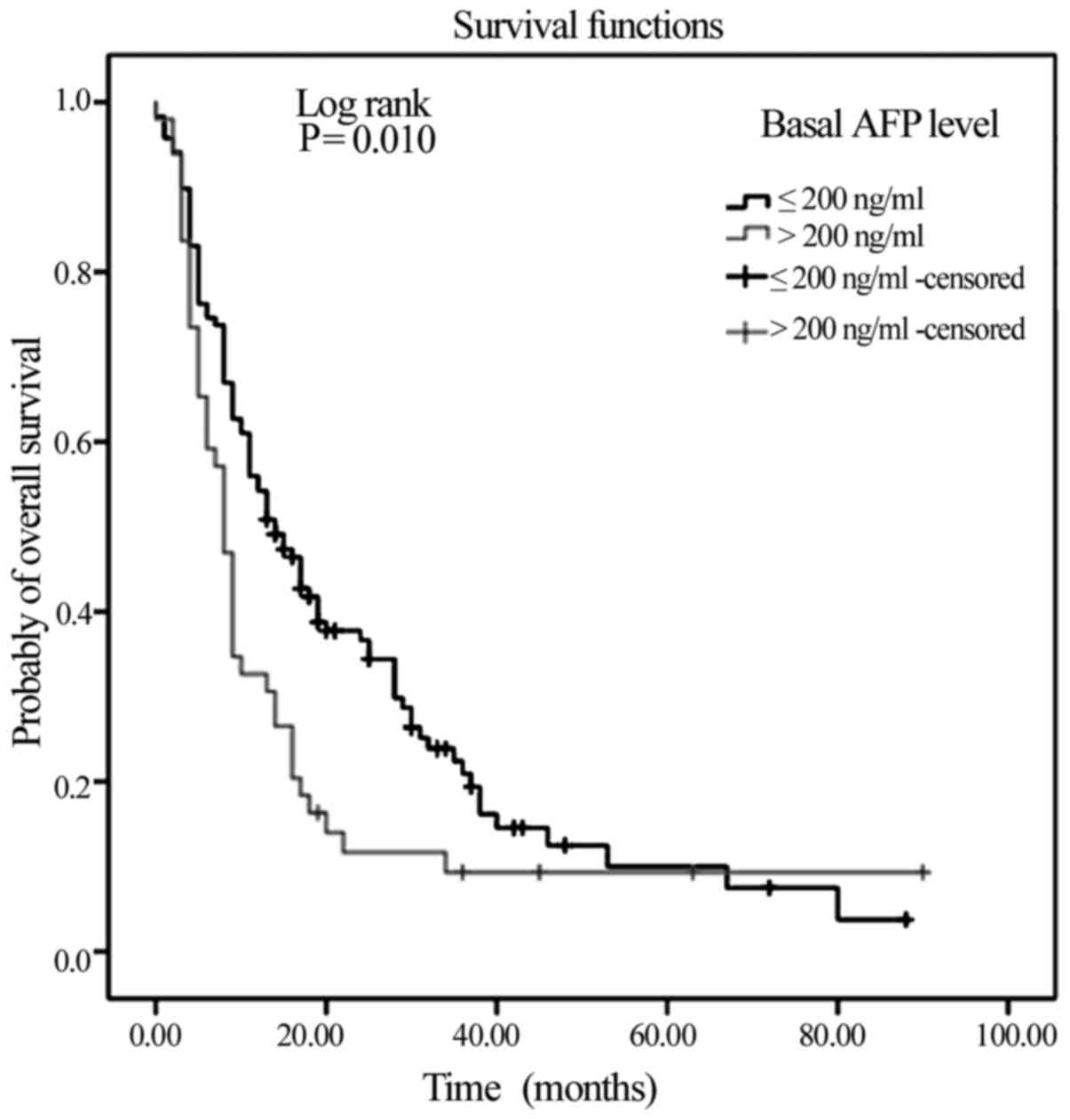
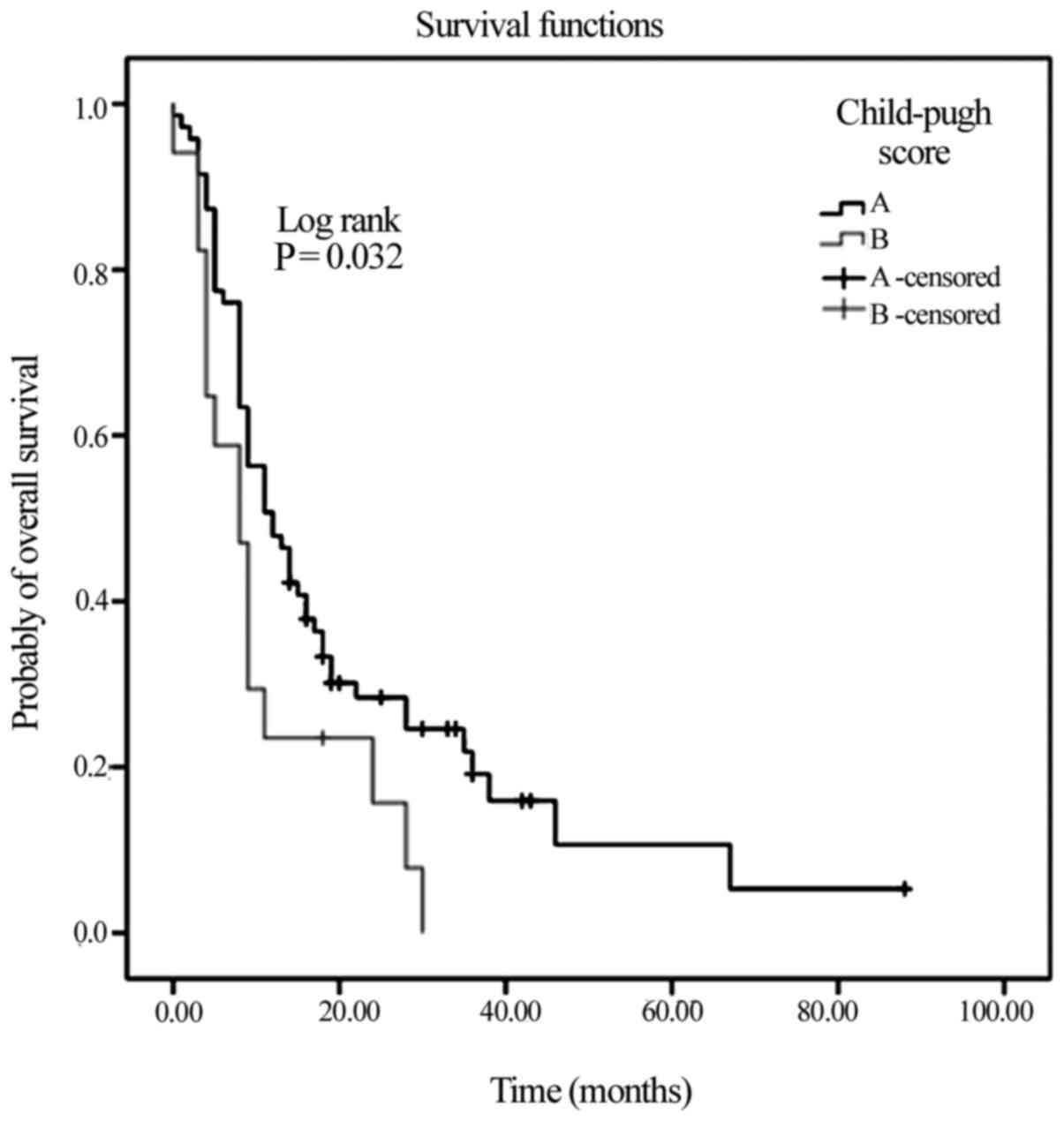
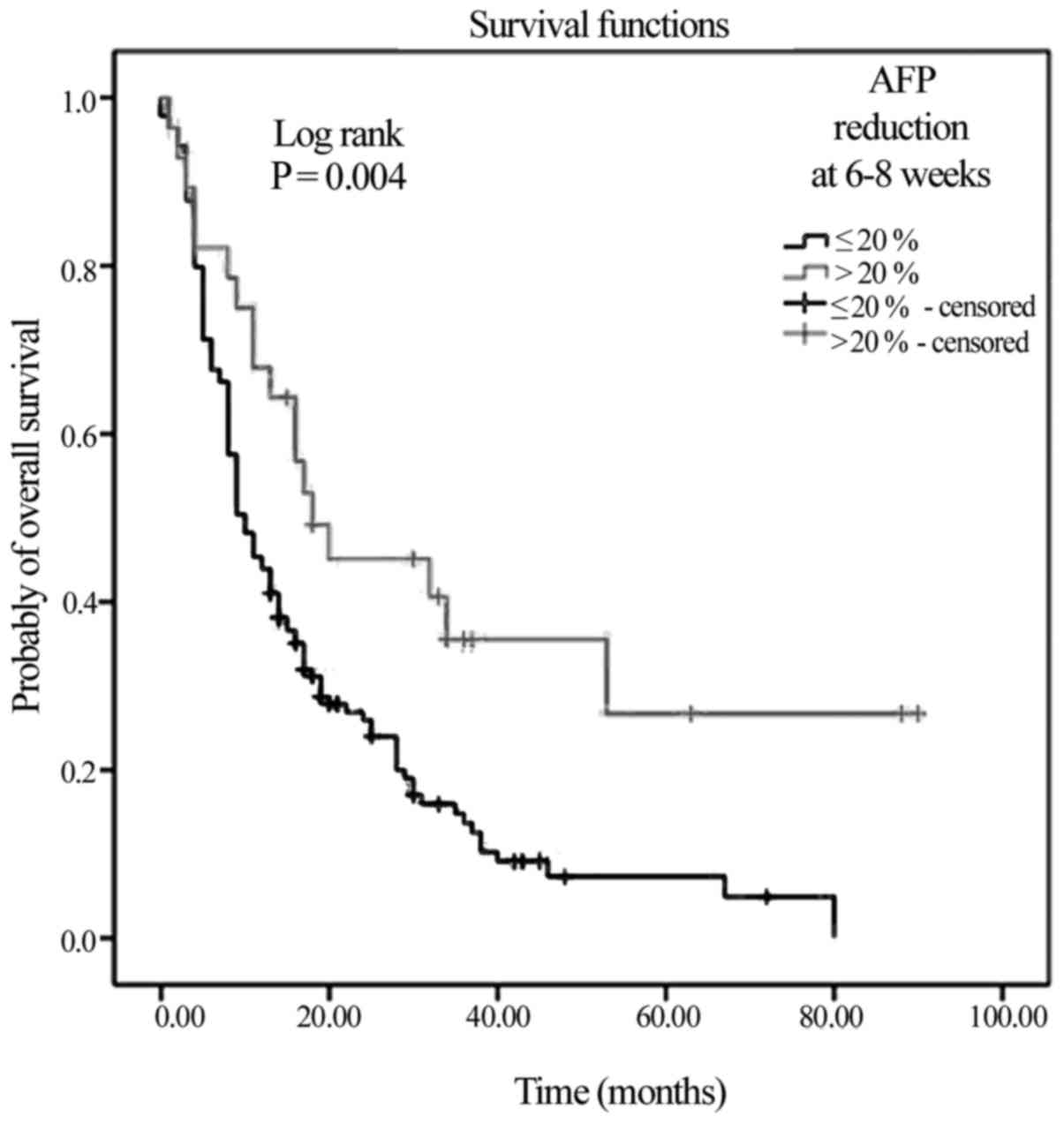
|
1
|
International Agency for Research on
Cancer (IARC): Globocan 2012: Estimated Cancer Incidence, Mortality
and Prevalence Worldwide in 2012. http://globocan.iarc.fr/Default.aspxApril
10–2015
|
|
2
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mittal S and El-Serag HB: Epidemiology of
hepatocellular carcinoma: Consider the population. J Clin
Gastroenterol. 47:S2–S6. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
European Association for the Study of The
Liver; European Organisation for Research and Treatment of Cancer:
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Omata M, Cheng AL, Kokudo N, Kudo M, Lee
JM, Jia J, Tateishi R, Han KH, Chawla YK, Shiina S, et al:
Asia-pacific clinical practice guidelines on the management of
hepatocellular carcinoma: A 2017 update. Hepatol Int. 11:317–370.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reig M, Matilla A, Bustamante J, Castells
L, de La Mata M, Delgado M, Moreno JM, Forner A and Varela M:
Recommendations for the management of Sorafenib in patients with
hepatocellular carcinoma. Gastroenterol Hepatol. 33:741–752.
2010.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bruix J and Sherman M: American
Association for the Study of Liver Diseases: Management of
hepatocellular carcinoma: An update. Hepatology. 53:1020–1022.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S,
Kim JS, Luo R, Feng J, Ye S, Yang TS, et al: Efficacy and safety of
sorafenib in patients in the asia-pacific region with advanced
hepatocellular carcinoma: A phase III randomised, double-blind,
placebo-controlled trial. Lancet Oncol. 10:25–34. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Llovet JM and Bruix J: Systematic review
of randomized trials for unresectable hepatocellular carcinoma:
Chemoembolization improves survival. Hepatology. 37:429–442. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dawson LA: The evolving role of radiation
therapy in hepatocellular carcinoma. Cancer Radiother. 12:96–101.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han KH, Seong J, Kim JK, Ahn SH, Lee DY
and Chon CY: Pilot clinical trial of localized concurrent
chemoradiation therapy for locally advanced hepatocellular
carcinoma with portal vein thrombosis. Cancer. 113:995–1003. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Colagrande S, Regini F, Taliani GG, Nardi
C and Inghilesi AL: Advanced hepatocellular carcinoma and
sorafenib: Diagnosis, indications, clinical and radiological
follow-up. World J Hepatol. 7:1041–1053. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Llovet JM, Ricci S, Mazzaferro V, Hilgard
P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A,
et al: Sorafenib in advanced hepatocellular carcinoma. N Engl J
Med. 359:378–390. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lencioni R and Llovet JM: Modified recist
(mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis.
30:52–60. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawaoka T, Aikata H, Murakami E, Nakahara
T, Naeshiro N, Tanaka M, Honda Y, Miyaki D, Nagaoki Y, Takaki S, et
al: Evaluation of the mrecist and α-fetoprotein ratio for
stratification of the prognosis of
advanced-hepatocellular-carcinoma patients treated with sorafenib.
Oncology. 83:192–200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takada J, Hidaka H, Nakazawa T, Kondo M,
Numata K, Tanaka K, Matsunaga K, Okuse C, Kobayashi S, Morimoto M,
et al: Modified response evaluation criteria in solid tumors is
superior to response evaluation criteria in solid tumors for
assessment of responses to sorafenib in patients with advanced
hepatocellular carcinoma. BMC Res Notes. 8:6092015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yau T, Yao TJ, Chan P, Wong H, Pang R, Fan
ST and Poon RT: The significance of early alpha-fetoprotein level
changes in predicting clinical and survival benefits in advanced
hepatocellular carcinoma patients receiving sorafenib. Oncologist.
16:1270–1279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nomura F, Ohnishi K and Tanabe Y: Clinical
features and prognosis of hepatocellular carcinoma with reference
to serum alpha-fetoprotein levels. Analysis of 606 patients.
Cancer. 64:1700–1707. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Matsumoto Y, Suzuki T, Ono H, Nakase A and
Honjo I: Evaluation of hepatoma chemotherapy by alpha-fetoprotein
determination. Am J Surg. 132:325–328. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chan SL, Mo FK, Johnson PJ, Hui EP, Ma BB,
Ho WM, Lam KC, Chan AT, Mok TS and Yeo W: New utility of an old
marker: Serial alpha-fetoprotein measurement in predicting
radiologic response and survival of patients with hepatocellular
carcinoma undergoing systemic chemotherapy. J Clin Oncol.
27:446–452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Personeni N, Bozzarelli S, Pressiani T,
Rimassa L, Tronconi MC, Sclafani F, Carnaghi C, Pedicini V,
Giordano L and Santoro A: Usefulness of alpha-fetoprotein response
in patients treated with sorafenib for advanced hepatocellular
carcinoma. J Hepatol. 57:101–107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Zhao Y, Jia J, Chen H, Bai W, Yang
M, Yin Z, He C, Zhang L, Guo W, et al: The prognostic value of
alpha-fetoprotein response for advanced-stage hepatocellular
carcinoma treated with sorafenib combined with transarterial
chemoembolization. Sci Rep. 6:198512016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okuyama H, Ikeda M, Kuwahara A, Takahashi
H, Ohno I, Shimizu S, Mitsunaga S, Senda S and Okusaka T:
Prognostic factors in patients with hepatocellular carcinoma
refractory or intolerant to sorafenib. Oncology. 88:241–246. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bruix J, Raoul JL, Sherman M, Mazzaferro
V, Bolondi L, Craxi A, Galle PR, Santoro A, Beaugrand M,
Sangiovanni A, et al: Efficacy and safety of sorafenib in patients
with advanced hepatocellular carcinoma: Subanalyses of a phase III
trial. J Hepatol. 57:821–829. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Iavarone M, Cabibbo G, Piscaglia F,
Zavaglia C, Grieco A, Villa E, Cammà C and Colombo M: SOFIA
(SOraFenib Italian Assessment) study group: Field-practice study of
sorafenib therapy for hepatocellular carcinoma: A prospective
multicenter study in Italy. Hepatology. 54:2055–2063. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kudo M, Imanaka K, Chida N, Nakachi K, Tak
WY, Takayama T, Yoon JH, Hori T, Kumada H, Hayashi N, et al: Phase
III study of sorafenib after transarterial chemoembolisation in
Japanese and Korean patients with unresectable hepatocellular
carcinoma. Eur J Cancer. 47:2117–2127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Køstner AH, Sorensen M, Olesen RK, Grønbæk
H, Lassen U and Ladekarl M: Sorafenib in advanced hepatocellular
carcinoma: A nationwide retrospective study of efficacy and
tolerability. ScientificWorldJournal. 2013:9319722013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
DA Fonseca LG, Barroso-Sousa R, Bento AD,
Blanco BP, Valente GL, Pfiffer TE, Hoff PM and Sabbaga J: Safety
and efficacy of sorafenib in patients with Child-Pugh B advanced
hepatocellular carcinoma. Mol Clin Oncol. 3:793–796. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nakano M, Tanaka M, Kuromatsu R, Nagamatsu
H, Tajiri N, Satani M, Niizeki T, Aino H, Okamura S, Iwamoto H, et
al: Kurume liver cancer study group of japan. sorafenib for the
treatment of advanced hepatocellular carcinoma with extrahepatic
metastasis: A prospective multicenter cohort study. Cancer Med.
4:1836–1843. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Z, Wu XL, Zeng WZ, Xu GS, Xu H, Weng
M, Hou JN and Jiang MD: Meta-analysis of the efficacy of sorafenib
for hepatocellular carcinoma. Asian Pac J Cancer Prev. 14:691–694.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shao YY, Lin ZZ, Hsu C, Shen YC, Hsu CH
and Cheng AL: Early alpha-fetoprotein response predicts treatment
efficacy of antiangiogenic systemic therapy in patients with
advanced hepatocellular carcinoma. Cancer. 116:4590–4596. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Raoul JL, Bruix J, Greten TF, Sherman M,
Mazzaferro V, Hilgard P, Scherubl H, Scheulen ME, Germanidis G,
Dominguez S, et al: Relationship between baseline hepatic status
and outcome, and effect of sorafenib on liver function: SHARP trial
subanalyses. J Hepatol. 56:1080–1088. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lee S, Kim BK, Kim SU, Park SY, Kim JK,
Lee HW, Park JY, Kim DY, Ahn SH, Tak WY, et al: Clinical outcomes
and prognostic factors of patients with advanced hepatocellular
carcinoma treated with sorafenib as first-line therapy: A korean
multicenter study. J Gastroenterol Hepatol. 29:1463–1469. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen LT, Liu TW, Chao Y, Shiah HS, Chang
JY, Juang SH, Chen SC, Chuang TR, Chin YH and Whang-Peng J:
Alpha-fetoprotein response predicts survival benefits of
thalidomide in advanced hepatocellular carcinoma. Aliment Pharmacol
Ther. 22:217–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Raoul JL, Park JW, Kang YK, Finn RS, Kim
JS, Yeo W, Polite BN, Chao Y, Walters I, Baudelet C and Lencioni R:
Using modified recist and alpha-fetoprotein levels to assess
treatment benefit in hepatocellular carcinoma. Liver Cancer.
3:439–450. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chou WC, Lee CL, Yang TS, Huang CY, Teng
W, Tseng YT, Chen JS, Lin YC, Hou MM, Chang HH and Hsieh Chia-Hsun
J: Changes in serum α-fetoprotein level predicts treatment response
and survival in hepatocellular carcinoma patients and literature
review. J Formos Med Assoc. 117:153–163. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xu XS, Qu K, Liu C, Zhang YL, Liu J, Song
YZ, Zhang P, Liu SN and Chang HL: Highlights for α-fetoprotein in
determining prognosis and treatment monitoring for hepatocellular
carcinoma. World J Gastroenterol. 18:7242–7250. 2012. View Article : Google Scholar : PubMed/NCBI
|