Introduction
The majority of colorectal cancer cases are
considered to arise from conventional adenomas, on the basis of the
concept of the adenoma-carcinoma sequence (1,2). By
contrast, serrated lesions, including hyperplastic polyps (HPs),
traditional serrated adenomas (TSAs), and sessile serrated
polyp/adenomas (SSA/Ps) (3–6), may be morphologically and genetically
distinct. Additionally, HPs, TSAs and SSA/Ps may be linked to
microsatellite unstable colorectal cancer. Therefore, the concept
of a HP-serrated adenoma-carcinoma pathway has been suggested
(7–9).
Colonoscopy is the only technique available for the
reliable detection and removal of colorectal lesions. A number of
advanced endoscopic imaging techniques, including chromoendoscopy
(CE) and narrow-band imaging (NBI), which enhance the detection and
discrimination of mucosal lesions, have been developed to improve
the accuracy of conventional endoscopy (10,11). Using
CE and NBI, the present authors and others have extensively
investigated the endoscopic characteristics of colorectal serrated
lesions, particularly lesions without a coexisting cancer component
(12–14).
There is growing evidence to show that serrated
lesions, particularly TSAs and SSA/Ps, demonstrated a significant
risk of developing colorectal cancer (15). Therefore, reliable discrimination of
serrated lesions with cancer from those without cancer has
important clinical implications and may condition therapeutic
attitudes. It has been hypothesized that serrated lesions with
cancer may be distinguishable using colonoscopy, particularly with
one of the advanced endoscopic imaging techniques. However, limited
data are available on the endoscopic features of serrated lesions
with and without cancer (16,17).
In the present study, it was hypothesized that a
detailed endoscopic analysis of serrated lesions with cancer may
permit delineation of their characteristic endoscopic features.
These observations may be used by endoscopists to improve the
endoscopic recognition of serrated lesions with cancer and assist
treatment selection.
Materials and methods
Patient selection and study
design
The present study was a retrospective analysis of
the data of 168 patients (109 males, 59 females; mean age, 63.8
years; range, 28–87 years) who had undergone total colonoscopy at
Kurume University Hospital (Kurume, Japan) between March 1999 and
September 2013. Lesions were excluded if postoperative
histopathological evaluation was impossible due to thermal or
mechanical tissue damage caused by endoscopic resection procedures
and if patients had other diseases at the same time. A total of 228
histologicalally confirmed serrated lesions were identified in the
patients. Of these 228 serrated lesions, there were 77 HPs, 58
TSAs, 84 SSA/Ps and 9 SSA/P plus TSAs. The ethics committee of
Kurume University Hospital approved the study protocol.
Endoscopic procedure
Bowel preparation for colonoscopy consisted of
ingesting polyethylene glycol-electrolyte solution (volume, 2 l) on
the morning of the procedure by all patients.
Colonoscopy was performed using white-light imaging
by experienced colonoscopists. When colorectal lesions were
detected, the leisions were examined by switching to the NBI mode
by the press of a button in the control head of the endoscope.
Subsequently, the lesions were observed by CE using indigo carmine
and the white-light imaging mode. Finally, magnifying endoscopy
with NBI and CE using crystal violet was performed.
Conventional endoscopic analysis
The lesions were classified on the basis of the
size, shape, site and other characteristic features, including
presence/absence of a reddish appearance, a central depression, a
two-tier raised appearance and a mucus cap (12,13). These
characteristics were determined once the images were reviewed by
two of the study investigators.
Pit pattern analysis
Using magnifying CE, the pit patterns were
classified into one of five types (I to V) according to
classification criteria described by of Kudo et al (18,19) and
Tobaru et al (20). Type I is
represented by regular round crypts, type II is represented by
stellar or papillary crypts, type III is represented by small
tubular or roundish crypts (IIIS) or large tubular or
roundish crypts (IIIL), type IV is represented by
branch- or gyrus-like crypts, and type V is represented by
irregular or non-structural crypts. The majority of type I and II
lesions are non-neoplastic, whereas type III, IV and V lesions are
typically neoplastic.
Capillary pattern analysis
Using NBI, the lesions were classified using Sano's
capillary pattern classification (21,22), with
type I (faintly visible microvessels surrounding the pits)
representing non-neoplastic lesions, type II (elongated vessels of
increased thickness surrounding the pits) representing adenomas,
and type III (type IIIa, unevenly sized vessels of increased
thickness with branching and curtailed irregularity and type IIIb
with nearly avascular or loose vessels with fragmentation)
indicative of cancer.
Histopathological analysis
All tissues were fixed in 10% neutral-buffered
formalin for 2 days at room temperature and embedded in paraffin.
The sections were cut into sections with a thickness of 4 µm and
stained using hematoxylin (1 min) and eosin (3 min) at room
temperature. The sections were assessed using a light microscope
(BX41; Olympus Corporation, Tokyo, Japan) by two pathologists who
were blinded to the aims of the study. In accordance with the World
Health Organization classification (6), each serrated lesion was classified as
HP, TSA or SSA/P. In regard to the evaluation of the
adenocarcinomatous component of the lesions, the lesions were
histologically rated as well-differentiated, moderately
differentiated or poorly differentiated, and the vertical depth of
invasion was also assessed as previously described (23). He present study defined a cancer
lesion as category 4 of the Vienna classification of
gastrointestinal epithelial neoplasia non-invasive high grade
neoplasia (24).
Immunohistochemistry
Immunohistochemistry was performed in 4-µm paraffin
sections using a peroxidase procedure with a Bond Polymer Refine
Detection kit (cat. no. DS9800; Leica Microsystems, Ltd., Milton
Keynes, UK). Peroxide blocking was performed using 3–4% hydrogen
peroxide at room temperature for 5 min. Hydrogen peroxide was
included in a Bond Polymer Refine Detection kit (cat. no. DS9800;
Leica Microsystems, Ltd.). A light microscope (BX41) was used for
analysis (×40 magnification). To delineate the proliferative zone
in the crypts, Ki-67 immunostaining was performed using monoclonal
antibodies against marker of proliferation Ki-67 (1:100, Dako;
Agilent Technologies, Inc., Santa Clara, CA, USA). Specific
antibodies to the mucins were used to define the gastric and
intestinal phenotypes, including antibodies against human gastric
mucin (HGM; 1:100; cat. no. 45M1; Novocastra Laboratories, Ltd.,
Newcastle, UK) and MUC5AC (1:200; cat. no. CLH2; Novocastra
Laboratories Ltd.) which detects the gastric foveolar type of
mucin, MUC6 (1:100; cat. no. CLH5; Novocastra Laboratories Ltd.)
which detects the pyloric gland type of mucin, and mucin (MUC)2
(Ccp58; Novocastra Laboratories Ltd.) which detects the mucin
produced by goblet cells and their precursors. Additionally, the
antibody against cluster of differentiation (CD)10 (1:100; cat. no.
56C6; Novocastra Laboratories Ltd.) was used to detect mucin
produced by the striated border on the luminal surface of the small
intestinal absorptive cells. Rabbit anti-mouse immunoglobulin G was
used as a secondary antibody (undiluted), and incubated at room
temperature for 8 min. The secondary antibody was included in a
Bond Polymer Refine Detection kit (cat. no. DS9800; Leica
Microsystems, Ltd.). Lesions were categorized as being of the
gastric type if they were positive for HGM, MUC5AC or MUC6, as the
small-intestinal type if they were positive for MUC2 and CD10, as
the large-intestinal type if they were positive for MUC2 and
negative for CD10, and as the mixed type when gastric and
intestinal markers were positive (25–27).
Statistical analysis
The results were analyzed using SPSS version 12.0
(SPSS, Inc., Chicago, IL, USA. Student's t-test was used for the
analysis of age and size, and the χ2 test for the
analysis of sex, shape, site, type V pit pattern, type III
capillary pattern, reddish appearance, two-tier raised appearance,
central depression and adherent mucus cap. P<0.05 was considered
to indicate a statistically significant difference.
Results
Clinicopathological
characteristics
The clinicopathological characteristics of the
serrated lesions with and without cancer are presented in Table I. Of the 228 serrated lesions, 2.6%
(2/77) HPs, 13.8% (8/58) TSAs, 10.7% (9/84) SSA/Ps and 0% (0/9) of
the SSA/P plus TSAs contained a carcinomatous component. The HPs,
TSAs and SSA/Ps with cancer were significantly increased in size,
compared with the HPs, TSAs and SSA/Ps without cancer: HPs (mean
size), 19.0 vs. 7.4 mm, P=0.0002; TSAs, 17.2 vs. 11.1 mm,
P=0.0079; SSA/P plus TSAs, 19.1 vs. 13.7 mm, P=0.0124. The shape
and site of the lesions did not differ significantly between the
HPs, TSAs and SSA/Ps with and without cancer. Furthermore, the HPs,
TSAs and SSA/Ps with and without cancer also showed no significant
differences in the size or shape. Notably, SSA/Ps with cancer, but
not HPs or TSAs with cancer, were preferentially located in the
right colon.
 | Table I.Clinicopathological features of
serrated lesions with and without cancer. |
Table I.
Clinicopathological features of
serrated lesions with and without cancer.
|
| HP | TSA | SSA/P | SSA/p plus TSA |
|---|
|
|
|
|
|
|
|---|
| Parameters | With cancer | Without cancer | With cancer | Without cancer | With cancer | Without cancer | With cancer | Without cancer |
|---|
| Age,
yearsa | 61.5 | 62.1 | 67.4 | 65.2 | 74 | 64.3 | – | 55.1 |
| Sex, n |
|
|
|
|
|
|
|
|
|
Male | 2 | 62 | 7 | 29 | 4 | 51 | 0 | 6 |
|
Female | 0 | 13 | 1 | 21 | 5 | 24 | 0 | 3 |
|
Lesions, n | 2 | 75 | 8 | 50 | 9 | 75 | 0 | 9 |
| Size,
mm | 19.0 | 7.5 | 17.8 | 11.1 | 19.1 | 13.3 | – | 15.0 |
| Shape, n |
|
|
|
|
|
|
|
|
|
Sessile | 2 | 68 | 3 | 27 | 9 | 73 | 0 | 5 |
|
Protruded | 0 | 7 | 5 | 23 | 0 | 2 | 0 | 4 |
| Site, n |
|
|
|
|
|
|
|
|
|
Cecum | 1 | 11 | 0 | 1 | 3 | 19 | 0 | 1 |
|
Ascending colon | 0 | 10 | 1 | 7 | 2 | 28 | 0 | 1 |
|
Transverse colon | 0 | 17 | 2 | 0 | 4 | 17 | 0 | 1 |
|
Descending colon | 0 | 2 | 0 | 4 | 0 | 1 | 0 | 0 |
| Sigmoid
colon | 1 | 20 | 1 | 18 | 0 | 7 | 0 | 5 |
|
Rectum | 0 | 15 | 4 | 20 | 0 | 3 | 0 | 1 |
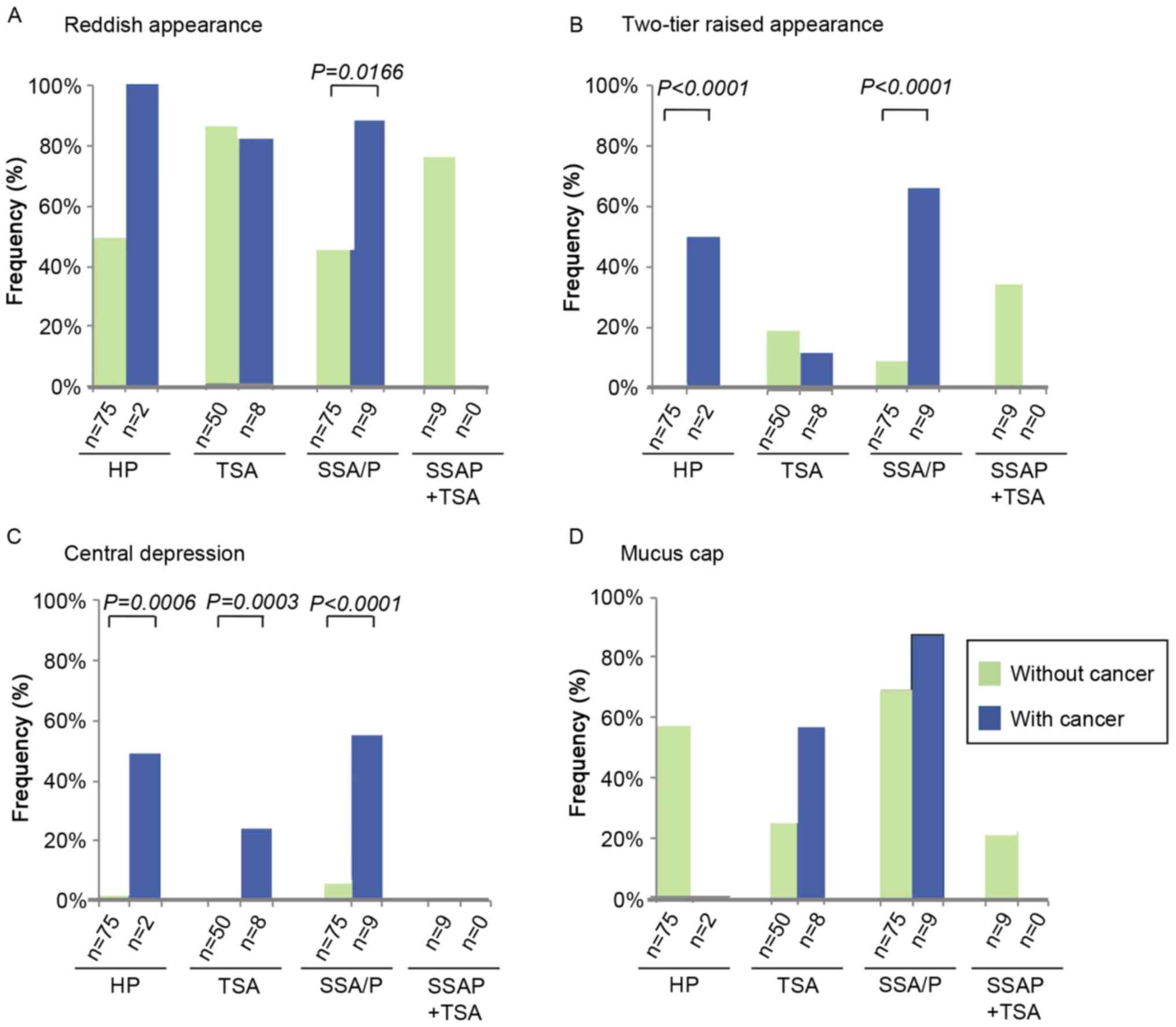
Conventional endoscopic features
A comparison of the conventional endoscopic features
between serrated lesions with and without cancer is shown in
Fig. 1. A reddish appearance of the
lesion was more frequently observed in SSA/Ps with cancer, compared
with lesions without cancer (88.9 vs. 46.7%; P=0.0166). A
two-tier raised appearance was identified preferentially in HPs and
SSA/Ps with cancer, compared with lesions without cancer (HP, 50.0
vs. 1.3%; P<0.0001; SSA/P, 66.7 vs. 9.3%, P<0.0001).
Furthermore, a central depression was observed more frequently in
HPs, TSAs and SSA/Ps with cancer compared with lesions without (HP,
50.0 vs. 2.7%, P=0.0006; TSA, 25.0 vs. 0.0%, P=0.0003; SSA/Ps, 55.6
vs. 6.7%, P<0.0001). By contrast, an adherent mucus cap was not
present at a higher frequency in HPs, TSAs or SSA/Ps with cancer
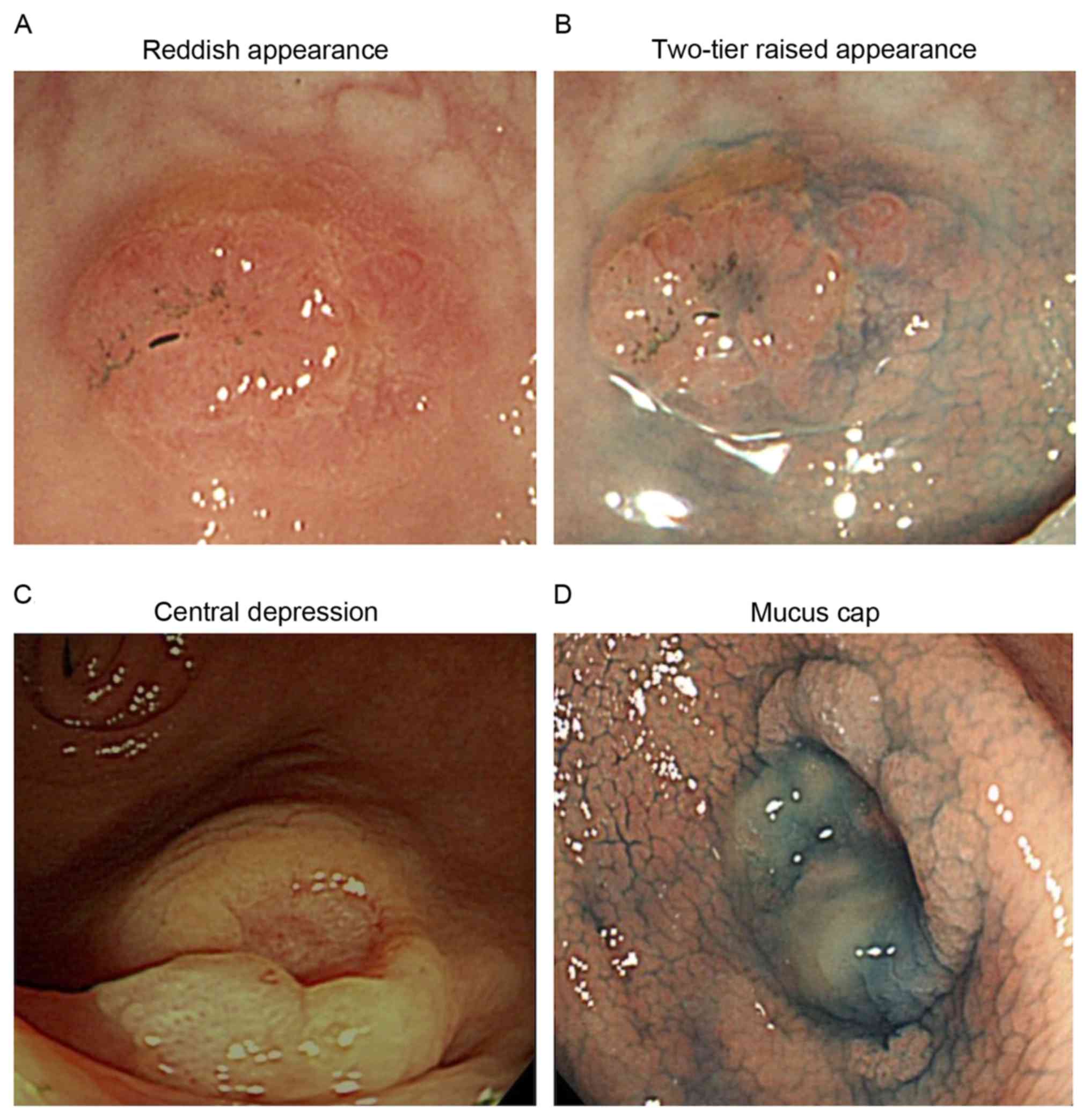
compared with lesions without cancer. Examples of the endoscopic
features of serrated lesions with cancer are presented in Fig. 2.
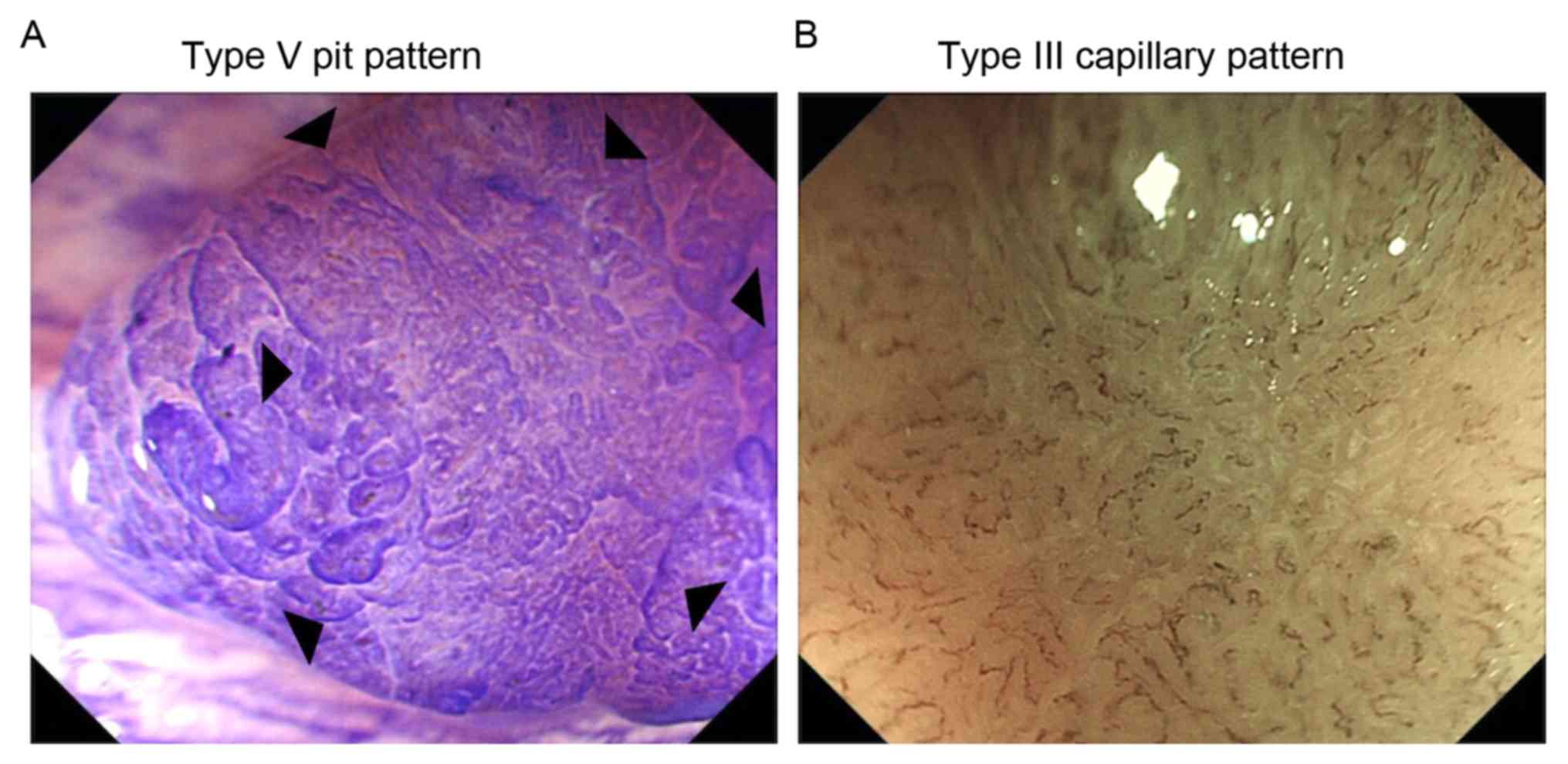
Advanced endoscopic features
The advanced endoscopic features of serrated lesions
with and without cancer were subsequently compared (Table II; Fig.
3). In the CE images, information was available for 64 lesions.
The type V pit pattern, represented by irregular crypts or
non-structural crypts, was identified at an increased frequency in
HPs, TSAs and SSA/Ps with cancer compared with lesions without
cancer (HP, 100 vs. 0%, P=0.00011; TSA, 100 vs. 0%, P=0.00031;
SSA/P, 100 vs. 0%, P<0.0001).
 | Table II.Advanced endoscopic features of
serrated lesions with and without cancer. |
Table II.
Advanced endoscopic features of
serrated lesions with and without cancer.
|
| HP | TSA | SSA/P | SSA/P plus TSA |
|---|
|
|
|
|
|
|
|---|
| Features | With cancer | Without cancer | With cancer | Without cancer | With cancer | Without cancer | With cancer | Without cancer |
|---|
| Type V pit
pattern |
|
|
|
|
|
|
|
|
|
Present | 1 | 0 | 1 | 0 | 4 | 0 | 0 | 0 |
|
Absent | 0 | 14 | 0 | 12 | 0 | 32 | 0 | 3 |
| Type III capillary
pattern |
|
|
|
|
|
|
|
|
|
Absent | 0 | 0 | 1 | 0 | 5 | 0 | 0 | 0 |
|
Present | 1 | 21 | 0 | 13 | 0 | 35 | 0 | 1 |
As for the features on NBI, information was
available for 76 lesions. The type III capillary pattern,
represented by unevenly sized vessels of increased thickness with
branching and curtailed irregularity, or nearly avascular lesions
or lesions containing loose vessels with fragmentation, was
observed more frequently in TSAs and SSA/Ps with cancer compared
with lesions without cancer (TSA, 100 vs. 0%, P=0.00018; SSA/P, 100
vs. 0%, P<0.0001).
Histological features
The histological features of serrated lesions with
cancer are presented in Table III.
As none of the 9 SSA/P plus TSA lesions exhibited any cancer
component, SSA/P plus TSA lesions were excluded from the table. In
regard to the cancer component, deep invasion (invasion of the
muscularis propria or deeper) was identified in 50.0% (1/2) HPs
with cancer, 12.5% (1/8) TSAs with cancer and 55.6% (5/9) SSA/Ps
with cancer. The histological differentiation grade of the cancer
was not significantly different among the HPs, TSAs and SSA/Ps.
 | Table III.Histological appearance in the cancer
areas and non-cancer areas of serrated lesions. |
Table III.
Histological appearance in the cancer
areas and non-cancer areas of serrated lesions.
|
| HP (n=2) | TSA (n=8) | SSA/P (n=9) |
|---|
|
|
|
|
|
|---|
| Features | Cancer area | Non-cancer
area | Cancer area | Non-cancer
area | Cancer area | Non-cancer
area |
|---|
| Depth of
invasion |
|
|
|
|
|
|
|
Intramucosal layer | 1 | – | 7 | – | 4 | – |
|
Submuscosal layer | 0 | – | 1 | – | 4 | – |
|
Muscularis propria or
deeper | 1 | – | 0 | – | 1 | – |
| Histological
differentiation grade |
|
|
|
|
|
|
|
Well-differentiated | 1 | – | 4 | – | 2 | – |
|
Moderately differentiated | 1 | – | 4 | – | 7 | – |
| Ki-67-positive
proliferation zone |
|
|
|
|
|
|
|
Upper | 0 | 0 | 0 | 5 | 0 | 1 |
|
Lower | 0 | 2 | 0 | 1 | 0 | 8 |
| Upper
to lower | 2 | 0 | 8 | 2 | 9 | 0 |
| Mucin
phenotype |
|
|
|
|
|
|
|
Large-intestinal | 0 | 0 | 3 | 1 | 0 | 0 |
|
Stomach | 1 | 0 | 0 | 0 | 0 | 0 |
|
Mixed | 1 | 2 | 5 | 7 | 9 | 9 |
The Ki-67-positive proliferative zone was
distributed diffusely (from the upper to the lower parts of the
crypts) within the cancer areas in all HPs (2/2; 100%), TSAs (8/8;
100%) and SSA/Ps (9/9; 100%). By contrast, the proliferative zone
within the non-cancer areas was partially distributed, as follows:
HP (total, 2 cases), 2 cases in the lower part (2/2; 100%); TSA
(total, 8 cases), 5 cases in the upper part (5/8; 62.5%), 1 case in
the lower part (1/8; 12.5%) and 2 cases in the upper to the lower
part (2/8; 25.0%); SSA/Ps (total, 9 cases), 1 case in the upper
part (1/9; 11.1%) and 8 cases in the lower part (8/9; 88.9%).
The lesion types in the cancer and non-cancer areas
were classified on the basis of the mucin phenotype. There were 2
cases of HP, with cancer area classified as gastric phenotype in 1
case (1/2; 50%) and mixed type (gastric plus intestinal type) in 1
case (1/2; 50%). Non-cancer area in HP was classified as mixed
phenotype in 2 cases (2/2; 100%). There were 8 cases of TSA with
cancer area classified as mixed phenotype in 5 cases (5/8; 62.5%)
and colonic phenotype in 3 cases (3/8; 37.5%). Non-cancer area in
TSA was classified as mixed phenotype in 7 cases (7/8; 87.5%) and
large intestinal phenotype in 1 case (1/8; 12.5%). Additionally,
there were 9 cases of SSA/P with mixed phenotype for cancer and
non-cancer areas in all cases (9/9; 100%).
Discussion
There is accumulating evidence to show that
colorectal serrated lesions are important precursors of colorectal
cancer (28,29). Previous studies by the present authors
and others have demonstrated the potential of endoscopic imaging to
discriminate between serrated lesions with and without cancer
(12–14). However, limited data are available in
regard to the characteristic endoscopic features of serrated
lesions with and without cancer (16,17).
Therefore, the potential of conventional and advanced endoscopic
imaging techniques was analyzed in the present study to delineate
the characteristic features of colorectal serrated lesions with
cancer.
In the present study, the cancer component was
observed most frequently in TSAs (13.8%), followed by SSA/Ps
(10.7%) and HPs (2.6%). Previous studies have reported varying
estimates of the frequency of cancer cases originating from each
subtype of serrated lesions (15).
This variability in frequency of cancer cases may reflect
inconsistent diagnostic criteria used, inconsistent histological
classification of the different subtypes of lesions, variations in
the lesion detection rate among endoscopists, variable application
of enhanced endoscopic modalities and different population
selection criteria. However, the finding that the risk of malignant
transformation was increased for TSAs and SSA/Ps compared with HPs
in the present study is consistent with previous studies (15,30). The
malignant potential of each serrated lesion subtype remains unknown
due to the lack of large-scale prospective studies.
The HPs, TSAs and SSA/Ps with cancer were increased
in size compared with the same lesions without cancer, and it is
hypothesized that there is an increased risk of malignant
transformation in larger lesions of all subtypes. At present, it is
not clear whether the threshold value of 10 mm to define advanced
conventional adenomas is applicable to SSA/Ps (13–15).
A number of endoscopic characteristics have been
identified as possible indicators for neoplastic lesions (12,13). In
the present study, a reddish appearance of SSA/Ps, a two-tier
raised appearance of HPs and SSA/Ps, and a central depression in
HPs, TSAs and SSA/Ps were observed more frequently in the presence
of a cancer component in the lesions. The results of the present
study were consistent with previously published studies (13,17), and
suggest that these characteristics are useful to discriminate
between serrated lesions with and without cancer. However, the
present study was unable to discriminate between serrated lesions
with and without cancer on the basis of the presence of a ‘mucus
cap’, in contradiction to previous studies (13,17). A
possible explanation for this discrepancy may be due to the methods
used for estimating the endoscopic appearance as electronic video
images were used in the present study whereas still images were
used in the aforementioned studies (13,17).
Electronic video imaging enables dynamic observation of colorectal
lesions, and hence, may provide additional details. A number of
features may have been underestimated by still image assessment in
previous studies.
In CE, it is now well-established that the pit
pattern is useful for diagnosing colorectal lesions (18–20). The
type V pit pattern (irregular crypts or non-structural crypts) is a
possible characteristic feature of colorectal cancer. Notably, in
the present study, the type V pit pattern was only identified in
HPs, TSAs and SSA/Ps with cancer, and not lesions without cancer,
indicating that the type V pit pattern may be a specific indicator
of cancer in all subtypes of serrated lesions.
NBI is an endoscopic technique that enables clear
visualization of the microvasculature of colorectal lesions
(21,22). The type III capillary pattern
(unevenly sized vessels of increased thickness with branching and
curtailed irregularity or nearly avascular lesions or lesions
containing loose vessels with fragmentation) represents a
characteristic feature of colorectal cancer on NBI. In the present
study, the type III capillary pattern was selectively identified in
TSAs and SSA/Ps with cancer. This finding strongly suggests that
the type III capillary pattern and the type V pit pattern are
useful for discriminating lesions with a cancer component.
The results of the present study indicate the
requirement for advanced endoscopic imaging techniques to
discriminate between serrated lesions with and without cancer.
However, the present study analyzed a limited number of cases by
advanced imaging techniques: 64/228 and 76/228 lesions by CE and
NBI, respectively. Additional studies including a larger number of
lesions are required to evaluate the significance of the two novel
imaging techniques.
Histological characteristics of the serrated lesions
with cancer were also examined in the present study. The depth of
cancer invasion is an important consideration in selecting the
appropriate therapeutic strategy. In the present study, deeply
invading cancer cases were observed more frequently in SSA/Ps
compared with TSAs, which is in accordance with previous reports
(30,31). These results prompted the hypothesis
that the transformation from SSA/P to invasive cancer may be rapid
and occur even when the lesions are still small.
A conceptual way to define the subgroup of serrated
lesions is on the basis of the location of the proliferation zones.
In non-cancer areas, the Ki-67-positive proliferative zone was
limited to a part of the crypts. Symmetric Ki-67 expression was
typically observed in the lower crypts in HPs. TSAs exhibited
symmetric expression in the upper crypts, and SSA/Ps exhibited
asymmetric expression predominantly in the lower crypts. The
results of the present study were consistent with previous reports
(32). In the cancer areas, the
positive proliferative zone was primarily distributed throughout
the crypts in the HPs, TSAs and SSA/Ps, which is a characteristic
feature of cancer lesions.
The mucin phenotype has been identified to be
associated with the development and progression of gastrointestinal
cancer (33). A previous study
suggested that colorectal serrated lesions exhibit variable degrees
of gastric and intestinal differentiation (26). In the present study, it was observed
that cancer and non-cancer areas in serrated lesions, particularly
SSA/Ps, were categorized as mixed type (gastric plus intestinal
types). This finding is consistent with a previous study by Gibson
et al (34), who demonstrated
that the levels of MUC1, MUC2, and MUC5AC expression were not
different between SSA/Ps with and without cancer. Therefore,
malignant transformation from SSA/Ps, but not from HPs or TSAs, may
occur without changes in the mucin phenotype.
Although the present study yielded a number of
important findings, its limitations were that it was a
retrospective single-center study. Therefore, prospective studies
in larger populations are required to validate the results of the
present study. In addition to using endoscopic imaging techniques,
it would also be useful if molecular or clinical markers were
identified that can predict which serrated lesions are at an
increased risk of progression to cancer.
In conclusion, the present retrospective analysis of
the endoscopic features of serrated lesions with cancer was useful
for delineating their characteristic endoscopic features and for
aiding in treatment selection.
Acknowledgements
The abstract was presented at the meeting of the
American Society for Gastrointestinal Endoscopy (May 21, 2016; San
Diego, CA, USA) and was published as abstract no. Su1619 in
Gastrointestinal Endoscopy 83 (Suppl): 2016.
Funding
The present study was supported in part by a
Grant-in-Aid from the Japanese Ministry of Education, Culture and
Science (grant no. 25460964).
Availability of data and materials
The datasets generated and analyzed in the present
study are included in this published article.
Authors' contributions
SN, KM, OT, and TT contributed to the concept and
design of the study. SN, HK, TN, YM, MM, HT, SY, KK, and OT
contributed to management of the patient. SN, KM, JA, and OT
contributed to analysis and interpretation of the data. SN and KM
contributed to writing the manuscript. All authors contributed to
the manuscript and read and approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Kurume University Hospital
approved the study protocol. Written informed consent was gained
from all participants.
Consent for publication
The study participants provided consent for the data
to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Morson BC: Precancerous and early
malignant lesions of the large intestine. Br J Surg. 55:725–731.
1968. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Muto T, Bussey HJ and Morson BC: The
evolution of cancer of the colon and rectum. Cancer. 36:2251–2270.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torlakovic E, Skovlund E, Snover DC,
Torlakovic G and Nesland JM: Morphologic reappraisal of serrated
colorectal polyps. Am J Surg Pathol. 27:65–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jass JR: Classification of colorectal
cancer based on correlation of clinical, morphological and
molecular features. Histopathology. 50:113–30. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cunningham KS and Riddell RH: Serrated
mucosal lesions of the colorectum. Curr Opin Gastroenterol.
22:48–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Snover D, Ahnen D, Burt R, et al: Serrated
polyps of the colon and rectum and serrated polyposisWHO
Classification of Tumours of the Digestive System. 4th edition.
Bosman F, Carneiro F, Hruban R and Theise N: IARC Press; Lyon: pp.
160–165. 2000
|
|
7
|
Kambara T, Simms LA, Whitehall VL, Spring
KJ, Wynter CV, Walsh MD, Barker M, Arnold S, McGivern A, Matsubara
N, et al: BRAF mutation is associated with DNA methylation in
serrated polyps and cancers of the colorectum. Gut. 53:1137–1144.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jass JR: Serrated adenoma of the
colorectum and the DNA-methylator phenotype. Nat Clin Pract Oncol.
2:398–405. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Brien M: Hyperpalastic and serrated
polyps of the colorectum. Gastroenterol Clin North Am. 36:947–968.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Axelrad AM, Fleischer DE, Geller AJ,
Nguyen CC, Lewis JH, Al-Kawas FH, Avigan MI, Montgomery EA and
Benjamin SB: High-resolution chromoendoscopy for the diagnosis of
diminutive colon polyps: Implications for colon cancer screening.
Gastroenterology. 110:1253–1258. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fu KI, Sano Y, Kato S, Fujii T, Nagashima
F, Yoshino T, Okuno T, Yoshida S and Fujimori T: Chromoendoscopy
using indigo carmine dye spraying with magnifying observation is
the most reliable method for differential diagnosis between
non-neoplastic and neoplastic colorectal lesions: A prospective
study. Endoscopy. 36:1089–1093. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hasegawa S, Mitsuyama K, Kawano H, Arita
K, Maeyama Y, Akagi Y, Watanabe Y, Okabe Y, Tsuruta O and Sata M:
Endoscopic discrimination of sessile serrated adenomas from other
serrated lesions. Oncol Lett. 2:785–789. 2011.PubMed/NCBI
|
|
13
|
Tadepalli US, Feihel D, Miller KM,
Itzkowitz SH, Freedman JS, Kornacki S, Cohen LB, Bamji ND, Bodian
CA and Aisenberg J: A morphologic analysis of sessile serrated
polyps observed during routine colonoscopy (with video).
Gastrointest Endosc. 74:1360–1368. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hazewinkel Y, López-Cerón M, East JE,
Rastogi A, Pellisé M, Nakajima T, van Eeden S, Tytgat KM, Fockens P
and Dekker E: Endoscopic features of sessile serrated adenomas:
Validation by international experts using high-resolution
white-light endoscopy and narrow-band imaging. Gastrointest Endosc.
77:916–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rosty C, Hewett DG, Brown IS, Leggett BA
and Whitehall VL: Serrated polyps of the large intestine: Current
understanding of diagnosis, pathogenesis, and clinical management.
J Gastroenterol. 48:287–302. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buda A, De Bona M, Dotti I, Piselli P,
Zabeo E, Barbazza R, Bellumat A, Valiante F, Nardon E, Probert CS,
et al: Prevalence of different subtypes of serrated polyps and risk
of synchronous advanced colorectal neoplasia in average-risk
population undergoing first-time colonoscopy. Clin Transl
Gastroenterol. 3:e62012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bouwens MW, van Herwaarden YJ, Winkens B,
Rondagh EJ, de Ridder R, Riedl RG, Driessen A, Dekker E, Masclee AA
and Sanduleanu S: Endoscopic characterization of sessile serrated
adenomas/polyps with and without dysplasia. Endoscopy. 46:225–235.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kudo S, Hirota S, Nakajima T, Hosobe S,
Kusaka H, Kobayashi T, Himori M and Yagyuu A: Colorectal tumours
and pit pattern. J Clin Pathol. 47:880–885. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kudo S, Tamura S, Nakajima T, Yamano H,
Kusaka H and Watanabe H: Diagnosis of colorectal tumorous lesions
by magnifying endoscopy. Gastrointest Endosc. 44:8–14. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tobaru T, Mitsuyama K, Tsuruta O, Kawano H
and Sata M: Sub-classification of type VI pit patterns in
colorectal tumors: Relation to the depth of tumor invasion. Int J
Oncol. 33:503–508. 2008.PubMed/NCBI
|
|
21
|
Sano Y, Ikematsu H, Fu KI, Emura F,
Katagiri A, Horimatsu T, Kaneko K, Soetikno R and Yoshida S: Meshed
capillary vessels by use of narrow-band imaging for differential
diagnosis of small colorectal polyps. Gastrointest Endosc.
69:278–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Uraoka T, Saito Y, Ikematsu H, Yamamoto K
and Sano Y: Sano's capillary pattern classification for narrow-band
imaging of early colorectal lesions. Dig Endosc. 1:112–125. 2011.
View Article : Google Scholar
|
|
23
|
Soetikno RM, Kaltenbach T, Rouse RV, Park
W, Maheshwari A, Sato T, Matsui S and Friedland S: Prevalence of
nonpolypoid (flat and depressed) colorectal neoplasms in
asymptomatic and symptomatic adults. JAMA. 299:1027–1035. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schlemper RJ, Riddell RH, Kato Y, Borchard
F, Cooper HS, Dawsey SM, Dixon MF, Fenoglio-Preiser CM, Fléjou JF,
Geboes K, et al: The Vienna classification of gastrointestinal
epithelial neoplasia. Gut. 47:251–255. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shiroshita H, Watanabe H, Ajioka Y,
Watanabe G, Nishikura K and Kitano S: Re-evaluation of mucin
phenotypes of gastric minute well-differentiated-type
adenocarcinomas using a series of HGM, MUC5AC, MUC6, M-GGMC, MUC2
and CD10 stains. Pathol Int. 54:311–321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fujita K, Hirahashi M, Yamamoto H,
Matsumoto T, Gushima M, Oda Y, Kishimoto J, Yao T, Iida M and
Tsuneyoshi M: Mucin core protein expression in serrated polyps of
the large intestine. Virchows Arch. 457:443–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shiroshita H, Watanabe H, Ajioka Y,
Watanabe G, Nishikura K and Kitano S: Re-evaluation of mucin
phenotypes of gastric minute well-differentiated-type
adenocarcinomas using a series of HGM, MUC5AC, MUC6, M-GGMC, MUC2
and CD10 stains. Pathol Int. 54:311–321. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jass JR, Whitehall VL, Young J and Leggett
BA: Emerging concepts in colorectal neoplasia. Gastroenterology.
123:862–876. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Leggett B and Whitehall V: Role of the
serrated pathway in colorectal cancer pathogenesis.
Gastroenterology. 138:2088–2100. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oono Y, Fu K, Nakamura H, Iriguchi Y,
Yamamura A, Tomino Y, Oda J, Mizutani M, Takayanagi S, Kishi D, et
al: Progression of a sessile serrated adenoma to an early invasive
cancer within 8 months. Dig Dis Sci. 54:906–909. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Horii J, Kato J, Nagasaka T, Hiraoka S,
Sun DS, Watanabe K, Fujita I, Toyokawa T, Tomoda J and Yamamoto K:
Development of invasive colon cancer with microsatellite
instability in a patient with hyperplastic polyposis syndrome. Jpn
J Clin Oncol. 42:451–454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Torlakovic EE, Gomez JD, Driman DK,
Parfitt JR, Wang C, Benerjee T and Snover DC: Sessile serrated
adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg
Pathol. 32:21–29. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oue N, Sentani K, Sakamoto N and Yasui W:
Clinicopathologic and molecular characteristics of gastric cancer
showing gastric and intestinal mucin phenotype. Cancer Sci.
106:951–918. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gibson JA, Hahn HP, Shahsafaei A and Odze
RD: MUC expression in hyperplastic and serrated colonic polyps:
Lack of specificity of MUC6. Am J Surg Pathol. 35:742–749. 2011.
View Article : Google Scholar : PubMed/NCBI
|