Introduction
Lymphoma is a type of malignant tumor derived from
the lymph and hematopoietic systems, including the subtypes of
Hodgkin lymphoma (HL) and non-Hodgkin lymphoma (NHL) (1,2). Although
HL and NHL are all neoplastic diseases of lymphoid tissues, the
clinical manifestations and histopathology is in contrast.
Reed-Sternberg cells are a characteristic feature of HL and their
presence is used for diagnosis. However, in NHL, Reed-Sternberg
cells are not utilised for diagnosis. Painless and progressive
swelling of lymph nodes is the typical initial symptom of HL;
however, extranodal invasion is the common symptom of NHL (3). Due to the high heterogeneity and
malignancy of lymphoma, the prognosis remains generally
unsatisfactory despite advances in conventional therapies,
including radiotherapy, chemotherapy and hematopoietic stem cell
transplantation (3–5). The necessity for the development and
clinical application of molecularly-targeted drugs has become
widely accepted, and the discovery of novel molecular therapeutic
targets is essential to improve patient prognosis.
Tumor necrosis factor receptor 2 (TNFR2) is a member
of the TNFR family. Previous studies have reported that TNFR2 is
mainly expressed in hematopoietic and endothelial cells, with
anti-inflammatory (6), protection
against lipopolysaccharide-induced lung damage (7) and bone fracture-healing (8) properties. TNFR2 has also been
demonstrated to promote the migration and invasion abilities of
malignant tumor cells, including cholangiocarcinoma and myeloma
cells (9,10). However, the role of TNFR2 in lymphoma
cells remains uncharacterized.
Anaplastic large-cell lymphoma (ALCL) is a type of
NHL exhibiting CD30+ expression. HL is similar to ALCL
in cellular morphology, and possesses pathogenic mechanisms
analogous to those of ALCL (11). An
HL cell line, L428, and an ALCL cell line, Karpas299, were used in
the present study to investigate the role of TNFR2 in the
proliferation and drug resistance of lymphoma cells. A potential
molecular mechanism underlying the proliferative and drug
resistance functions of TNFR2 was also investigated.
Materials and methods
Cell lines and cell culture
L428 and Karpas299 cells were obtained from the
American Type Culture Collection (Manassas, VA, USA). Both cell
lines were cultured in RPMI-1640 medium containing 10% fetal bovine
serum (both Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA at 3°C in 5% CO2.
Cell transfection
A PcDNA3.1/TNFR2-vector plasmid (Shanghai GenePharma
Co., Ltd., Shanghai, China) was transfected into L428 cells using a
Cell Line Nucleofector kit T (Amaxa; Lonza Group, Ltd., Basel,
Switzerland) in order to upregulate TNFR2 expression; a
GPU6/FAP-shRNA plasmid (Shanghai GenePharma Co., Ltd.) was
transfected into Karpas299 cells to silence TNFR2 expression. Empty
plasmids were used as controls, and transfection was performed
according to the manufacturer's instructions. At 48 h, the
transfected cells were harvested for use in further procedures.
Reverse transcription-polymerase chain
reaction (RT-PCR)
Total RNA was extracted from cells using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.). cDNA was synthesized
using a PrimeScript RT-PCR kit (Takara Biotechnology Co., Ltd.,
Dalian, China). All procedures were performed according to the
manufacturer's protocols. PCR was performed using the kit and the
results were analyzed by labwork software, version 4.0 (UVP,
Upland, CA, USA). The primers were as follows: TNFR2: Forward,
5′-ACGTTCTCCAACACGACTTCATC-3′; Reverse,
5′-ATGATGACACAGTTCACCACTCC-3′. GAPDH: Forward,
5′-AGAAGGCTGGGGCTCATTTG-3′ and Reverse, 5′-AGGGGCCATCCACAGTCTTC-3′.
The experiment was performed 3 times.
Western blotting
Total protein was extracted and its concentration
was measured using a bicinchoninic assay kit (Pierce; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A total
of 300 µg protein per lane was separated by 10% SDS-PAGE and
transferred to a nitrocellulose membrane. After being Blocked using
TBS with Tween 20 containing 5% non-fat dried milk for 1 h at room
temperature, the membrane was incubated in primary antibody
solutions, including antibodies against TNFR2 (cat. no. 19272-1-AP;
dilution, 1:1,000; Proteintech Group, Inc., Wuhan Sanying
Biotechnology, Wuhan, China), phospho-extracellular signal-related
kinase 1/2 (ERK1/2), ERK1/2, phospho-Akt, Akt, β-catenin (cat. nos.
4370, 4695, 9611, 4691 and 8480, respectively; all dilution,
1:1,000; Cell Signaling Technology, Danvers, MA, USA) and GAPDH
(cat. no. 13937-1-AP; dilution, 1:5,000; Proteintech Group, Inc.)
at 4°C overnight, followed by an peroxidase-linked goat anti-rabbit
-IgG (cat. no. 4413; dilution, 1:4,000; Cell Signaling Technology)
at room temperature for 1 h. Immunoreactive signals were detected
using an enhanced chemiluminescence reagent (Pierce; Thermo Fisher
Scientific, Inc.).
Proliferation assay
Cells were counted and plated at 5×103
cells in 100-µl medium per well into a 96-well plate, in
triplicate. At 24, 48, 72 and 96 h after plating, 10 µl Cell
Counting kit-8 (CCK-8; Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) solution was added. After 2 h, the OD
value was read at a wavelength of 450 nm. The experiment was
repeated 3 times.
Drug resistance assay
Cells were counted and plated into a 96-well plate
in triplicate at 8×103 cells per well. At 24 h, the
medium was replaced with medium containing different concentrations
of Adriamycin (ADM; HarveyBio, Inc., Beijing, China; 0.2, 0.4, 0.8,
1.6, 3.2 and 6.4 µmol/l). Cells treated with 0 µmol/l served as the
control. At 48 h later, 10 µl CCK-8 solution was added. After 2 h,
the OD value was read at a wavelength of 450 nm. Survival curves
were constructed, and the 50% inhibitory concentration
(IC50) was calculated. Cell inhibition rate=OD value at
each concentration of ADM/OD value at 0 µmol/l ADM (%). To study
potential molecular mechanism, AKT inhibitor LY294002 or
WNT/β-catenin inhibitor DKK1 was added into lymphoma cells 1 h
before ADM and cells were cultured in this system for 48 h. The
final concentrations of LY294002 and DKK1 were 50 µM and 50 ng/ml
respectively. The experiment was repeated ≥3 times.
Statistical analysis
All results are expressed as mean ± standard
deviation, and SPSS 13.0 software (SPSS, Inc., Chicago, IL, USA)
was used for all statistical analysis. IC50 was
calculated using regression analysis. The differences between
groups were analyzed using two-tailed t tests. P<0.05 was
considered to indicate a statistically significant difference.
Results
TNFR2 expression is associated with
the proliferation and drug resistance of lymphoma cells
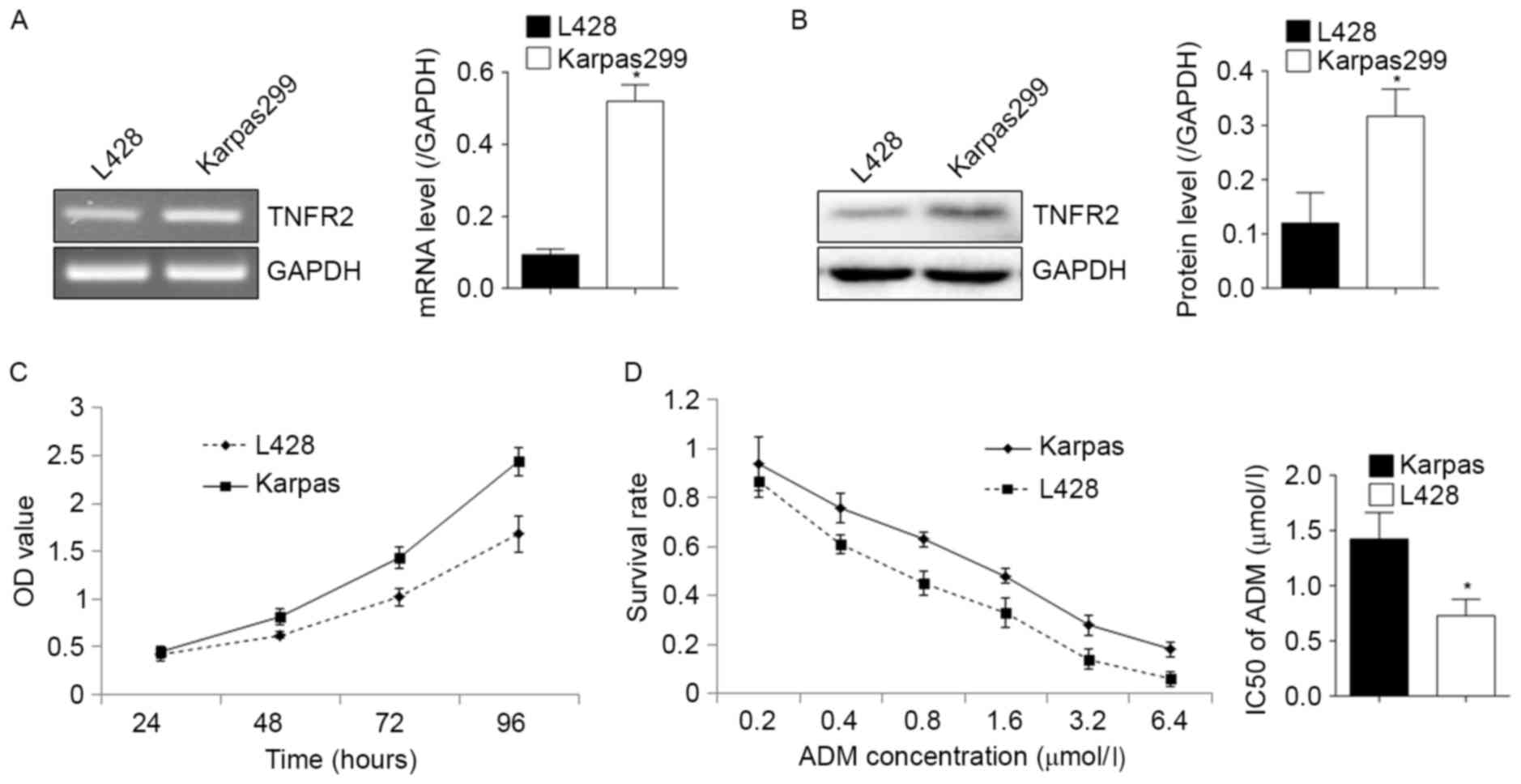
As illustrated in Fig.
1, RT-PCR and western blot analysis confirmed the expression of
TNFR2 in L428 and Karpas299 cells at the mRNA (Fig. 1A) and protein (Fig. 1B) levels. Karpas299 cells expressed
relatively more TNFR2 and demonstrated a greater proliferative
ability (Fig. 1C), and an increased
resistance to ADM, with an IC50 of 1.42±0.24 µmol/l in
Karpas299 cells, compared with 0.728±0.15 µmol/l in L428 cells
(Fig. 1D). This suggests that there
may be a positive association between TNFR2 expression, and the
proliferative and drug resistance abilities of lymphoma cells.
TNFR2 promotes the proliferation and
drug resistance of lymphoma cells
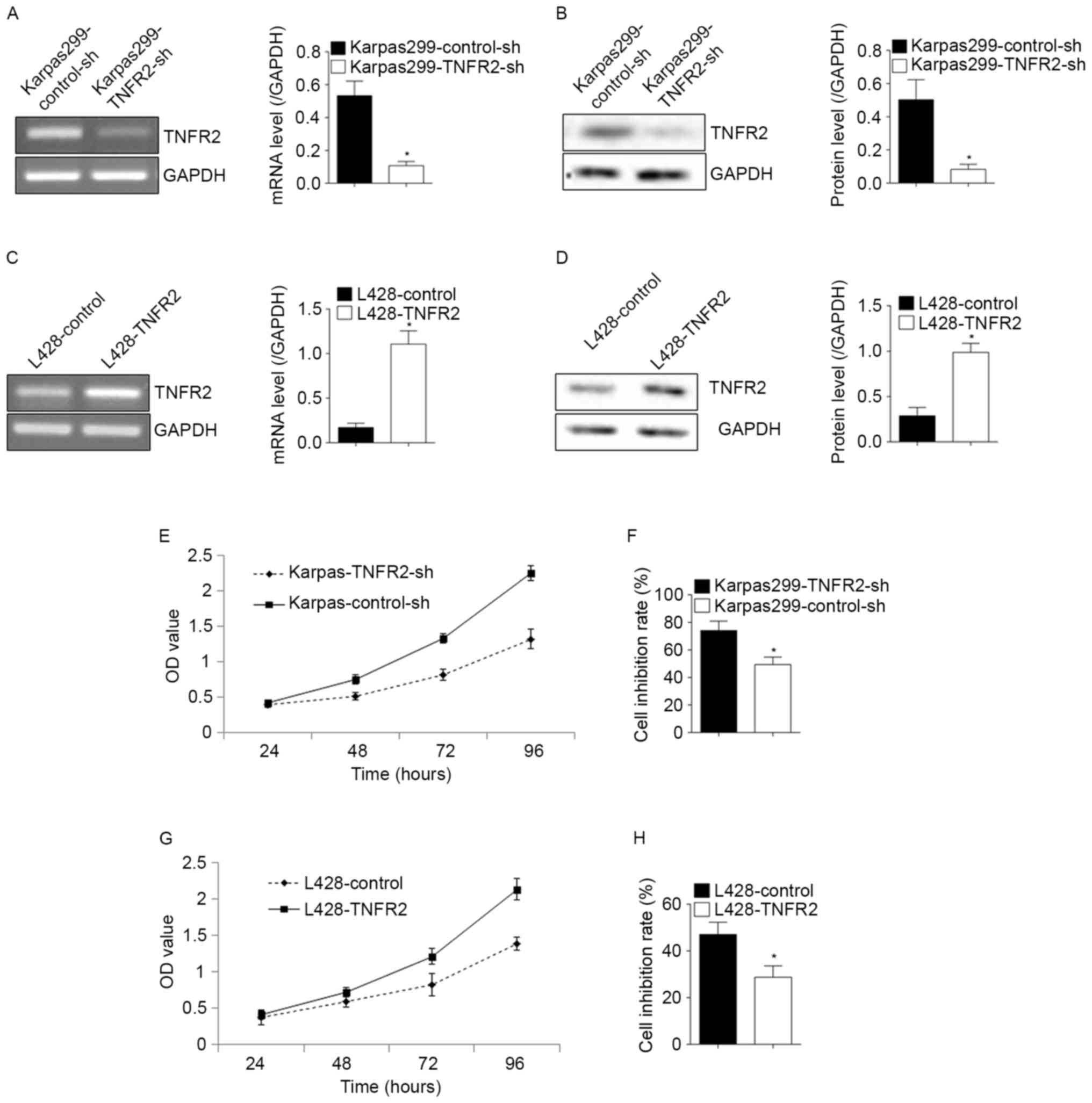
To confirm whether TNFR2 affected the proliferation
or contributed to the drug resistance of lymphoma cells, the
expression of TNFR2 was decreased by transfection with shRNA
against TNFR2, or increased by transfection with a TNFR2 plasmid.
The shRNA-mediated knockdown in Karpas299 was verified at the mRNA
and protein level (Fig. 2A and B;
P<0.05), in addition to the TNFR2 upregulation in L428 (Fig. 2C and D; P<0.05). The knockdown of
TNFR2 in Karpas299 cells induced a significant decline in the
proliferative ability (Fig. 2E) and a
significant increase in the cell inhibition rate following ADM
treatment, from 49.34±5.42% to 74.13±6.81% (P<0.05; Fig. 2F). Following the upregulation of TNFR2
expression in L428 cells, the proliferative ability increased
significantly (Fig. 2G) and the cell
inhibition rate upon ADM treatment declined from 47.03±5.25% to
28.71±4.90% (P<0.05; Fig. 2H).
These results confirmed the roles of TNFR2 in the proliferation and
drug resistance of lymphoma cells.
AKT and WNT signaling pathways
function in the regulation of proliferation and drug resistance of
lymphoma cells by TNFR2
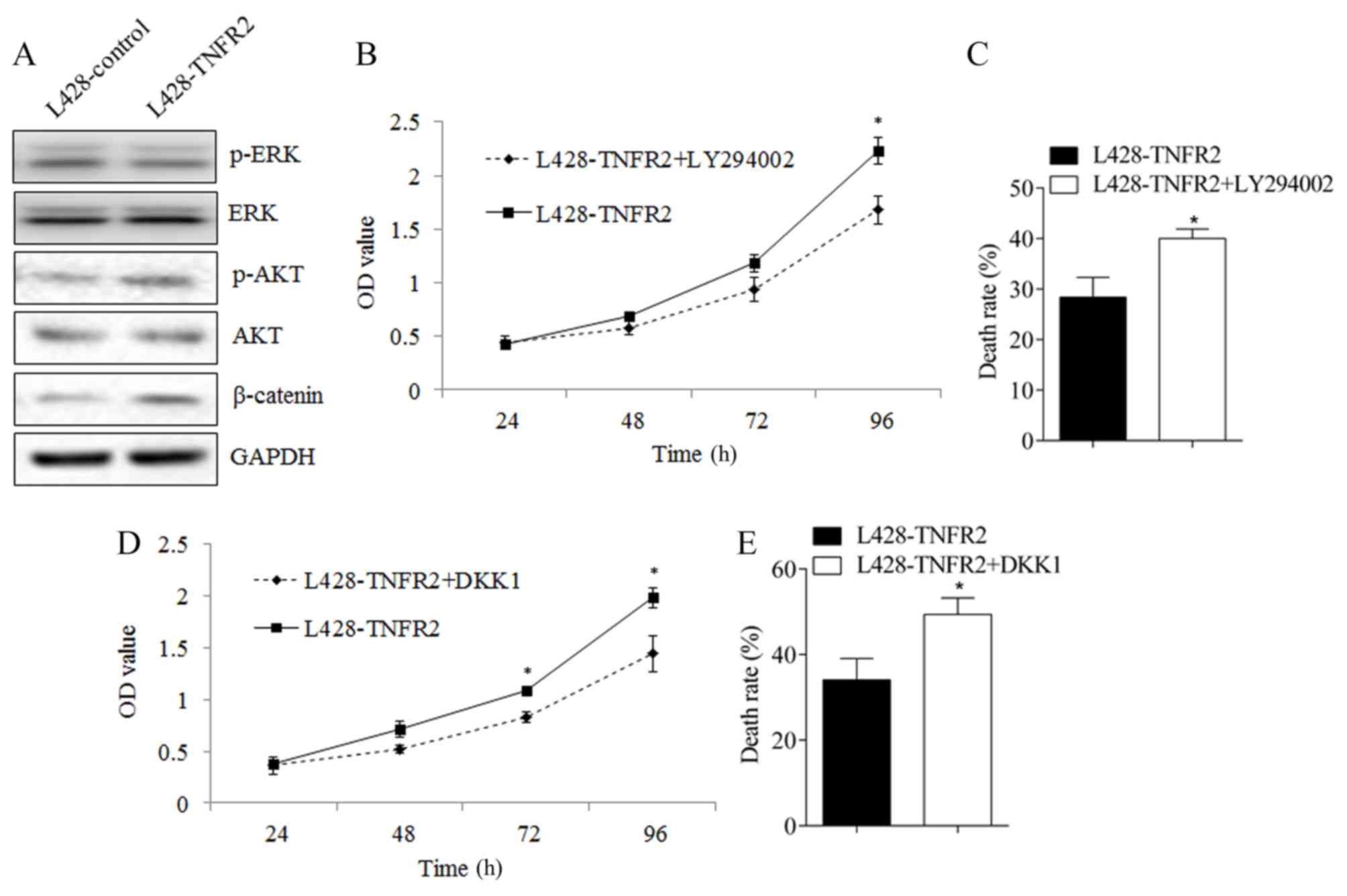
AKT, ERK and WNT/β-catenin are important to the
proliferation and drug resistance of tumor cells (12–14). To
investigate the potential molecular mechanism underlying the effect
of TNFR2 up- and downregulation, the expression of AKT, ERK and
WNT/β-catenin was examined. Following the upregulation of TNFR2
expression in L428 cells, the phosphorylation of AKT and the
expression of β-catenin were significantly increased (Fig. 3A). No difference was observed in the
phosphorylation of ERK. Following the addition of the AKT
inhibitor, LY294002, the proliferation ability of L428 cells
overexpressing TNFR2 declined significantly (P<0.05 at 96 h;
Fig. 3B) and the cell inhibition rate
following treatment with ADM increased from 28.38±3.94% to
40.03±1.88% (P<0.05; Fig. 3C). On
the application of a WNT/β-catenin inhibitor, DKK1, the
proliferative ability of L428 cells overexpressing TNFR2 also
declined significantly (P<0.05 at 72 and 96 h; Fig. 3D) and the cell inhibition rate
following treatment with ADM increased from 34.07±4.99% to
49.36±3.86% (P<0.05; Fig. 3E).
These results indicate that the AKT and WNT/β-catenin signaling
pathways contribute to the proliferative and drug resistance
abilities of lymphoma cells, and that the mechanism is regulated by
TNFR2.
Discussion
TNFR2 is encoded by the tumor necrosis factor
receptor superfamily 1B (TNFRSF1B) gene (15). TNFR2 occurs as membrane binding TNFR2
(mTNFR2) and soluble TNFR2 (sTNFR2). To the best of our knowledge,
the existing publications regarding TNFR2 in lymphoma are confined
to the clinical significance of sTNFR2 in the blood. In 1998,
Warzocha et al (16) reported
that high levels of sTNFR2 were associated with clinical
characteristics and a relatively poor prognosis for patients with
Hodgkin's lymphoma. In 2012, Heemann et al (17) reported that patients with circulating
levels of sTNFR2 ≥2.16 ng/ml exhibited a 2.07-fold higher relative
risk for a reduced overall survival time, and a 2.49-fold higher
risk for reduced event-free survival time. In 2013, Nakamura et
al (18) reported that high
levels of sTNFR2 in the blood were associated with a relatively
poor prognosis for patients with diffuse large B-cell lymphoma
treated with the R-CHOP regimen. All of these studies consolidate a
role for sTNFR2 in lymphoma. However, the role of TNFR2 in lymphoma
cells remains uncharacterized.
ADM is a conventional chemotherapy drug currently
used to treat lymphoma, with anti-tumor functions mediated by the
inhibition of DNA synthesis (19,20). In
the present study, TNFR2 was expressed in L428 and Karpas299 cells;
Karpas299 cells expressed relatively more TNFR2 and exhibited a
greater proliferative ability and ADM resistance, indicating that
TNFR2 may be associated with the proliferation and drug resistance
of lymphoma cells. Further experimentation revealed that TNFR2
overexpression enhanced the proliferation and drug resistance of
L428 cells, whereas the silencing of TNFR2 in Karpas299 cells
inhibited the proliferation and drug resistance of lymphoma cells.
This confirms the role of TNFR2 in promoting lymphoma progression,
and corroborates with the previous reports regarding circulating
sTNFR2 and prognosis for patients with lymphoma.
The AKT- and ERK-associated pathways are critical
for mediating various physiological and pathological conditions. In
tumors, their roles in proliferation, survival, adhesion, drug
resistance and migration are well established (12,13,21–23).
It has been reported that inhibiting TNFR2 using a neutralizing
antibody may block the activation of the AKT signal pathway in
cholangiocarcinoma cells (9). The
Wnt/β-catenin signaling cascade is considered to be central to
carcinogenesis; it can affect a number of traits associated with
cell malignancy, including proliferation, migration, drug
resistance and even the maintenance of stemness (14,24). In
the present study, it was demonstrated that TNFR2 could function in
proliferation and drug resistance via the AKT and WNT/β-catenin
signaling pathways. This is consistent with the roles of AKT,
WNT/β-catenin and TNFR2 in tumor progression that have been
reported in the past. However, the question remains of whether the
drug resistance role of TNFR2 could be partly or wholly attributed
to its pro-proliferative role. Further in vitro
experimentation will be required to answer this question.
In conclusion, TNFR2 promoted proliferation and drug
resistance via AKT and WNT/β-catenin signaling pathways in lymphoma
cells. This may contribute to the understanding of TNFR2 function
in lymphoma, and provides a basis for discovering novel therapeutic
targets against lymphoma.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YL performed cell experiments and revised the
present study. YW revised the present study. MX performed cell
experiments. YH performed cell experiments. YZ performed data
analysis. SL designed the present study and wrote the present
paper.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kewitz S, Kurch L, Volkmer I and Staege
MS: Stimulation of the hypoxia pathway modulates chemotherapy
resistance in Hodgkin's lymphoma cells. Tumour Biol. 37:8229–8237.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fino P, Spagnoli AM, Ruggieri M,
Marcasciano M and Scuderi N: Bilateral hand squamous-cells
carcinoma in patient affected with non-Hodgkin lymphoma. Case
report and literature review. G Chir. 36:172–182. 2015.PubMed/NCBI
|
|
3
|
Hu RH, Sun WL, Zhao H, Hui WH, Guo YX, Wan
SG and Su L: Rituximab combined with EPOCH regimen for treatment of
diffuse large B cell lymphoma of the gastrointestinal tract:
Analysis of 4 cases. Nan Fang Yi Ke Da Xue Xue Bao. 36:1291–1294.
2016.(In Chinese). PubMed/NCBI
|
|
4
|
Khandelwal A, Trinkaus MA, Ghaffar H,
Jothy S and Goldstein MB: A case report of unusually long lag time
between immunotactoid glomerulopathy (itg) diagnosis and diffuse
large B-cell lymphoma (DLBCL) development. BMC Nephrol. 17:1402016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang D, Fu X, Zhang X, Li W and Zhang M:
Therapy-related acute myeloid leukemia in patients with lymphoma: A
report of four cases and review of the literature. Oncol Lett.
10:3261–3265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang W, Lu Y, Tian QY, Zhang Y, Guo FJ,
Liu GY, Syed NM, Lai Y, Lin EA, Kong L, et al: The growth factor
progranulin binds to TNF receptors and is therapeutic against
inflammatory arthritis in mice. Science. 332:478–484. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo Z, Li Q, Han Y, Liang Y, Xu Z and Ren
T: Prevention of LPS-induced acute lung injury in mice by
progranulin. Mediators Inflamm. 2012:5407942012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhao YP, Tian QY, Frenkel S and Liu CJ:
The promotion of bone healing by progranulin, a downstream molecule
of BMP-2, through interacting with TNF/TNFR signaling.
Biomaterials. 34:6412–6421. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tanimura Y, Kokuryo T, Tsunoda N, Yamazaki
Y, Oda K, Nimura Y, Mon Naing N, Huang P, Nakanuma Y, Chen MF, et
al: Tumor necrosis factor alpha promotes invasiveness of
cholangiocarcinoma cells via its receptor, TNFR2. Cancer Lett.
219:205–213. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johrer K, Janke K, Krugmann J, Fiegl M and
Greil R: Transendothelial migration of myeloma cells is increased
by tumor necrosis factor (TNF)-alpha via TNF receptor 2 and
autocrine up-regulation of MCP-1. Clin Cancer Res. 10:1901–1910.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Minich SS: Brentuximab vedotin: A new age
in the treatment of hodgkin lymphoma and anaplastic large cell
lymphoma. Ann Pharmacother. 46:377–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dahlmann M, Okhrimenko A, Marcinkowski P,
Osterland M, Herrmann P, Smith J, Heizmann CW, Schlag PM and Stein
U: RAGE mediates S100A4-induced cell motility via MAPK/ERK and
hypoxia signaling and is a prognostic biomarker for human
colorectal cancer metastasis. Oncotarget. 5:3220–3233. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miao B and Degterev A: Targeting
phospshatidylinositol 3-kinase signaling with novel
phosphatidylinositol 3,4,5-triphosphate antagonists. Autophagy.
7:650–651. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sebio A, Kahn M and Lenz HJ: The potential
of targeting Wnt/β-catenin in colon cancer. Expert Opin Ther
Targets. 18:611–615. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu F, Zhou G, Han S, Yuan W, Chen S, Fu Z,
Li D, Zhang H, Li D and Pang D: Association of TNF-α, TNFRSF1A and
TNFRSF1B gene polymorphisms with the risk of sporadic breast cancer
in northeast Chinese han women. PLoS One. 9:e1011382014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Warzocha K, Bienvenu J, Ribeiro P, Moullet
I, Dumontet C, Neidhardt-Berard EM, Coiffier B and Salles G: Plasma
levels of tumour necrosis factor and its soluble receptors
correlate with clinical features and outcome of Hodgkin's disease
patients. Br J Cancer. 77:2357–2362. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heemann C, Kreuz M, Stoller I, Schoof N,
von Bonin F, Ziepert M, Löffler M, Jung W, Pfreundschuh M, Trümper
L and Kube D: Circulating levels of TNF receptor II are prognostic
for patients with peripheral T-cell non-Hodgkin lymphoma. Clin
Cancer Res. 18:3637–3647. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura N, Goto N, Tsurumi H, Takemura M,
Kanemura N, Kasahara S, Hara T, Yasuda I, Shimizu M, Sawada M, et
al: Serum level of soluble tumor necrosis factor receptor 2 is
associated with the outcome of patients with diffuse large B-cell
lymphoma treated with the R-CHOP regimen. Eur J Haematol.
91:322–331. 2013.PubMed/NCBI
|
|
19
|
Engert A: ABVD or BEACOPP for advanced
Hodgkin lymphoma. J Clin Oncol. 34:1167–1169. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ajish JK, Kumar Ajish KS, Chattopadhyay S
and Kumar M: Glycopolymeric gel stabilized N-succinyl chitosan
beads for controlled doxorubicin delivery. Carbohydr Polym.
144:98–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu SY, Tai CC, Li YH and Wu JL:
Progranulin compensates for blocked IGF-1 signaling to promote
myotube hypertrophy in C2C12 myoblasts via the PI3K/Akt/mTOR
pathway. FEBS Lett. 586:3485–3492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bai T, Lian LH, Wu YL, Wan Y and Nan JX:
Thymoquinone attenuates liver fibrosis via PI3K and TLR4 signaling
pathways in activated hepatic stellate cells. Int Immunopharmacol.
15:275–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li N, Cui J, Duan X, Chen H and Fan F:
Suppression of type I collagen expression by miR-29b via PI3K, Akt,
and Sp1 pathway in human Tenon's fibroblasts. Invest Ophthalmol Vis
Sci. 53:1670–1678. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sawa M, Masuda M and Yamada T: Targeting
the Wnt signaling pathway in colorectal cancer. Expert Opin Ther
Targets. 20:419–429. 2016. View Article : Google Scholar : PubMed/NCBI
|