supplemented with 15% FBS at 37°C for a week. THP-1 cells were
treated with phorbol-12-myristate-13-acetate at 100 ng/ml for 48 h
to generate macrophages.
Western blot analysis
HCCC-9810 cells were lysed using lysis buffer
containing 1 mM phenylmethanesulfonyl-fluoride (ST506; Beyotime,
Shanghai, China) on ice for 5 min, then total protein of HCCC-9810
and supernatant content was evaluated using a bicinchoninic acid
quantitative kit. Quantified protein lysates (5 µg per lane) were
resolved using SDS-PAGE (7.5% gels), transferred onto a
polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA,
USA). The membrane was blocked with 5% non-fat milk in TBS with
Tween 20 for 30 min at 25°C and immunoblotted with primary
antibodies against POSTN (TA500070; 1:500; OriGene Technologies,
Inc., Beijing, China) at 4°C overnight. Following this, the
membrane was incubated with horseradish peroxidase-conjugated
secondary antibody (ab6789; 1:4,000; Abcam) at 4°C overnight. The
blots were visualized using an enhanced chemiluminescence kit
(Vazyme, Piscataway, NJ, USA). β-actin (ab13822; 1:1,000; Abcam)
was used as a loading control at 4°C overnight. Protein was
visualized using FluorChem FC3 (ProteinSimple, San Jose, CA, USA)
and ImageJ software (version 1.51p; National Institutes of Health,
Bethesda, MD, USA) quantified the band density.
Cell migration assays
Transwell chamber assays were used to compare the
migratory ability of THP-1-derived macrophages using conditional
medium (CM) [NSCCs CM, ICSCs CM, ICSCs CM with POSTN-neutralizing
antibody (α-POSTN) and IgG, NSCCs CM with recombinant POSTN
(rPOSTN)]. Briefly, phorbol-12-myristate-13-acetate (PMA) treated
THP-1-derived macrophages were resuspended in serum-free RPMI-1640
(5×104 cells/200 µl). BSA (2%)-RPMI-1640 (500 µl) was
added to the upper chambers as the control. Conditioned medium [CM;
NSCCs CM, ICSCs CM, ICSCs CM with α-POSTN (10 µg/ml; cat no.
TA600528; OriGene Technologies, Inc., Beijing, China) or IgG and
NSCCs CM with rPOSTN (0.2 µg/ml)] with 10% FBS was added to the
lower chambers. Following a 24-h incubation at 37°C, the migratory
cells to the lower surface of the membrane were fixed with 4%
paraformaldehyde at room temperature for 5 min, stained with Wright
Giemsa for 20 min at room temperature, and counted and imaged using
a microscope (BX41; Olympus Corporation, Tokyo, Japan;
magnification, ×400).
Statistical analysis
Data were presented as the mean ± standard error of
the mean from at least three samples or experiments per data point.
Differences between the groups were analyzed by one-way analysis of
variance with Fisher's Least Significant Difference test as a
post-hoc using SPSS version 15.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
POSTN is secreted by ICSCs in ICC
tissues and HCCC-9810 cells
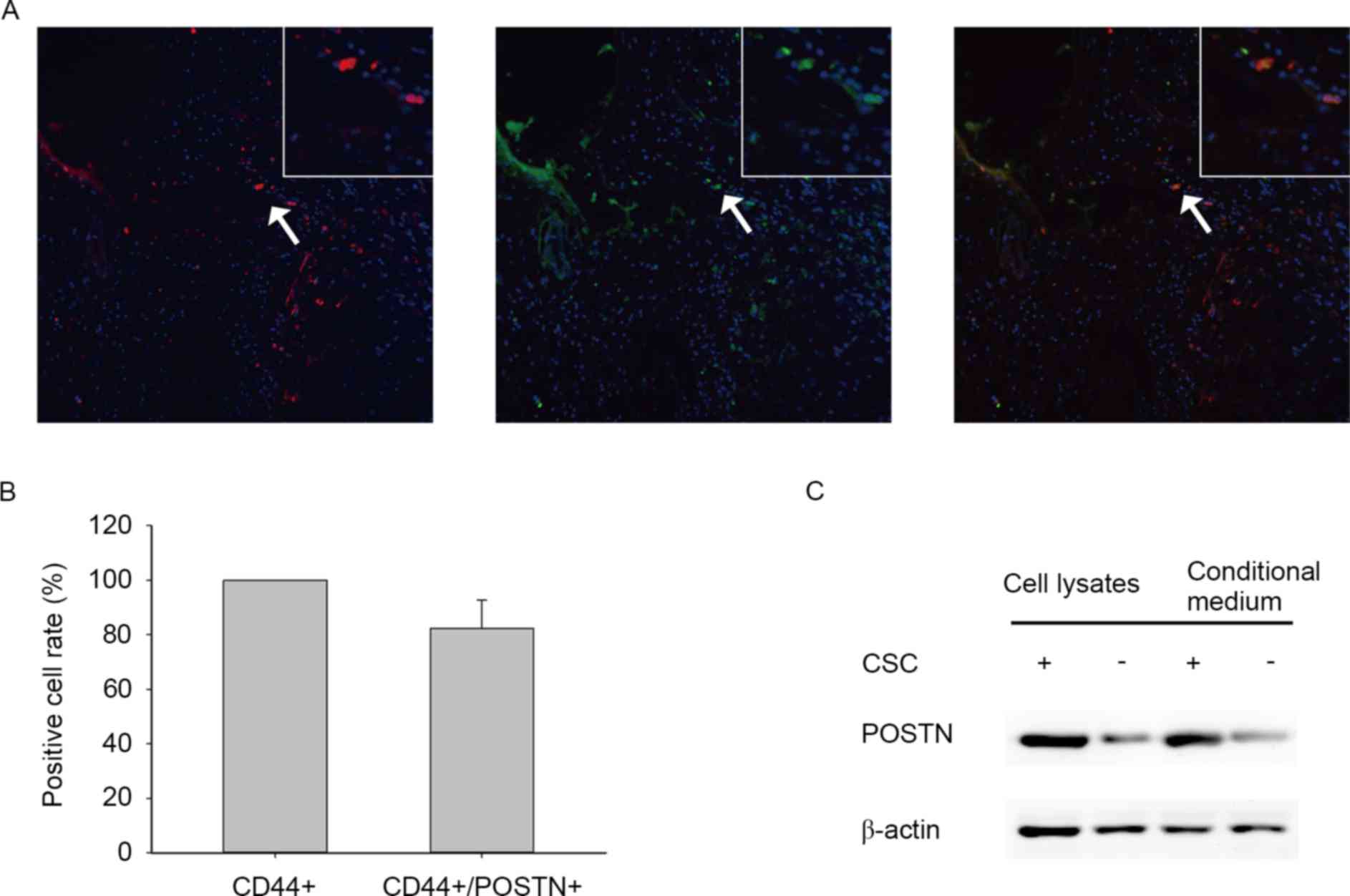
To investigate the latency association between POSTN
expression and distribution of CSCs in cholangiocarcinoma, the
present study determined the expression levels of POSTN and CSC
marker CD44 in human primary ICC samples by evaluating
immunofluorescence. The findings revealed that POSTN is
preferentially expressed by CD44+ cancer cells and
located in the area around ICSCs (Fig. 1A
and B). To determine the differential expression of POSTN
between ICSCs and non-stem cancer cells (NSCCs), the present study
examined the expression levels of POSTN in ICSCs and NSCCs of the
HCCC-9810 cell line by western blot analysis (Fig. 1C). The results demonstrated that ICSCs
expressed higher levels of POSTN compared with NSCCs. Furthermore,
ICSC conditioned medium also contained higher POSTN protein levels
compared with matched NSCC conditioned medium (Fig. 1C). These results suggest that POSTN is
preferentially secreted by ICSCs.
POSTN is associated with TAM density
in primary ICC
The association between POSTN expression level and
TAM density was first evaluated by immunofluorescence. The results
demonstrated that TAM-labeled marker (CD206) was accumulated in a
POSTN-abundant location (Fig. 2A).
Immunohistochemistry demonstrated that high levels of POSTN and
high levels of TAM markers were identified in ICC (Fig. 2B and C). These results suggest that
POSTN expression levels had a positive association with the number
of TAMs in ICC.
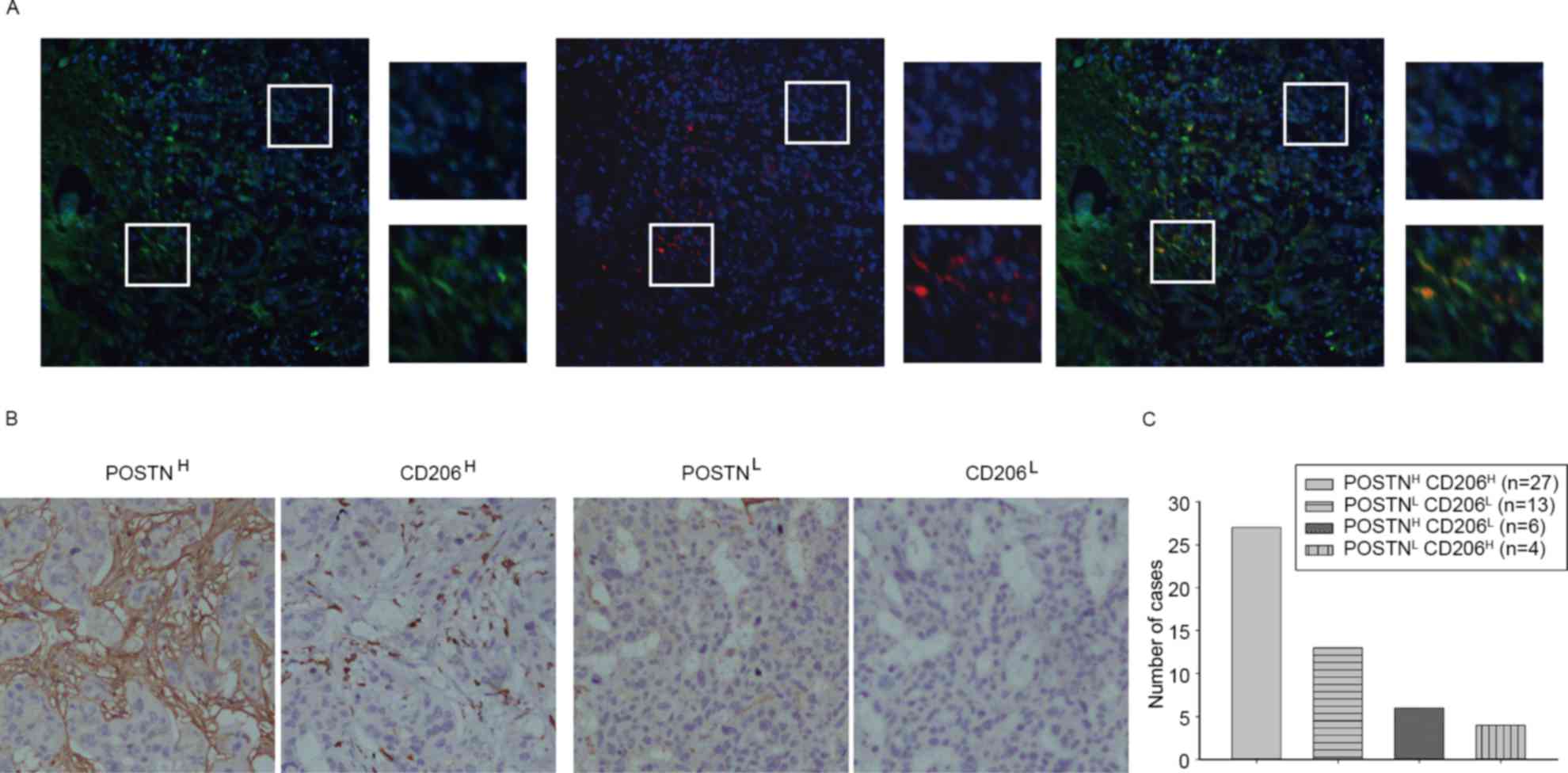 | Figure 2.POSTN is associated with TAM density
in primary ICC. (A) Representative immunofluorescence images
showing POSTN (green) and TAM marker CD206 (red) expression in ICC
tissues (magnification, ×200) and selected areas (magnification,
×200). (B) Representative immunohistochemical images showing POSTN
and CD206 staining (magnification, ×200). (C) A total of 54% of ICC
cases presented POSTNH and CD206H staining,
and 26% of ICC cases presented POSTNL and
CD206L staining; however, 12% of ICC cases presented
POSTNH and CD206L staining, and 8% of ICC
cases presented POSTNL and CD206H staining.
The majority (80%) of ICC cases revealed that POSTN expression was
positively associated with TAM density. POSTN, periostin; TAM,
tumor-associated macrophage; ICC, intrahepatic cholangiocarcinoma;
CD, cluster of differentiation; H, high level; L, low level. |
POSTN promotes migratory ability of
TAMs derived from human macrophage-like THP-1 cells
To clarify the mechanism underlying POSTN action as
an effective ICSC-secreted chemotaxin, migration of PMA-primed
macrophage-like THP-1 cells were evaluated by Transwell assays.
Conditioned medium from ICSCs attracted significantly more TAMs
than the medium from matched NSCCs (Fig.
3A and B). Subsequently, the present study used α-POSTN to
deplete POSTN expression. As presented in Fig. 3C and D, the depletion of POSTN in
ICSC-CM suppressed the promoting effect of macrophage migration
in vitro. The capacity of POSTN to increase invasiveness of
human monocytes was also demonstrated in the present study
(Fig. 3E and F). Collectively, these
results demonstrate that POSTN preferentially secreted by ICSCs had
an effective capacity to attract macrophages.
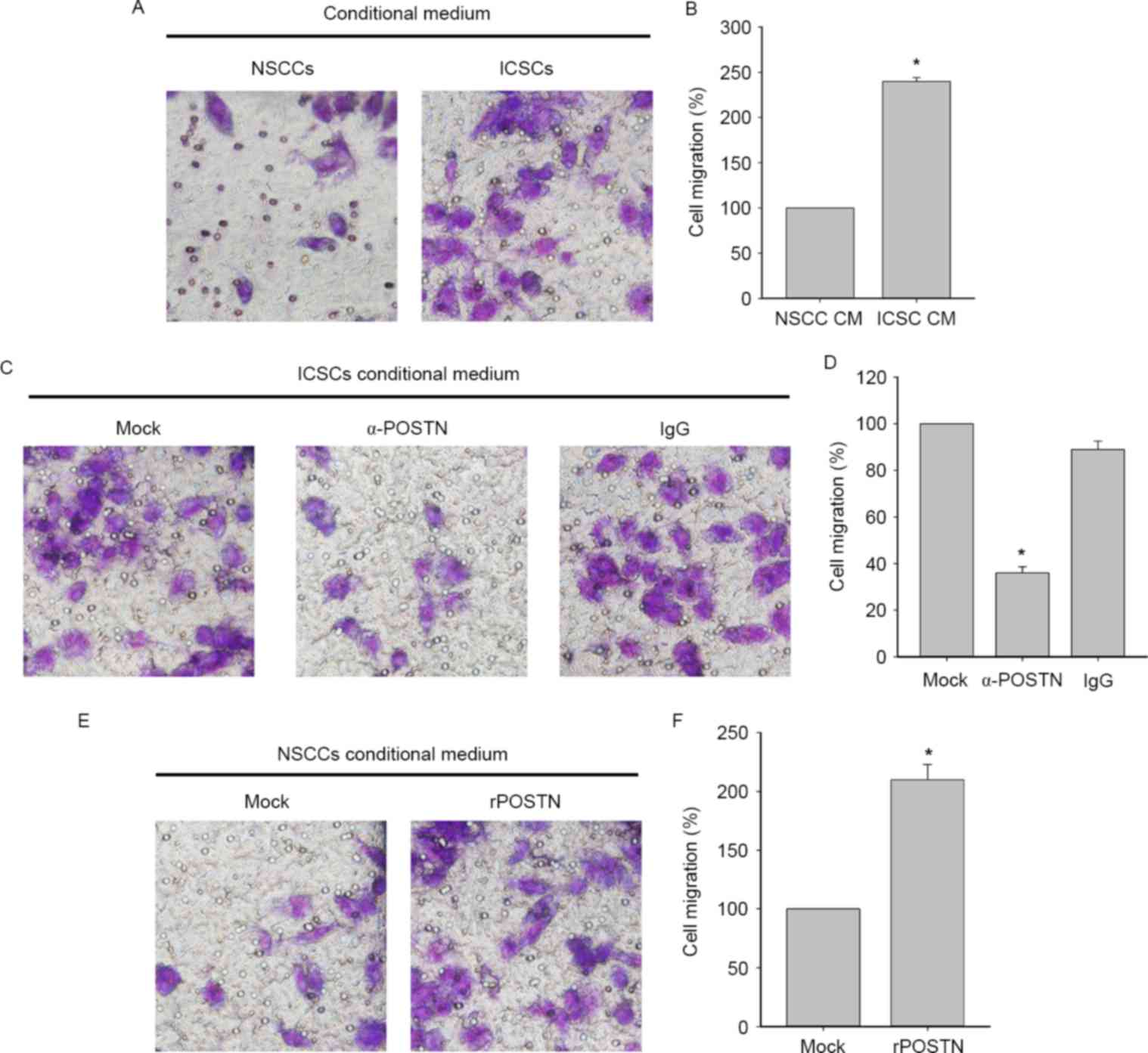 | Figure 3.POSTN promotes the migration of TAMs.
(A) Transwell assay showing comparison of TAMs migration toward CM
from NSCCs and ICSCs in HCCC-9810 cells (magnification, ×400) and
(B) the graphical analysis. Data are presented as mean ± SD (n=3).
*P<0.05, migrated TAMs towards ICSC CM vs. NSCC CM. The analysis
identified that the increased TAMs cell migration toward ISCCs CM
relative to NCSCs CM. (C) Comparison of TAMs migration toward ICSC
CM or following treatment with anti-POSTN (10 µg/ml) antibody or
IgG (magnification, ×400) and (D) its graphical analysis. Data are
presented as mean ± SD (n=3). *P<0.05, migrated TAMs towards to
α-POSTN ICSC CM vs. ICSC CM and IgG ICSC CM. (E) Comparison of
invading TAMs toward NSCC CM or following treatment with rPOSTN
(0.2 µg/ml) or IgG (magnification, ×400) and the (F) graphical
analysis. Data are presented as mean ± SD (n=3). *P<0.05,
migrated TAMs towards to rPOSTN NSCC CM vs. NSCC CM. POSTN,
periostin; TAM, tumor-associated macrophage; NSCCs, non-stem cancer
cells; ICSCs, intrahepatic cholangiocarcinoma stem cells; IgG,
immunoglobulin G; r, recombinant; CM, conditional medium; SD,
standard deviation; Ig, immunoglobulin. |
Discussion
The present study observed a large level of
CD206+ macrophage infiltration in parts of the ICC tumor
niche. TAMs in cancerous tissues are regarded as immunosuppressive
cells that have a tumor supportive role (27). Therefore, investigating the molecular
mechanisms underlying TAM recruitment may contribute to improvement
of ICC treatment.
It has been reported that tumors recruit TAMs by
secreting the CC chemokine ligand 2 and soluble colony-stimulating
factor 1 in tumors (28–30). The present study revealed that TAMs
were concentrated in POSTN-abundant regions in ICCs. Similarly,
immunohistochemistry analysis demonstrated the following: In ICC,
tumor tissues with higher expression levels of POSTN contained
higher densities of TAMs, revealing a positive association between
POSTN levels and TAM density in human ICCs. The present study also
revealed that POSTN was secreted by ICSCs. In order to determine
the differential expression of POSTN in CD44+ ICSCs, the
present study observed the expression of POSTN in matched ICSCs and
NSCCs. These results demonstrated that ICSCs preferentially
expressed markedly higher POSTN levels compared with NSCCs.
Consistently, CM from ICSCs contained higher levels of POSTN
protein compared with that from matched NSCCs. These results
indicated that POSTN was preferentially produced by ICSCs rather
than NSCCs.
To further elucidate whether ICSCs secreting POSTN
had potent capacity to recruit TAMs, cell migration assays were
performed in vitro. The Transwell assay identified that TAMs
of the CM group had higher migratory ability compared with the
NSCCs group. The present study also revealed the migratory ability
of the CM group with anti-POSTN antibody exhibited a decreased
migratory ability. Subsequently, the present study demonstrated
that the migratory ability of NSCCs was increased by rPOSTN. These
results revealed that POSTN preferentially secreted by ICSCs
displays potent ability to attract TAMs.
Trabectedin has demonstrated antitumor activity by
targeting TAMs (31). The present
study revealed that the underlying mechanisms of TAM recruitment by
ICSC-secreted POSTN may be responsible for the crosstalk of TAMs
and ICSCs. In addition, therapeutic targeting of the immune TME may
synergize with current immunotherapies to effectively increase
survival of ICC patients.
Acknowledgements
The present study was supported by the Project of
the National Natural Science Foundation of China (grant no.
81001107), the Project of the Scientific Research Fund of Hunan
Provincial Education Department (grant no. 15A114) and the Project
of Scientific Research Fund of Hunan Science and Technology
Education Department (grant no. 2015SK2050).
References
|
1
|
Goodman ZD: Neoplasms of the liver. Mod
Pathol. 20:S49–S60. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakanuma Y, Harada K, Ishikawa A, Zen Y
and Sasaki M: Anatomic and molecular pathology of intrahepatic
cholangiocarcinoma. J Hepatobiliary Pancreat Surg. 10:265–281.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Okuno M, Ebata T, Yokoyama Y, Igami T,
Sugawara G, Mizuno T, Yamaguchi J and Nagino M: Appraisal of
inflammation-based prognostic scores in patients with unresectable
perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci.
23:636–642. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shaib Y and El-Serag HB: The epidemiology
of cholangiocarcinoma. Semin Liver Dis. 24:115–125. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Patel T: Worldwide trends in mortality
from biliary tract malignancies. BMC Cancer. 2:102002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu
SJ, Shi RY, Hu B, Zhou J and Fan J: Circulating stem cell-like
epithelial cell adhesion molecule-positive tumor cells indicate
poor prognosis of hepatocellular carcinoma after curative
resection. Hepatology. 57:1458–1468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chiba T, Zheng YW, Kita K, Yokosuka O,
Saisho H, Onodera M, Miyoshi H, Nakano M, Zen Y, Nakanuma Y, et al:
Enhanced self-renewal capability in hepatic stem/progenitor cells
drives cancer initiation. Gastroenterology. 133:937–950. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu XZ and Yu XH: Bone marrow cells: The
source of hepatocellular carcinoma? Med Hypotheses. 69:36–42. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Borovski T, De Sousa E, Melo F, Vermeulen
L and Medema JP: Cancer stem cell niche: The place to be. Cancer
Res. 71:634–639. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schiavoni G, Gabriele L and Mattei F: The
tumor microenvironment: A pitch for multiple players. Front Oncol.
3:902013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Biswas SK, Allavena P and Mantovani A:
Tumor-associated macrophages: Functional diversity, clinical
significance, and open questions. Semin Immunopathol. 35:585–600.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cook J and Hagemann T: Tumour-associated
macrophages and cancer. Curr Opin Pharmacol. 13:595–601. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sielska M, Przanowski P, Wylot B,
Gabrusiewicz K, Maleszewska M, Kijewska M, Zawadzka M, Kucharska J,
Vinnakota K, Kettenmann H, et al: Distinct roles of CSF family
cytokines in macrophage infiltration and activation in glioma
progression and injury response. J Pathol. 230:310–321. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Staudt ND, Jo M, Hu J, Bristow JM, Pizzo
DP, Gaultier A, VandenBerg SR and Gonias SL: Myeloid cell receptor
LRP1/CD91 regulates monocyte recruitment and angiogenesis in
tumors. Cancer Res. 73:3902–3912. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang G, Guo L, Yang C, Liu Y, He Y, Du Y,
Wang W and Gao F: A novel role of breast cancer-derived hyaluronan
on inducement of M2-like tumor-associated macrophages formation.
Oncoimmunology. 5:e11721542016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Oishi K, Sakaguchi T, Baba S, Suzuki S and
Konno H: Macrophage density and macrophage colony-stimulating
factor expression predict the postoperative prognosis in patients
with intrahepatic cholangiocarcinoma. Surg Today. 45:715–722. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mantovani A and Sica A: Macrophages,
innate immunity and cancer: Balance, tolerance, and diversity. Curr
Opin Immunol. 22:231–237. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu JY, Yang XJ, Geng XF, Huang CQ, Yu Y
and Li Y: Prognostic significance of tumor-associated macrophages
density in gastric cancer: A systemic review and meta-analysis.
Minerva Med. 107:314–321. 2016.PubMed/NCBI
|
|
20
|
Bao S, Ouyang G, Bai X, Huang Z, Ma C, Liu
M, Shao R, Anderson RM, Rich JN and Wang XF: Periostin potently
promotes metastatic growth of colon cancer by augmenting cell
survival via the Akt/PKB pathway. Cancer Cell. 5:329–339. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Baril P, Gangeswaran R, Mahon PC, Caulee
K, Kocher HM, Harada T, Zhu M, Kalthoff H, Crnogorac-Jurcevic T and
Lemoine NR: Periostin promotes invasiveness and resistance of
pancreatic cancer cells to hypoxia-induced cell death: Role of the
beta4 integrin and the PI3k pathway. Oncogene. 26:2082–2094. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Malanchi I, Santamaria-Martínez A, Susanto
E, Peng H, Lehr HA, Delaloye JF and Huelsken J: Interactions
between cancer stem cells and their niche govern metastatic
colonization. Nature. 481:85–89. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou W, Ke SQ, Huang Z, Flavahan W, Fang
X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, et al: Periostin
secreted by glioblastoma stem cells recruits M2 tumour-associated
macrophages and promotes malignant growth. Nat Cell Biol.
17:170–182. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Michaylira CZ, Wong GS, Miller CG,
Gutierrez CM, Nakagawa H, Hammond R, Klein-Szanto AJ, Lee JS, Kim
SB, Herlyn M, et al: Periostin, a cell adhesion molecule,
facilitates invasion in the tumor microenvironment and annotates a
novel tumor-invasive signature in esophageal cancer. Cancer Res.
70:5281–5292. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, Li F, Gao F, Xing L, Qin P, Liang
X, Zhang J, Qiao X, Lin L, Zhao Q and Du L: Periostin promotes
tumor angiogenesis in pancreatic cancer via Erk/VEGF signaling.
Oncotarget. 7:40148–40159. 2016.PubMed/NCBI
|
|
26
|
Nathan H, Aloia TA, Vauthey JN, Abdalla
EK, Zhu AX, Schulick RD, Choti MA and Pawlik TM: A proposed staging
system for intrahepatic cholangiocarcinoma. Ann Surg Oncol.
16:14–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mantovani A, Sica A, Sozzani S, Allavena
P, Vecchi A and Locati M: The chemokine system in diverse forms of
macrophage activation and polarization. Trends Immunol. 25:677–686.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Popivanova BK, Kostadinova FI, Furuichi K,
Shamekh MM, Kondo T, Wada T, Egashira K and Mukaida N: Blockade of
a chemokine, CCL2, reduces chronic colitis-associated
carcinogenesis in mice. Cancer Res. 69:7884–7892. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pyonteck SM, Gadea BB, Wang HW, Gocheva V,
Hunter KE, Tang LH and Joyce JA: Deficiency of the macrophage
growth factor CSF-1 disrupts pancreatic neuroendocrine tumor
development. Oncogene. 31:1459–1467. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Germano G, Frapolli R, Belgiovine C,
Anselmo A, Pesce S, Liguori M, Erba E, Uboldi S, Zucchetti M,
Pasqualini F, et al: Role of macrophage targeting in the antitumor
activity of trabectedin. Cancer Cell. 23:249–262. 2013. View Article : Google Scholar : PubMed/NCBI
|