Introduction
Cervical cancer is the most common type of
gynecological cancer. There are ~500,000 newly diagnosed cases, and
>250,000 associated deaths worldwide each year (1). The incidence of cervical cancer has
decreased in developed countries, while continuing to increase in
developing countries (2). The
currently available treatment routes for cancer include surgery,
radiotherapy and chemotherapy, but the efficiency of these
treatments is low and they involve various adverse effects
(3). Therefore, we need to develop
new low-toxicity drugs for cancer treatment.
Fomitopsis pinicola (F. pinicola; Sw.
Ex Fr.) Karst is one of the most common wood-rooting fungi in the
northern hemisphere (4). It is widely
distributed in Japan, Korea, China and Sweden (5). F. pinicola has a long history of
medicinal use for the treatment of poor leg circulation in the
elderly in Northeast China, and has been used for diabetes in Japan
(6). F. pinicola is a
non-toxic natural product that is becoming increasingly more
attractive in academia. The extracts of F. pinicola have
numerous pharmacological effects, including anti-inflammatory,
antimicrobial, antifungal and anti-obesity properties (7–10). Wu
et al (11), reported that the
ethanol extract of F. pinicola could induce apoptosis in
A549, HCT-116 and MDA-MB-231 cells, and inhibit SW180 cell growth
in vivo. Chemical analysis showed that there were no
ergosterol, ergosterol derivatives or lanostane triterpenes in the
F. pinicola extract (12,13).
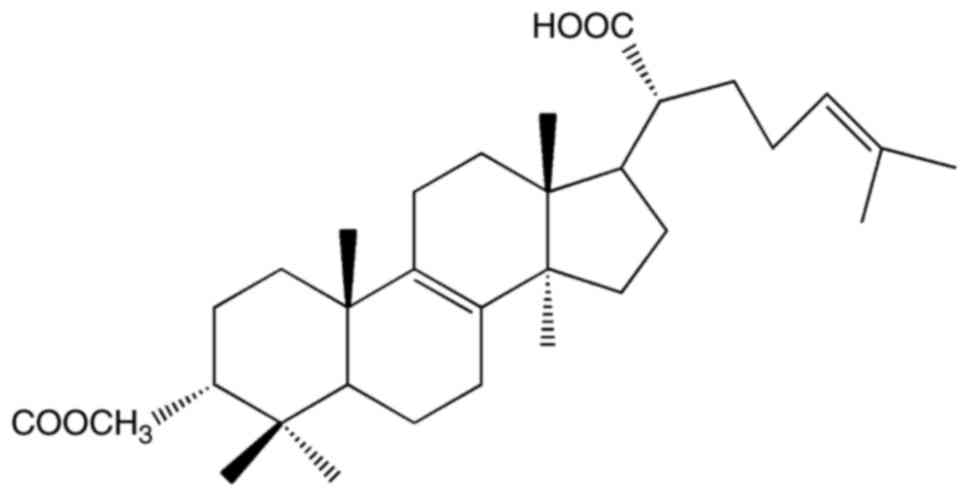
We isolated the triterpenoid
3-acetoxylanosta-8,24-dien-21-oic acid (FPOA; Fig. 1) from the fruiting body of F.
pinicola, and determined that it was the primary active
ingredient (14). In this study, we
aimed to elucidate whether FPOA could inhibit the growth of HeLa
cells, as well as to investigate its underlying mechanisms.
Materials and methods
Materials
The fruiting bodies were obtained in Changbai
Mountain, Jilin Province, P.R.China. The
3-acetoxylanosta-8,24-dien-21-oic acid (FPOA) was extracted from
the fruiting bodies of Fomitopsis pinicola. The fruiting
bodies were extracted with ethanol and separated by silica gel
column, elution with a mixture of petroleum ether and ethyl acetate
to get FPOA. The purity of FPOA was >95%, as detected via HPLC.
Antibodies against caspase-3, −9, Bcl-2, PARP and Bax were
purchased from Cell Signaling Technology, Inc., (Danvers, MA, USA).
β-actin was obtained from Wuhan Sanying Biotechnology, (Wuhan,
China). BCA protein assay kit was purchased from Beijing Leagene
Biotech, Co., Ltd., (Beijing, China). An Annexin V-FITC Apoptosis
Detection kit was obtained from Tianjin Sungene Biotech Co., Ltd.,
(Tianjin, China). MTT and all other reagents were obtained from
Sigma-Aldrich; Merck KGaA (Darmstadt, Germany).
Cell culture
Human cervical cancer HeLa cells were obtained from
the Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China). The HeLa cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (Sijiqing; Zhejiang
Tianhang Biotech Co., Ltd., Huzhou, China) and 1%
penicillin-streptomycin.
MTT assay
Cell viability was evaluated using an MTT assay, as
previously described (15). Briefly,
HeLa cells were seeded into 96-well plates with 4,500 cells/well
and treated with different concentrations of FPOA (12.5, 25, 50,
100 and 200 µg/ml). After incubation for 20, 44 and 68 h, 37°C, 10
µl MTT (5 mg/ml) was added to each well and then incubated for
another 4 h, 37°C. Subsequently, dimethyl sulfoxide (100 µl) was
added to each well and the plates were agitated for 10 min. The
absorbance was read at a wavelength of 570 nm using a microplate
reader. The inhibitory concentration 50% (IC50) was
defined as the concentration of experimental compound required to
inhibited 50% cell proliferation, as compared with the untreated
cells (16). The IC50 was
calculated with GraphPad Prism v5.0 (GraphPad Software, Inc., La
Jolla, CA, USA).
DAPI staining
DAPI staining was performed as previously described
(17). Briefly, HeLa cells were
seeded into 6-well plates with 140,000 cells/well and treated with
FPOA for 24 h. The cells were fixed with 4% polyoxymethylene at
room temperature for 10 min. The coverslips were equilibrated in
PBS, following which 300 µl DAPI (300 nM) staining solution was
added to the coverslips and incubated for 5 min at room
temperature. The coverslips were rinsed two times in PBS and viewed
using a fluorescence microscope (Nikon TE-2000 U; Nikon
Corporation, Tokyo, Japan). The normal cell nuclei were faint
staining (cells were alive). The apoptotic cell nuclei were
brightness (18).
Apoptosis assay
Annexin V-FITC/PI staining was performed, followed
by flow cytometry as previously described (15). Briefly, HeLa cells were seeded into
6-well plates with 140,000 cells/well and treated with FPOA for 24
h, after which the cells were collected and washed with cold PBS.
Then, the cells were resuspended in 300 µl binding buffer. The
cells were incubated with 5 µl Annexin V-FITC and 5 µl propidium
iodide (PI) in the dark for 15 min at room temperature. Finally,
the cells were analyzed via flow cytometry (BD FASCCalibur; BD
Biosciences, Franklin Lakes, NJ, USA). The total percentage of
apoptotic cells was defined as the sum of both early apoptosis
(annexin V-FITC positive, PI negative) and late apoptosis (annexin
V-FITC PI positive), top and bottom right quadrants in a flow
cytometric dot plots, respectively (19). For each sample, 10,000 events were
collected and analyzed by CellQuest™ Pro (v6.0) software (BD
Biosciences).
ROS detection
ROS generation was assessed as previously described
(20). Briefly, HeLa cells were
seeded into 6-well plates with 140,000 cells/well and treated with
FPOA for 24 h. Following this, HeLa cells were incubated with
non-fluorescent probe 2′,7′-dichlorofluorescein diacetate (DCFH-DA)
for 20 min at 37°C, and then cells were washed two times with
medium without serum. ROS was examined by fluorescence microscopy
or flow cytometry.
Western blot analysis
HeLa cells were seeded into 6-well plates with
140,000 cells/well and treated with FPOA for 24 h, following which
the cells were harvested and lysed on ice with RIPA buffer (150 mM
NaCl, 1% Triton-X-100, 0.5% sodium deoxycholate, 0.1% SDS, 50 mM
Tris-HCl, pH 7.4) for 30 min. After centrifugation at 13,000 × g
for 15 min, the protein concentration was determined using a BCA
protein assay kit. Then, 20 µg cell lysate proteins were loaded
onto 12% SDS-polyacrylamide gels, resolved via electrophoresis and
then electrophoretically transferred to a polyvinylidene difluoride
membrane. The membrane was blocked with 5% (w/v) non-fat milk for 1
h. After blocking, the membrane was incubated overnight at 4°C with
primary antibodies: caspase-3 (cat. no. 9662, 1:1,000 dilution),
caspase-9 (cat. no. 9508, 1:1,000 dilution), Bcl-2 (cat. no. 2876,
1:1,000 dilution), PARP (cat. no. 9542, 1:1,000 dilution) and Bax
(cat. no. 2772, 1:1,000 dilution) supplied by Wuhan Sanying
Biotechnology. β-actin (20536-1-AP, 1:1,000 dilution; Wuhan Sanying
Biotechnology), was also used. Membranes were then washed twice
with PBST and incubated with horseradish peroxidase
(HRP)-conjugated anti-rabbit (cat. no. IH-0011, 1:5,000 dilution)
and anti-mouse antibodies (cat. no. IH-0031, 1:5,000 dilution)
supplied by Dingguo Changsheng Biotechnology, (Beijing, China) at
room temperature for 1 h. Then the membranes were then washed twice
with PBST at room temperature. Western blot bands were detected
using Odyssey v1.2 software.
Statistical analysis
Results are expressed as the mean ± SD. Statistical
comparisons were evaluated by SPSS v21.0 (IBM SPSS, Armonk, NY,
USA) using the Student's t-test or one-way analysis of variance
with Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
FPOA inhibits cell proliferation
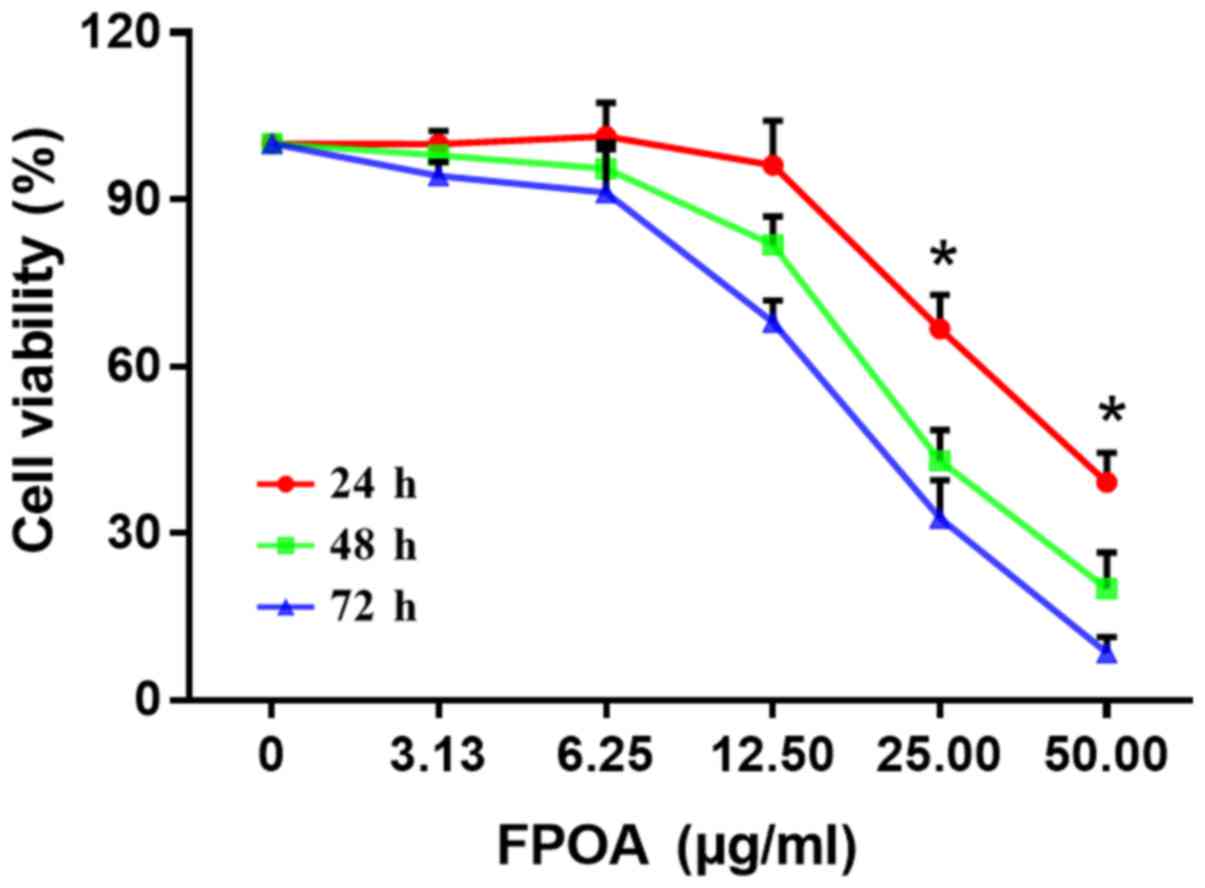
We investigated the growth inhibitory effect of FPOA
on HeLa cells using an MTT assay. As shown in Fig. 2, FPOA could inhibit the proliferation
of HeLa cells in a dose- and time-dependent manner. IC50
values of 25.28, 15.30 and 11.79 µg/ml were calculated at 24, 48
and 72 h, respectively.
FPOA induces the apoptosis of HeLa
cells
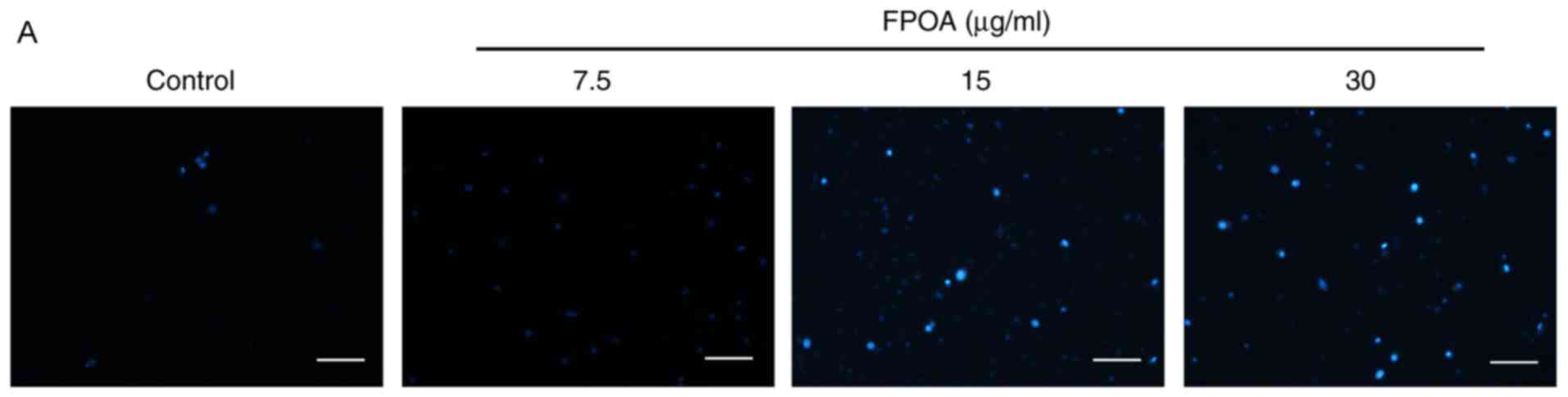
In order to elucidate whether FPOA induces
apoptosis, DAPI staining was performed. As shown in Fig. 3, numerous apoptotic bodies containing
unclear fragments were observed following FPOA treatment, but
comparatively few were present in the control group. These results
indicated that FPOA could induce the apoptosis of HeLa cells.
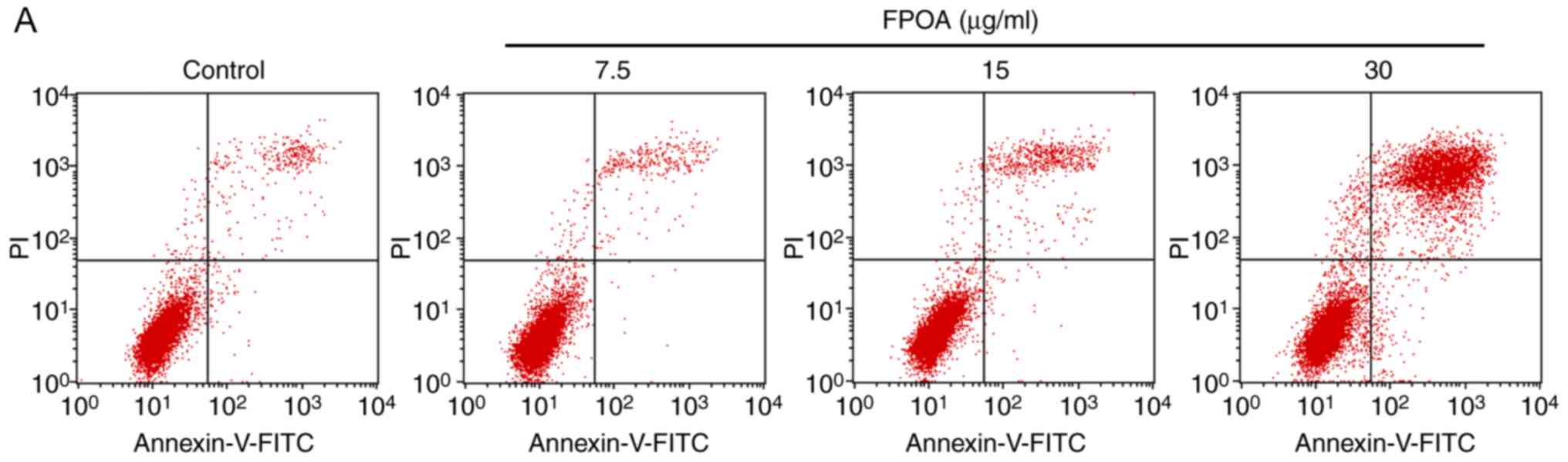
Furthermore, in order to quantify the rate of
apoptosis, Annexin V-FITC/PI staining was performed. As shown in
Fig. 4, the percentage of apoptotic
cells was 3.00, 3.12, 6.18 and 32.28% following treatment with 0,
7.5, 15 and 30 µg/ml FPOA, respectively.
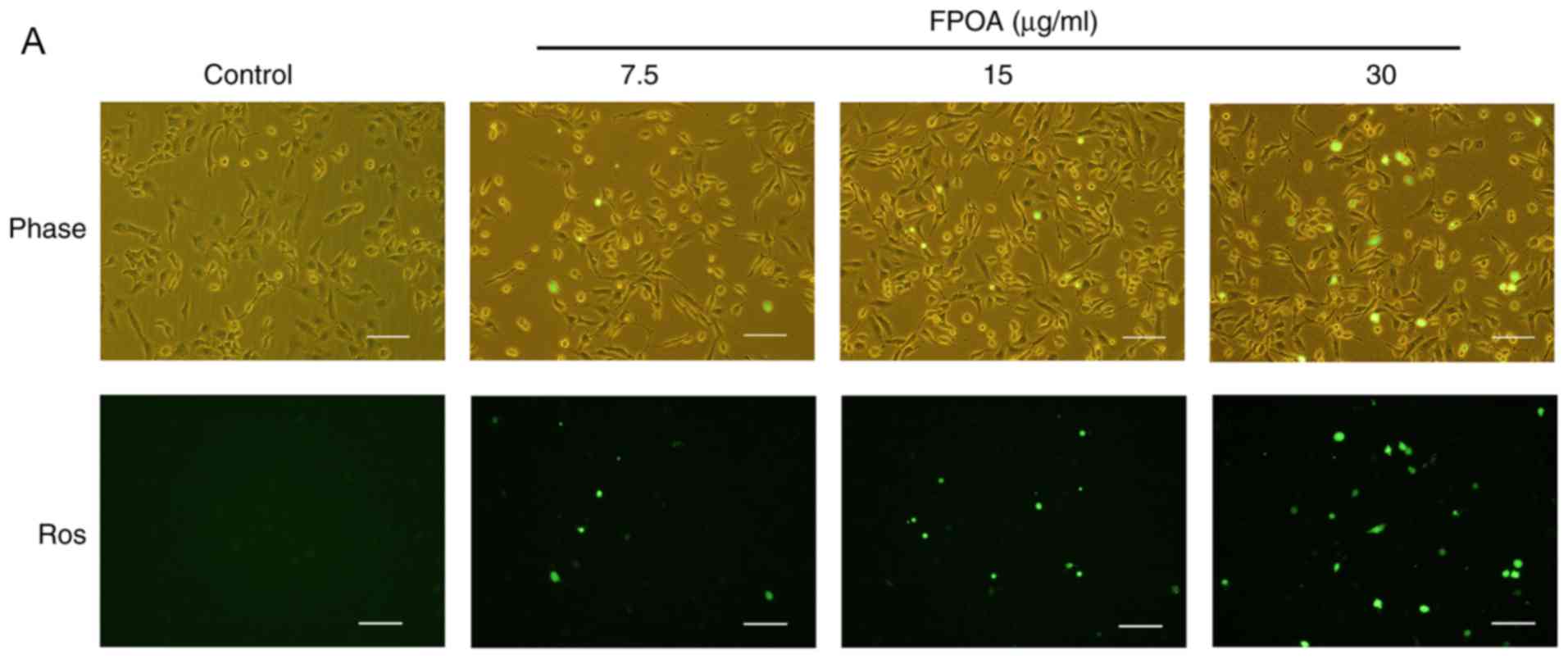
FPOA induces ROS in HeLa cells
In order to test whether mitochondria play a role in
FPOA-induced apoptosis, we performed ROS generation assays. As
shown in Fig. 5, FPOA could increase
ROS production in a dose-dependent manner.
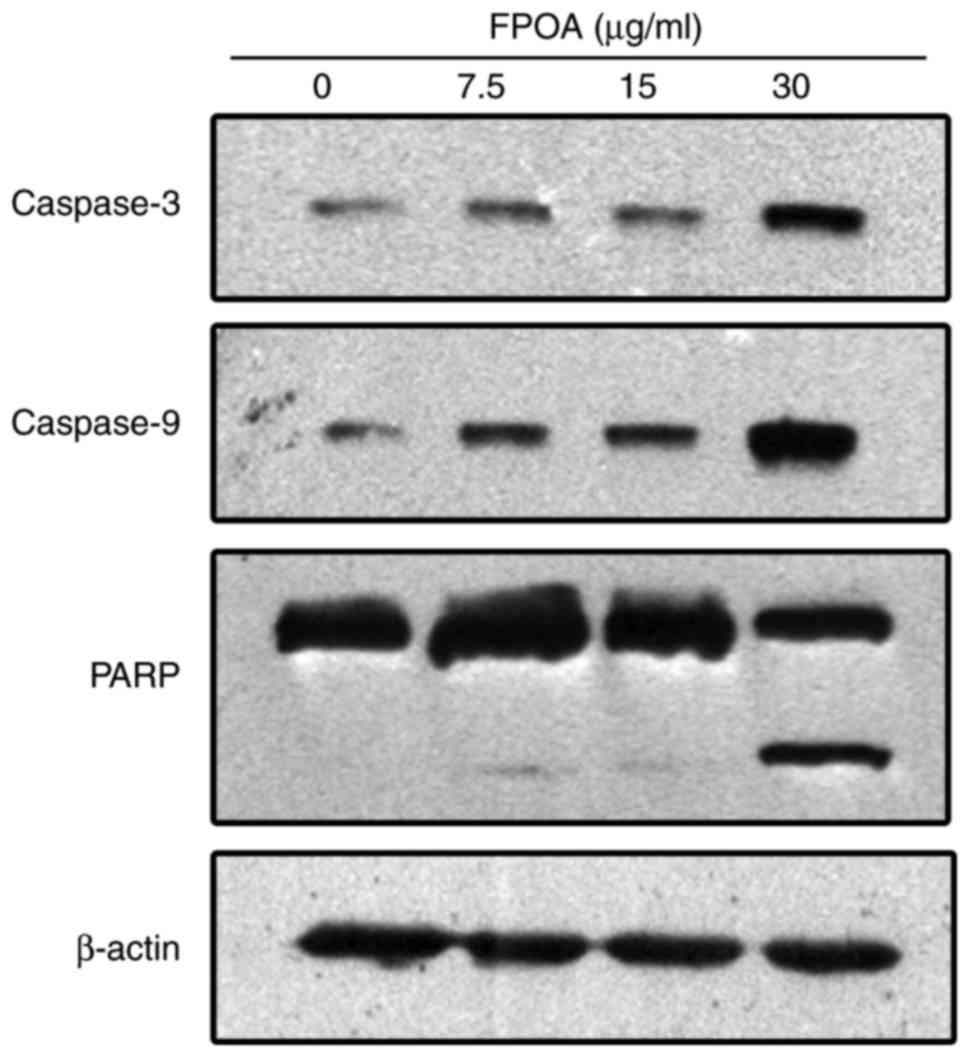
Induction of apoptosis via the caspase cysteine
protease familyIt is known that the cysteine protease family is an
integral part of the apoptotic signaling cascade. As shown in
Fig. 6, the levels of cleaved
caspase-9 and caspase-3 were increased following treatment with
FPOA. PARP is a substrate of caspase-3 (21). After treatment with FPOA, PARP was
hydrolyzed to an 89-kDa fragment, corresponding to the activation
of caspase-3.
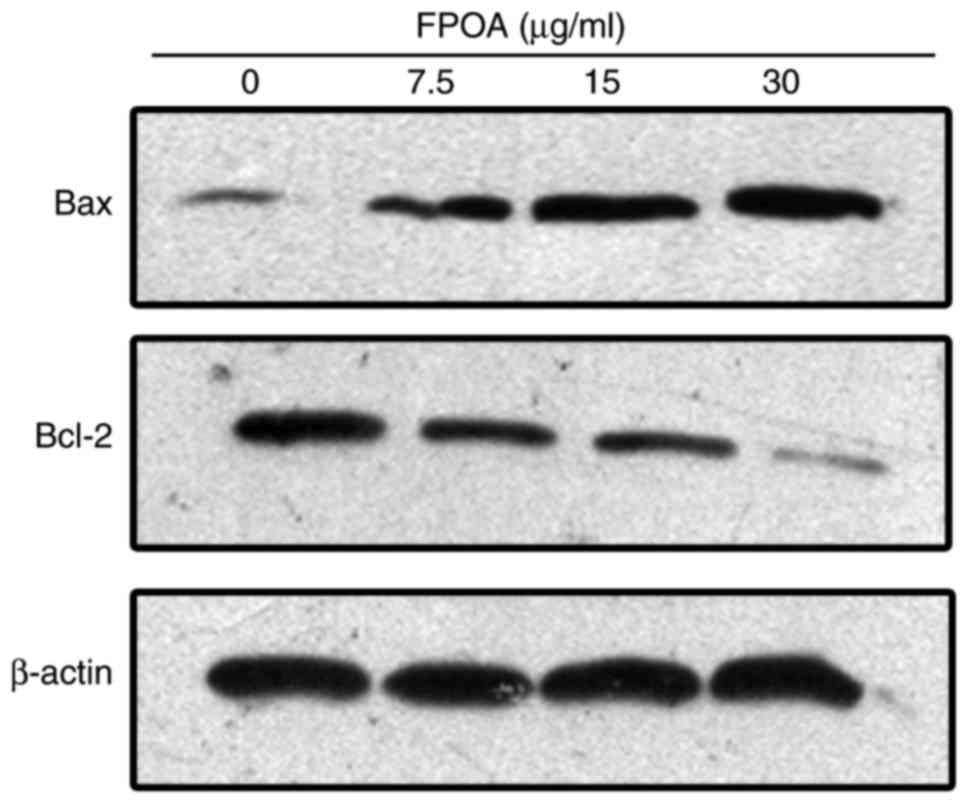
FPOA regulates the expression of Bcl-2
family proteins
Bcl-2 family members are critical regulators of the
apoptotic cascade (15). We further
investigated the effect of FPOA on the Bcl-2 protein family. As
shown in Fig. 7, the expression of
Bcl-2 was decreased while the expression of Bax was increased
following treatment with FPOA. These results suggested that FPOA
induced apoptosis by regulating the ratio of Bcl-2 to Bax.
Discussion
In a previous study, the fungal extracts exhibited
anti-cancer effects in multiple tumor types (11). FPOA, isolated from the fruiting body
of F. pinicola, was determined to be the main active
ingredient (14). Here, we studied
the anticancer activity of FPOA, and found that FPOA could inhibit
the proliferation of HeLa cells in a dose-dependent manner.
Morphological examination revealed that HeLa cells exhibited
cellular alterations after treatment with FPOA. Chromatin
condensation, a change typically observed during apoptosis, was
revealed by DAPI staining. In addition, the Annexin V-FITC/PI
double-staining assay confirmed the apoptosis induced by FPOA
(Fig. 4). The results verified FPOA
exerted anticancer activity through inducing apoptosis.
Apoptosis is an important mechanism for regulating
the development of organisms, the renewal of cells and the
stability of the intracellular environment (22,23). At
present, there are two pathways involved in the apoptosis cascade,
including the mitochondrial-mediated intrinsic pathway, and the
endoplasmic reticulum pathway; these pathways are interlinked and
interact to promote apoptosis (24).
The Bax/Bcl-2 protein family is involved in the
mitochondrial-mediated intrinsic pathway. Specifically, Bcl-2 and
Bax can regulate the permeability of mitochondrial membranes. When
the Bax/Bcl-2 ratio increases, the permeability of the
mitochondrial membrane increases, which induces apoptosis. We
detected the expression of certain Bcl-2 family members via western
blotting; the data suggested that FPOA treatment leads to elevated
Bax levels, accompanied by a decrease in Bcl-2 levels (Fig. 7).
Activation of the caspase protease family has an
important role in the apoptosis regulated by the Bcl-2 family.
Caspases are produced as inactive zymogens and undergo proteolytic
activation during apoptosis. Caspase-9 is a regulator in the
caspase cascade reaction and is involved in the
mitochondrial-mediated intrinsic pathway; and caspase-3 is a
primary effector that triggers the initiation of apoptosis
(25). Our results indicated that
FPOA treatment induced the proteolytic activation of caspase-9 and
−3 in a dose-dependent manner (Fig.
6). PARP has a protective effect on DNA; as a substrate for
caspase-3 involved in apoptosis, PARP is cleaved by caspase-3,
leading to DNA damage and apoptosis. We found that FPOA also
resulted in the cleavage of 116 kDa PARP into an 89-kDa fragment in
HeLa cells (Fig. 6). Taken together,
our data suggested that FPOA induced the mitochondrial-mediated
apoptosis of HeLa cells by activating the caspase cascade.
In conclusion, this study demonstrated that FPOA
could inhibit the proliferation of HeLa cells by inducing
apoptosis. Furthermore, our results demonstrated that FPOA induces
apoptosis in HeLa cells via the mitochondrial-mediated intrinsic
pathway. The present results suggested that FPOA could be a
potential anticancer agent for human cervical cancer.
Acknowledgements
The authors would like to thank Professor Dayun Sui
from the Department of Pharmacology, School of Pharmaceutical
Sciences, Jilin University (Changchun, China) for providing
technical guidance. The technical assistance of Professor Huali Xu,
Dr Yuchen Wang and Dr Zeyuan Lu from the Department of
Pharmacology, School of Pharmaceutical Sciences, Jilin University
(Changchun, China) is also acknowledged.
Funding
The present study was supported by the Science and
Technology Development Plan Project of Jilin Province, China (grant
no. 20150311016YY), and the National Natural Science Foundation of
China (grant no. 31270088).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TB and HB conceived and designed the study. XL
performed the experiments. XL and HB wrote the paper. TB, HB and XL
reviewed and edited the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bayu H, Berhe Y, Mulat A and Alemu A:
Cervical cancer screening service uptake and associated factors
among age eligible women in mekelle zone, northern ethiopia, 2015:
A community based study using health belief model. PLoS One.
11:e01499082016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cragg GM and Newman DJ: Plants as a source
of anti-cancer agents. J Ethnopharmacol. 100:72–79. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gilbertson RL and Ryvarden L: North
american polypores, vol. 2, megasporoporia-wrightoporia. Mycologia.
81:437–885. 1987.
|
|
5
|
Ying JZ MX, Ma QM, Zong YC and Wen HA:
Illustrated handbook for medicinal fungi from China. Beijing:
Science Press. 120, 128, 172; pp. 2181987
|
|
6
|
Usui T SK, Satoh H, Iwasaki Y and Mizuno
T: Studies on host mediated antitumor polysaccharides. Part V.
Chemical structure and antitumor activity of a water-soluble Glucan
isolated from Tsugasarunokoshikake, the fruit body of Fomitopsis
pinicola. Shizuoka Daigaku Nogakubu Kenkyu Hokoku. 29–40. 1982.
|
|
7
|
Yoshikawa K, Inoue M, Matsumoto Y,
Sakakibara C, Miyataka H, Matsumoto H and Arihara S: Lanostane
triterpenoids and triterpene glycosides from the fruit body of
Fomitopsis pinicola and their inhibitory activity against COX-1 and
COX-2. J Nat Prod. 68:69–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Keller AC, Maillard MP and Hostettmann K:
Antimicrobial steroids from the fungus Fomitopsis pinicola.
Phytochemistry. 41:1041–1046. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guler P, Akata I and Kutluer F: Antifungal
activities of fomitopsis pinicola (Sw.:Fr) Karst and Lactarius
vellereus (Pers.) Fr. African J Biotechnol. 8:3811–3813. 2009.
|
|
10
|
Jung HY, Ji Y, Kim NR, Kim DY, Kim KT and
Choi BH: A fomitopsis pinicola jeseng formulation has an
antiobesity effect and protects against hepatic steatosis in mice
with high-fat diet-induced obesity. Evid Based Complement Alternat
Med. 2016:73124722016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu HT, Lu FH, Su YC, Ou HY, Hung HC, Wu
JS, Yang YC and Chang CJ: In vivo and in vitro anti-tumor effects
of fungal extracts. Molecules. 19:2546–2556. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rösecke J and König WA: Steroids from the
fungus Fomitopsis pinicola. Phytochemistry. 52:1621–1627. 1999.
View Article : Google Scholar
|
|
13
|
Rosecke J and König WA: Constituents of
various wood-rotting basidiomycetes. Phytochemistry. 54:603–610.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren G, Liu XY, Zhu HK, Yang SZ and Fu CX:
Evaluation of cytotoxic activities of some medicinal polypore fungi
from China. Fitoterapia. 77:408–410. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu HL, Yu XF, Qu SC, Zhang R, Qu XR, Chen
YP, Ma XY and Sui DY: Anti-proliferative effect of Juglone from
Juglans mandshurica Maxim on human leukemia cell HL-60 by inducing
apoptosis through the mitochondria-dependent pathway. Eur J
Pharmacol. 645:14–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Oldham ED, Nunes LM, Varela-Ramirez A,
Rankin SE, Knutson BL, Aguilera RJ and Lehmler HJ: Cytotoxic
activity of triazole-containing alkyl β-D-glucopyranosides on a
human T-cell leukemia cell line. Chem Cent J. 9:32015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang H, Xu HL, Fu WW, Xin Y, Li MW, Wang
SJ, Yu XF and Sui DY: 20(S)-protopanaxadiol induces human breast
cancer MCF-7 apoptosis through a caspase-mediated pathway. Asian
Pac J Cancer Prev. 15:7919–7923. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen W, Du J, Li X, Su J, Huang Y, Ding N,
Zhang M and Jiang S: miR-509-3p promotes cisplatin-induced
apoptosis in ovarian cancer cells through the regulation of
anti-apoptotic genes. Pharmacogenomics. 18:1671–1682. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schorr GS, Falcone EA, Moretti DJ and
Andrews RD: First long-term behavioral records from Cuvier's beaked
whales (Ziphius cavirostris) reveal record-breaking dives. PLoS
One. 9:e926332014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deeb D, Gao X, Jiang H, Janic B, Arbab AS,
Rojanasakul Y, Dulchavsky SA and Gautam SC: Oleanane triterpenoid
CDDO-Me inhibits growth and induces apoptosis in prostate cancer
cells through a ROS-dependent mechanism. Biochem Pharmacol.
79:350–360. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi BH, Kim W, Wang QC, Kim DC, Tan SN,
Yong JW, Kim KT and Yoon HS: Kinetin riboside preferentially
induces apoptosis by modulating Bcl-2 family proteins and caspase-3
in cancer cells. Cancer Lett. 261:37–45. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hail N Jr, Carter BZ, Konopleva M and
Andreeff M: Apoptosis effector mechanisms: A requiem performed in
different keys. Apoptosis. 11:889–904. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aragane Y, Kulms D, Metze D, Wilkes G,
Pöppelmann B, Luger TA and Schwarz T: Ultraviolet light induces
apoptosis via direct activation of CD95 (Fas/APO-1) independently
of its ligand CD95L. J Cell Biol. 140:171–182. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abraham MC and Shaham S: Death without
caspases, caspases without death. Trends Cell Biol. 14:184–193.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salvesen GS and Dixit VM: Caspase
activation: The induced-proximity model. Proc Natl Acad Sci USA.
96:10964–10967. 1999. View Article : Google Scholar : PubMed/NCBI
|