Introduction
Osteosarcoma is the most commonly occurring sarcoma
in bones. It is derived from the progenitor cells in the osteoblast
lineage with accumulated mutations to evade cell cycle checkpoints,
resulting in excessive proliferation and defects in the ability to
appropriately differentiate into mature bone-forming osteoblasts
(1). The majority of osteosarcoma
cases occur in young individuals, particularly children and
adolescents (2). It is the second
leading cause of cancer-associated mortality in children and young
adults worldwide (3). As an
aggressive sarcoma, patients with osteosarcoma are prone to relapse
and the prognosis of osteosarcoma remains poor despite advances in
therapeutic strategies, including surgery and chemotherapy
(4). Statistics indicate that modern,
multi-agent therapeutic strategies, for example dose-intensive
chemotherapy in conjunction with surgery, can only achieve a 3-year
event-free survival of 60–70% in particularly localized,
non-metastatic disease (5). However,
this aggressive sarcoma has a high metastatic potential,
particularly to the lungs, with ~20% of patients presenting with
lung metastases at initial diagnosis (6). It is reported that ~80% of patients with
osteosarcoma eventually develop metastatic disease following
treatment, which is the major cause of treatment failure and
contributor to mortality rates (7).
Fortunately, it has been noted that tumorigenesis, progression and
the efficacy of osteosarcoma treatment are closely associated with
gene expression (8). According to
evidence, malignant transformation and tumorigenesis can result
from the dysregulation of tumor suppressor genes (9), and previous studies have indicated that
the dysregulation of tumor suppressor genes is a key contributor
for the initiation and development of osteosarcoma (8,10).
Therefore, tumor gene therapy, which is a form of tumor biotherapy,
has promising potential in osteosarcoma treatment.
MicroRNAs (miRNAs) are single-stranded RNAs with a
length of 20–23 nucleotides, which can control gene expression in a
variety of cellular processes (11).
The primary function of miRNAs is to act as negative regulators of
gene expression at the post-transcriptional level (12). miRNAs typically reduce the translation
and stability of mRNA, affect the output of numerous protein-coding
genes and mediate processes in tumorigenesis, including cell cycle
regulation, cell differentiation, invasion, apoptosis and
inflammation (13). It has been found
that >4,000 mature miRNAs in humans regulate ~30% of mammalian
protein-coding genes (14). miRNA-128
(miR-128) is an important member of the miRNA family. It is a
brain-enriched miRNA with tissue-specific and
developmental-specific expression patterns, predominantly in
neurons (14). However, in addition
to tissues, the aberrant expression of miR-128 is detected in the
blood of patients with types of malignant tumor, including
leukemia, glioblastoma and prostate cancer (14,15).
Studies have shown that miR-128 can regulate the proliferation,
differentiation and apoptosis of various tumor cells through
targeting several genes, indicating its importance in tumorigenesis
and development (14,16). It has been reported that the
expression of miR-128 is significantly increased in osteosarcoma
tissues (17,18). However, the specific correlation
between this ectopic overexpression of miR-128 and osteosarcoma,
and the regulatory mechanism of the expression of miR-128 towards
osteosarcoma cells remain to be fully elucidated.
In the present study, the regulatory mechanism of
miR-128 in the progression of osteosarcoma cells through targeting
SAM and SH3 domain-containing 1 (SASH1) was examined. Multifarious
methods were applied to measure the expression of miR-128 and SASH1
in osteosarcoma cells, and to examine the effects of miR-128 on
osteosarcoma cell proliferation, apoptosis, and the expression of
SASH1 and certain associated proteins. The present study on the
potential mechanism of miR-128 regulation through targeting SASH1
may offer a foundation for the clinical therapeutic treatment of
osteosarcoma.
Materials and methods
Specimens
A total of 35 fresh osteosarcoma tissue and adjacent
normal bone tissue specimens were collected from patients at the
Second Hospital of Xiangya Medical College of Central South
University (Changsha, China) from December 2015 to January 2017.
The patients included 20 men and 15 women, 25 of which were aged
<60 years and 10 were aged >60 years. All 35 cases were
pathologically diagnosed as osteosarcoma postoperatively without
any preoperative chemotherapy and radiotherapy. The present study
was approved by the Second Hospital of Xiangya Medical College of
Central South University (Changsha, China). Informed consent was
obtained from each patient.
Cell culture
The MG-63 human osteoblastic cell line (Type Culture
Collection of the Chinese Academy of Sciences, Shanghai, China) was
cultivated in RPMI-1640 medium supplemented with 20% fetal bovine
serum (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and incubated at 37°C in a 5% CO2 humidified
incubator. The cells were divided into three groups: i)
miR-128-inhibited group (transfected with miR-128 inhibitor); ii)
blank group (without miR-128 inhibitor); and iii) negative control
(NC) group (to eliminate non-sequence specific effects). The
logarithmic growth phase cells were trypsinized, counted and seeded
in six-well plates at 1×106 cells/well. The miR-128
inhibitors or NC vectors were then transfected into cells with
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The transfection
efficiency was measured using reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis. The miR-128 mimics,
inhibitor and NC oligonucleotides were purchased from Genepharma
Co., Ltd. (Shanghai, China), and the sequences of the
oligonucleotides were as follows: miR-128 mimics,
5′-UCACAGUGAACCGGUCUCUUU-3′; miR-128 inhibitor,
5′-AAAGAGACCGGUUCACUGUGA-3′; NC, 5′-UGUCCUCCUGGAAUUACACGU-3′.
Analysis of cell proliferation
At 72 h post-transfection, the cells were washed
with PBS (pH 7.4), harvested by trypsinization, reseeded into a
96-well plate at a density of 105 cells/well and
cultured in RPMI-1640 medium. The cells were then incubated for
6–48 h for the MTT cell proliferation assay. Briefly, 10 µl MTT
reagent was added to the cells and incubated for 4 h at 37°C until
purple precipitate was visible. Subsequently, 100 µl detergent
reagent was added and left at room temperature in the dark for 2 h.
The absorbance in each well, including the blanks, was measured at
570 nm in a microtiter plate reader.
Analysis of cell apoptosis
The apoptosis of MG-63 human osteoblastic cells from
the three groups was analyzed with an Annexin V-fluorescein
isothiocyanate (FITC) apoptosis kit (BD Biosciences, San Diego, CA,
USA) using the flow cytometry method according to the
manufacturer's protocol. Briefly, following transfection for 72 h,
the cells were trypsinized and washed three times with pH 7.4 PBS
buffer, followed by centrifugation at 1,000 × g for 5 min at room
temperature and then dispersal in the buffer provided with the
apoptosis kit. Subsequently, 5 µl each of Annexin V-FITC and
propidium iodide (PI) solutions were added and mixed gently at room
temperature. Following incubation on ice in the dark for 30 min,
the cells were quantified by flow cytometry. Cells showing positive
Annexin V-FITC and negative PI results were considered to be
apoptotic. The basal apoptosis and necrosis were also determined in
untreated cells.
Analysis of the expression of miR-128
via RT-qPCR analysis
Total RNA was extracted using an miRNeasy kit
according to the manufacturer's protocol (Invitrogen; Thermo Fisher
Scientific, Inc.). To detect the expression of miR-128 in 35 paired
tumor and adjacent tissues, as well as MG-63 cells, RT-qPCR
analysis was performed using the TaqMan MicroRNA RT kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Briefly, 2 µl RNA isolated from tissue
samples or MG-63 cells was reverse transcribed into cDNA. The PCR
reaction was subsequently performed with 2 µl cDNA, 10 µl TaqMan
Universal Master Mix (2X), 1 µl primers and nuclease-free
H2O, with a total volume of 20 µl. The PCR conditions
were as follows: Initial denaturing at 95°C for 3 min, denaturing
at 94°C for 30 sec, annealing at 56°C for 30 sec, and extension at
72°C for 30 sec. After 30 cycles, the final extension was performed
at 72°C for 10 min. The PCR products were detected using 1.5%
agarose gel electrophoresis. U6 small nuclear RNA was used as an
endogenous control. The sequences of the primers were as follow: U6
forward, CTCGCTTCGGCAGCACA and reverse, AACGCTTCACGAATTTGCGT;
miR-128 forward, GGCTCACAGTGAACCGG and reverse, GTGCAGGGTCCGAGGT.
The relative expression level of miR-128 was normalized to that of
internal control U6 according to the 2−ΔΔCq method
(19).
Protein expression analysis by western
blot analysis
The cells were lysed using M-PER protein extraction
reagent supplemented with protease inhibitor cocktail (Thermo
Fisher Scientific, Inc.). The Bradford method was used to determine
the concentration of proteins in the supernatant.
Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as the
internal control. Protein (10 µg/lane) was separated by 10%
SDS-PAGE and then transferred to a polyvinylidene fluoride (PVDF)
membrane. The PVDF membrane was blocked and incubated with rabbit
anti-SASH1 (1:500; cat. no. A302-265A-1; Bethyl Laboratories, Inc.,
Montgomery, TX, USA), rabbit anti-B-cell lymphoma 2 (Bcl-2;
1:1,000; cat. no. ab59348; Abcam, Cambridge, MA, USA), rabbit
anti-Bcl-2-associated X protein (Bax; 1:1,000; cat. no. ab182733;
Abcam), rabbit anti-caspase-3 (1:1,000; cat. no. ab13847; Abcam)
and rabbit anti-GAPDH (1:2,500; cat. no. ab9485; Abcam) at 4°C
overnight, and subsequently labeled with horseradish
peroxidase-coupled secondary antibodies (1:5,000; cat. no. A0208;
Beyotime Institute of Biotechnology, Shanghai, China) at room
temperature for 2 h. Following washing with Tris-buffered
saline/Tween-20 for 10 min three times, autoradiography was
performed with chemiluminescence reagents. The relative protein
expression levels of SASH1, Bax, Bcl-2 and caspase-3 were
semi-quantified through the gray value ratio of each protein and
internal control using ImageJ software (version 1.49; National
Institutes of Health, Bethesda, MD, USA).
Dual luciferase reporter assay
The 293 cells (Type Culture Collection of the
Chinese Academy of Sciences) were transfected with the wild-type
SASH1 3′-UTR (SASH1-3′UTR) or mutant SASH1 3′-UTR (SASH1-MUT) were
cloned into the p-MIR-reporter plasmid (Thermo Fisher Scientific,
Inc.) together with either miR-128 mimics or NC using Lipofectamine
RNAi Max (Thermo Fisher Scientific, Inc.). The cells were cultured
at 37°C for 48 h and the activities of the luciferases were
measured using a dual-luciferase reporter kit (Beyotime Institute
of Biotechnology).
In vivo xenograft tumor model
The MG-63 human osteoblastic cells were transfected
with miR-128 inhibitor, and implanted into ten C57BL/6 female nude
mice (4-week-old; weight range, 18.6–20.4 g). Mice were housed in
specific pathogen-free conditions at 23±2°C and 50±10% humidity,
under a 12 h light/dark cycle with access to food and water ad
libitum. The formation of tumors was examined weekly. The mice
were sacrificed 4 weeks following implantation, and the tumor
volumes and weights were measured.
Statistical analysis
SPSS 22.0 (IBM SPSS, Armonk, NY, USA) was used to
analyze the data. Data are presented as the mean ± standard error
of the mean. Statistical comparisons among groups were calculated
using an independent t-test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Expression of miR-128 in osteosarcoma
and normal tissues
In order to investigate the biological roles of
miR-128 in the development of osteosarcoma, the expression of
miR-128 in human osteosarcoma tumor tissues and human normal
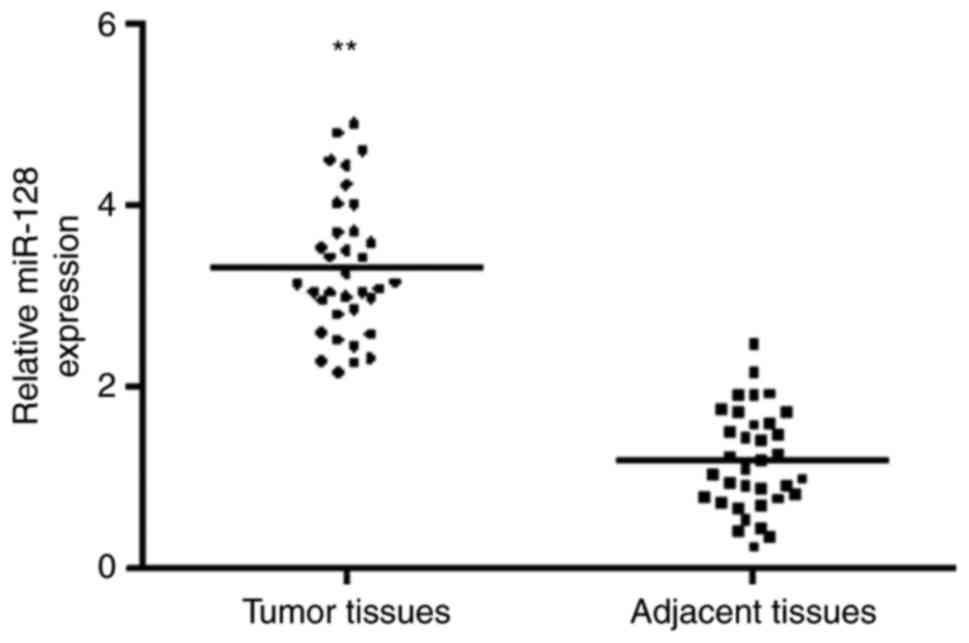
osteoblast tissues was compared. As shown in Fig. 1, the results indicated that the
expression of miR-128 was increased significantly in tumor tissues,
compared with that in normal tissues.
Expression of miR-128 and SASH1 in
clinical pathology
To investigate the association between miR-128 and
its target gene, SASH1, in osteosarcoma, and whether their
expression levels are correlated with the progression of
osteosarcoma, the present study evaluated the expression of miR-128
and SASH1 in 35 samples from patients with osteosarcoma (Table I). These patients included 20 men and
15 women, 25 of which were aged <60 years and 10 were aged
>60 years. However, the results demonstrated that the expression
of miR-128 in the osteosarcoma samples was independent of certain
factors, including sex, age and histological grade (P=0.409,
P=0.362 and P=0.172, respectively). The expression of SASH1 was
also independent of these three factors (P=0.391, P=0.089 and
P=0.192). By contrast, the expression of miR-128 and SASH1 showed
significant correlation with tumor size (P=0.035 and P=0.044,
respectively), invasion depth (P=0.034 and P=0.018, respectively),
lymph node metastasis (P=0.026 and P=0.023, respectively) and TNM
stage (P=0.015 and P=0.029, respectively). The expression value of
miR-128 in the T3-T4 invasion depth group was 4.356±1.92, which was
markedly higher than that in the T1-T2 invasion depth group, which
had a value of 2.832±0.98. However, the expression value of SASH1
in the T3-T4 invasion depth group was 1.432±0.34, which was
markedly lower than that in the T1-T2 invasion depth group, which
had a value of 2.105±0.42. Similar trends were found in the
different tumor size groups and different lymph node metastasis
groups.
 | Table I.Expression of miR-128 and SASH1 in
patient osteosarcoma tissues. |
Table I.
Expression of miR-128 and SASH1 in
patient osteosarcoma tissues.
| Factor | Cases (n) | miR-128 (mean) | P-value | SASH1 (mean) | P-value |
|---|
| Sex |
|
| 0.409 |
| 0.391 |
| Male | 20 | 2.982±1.27 |
| 2.052±0.28 |
|
|
Female | 15 | 3.281±1.67 |
| 1.992±0.36 |
|
| Age (years) |
|
| 0.362 |
| 0.089 |
|
<60 | 25 | 3.064±1.03 |
| 1.468±0.34 |
|
| ≥60 | 10 | 2.763±1.32 |
| 1.336±0.37 |
|
| Tumor size
(cm) |
|
| 0.035 |
| 0.044 |
| ≥5 | 18 | 3.252±1.12 |
| 1.691±0.30 |
|
|
<5 | 17 | 2.878±1.03 |
| 1.877±0.46 |
|
| Histological
grade |
|
| 0.172 |
| 0.192 |
|
Well/intermediately
differentiated | 15 | 3.481±1.16 |
| 1.646±0.33 |
|
| Poor
differentiation | 20 | 3.925±1.55 |
| 1.671±0.40 |
|
| Invasion depth |
|
| 0.034 |
| 0.018 |
|
T1-T2 | 16 | 2.832±0.98 |
| 2.105±0.42 |
|
|
T3-T4 | 19 | 4.356±1.92 |
| 1.432±0.34 |
|
| Lymph node
metastasis |
|
| 0.026 |
| 0.023 |
| N0 | 27 | 2.696±1.55 |
| 1.993±0.38 |
|
|
N1-N3 | 8 | 3.089±1.20 |
| 1.513±0.33 |
|
| Distant
metastasis |
|
| 0.467 |
| 0.356 |
| N0 | 13 | 2.560±1.17 |
| 1.236±0.22 |
|
| M1 | 22 | 3.170±1.64 |
| 1.712±0.39 |
|
| TNM stage |
|
| 0.015 |
| 0.029 |
| I,
II | 21 | 4.695±2.04 |
| 2.052±0.35 |
|
| III,
IV | 14 | 2.647±1.11 |
| 1.443±0.29 |
|
Cell growth inhibition
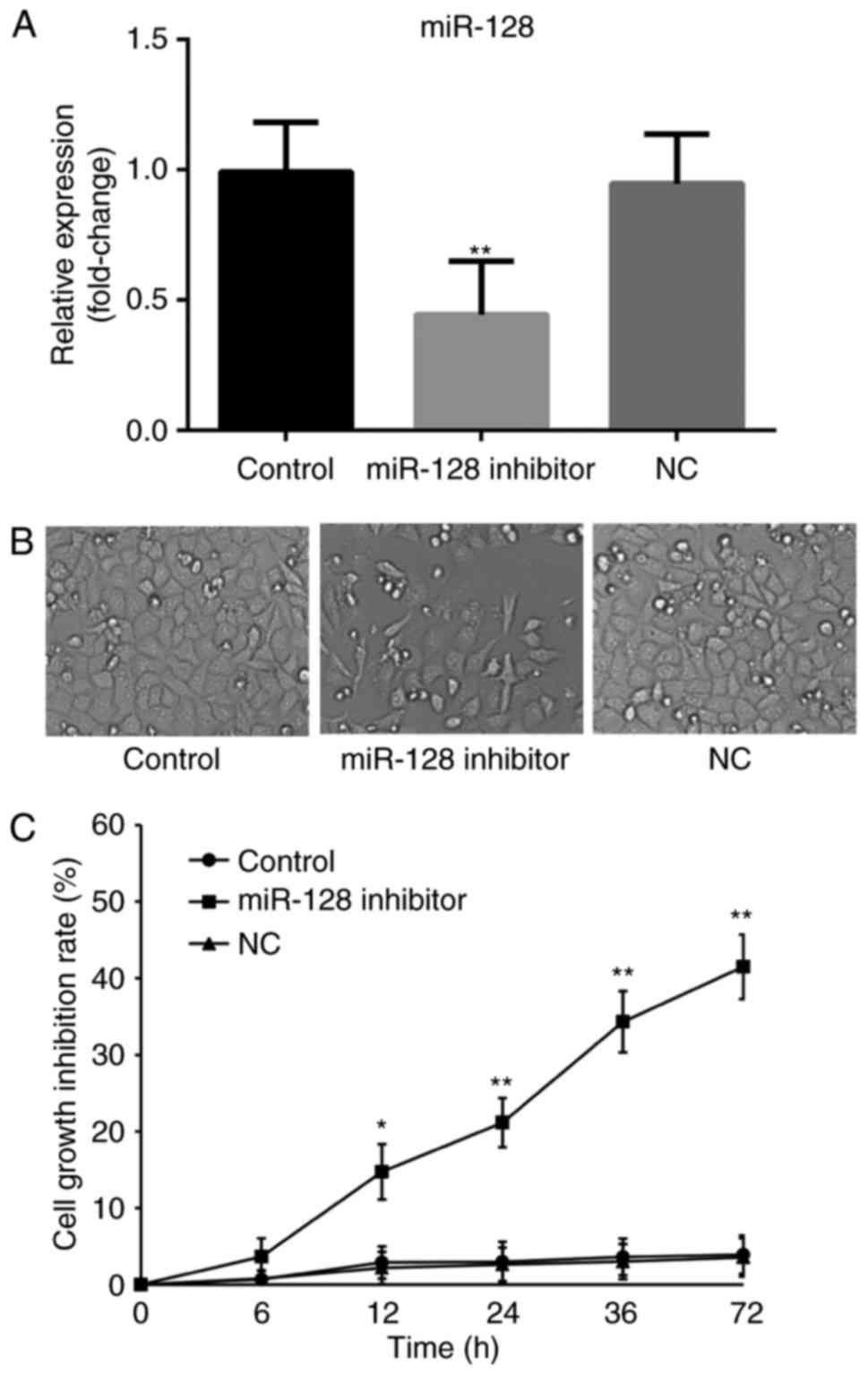
The cells were transfected with miR-128 inhibitors,
and the transfection efficiency was measured using RT-qPCR analysis
(Fig. 2A). Subsequently, an MTT assay
was used to monitor the effect of miR-128 on osteosarcoma cell
growth (Fig. 2A and B). The results
showed no significant difference in cell growth inhibition between
the blank control and NC groups as time increased (P>0.05). As
time increased, the miR-128-inhibited group showed significantly
decreased cell viability, compared with those in the control groups
(P<0.05), indicating that the absence of miR-128 may be able to
inhibit the viability of osteosarcoma cells.
Cell apoptosis
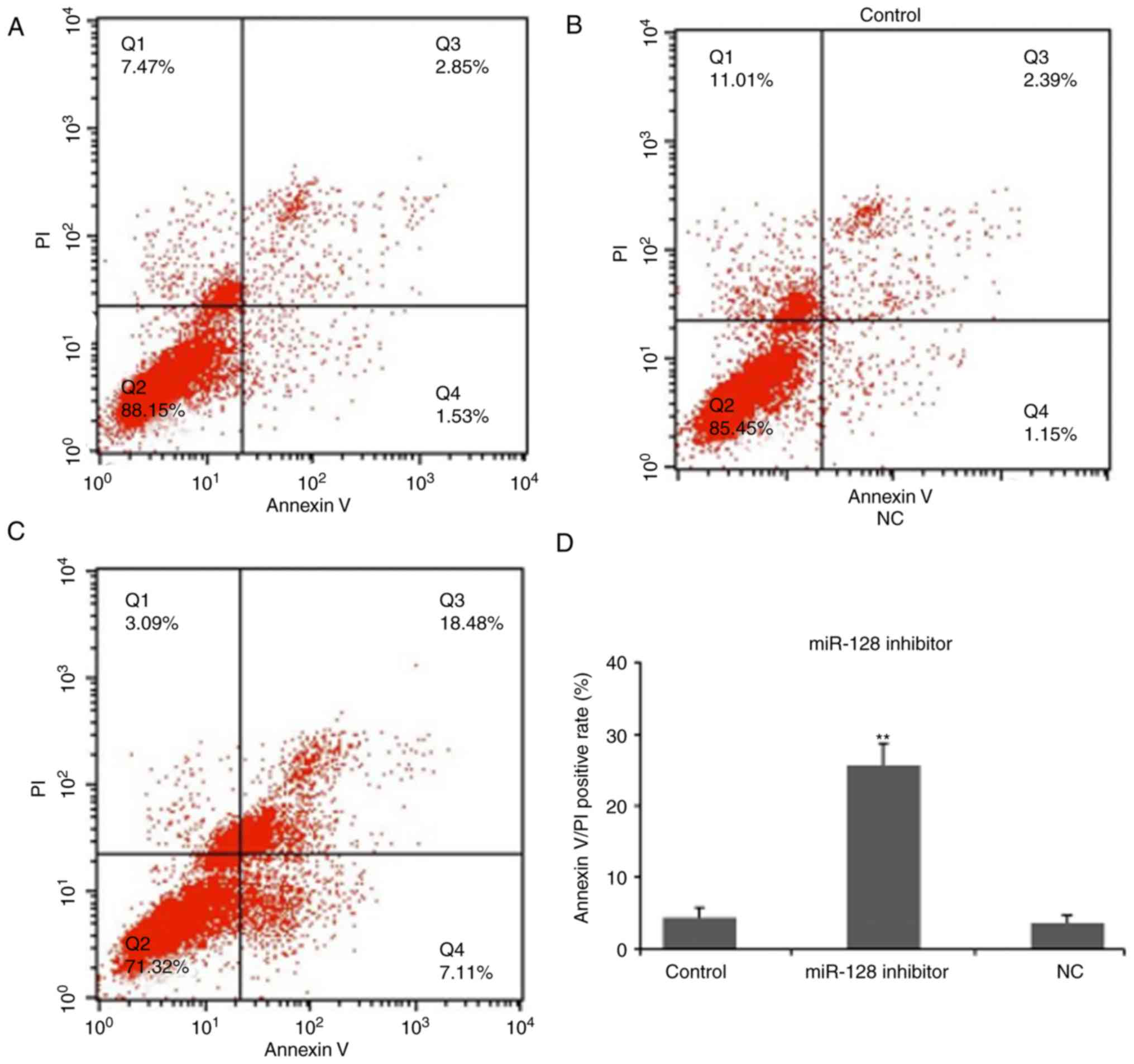
To examine the effect of miR-128 on osteosarcoma
cell apoptosis, an Annexin V-FITC apoptosis kit was used to analyze
apoptotic cells (Fig. 3). The blank
control and NC groups had apoptotic rates of 1.53% and 1.15%,
respectively (Fig. 3A and B). The
apoptotic rate of cells in the miR-128-inhibited group was 7.11%,
which was higher, compared with the rate in the control groups
(Fig. 3C), indicating that the
absence of miR-128 may be able to promote osteosarcoma cell
apoptosis.
Western blot analysis
To investigate the correlation between the miR-128
and SASH1 in the signaling pathway, the protein expression levels
of SASH1 and associated proteins were measured using western blot
analysis. The results showed no marked variation in the protein
expression levels of SASH1, Bax, Bcl-2 or caspase-3 between the NC
and blank groups (P>0.05; Fig.
4A-F). In the miR-128-inhibited group, the protein expression
levels of SASH1, Bax and caspase-3 were significantly increased,
whereas the expression level of Bcl-2 was significantly reduced,
compared with the expression in the control groups (P<0.05).
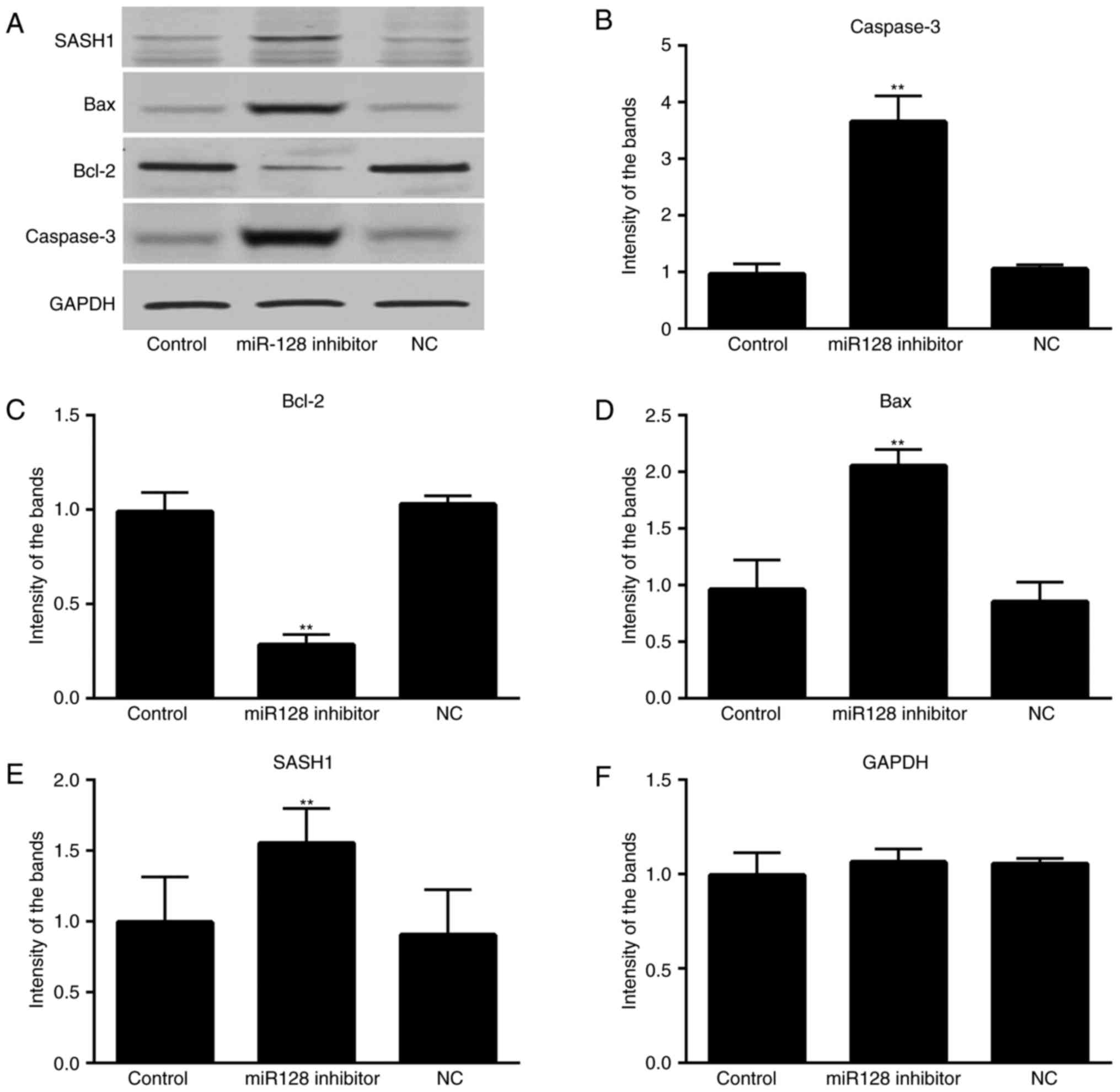 | Figure 4.Effect of miR-128 on the expression of
SASH1, Bcl-2, Bax and caspase-3 in MG-63 cells. (A) Protein
expression of SASH1, Bcl-2, Bax, Caspase-3 and GAPDH in different
groups; (B) Relative intensity of the Caspase-3 band; (C) Relative
intensity of the Bcl-2 band; (D) Relative intensity of the BAX
band; (E) Relative intensity of the SASH1 band; (F) Relative
intensity of the GAPDH band; miR, microRNA; control, untransfected
cells; miR-128 inhibitor, miR-128 inhibitor-transfected cells; NC,
miR-128 negative control-transfected cells; SASH1, SAM-and
SH3-domain containing 1; Bcl-2, B-cell lymphoma-2; Bax,
Bcl2-associated X protein; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase. |
SASH1 is a direct target of miR-128 in
osteosarcoma
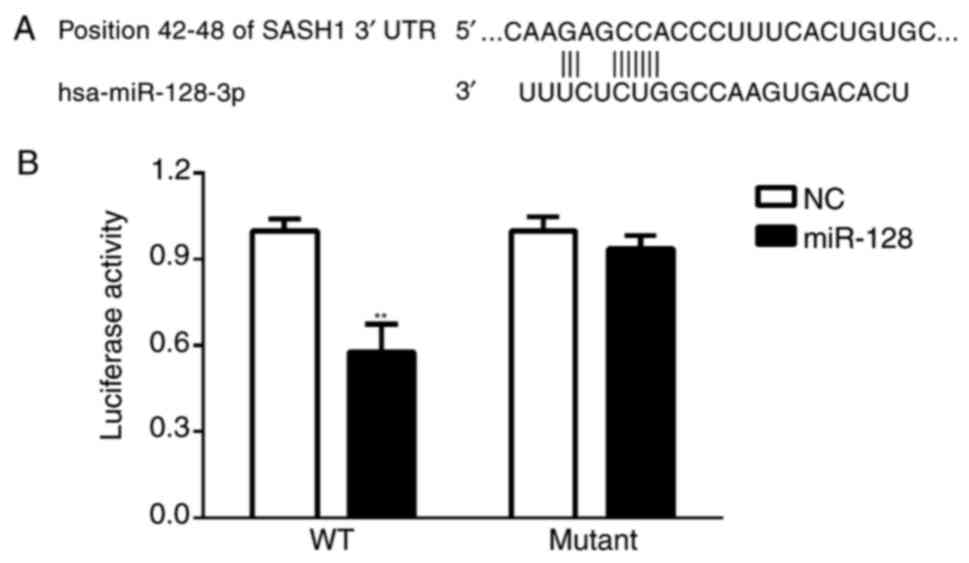
Using online bioinformatics tools TargetScan
(20) and miRanda (21), SASH1 has been predicted as a target
gene of miR-128, therefore, the present study further examined the
association between SASH1 and miR-128 using a dual luciferase gene
reporter assay. As shown in Fig. 5A and
B, transfection in the miR-128 group showed significantly
suppressed luciferase activity in the wild-type SASH1 3′UTR
(SASH1-3′UTR) plasmid-transfected cells, whereas miR-128 had no
effect on the mutant SASH1 3′UTR (SASH1-MUT) plasmid-transfected
cells (P<0.01; Fig. 5B),
suggesting that miR-128 directly targeted SASH1 in
vitro.
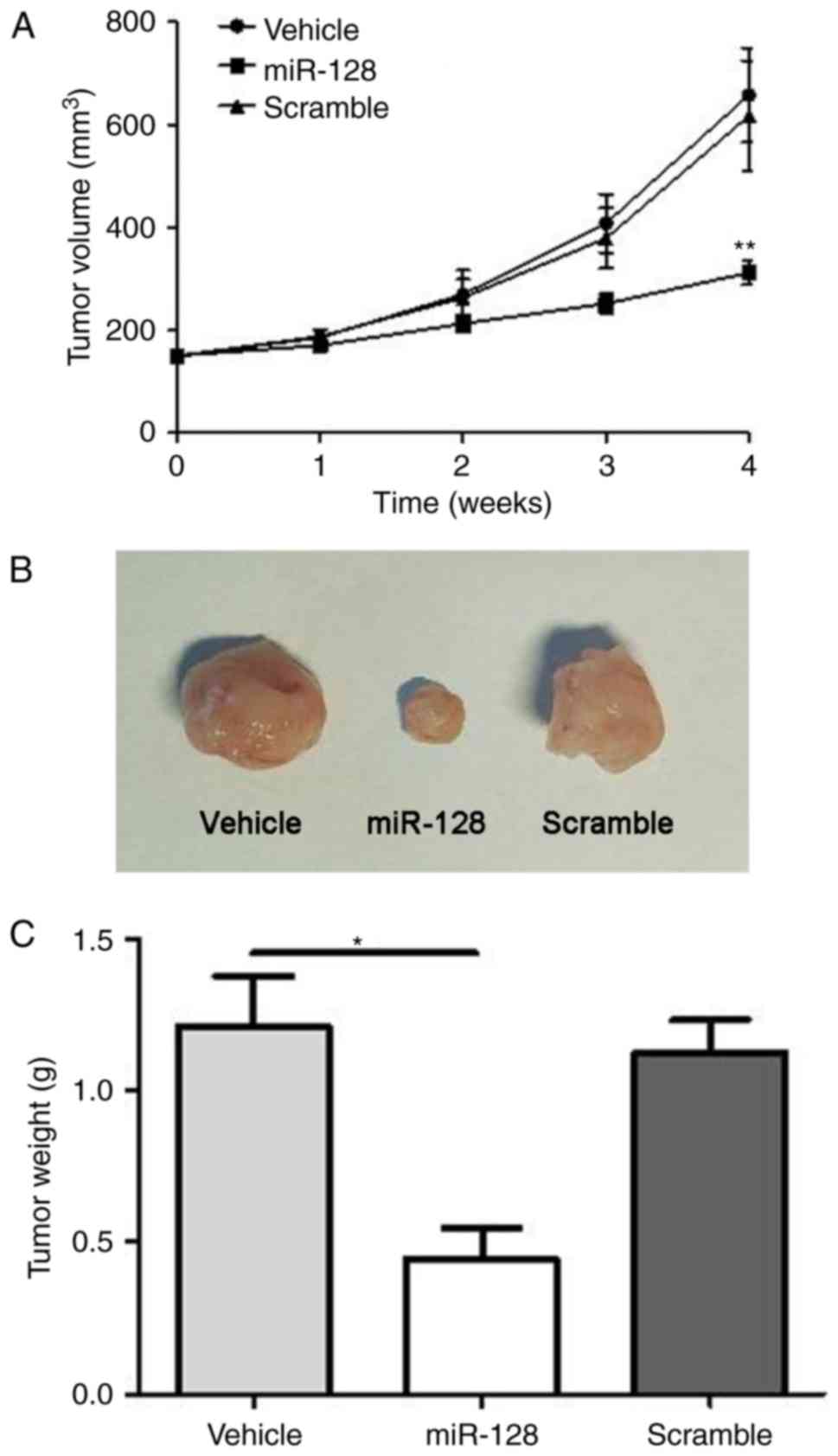
Knockdown of miR-128 induces tumor
suppressive effects on xenograft mice tumor models of osteosarcoma
in vivo
Finally, the present study examined the roles of
miR-128 in xenograft mouse osteosarcoma models. As shown in
Fig. 6A-C, transplantation of the
mice with MG-63 human osteoblastic cells, which had been
transfected with miR-128 inhibitor, led to significant decreases in
tumor volume and weight (P<0.05).
Discussion
Osteosarcoma is the most commonly occurring sarcoma
in young individuals, accounting for almost 60% of all bone
sarcomas (21,22). This malignant bone tumor is an
osteoid-producing solid tumor, which develops rapidly, particularly
in large bones close to epiphyseal regions and has shown a high
mortality rate in recent years (22).
Despite studies reporting the importance of miR-128 in several
types of malignant tumors, the potential mechanism and signaling
pathway of miR-128 in regulating the development of osteosarcoma
remain to been fully elucidated. Osteosarcoma is known for its high
invasive ability and early metastasis, which contribute most to
treatment failure and mortality rates (23). Therefore, it is crucial to investigate
the molecular mechanism of miRNAs and signaling proteins in the
development, invasion and metastasis of osteosarcoma in order to
identify effective treatments. The present study demonstrated the
overexpression of miR-128 in osteosarcoma tissue and suggested the
regulatory effect of miR-128 in the pathogenesis of osteosarcoma
through targeting SASH1. Studies have reported similar
overexpression of miR-128 in other types of cancer(24). Zhu et
al (25) found that miR-128 was
expressed at high levels in the blood of 147 patients newly
diagnosed with acute leukemia. It was reported that miR-128 was
associated with to the prognosis of acute leukemia. According to
another report, the expression level of miR-128 was increased in
samples from patients with glioblastoma (26). Abnormal expression levels of miR-128
in other types of tumor tissue have also been reported (27,28).
In the present study, the effects of miR-128 on the
progression of osteosarcoma at the cellular level were examined
through the transfection of miR-128 inhibitor into MG-63 cells. The
results showed that the absence of miR-128 markedly inhibited the
growth and promoted the apoptosis of MG-63 cells. In addition, the
knockdown of miR-128 induced tumor suppressive effects on xenograft
tumor models of osteosarcoma in mice in vivo. Therefore,
miR-128 may be able to promote osteosarcoma cell growth and prevent
osteosarcoma cell apoptosis, indicating that miR-128 is important
in tumorigenesis and development. SASH1 is a tumor suppressor
candidate (29). According to
previous studies, the decrease of SASH1 is linked to tumorigenesis,
metastasis and poor prognosis in different types of cancer
(10,29,30).
Studies have demonstrated that SASH1 can inhibit cell proliferation
(10,30). These studies suggested that the
downregulation of SASH1 may be an important factor in tumorigenesis
and evolution. Former studies have reported that functions,
including the inhibition of cell growth, repression of invasive
ability and promotion of apoptosis can suppress the metastasis of
tumors (31). The data in the present
study showed that the expression level of SASH1 was higher in the
miR-128-inhibited group, compared with that in the negative control
group and blank group, indicating that miR-128 may be able to
downregulate the expression of SASH1 and prevent its inhibitory
effect on tumor cell proliferation. The direct target association
between SASH1 and miR-128 was confirmed using a dual luciferase
gene reporter assay. Furthermore, the results of the present study
revealed that the protein expression level of Bcl-2 was decreased
in the miR-128-inhibited group, compared with that in the negative
control group and blank group, whereas the expression levels of Bax
and caspase-3 were markedly increased in the miR-128-inhibited
group. This suggested that miR-128 may be able to upregulate the
expression of Bcl-2 and downregulate the expression levels of Bax
and caspase-3. Bcl-2 protein is well-known for its ability to
repress a number of cell apoptotic processes (32), whereas Bax and caspase-3 are key
factors in cell apoptotic processes (33,34). This
further confirmed the hypothesis that miR-128 regulates the
tumorigenesis and evolution of osteosarcoma through targeting SASH1
and correlative signal proteins. The clinical patient osteosarcoma
samples also showed marked correlation between the expression of
miR-128 and SASH1, particularly with tumor size, invasion depth,
lymph node metastasis and TNM stage. With increased tumor size and
more marked invasion and metastasis, there were higher expression
levels of miR-128 and lower expression levels of SASH1 in the
clinical osteosarcoma samples.
In conclusion, the present study investigated the
role of miR-128 in the progression of osteosarcoma and the possible
regulatory mechanism. The present study revealed that miR-128
downregulated the expression of SASH1 in osteosarcoma tumor cells,
promoted osteosarcoma tumor cell proliferation and inhibited
osteosarcoma tumor cell apoptosis. Therefore, gene therapy
targeting miR-128 may offer a promising treatment method for
osteosarcoma through inhibiting cell growth, repressing invasion
and promoting apoptosis via the downregulation of miR-128 and
upregulation of SASH1. Finally, the results of the in vivo
animal experiment confirmed that the downregulation of miR-128
induced tumor suppressive effects on xenograft tumor mouse models
of osteosarcoma. The findings of the present study may offer a
foundation for future clinical therapeutics in osteosarcoma.
However, further detailed investigations are required to thoroughly
examine the regulatory mechanisms of miR-128 in osteosarcoma.
Acknowledgements
This study was sponsored by the National Natural
Science Foundation of China (grant no. 81302338).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Basu-Roy U, Basilico C and Mansukhani A:
Perspectives on cancer stem cells in osteosarcoma. Cancer Lett.
338:158–167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lin PP and Patel S: Osteosarcoma. Bone
Sarcoma Springer. 75–97. 2013. View Article : Google Scholar
|
|
3
|
Di Fiore R, Guercio A, Puleio R, Di Marco
P, Drago-Ferrante R, D'Anneo A, De Blasio A, Carlisi D, Di Bella S,
Pentimalli F, et al: Modeling human osteosarcoma in mice through
3AB-OS cancer stem cell xenografts. J Cell Biochem. 113:3380–3392.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ziyan W, Shuhua Y, Xiufang W and Xiaoyun
L: MicroRNA-21 is involved in osteosarcoma cell invasion and
migration. Med Oncol. 28:1469–1474. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ren Z, Liang S, Yang J, Han X, Shan L,
Wang B, Mu T, Zhang Y, Yang X, Xiong S and Wang G: Coexpression of
CXCR4 and MMP9 predicts lung metastasis and poor prognosis in
resected osteosarcoma. Tumor Biol. 37:5089–5096. 2016. View Article : Google Scholar
|
|
7
|
Meazza C and Scanagatta P: Metastatic
osteosarcoma: A challenging multidisciplinary treatment. Expert Rev
Anticancer Ther. 16:543–556. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Meng Q, Zheng M, Liu H, Song C, Zhang W,
Yan J, Qin L and Liu X: SASH1 regulates proliferation, apoptosis,
and invasion of osteosarcoma cell. Mol Cell Biochem. 373:201–210.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Luo H, Zou J, Dong Z, Zeng Q, Wu D and Liu
L: Up-regulated miR-17 promotes cell proliferation, tumour growth
and cell cycle progression by targeting the RND3 tumour suppressor
gene in colorectal carcinoma. Biochem J. 442:311–321. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Martini M, Gnann A, Scheikl D, Holzmann B
and Janssen KP: The candidate tumor suppressor SASH1 interacts with
the actin cytoskeleton and stimulates cell-matrix adhesion. Int J
Biochem Cell Biol. 43:1630–1640. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in human cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Filipowicz W, Bhattacharyya SN and
Sonenberg N: Mechanisms of post-transcriptional regulation by
microRNAs: Are the answers in sight? Nat Rev Genet. 9:102–114.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang C, Chen X, Alattar M, Wei J and Liu
H: MicroRNAs in tumorigenesis, metastasis, diagnosis and prognosis
of gastric cancer. Cancer Gene Ther. 22:291–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li M, Fu W, Wo L, Shu X, Liu F and Li C:
miR-128 and its target genes in tumorigenesis and metastasis. Exp
Cell Res. 319:3059–3064. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sun X, Yang Z, Zhang Y, He J, Wang F, Su
P, Han J, Song Z and Fei Y: Prognostic implications of tissue and
serum levels of microRNA-128 in human prostate cancer. Int J Clin
Exp Pathol. 8:8394–8401. 2015.PubMed/NCBI
|
|
16
|
Liu P, Cheng H, Roberts TM and Zhao JJ:
Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev
Drug Discov. 8:627–644. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Botter SM, Neri D and Fuchs B: Recent
advances in osteosarcoma. Curr Opin Pharmacol. 16:15–23. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen L, Chen XD and Zhang YH: MicroRNA-128
promotes proliferation in osteosarcoma cells by downregulating
PTEN. Tumor Biol. 35:2069–2074. 2014. View Article : Google Scholar
|
|
19
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar
|
|
21
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human microRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Heare T, Hensley MA and Dell'Orfano S:
Bone tumors: Osteosarcoma and Ewing's sarcoma. Curr Opin Pediatr.
21:365–372. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Basuroy U, Basilico C and Mansukhani A:
Perspectives on cancer stem cells in osteosarcoma. Cancer Lett.
338:158–167. 2011. View Article : Google Scholar
|
|
24
|
Zwaga T, Bovée JV and Kroon HM:
Osteosarcoma of the femur with skip, lymph node, and lung
metastases. Radiographics. 28:277–283. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu YD, Wang L, Sun C, Fan L, Zhu DX, Fang
C, Wang YH, Zou ZJ, Zhang SJ, Li JY and Xu W: Distinctive microRNA
signature is associated with the diagnosis and prognosis of acute
leukemia. Med Oncol. 29:2323–2331. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roth P, Wischhusen J, Happold C, Chandran
PA, Hofer S, Eisele G, Weller M and Keller A: A specific miRNA
signature in the peripheral blood of glioblastoma patients. J
Neurochem. 118:449–457. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pang CS, Kwok WK, Chen Z and Ng HK:
Oncogenic role of microRNAs in brain tumors. Acta Neuropathol.
117:599–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katada T, Ishiguro H, Kuwabara Y, Kimura
M, Mitui A, Mori Y, Ogawa R, Harata K and Fujii Y: MicroRNA
expression profile in undifferentiated gastric cancer. Int J Oncol.
34:537–542. 2009.PubMed/NCBI
|
|
29
|
Woo HH, László CF, Greco S and Chambers
SK: Regulation of colony stimulating factor-1 expression and
ovarian cancer cell behavior in vitro by miR-128 and miR-152. Mol
Cancer. 11:582012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rimkus C, Martini M, Friederichs J,
Rosenberg R, Doll D, Siewert JR, Holzmann B and Janssen KP:
Prognostic significance of downregulated expression of the
candidate tumour suppressor gene SASH1 in colon cancer. Br J
Cancer. 95:1419–1423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen H, Wang D and Liu Y: SASH1 inhibits
cervical cancer cell proliferation and invasion by suppressing the
FAK pathway. Mol Med Rep. 13:3613–3618. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He P, Zhang HX, Sun CY, Chen CY and Jiang
HQ: Overexpression of SASH1 inhibits the proliferation, invasion,
and EMT in hepatocarcinoma cells. Oncol Res. 24:25–32. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Delbridge ARD and Strasser A: The BCL-2
protein family, BH3-mimetics and cancer therapy. Cell Death Differ.
22:1071–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Czabotar PE, Westphal D, Dewson G, Ma S,
Hockings C, Fairlie WD, Lee EF, Yao S, Robin AY, Smith BJ, et al:
Bax crystal structures reveal how BH3 domains activate Bax and
nucleate its oligomerization to induce apoptosis. Cell.
152:519–531. 2013. View Article : Google Scholar : PubMed/NCBI
|