Introduction
The solitary fibrous tumor (SFT) is a rare type of
spindled cell tumor of the mesenchymal tissue (1) whose identity was first proposed by
Wagner in 1870 (2). In 1931,
Klemperer and Coleman (3) first
reported their pathological characteristics (4). The tumors often originate in the pleural
space (5,6). Although they can occur anywhere in the
body, SFTs of the central nervous system (CNS) are rare (7), with Carneiro et al (8) first reporting them in 1996. Due to the
distinct pathological type, the incidence of this disease is low
and forming a preoperative diagnosis is difficult (9). Furthermore, an SFT is often misdiagnosed
as another type of tumor (10), such
as a fibrous meningioma. The features of meningeal SFTs are similar
to those of other tumors in the CNS in certain respects, such as
upon reviews of the pathology or from the imaging perspective.
Globally, there are currently few studies on meningeal SFT
characteristics in magnetic resonance imaging (MRI) (11,12). At
present, there are also few cases reported (13) domestically in China. In order to
improve the recognition of the tumors for the diagnosis of an SFT,
the data from 12 cases of meningeal SFTs that were treated in the
Second Affiliated Hospital of Guangzhou Medical University
(Guangzhou, China) and whose diagnosis was confirmed by surgery and
pathology, were collected between January 2006 and January 2016.
The MRI signs of the SFTs were observed and analyzed. The present
study aimed to determine valuable features for a differential
diagnosis to separate an SFT from other tumors using preoperative
MRI.
Materials and methods
Case data
In total, 12 cases of meningeal SFTs with complete
clinical and MRI data were reviewed in the present study. All
patients were treated in the Neurosurgery Unit of the Second
Affiliated Hospital of Guangzhou Medical University between January
2006 and January 2016. The inclusion criterion used was that
patients had undergone partial or complete surgical resection and
were confirmed by pathology as having meningeal SFTs. According to
the 2007 World Health Organization (WHO) classification of central
nervous system tumors, ISFT was classified as meningeal mesenchymal
tumor (WHO Grade 1) (Table I)
(14). The study cohort consisted of
6 men and 6 women aged between 20 and 71 years, with a mean age of
48.8 years. This study was conducted in accordance with the
declaration of Helsinki and with approval from the Ethics Committee
of Guangzhou Medical University. Written informed consent for
publication of this study was obtained from the patient or their
guardian/family members.
 | Table I.Case data. |
Table I.
Case data.
| Case no. | Sex/age, years | Location and MRI
findings | Size,
mma | MRI preoperative
diagnosis |
|---|
| 1 | Male/67 | Left anterior
cranial fossa, across the falx, the deep lobulation, more cystic
areas, inhomogeneous signal, mild edema | 60×75×62 | Meningioma |
| 2 | Male/59 | Left parietal, with
adjacent bony destruction, certain cystic areas, inhomogeneous
signal, no edema | 55×53×59 | Meningioma |
| 3 | Male/53 | Right tentorium
cerebelli, across the tentorium cerebelli, inhomogeneous signal,
mild edema | 45×44×48 | Meningioma |
| 4 | Male/36 | Right
temporal-parietal-occipital junction, with adjacent bony
destruction, certain patches of cystic areas, inhomogeneous signal,
moderate edema | 63×64×67 | Gliobastona |
| 5 | Female/52 | Left occipital and
left cerebellum, across the tentorium cerebelli, more cystic area,
inhomogeneous signal, no edema | 44×60×62 | Identify meningioma
and SFT |
| 6 | Female/40 | Frontal on each
side of the cerebral falx, across the falx, more cystic areas,
inhomogeneous signal, mild edema | 71×60×43 | Meningioma |
| 7 | Female/20 | Right parietal
areas beside sagittal sinus-the falx, deep lobulation,
inhomogeneous signal, with adjacent bony destruction, no edema | 36×33×33 | Meningioma |
| 8 | Male/50 | Frontal on each
side of the cerebral falx, across the falx, deep lobulation, more
cystic areas, inhomogeneous signal, close to the adjacent dura,
with adjacent bony destruction, moderate edema, numerous small
vesicles | 77×55×50 | Meningioma |
| 9 | Female/45 | Across the right
tentorium cerebelli, at the occipital and right cerebellum, shallow
lobe, few cystic areas, mild inhomogeneous signal, mild edema, low
signal on T2-weighted imaging | 53×45×42 | Meningioma |
| 10 | Male/54 | Right lateral
ventricles triangle, deep lobulation, few cystic areas, mild
inhomogeneous signal, moderate edema, a little bleeding | 51×43×51 | Identify meningioma
and papilloma choroideum |
| 11 | Female/71 | Right temporal
region, moderate edema, low signal on T2-weighted imaging,
inhomogeneous signal, shallow lobe, less cystic regions, moderate
edema | 48×55×53 | Meningioma |
| 12 | Female/39 | Left frontal
bottom, a little partial cystic region, shallow lobe, severe edema,
close to the falx, low signal on T2-weighted imaging | 35×40×33 | Meningioma |
Methods and parameters
A 1.5T superconducting twin-speed magnetic resonance
scanner (SignaHDxt 1.5T; GE Healthcare, Chicago, IL, USA) was used
for MR examination.
The patients were scanned head first in the supine
position. Imaging assessments included plain scanning (without an
injection of any contrast medium) and enhancement scanning
(injection with a contrast medium). There were two sequences on the
plain scan: Axial plane T1-weighted imaging (T1WI) and T2WI. There
were three sequences on the enhanced scan: Axial plane
fat-suppression (FS)-T1WI, coronal plane FS-T1WI and sagittal plane
FS-T1WI.
For the axial plane on the plain scan, the time of
repetition/time of echo (TR/TE) was 500/20 msec in T1WI and
4,000/102 msec in T2WI. The enhanced scanning parameters were the
same as that of plain scan in the axial plane, while FS technology
was used. In the coronal plane FS-T1WI, the TR/TE was 400–500/20–24
msec. The TR/TE was 400–500/20–24 msec for the sagittal plane
FS-T1WI. The field of view was 240×240 mm. The layer thickness was
5 mm and the layer distance was 10%.
The scan was commenced once the patient had been
injected with the contrast medium. The contrast medium was a
gadopentetatedimeglumine intravenous injection for adults and
children aged over 2, with 0.2 ml/kg body weight (or 0.1
mmol/kg).
Evaluation
Preoperative diagnosis and postoperative evaluation
of the MRI were performed by 2 to 3 radiologists with the title of
Associate Chief Physician and above. This was initially performed
in a blinded manner, then the results of the cases were discussed
and a consensus was reached.
Evaluation of tumor peripheral
edema
The peripheral edema around the tumor was measured
in the MR images, with an evident edema found around the lesion.
The image was divided into four degrees according to the maximum
width of the peripheral edema as follows: i) No edema; ii) mild
edema, the maximum width of the peripheral edema was ≤2 cm; iii)
moderate edema, the maximum width of the peripheral edema was >2
cm and ≤4 cm; and iv) severe edema, the maximum width of the
peripheral edema >4 cm.
Assessment of the lobulated margin of
focus
According to the degree of protrusion at the edge of
the lesion, the lobulated margin was divided into two types as
follows: i) Deep lobulation, the arc chord distance/chord length
ratio was ≥0.4; and ii) shallow lobulation, the arc chord
distance/chord length ratio was <0.4.
Results
MRI preoperative diagnosis
A total of 12 cases were assessed in the present
study, of which, 9 cases were originally diagnosed as meningioma
preoperatively. Among these, 5 cases were suggested to possibly be
malignant meningioma and 4 cases were benign. A single case was
originally believed to have a differential diagnosis of a
meningioma and papilloma of the choroid. Another case was
originally diagnosed as a glioblastoma, and in only 1 case was a
meningioma distinguished as an SFT. For the postoperative
diagnosis, all 12 cases were meningeal SFTs. Of the cases, 3 were
malignant, 7 were benign and 2 were a borderline tumor.
Growth pattern of the tumor
As observed from MRI, 3 cases spanned the tentorium
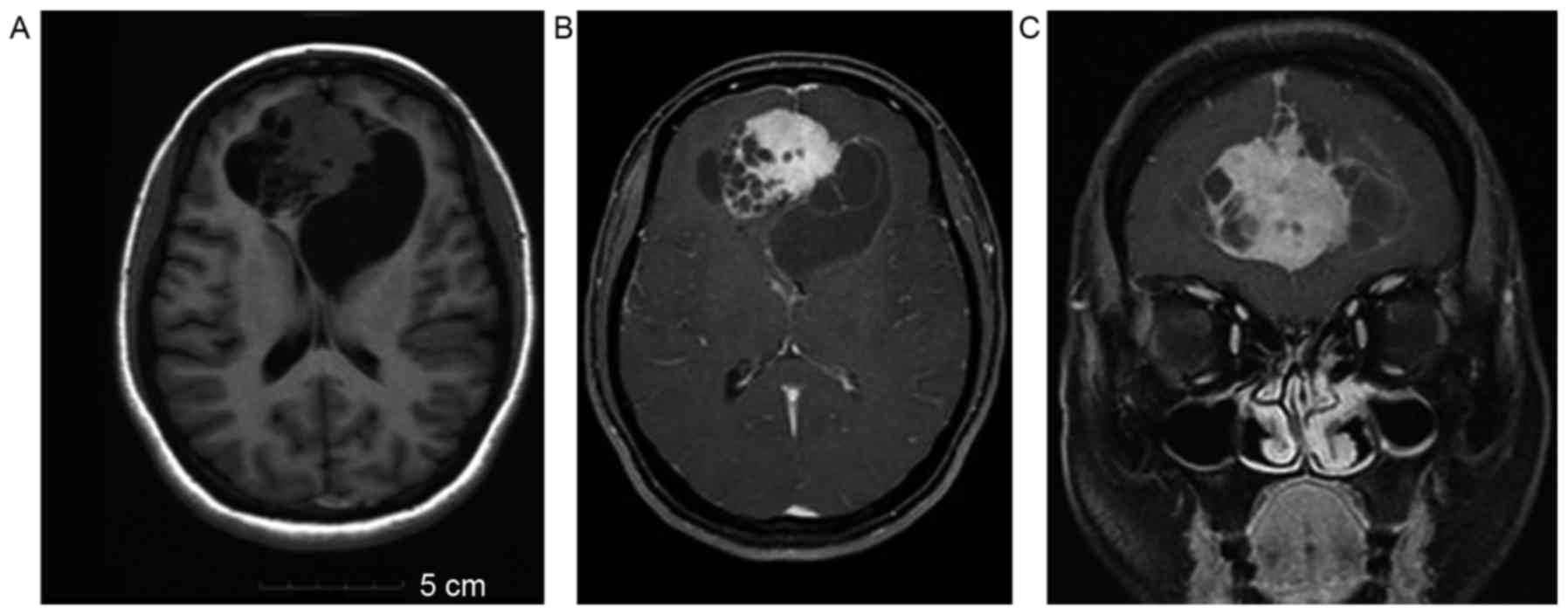
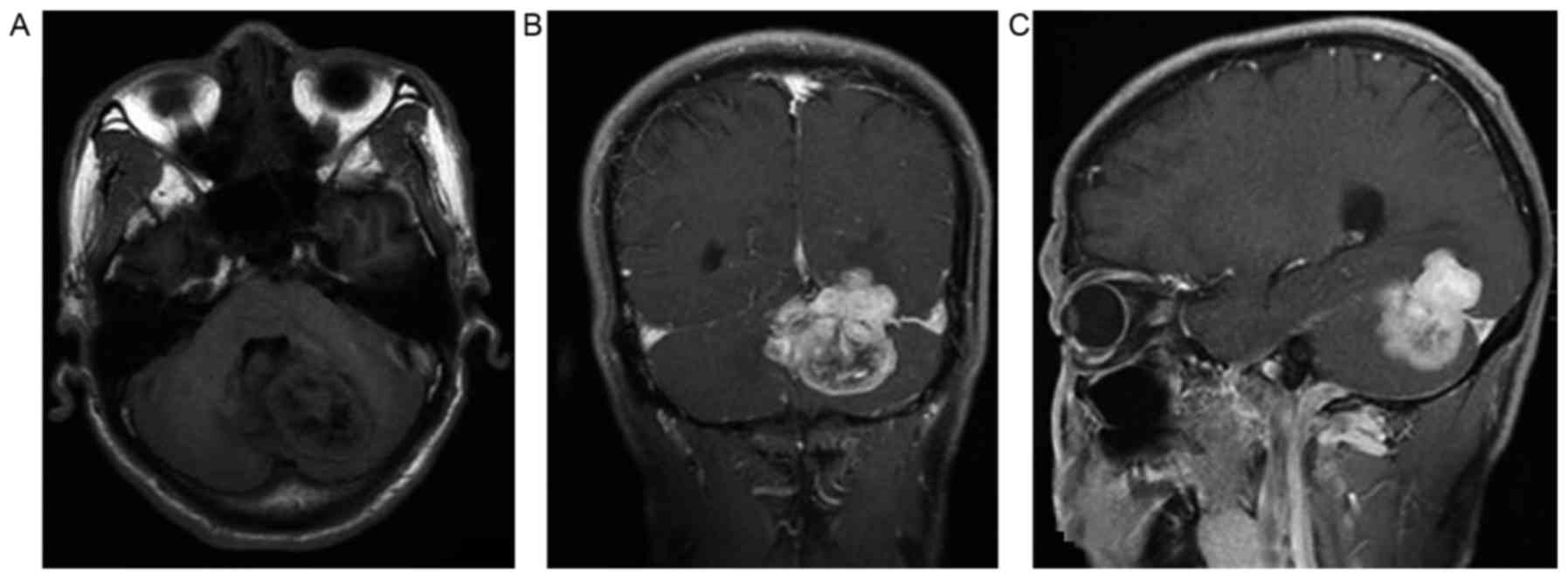
cerebelli and 3 cases spanned the falx (Figs. 1A-C and 2A-C). A total of 4 superficial tumors were
accompanied by adjacent bony destruction, but did not span the
tentorium cerebelli or falx. A single case was close to the falx,
and another case was located at the triangular area created by the
right lateral ventricles, without bone destruction and not crossing
the falx or tentorium. All 12 cases invaded and involved a wide
range of tissues. The margins of the lesions were irregular and 11
cases had distinct interfaces with the surrounding structures; only
1 case did not.
Shape and lobulation
Observed from MRI, the shapes of the 12 cases were
irregular. The margins of all the cases were not smooth, with 7
cases of deep lobulation and 5 cases of shallow lobulation.
MRI signal change
In the plain scans, the lesions of the 12 cases
usually exhibited inhomogeneous signals. The tumors were of low to
intermediate mixed signal intensity on the T1WI, and 3 had a medium
to slightly higher signal (which may be associated with a small
quantity of bleeding). A substantial region of the tumor was of
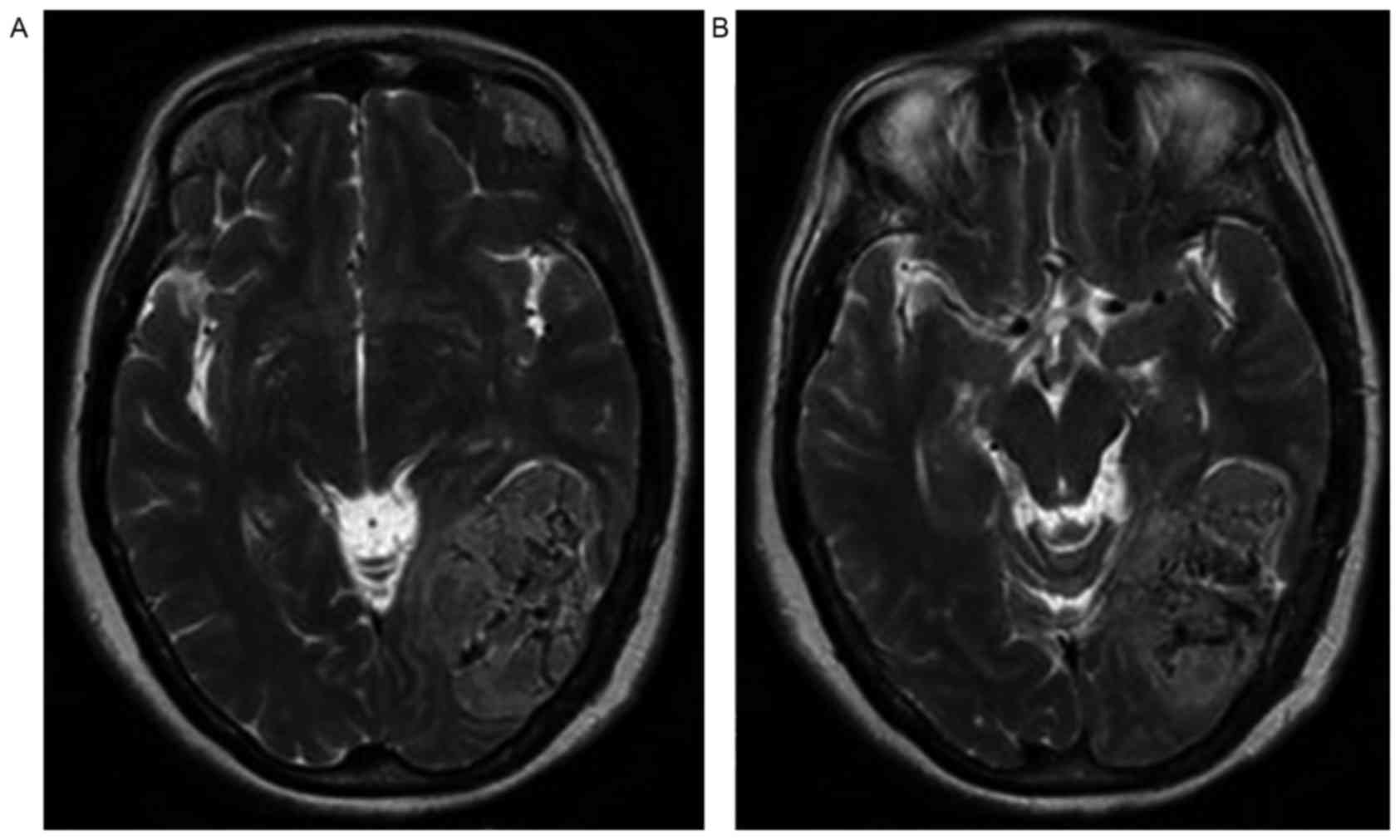
medium low or high signal on the T2WI (Fig. 3A and B). An irregular low signal
intensity was observed in the lesions of 7 cases. Combined with the
T1WI and T2WI plain scans, the outer limits of the substantial
region of the tumor also showed larger cystic areas in 5 cases.
Cystic vacuole lesions were modified in the cystic areas in 2
cases, a marked ‘capsule’ change was observed in the2 cases. In the
enhanced scans, a substantial region of the tumor was markedly
enhanced (Figs. 1A-C and 2A-C). There was no enhancement in the cystic
region. The cyst wall was circularly enhanced. The signal of the
tumor was extremely uneven in the 12 cases of the group.
Non-enhanced zones, largely in the tumors, shown as multiple small
strips, where patchy tracts could be found. The signal in the focus
became patchy and inhomogeneous in 10 cases. Non-enhanced zones in
the focus were shown as layered, with low signal, in 2 cases.
Tumor hemorrhage
Tumor hemorrhage occurred in only 3 cases, and could
be observed as a slightly higher signal on the plain T1WI scan.
There was no bleeding in the remaining 9 cases.
Tumor peripheral edema
Observed from MRI, there was mostly no or mild to
moderate edema around the lesion. No edema was present around the
lesions in 3 cases, while 4 cases presented with mild edema and 4
cases with moderate edema around the lesions. Only 1 case exhibited
severe edema around the lesion.
Representative cases
Case 1
In the representative case of a 40-year-old woman
shown in Fig. 1, the MR plain scan
showed an inhomogeneous signal bilateral soft-tissue mass of the
falx. The tumor spanned the falx, with deep lobulation, a
polycystic variable region and a clear edge. There was no edema
around the lesion, and the mass showed inhomogeneous enhancement
(Fig. 1).
Case 2
In the representative case of a 53-year-old man
shown in Fig. 2, MR enhancement
showed that there was an inhomogeneous signal soft-tissue mass
spanning the tentorium cerebelli. The tumor had deep lobulation,
partial cystic areas and a clear edge. There was mild peripheral
edema around the mass. A substantial region of the tumor was
markedly enhanced. Inhomogeneous enhancement was also present
(Fig. 2).
Case 3
In the representative case of a 52-year-old woman
shown in Fig. 3, the lesion was
located at the left occipital lobe and left cerebellum. The tumor
spanned the tentorium cerebelli and presented with an inhomogeneous
signal. The mass exhibited an uneven low signal on T2WI. An
irregular low signal intensity was observed in the lesion (Fig. 3).
Change in adjacent tissues
The lesions were close to the dura mater in 11
cases. The adjacent dura had no typical characteristics of the
‘dural tail sign’. Only 1 case was at the triangular area created
by the right lateral ventricles. There was no clear association
with the meninges in the MR images.
Discussion
The SFT has gradually been recognized as an
independent tumor since it was first reported in 1931. The tumor
was once believed to occur only in the pleura and to originate from
the tissue of the mesothelium (15).
Later, it was revealed that an SFT could occur almost anywhere in
the body, including the dura, orbital cavity, lungs, liver, kidneys
and particularly the pleura. Approximately 80% of SFTs originate in
the pleural space (2,13). Another previous study suggested that
60% were located outside of the pleura (16), as SFTs have occurred on the
falxcerebri, the convexity of the brain, the saddle area, the
posterior cranial fossa, the dura mater, the encephala and in the
CNS. SFTs often originate in the dura mater (17) and have been reported to be ubiquitous
neoplasms of mesenchymal origin (18). SFT originating from the meninges are
rare overall; the world literature on solitary fibrous tumors of
the central nervous system (CNS) between August 1996 and July 2011
were reviewed, only 220 cases were reported in CNS, and 170 cases
of SFT were originated from the meninges (19) ISFT is rare, accounting for ~0.6% of
all intracranial tumors (13,20,21).
Reports of meningeal SFTs have gradually increased, however, an
understanding of its image characteristics is lacking. In the
present study, the imaging characteristics of 12 cases were
discussed in order to assist the recognition and understanding of
this disease.
With regard to sex and age, the ages of the patients
at the diagnosis of SFT range greatly, for example, being reported
as between 5 and 87 years in one study (22). Another literature review reported a
median age of 44.5–55.0 years (23).
The ages of other reported cases have been within this range
(24–27). In the present study, the ages of the
patients ranged between 20 and 71 years, with a mean age of 48.8
years. The results from this group were similar to those from the
literature review. Viewpoints on the incidence in males and females
were highly varied. Certain studies identified no gender
differences (13,21,28).
However, another study (29) deemed
that the proportion of male patients with SFT was slightly higher
than that of female patients. Meningiomas were also reported to be
more common in middle-aged women. Other reports showed that the
male to female ratio of cases was 3:1, 4:14 and 23:20 (30–32). There
were 12 cases in the present study, with 6 men and 6 women, giving
a 1:1 ratio and no difference in sex predominance. The research
results in the present study were similar to that of certain
aforementioned studies and the study by Nawashiro et al
(29), but the number of patients in
these studies is small, and further observations are therefore
required to confirm the ratio between men and women.
The shape of an SFT has been reported as round, oval
or irregular (14). The result in the
present group is similar to that reported previously (13); that is, there were 4 oval cases and 8
irregular cases in the present group. All showed different degrees
of lobulation, namely, 7 cases presented with deep lobulation and 5
cases with shallow lobulation. With regard to the size, one
previous study (29) reported that
the tumor size was not the same in intracranial-SFT (I-SFT). The
smallest reported diameter was ~2 cm and the largest was 6 cm. The
sizes of the lesions differed greatly within the present study. The
majority of the lesions were large, with the largest diameter at
>7 cm, and the smallest was >3 cm in diameter. In addition,
the volume of the lesions was larger in all cases and the span of
the size of the lesions was also larger. However, the results of
the present study were similar to those of previous reports.
Meningeal SFT originates in the dura materand shares
characteristics of intracranial-extracerebral tumors. Therefore,
observations of the boundary between the tumor and surrounding
brain tissue show that the boundary of the lesion is clear
(21). The result of the present
study was similar to these findings. The boundaries in 11 cases
were clear, with an unclear boundary in 1 case only.
The pathological characteristics of the tumor tissue
in an SFT under the microscope show tumor cells that are spindled,
and cell arrangement that is sparse and densely interphase
(23). Different proportions of
collagenous fibers are present. The pathological characteristics of
the tumor are similar to that of a fibrous-type (fibroblastic)
meningioma. SFT is often clinically misdiagnosed as a meningioma or
another extra-axial tumor. Certain studies have, however, indicated
an SFT in the CNS at the preoperative diagnosis. The correct rate
of diagnosis of an SFT has been reported as almost 0 in other areas
of the pleura (13,14,21). The
present result was similar to this; 11 cases were not considered to
be an SFT based on the original preoperative MRI. Only 1 case was
considered to have a differential diagnosis of meningioma and SFT,
however, it was also originally misdiagnosed as a meningioma. The
preoperative diagnosis from MRI in the present study was consistent
with that reported in the literature. As there are a number of
similarities between the features of an SFT and that of certain
other tumors (particularly fibrous-type meningioma) with regard to
image changes and pathological features, the SFTs were easily
misdiagnosed upon imaging. Moreover, there was no specificity of
clinical symptoms and the tumors were mostly associated with the
dura mater. Therefore, it was often misdiagnosed as meningioma.
The T1WI features of a SFT in the CNS and meningioma
have been considered to be similar (22,31–33), with
the SFT displaying a slightly lower T1WI signal and a high or mixed
T2WI signal. The MR findings of I-SFT were described in the study
by Wang et al (24). It was
reported that I-SFT was usually a solid lesion with clear edges,
which exhibited a medium T1WI signal, a low T2WI signal and was
significantly enhanced upon contrast-enhanced scanning. Nawashiro
et al (29) suggested that SFT
was characterized by high and low hybrid signals in the T2
sequence, which is known as the ‘black and white sign’. Clarençon
et al (34) described the
specific performance of intracranial SFTs as a ‘yin-yang sign’, as
there were diametric boundaries between the low signal area and a
slightly high signal area. When enhanced, the low signal area on
plain scan was markedly enhanced.
Overall, the results were similar in the present
group, but there were a few differences. In the 12 cases in the
study, the signal was mainly a low or medium mixed T1WI signal.
There were a few patchy uneven medium and slightly higher signals
in 3 cases that may have been associated with a small quantity of
bleeding. When observing the cases using T2WI, the solid region of
the tumors showed high and low mixed signals. Among those, the
lesions in 7 cases could be clearly observed with a cord-like or
patchy low signal, referred to previously as the ‘black and white
sign’ or the ‘yin-yang sign’ (21).
Further detail was also observed in the present study and this may
be associated with the different collagen fiber content of the
tumor. The cystic lesion area presented with a low T1WI signal and
a high T2WI signal.
Signal changes of the tumor in the present study
were consistent with previous reports (22,29,33).
However, the results were slightly different from the ‘usually
solid lesions’ described in the study by Wang et al
(24). There were large cystic
regions in the certain tumors of the present study (5/12 cases,
41.67%), and there were small cystic areas in others (3/12 cases,
25.00%). Moreover, no fluid-attenuation fluid inversion recovery
(FLAIR) sequence imaging was performed in the present study, so the
characteristics of an SFT using FLAIR could not be compared. This
will require further studies, in which a greater number of cases
should be compared and analyzed.
Another MR sign is cystic degeneration and necrosis
in the lesion. There were large cystic areas in the tumor in 5
cases; a marked ‘capsule’ change was observed in 2 of the cases.
The result was the same as that in a previous study (2). The fact that there are large cystic
areas or ‘capsule’ changes in the tumor could be a potential
important feature of an SFT upon MRI.
Spindled fibroblast-like cells have been reported in
the SFT pathology (24). The tumors
contain a significant amount of collagen fiber and scar tissue. The
cell sparse zone and dense region is arranged in phases, and is
hypervascular. Large amounts of collagen fibers are present in the
cell sparse zone, so that a low T2WI signal is evident. Less
slender collagen fibers are apparent in the cell dense region, so a
moderate or slightly high T2WI signal is evident (2). Nawashiro et al (29) also analyzed the signal of the collagen
fibers in the tumor and found that they showed a low signal on the
T2 sequence and a high signal in the area of blood vessels. A
characteristic feature was a high and low mixed T2WI signal,
referred to as the ‘black and white sign’ in the aforementioned
text. This sign was often apparent in an SFT, but less so in
meningioma, and may be another important feature that may
contribute to the diagnosis of an SFT upon MRI.
The different contents of the tumor tissue influence
the signal changes found by MRI. Different contents of different
tissues lead to the different signal features in the images.
Therefore, the signal changes of an SFT are associated with the
different contents of different tissues (2,14). In the
present study, an abundant number of spindle cells was found upon
pathological examination. The tumor cells were spindle-shaped or
oval. SFT cells were small and present in spiral, woven and radial
arrangements. The cells were sparsely and densely arranged in
alternate phases. Proliferation of collagen fibers was found in the
tumor cells. Certain cases exhibited degeneration, including cystic
changes in the tumor. Mucus changes were also observed focally. The
T1WI and T2WI signal features of the cases were consistent with the
pathological characteristics, so inhomogeneous signals in an SFT
were often observed.
Due to an abundance of collagen fibers rich in blood
vessels, and the different contents of different tissues, the
degree of enhancement of the lesions varied with the contents of
the types of tissues. The degree of tumor enhancement was different
in the report literature (33), with
mild, moderate and significant uniform or uneven enhancement. The
degree of tumor enhancement was reported to be associated with the
collagen fibers, matrix and blood vessel content in the study by
Chong et al (35). As SFTs are
tumors with a rich blood supply, with a different organizational
structure, they were often noted as heterogeneous. Significantly
tumor enhancement was noted in the cell dense regions and blood
vessel-rich regions. However, enhancement was not evident in cell
sparse regions and areas lacking vascular structure (2), similar to the results in the present
study. The essence of the tumor (the cell dense region and blood
vessel-rich region) was markedly enhanced in the present study.
This phenomenon suggested that the SFT was rich in vascularity and
in accordance with the pathological findings. Moreover, the signal
in the tumor was uneven. The non-enhancement zone was visible in
the shape of a cord-like patch. The majority of the lesions were
significantly uneven in the enhanced scanning. Now, the changes in
the tumor were non-uniform. For certain tumors, the low signal
areas exhibited layered signal intensity. The non-uniform
distribution change was visible in 10 cases. The layered
non-enhancement low signal was found in the lesions of 2 cases in
the cell sparse region. These results were also consistent with the
pathological characteristics. The cystic areas were non-enhanced,
but the cyst wall showed a moderate circular enhancement. The
difference in enhancement in the cystic region and cystic wallof
the SFT also hinted at the diversity of the histological
compositions and enhancement patterns.
Dynamic enhancement was observed in a previous study
(36), but the dynamically enhanced
features of the SFT were influenced by a number of factors. Due to
the SFT being a tumor containing a rich blood supply with a large
number of collagen tissues and all types of modified ingredients,
its proportion of cells and collagen was different. Therefore, the
dynamically enhanced features of the SFT were influenced by a
number of factors. When the dynamic enhancement of the arterial
phase to the delayed phase was observed in certain reports, the
essence of the tumor showed a continuous filling type (13). Another study (34) reported that the low T2WI signal in the
tumor with significantly progressive enhancement was associated
with the density of the collagen fibers. The feature was a hint
that the tumor would later be diagnosed as an SFT. Due to the lack
of experience, only routine enhanced imaging was performed in the
preoperative imaging of the present study, and dynamic enhancement
was not performed, so these features could not be observed. This
therefore requires further research.
The literature does not include much discussion on
the growth mode of an SFT. MRI findings of an I-SFT were observed
in a study by Dai et al (28),
which found that the lesions crossed the tentorium cerebelli in 2
cases (2/8 cases), similar to the present study in which it crossed
the tentorium cerebelli in 3 cases (3/12 cases). By observing the
growth method of an SFT on MRI, it was found that the tumor was
often spanning growth (6/12 cases, 50%), meaning thatthe lesion
spanned both sides of the tentorium cerebella or the cerebral falx;
3 cases spanned the tentorium cerebelli and 3 cases spanned the
cerebral falx. Wide ranges were involved.
Literature information is lacking on the changes to
the adjacent skull bones, and the results presented vary. One study
(37) indicated that the adjacent
bone, nerve and brain parenchyma near the tumor were involved when
51 cases of CNS SFTs from the literature were reviewed. Other
studies (38,39) reported structural changes of the tumor
of the orbiton computed tomography (CT) scans. However, compression
bone remodeling was rare and without bony erosion in the imaging.
If bone destruction was observed clearly on CT, it would reflect
the malignant biological behavior of the tumor. However, other
studies (38,39) showed that the adjacent bone was
evidently damaged or corroded in the orbital SFT. The present
results were roughly consistent with the aforementioned studies in
regard to identification of bone structure alterations and done
destruction, 4 cases (4/12 cases) with adjacent bony destruction
were detected. The lesions in these 4 cases were located on the
convexity of the brain and were superficial tumors. Among the 4
cases, there was 1 case spanning the falx with adjacent bony
destruction. The other 3 cases did not span the tentorium or falx,
but invaded the adjacent skull. All the lesions in this group had a
large size and a wide range of tissues involved. Although the
boundary between the lesion and surrounding tissue was clear, the
edge of the lesion was mostly not neat.
Nawashiro et al (29) studied the changes in the adjacent
meninges. The study suggested that the SFT was attached to the dura
mater, but the ‘dural tail sign’ was rarely observed. It was
suggested that the formation mechanism of the SFT was different
from that of meningioma, but other studies presented different
views (21,29): It was discussed that the enhancement
was markedly inhomogeneous and the ‘dural tail sign’ may
occasionally be visible. In the present study, the tumor was
attached to the dura mater, but there was no typical ‘dural tail
sign’. This result was similar to that found by Nawashiro et
al (29).
I-SFT is often derived from the meninges; it has
characteristics of extra cerebral tumors, and is coated by a
fibrous capsule. Edema in the peripheral brain parenchyma is rare
(21). In the present study, mild or
no edema could be commonly found. The cases were as follows: 3
cases without edema and 4 cases with mild edema. Moderate edema was
observed in 4 cases and only 1 case presented with severe edema.
This last case may have been associated with the size of the lesion
and oppression of the adjacent vein or sinus. This situation was
consistent with the experimental results reported in other studies
(40).
The occurrence rate of an SFT is low, and
clinically, doctors are unfamiliar with the tumor (28). The characteristics of the tumor are
similar to other tumors upon imaging. Moreover, the tumor has no
specificity in clinical symptoms, so it is often misdiagnosed as
other tumors (28). While SFT should
be differentiated from other tumors occurring in the meninges, the
diagnosis may easily be confused as an SFT is morphologically
similar to other tumors, such as meningioma, particularly fibrous
(fibroblastic) meningioma, angiomatous-type meningioma,
hemanyiopericytoma, neurilemmoma and hemangiopericytoma (28). The differential diagnosis requires
further research. Although the signal changes could reflect more
pathological changes in the lesions, the final diagnosis and
differential diagnosis must be confirmed by pathological and
immunohistochemical analysis (41).
In summary, intracranial SFTs often occur in or
close to the meninges. The MRI features of a meningeal SFT are
similar to other tumors in the CNS, such as fibrous meningioma.
However, certain individual characteristics are also present. The
present study and literature review indicated that meningeal SFTs
were often solitary, large, soft-tissue masses with an irregular
edge and a clear boundary, with a lobulated contour and involving a
wide range of tissues. The tumor often grew spanning the falx or
the tentorium cerebelli. A significantly inhomogeneous signal was
often found upon MRI, particularly where the T2WI signal was low or
low-high mixed. This characteristic is often called the ‘black and
white sign’ or ‘yin-yang sign’. The majority of the lesions were
significantly uneven in the enhanced scanning. The changes in the
tumor were map-like (‘map sign’). For certain tumors, the low
signal areas looked like a ‘chrysanthemum’ (called ‘chrysanthemum
sign’), lacking the typical ‘dural tail sign’. There was a large
cystic area in the tumor, particularly in the periphery of the
lesion, with little or no bleeding. The aforementioned features are
useful for the identification of SFTs and other tumors, such as
fibrous meningioma.
In addition, only MR scans were performed, not CT
scans, in the present study. No dynamically enhanced scan was
performed and the characteristics of changes in gradual enhancement
in dynamic scanning requires additional investigation. Moreover,
the number of cases in this study was small and therefore
insufficient imaging characteristics may have been studied. In
future studies, the characteristics of an SFT in MRI require
further analysis.
References
|
1
|
Alawi F, Stratton D and Freedman PD:
Solitary fibrous tumor of the oral soft tissues: A
clinicopathologic and immunohistochemical study of 16 cases. Am J
Sur Pathol. 25:900–910. 2001. View Article : Google Scholar
|
|
2
|
Wagner E: Das tuberkelahnliche lymphadenom
(der cytogene oder reticulirte tuberkel). Arch Heilk (Leipzig).
11:4971870.(In German).
|
|
3
|
Klemperer P and Coleman BR: Primary
neoplasms of the pleura: A report of five cases. Am J Ind Med.
22:1–31. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
de Perrot M, Fischer S, Bründler MA,
Sekine Y and Keshavjee S: Solitary fibrous tumors of the pleura.
Ann Thorac Surg. 74:285–293. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guinee DG and Allen TC: Primary pleural
neoplasia: Entities other than diffuse malignant mesothelioma. Arch
Pathol Lab Med. 132:1149–1170. 2008.PubMed/NCBI
|
|
6
|
Zhang ZL, Gu XW, Xiao Q and Wang CM:
Clinicopathological analysis of solitary fibrous tumor in 11
patients. Cancer Res Clin. 27(107–108): 1122015.(In Chinese).
|
|
7
|
Mekni A, Kourda J, Hammouda KB, Tangour M,
Kchir N, Zitouna M and Haouet S: Solitary fibrous tumour of the
central nervous system: Pathological study of eight cases and
review of the literature. Pathology. 41:649–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carneiro SS, Scheithauer BW, Nascimento
AG, Hirose T and Davis DH: Solitary fibrous tumour of the meninges:
A lesion distinct from fibrous meningioma. A clinicopathologic and
immunohistochemical study. AM J Clin Pathol. 106:217–224. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lococo F, Cesario A, Cardillo G, Filosso
P, Galetta D, Carbone L, Oliaro A, Spaggiari L, Cusumano G,
Margaritora S, et al: Malignant solitary fibrous tumors of the
pleura: Retrospective review of a multicenter series. J Thorac
Oncol. 7:1698–1706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Val-Bernal JF, Mayorga M, Fernández F,
Parra A, Crespo J and García-Polavieja M: Solitary fibrous tumors
arising from the mesentery of adult patients. Report of two cases
and review of the literature. Rom J Morphol Embryol. 55:203–207.
2014.PubMed/NCBI
|
|
11
|
Bisceglia M, Dimitri L, Giannatempo G,
Carotenuto V, Bianco M, Monte V, D'Angelo V and Magro G: Solitary
fibrous tumor of the central nervous system: Report of an
additional 5 cases with comprehensive literature review. Int J Surg
Pathol. 19:476–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yan J, Ahl KL, Manning KA, Mann FA and
Lewis DH: Radiology-pathology conference: 18F-FDG PET-CT imaging of
solitary fibrous tumor of the pleura. Clin Imaging. 37:598–601.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cui J, Han LX, Cao HX, Du WQ, Zhang L,
Wang J and Chen G: MRI characteristic of intracranial solitary
fibrous tumors. Radiol Pract. 31:224–227. 2016.(In Chinese).
|
|
14
|
Liu H, Yang ZQ, Wang YT, Liu X, Chen H,
Zhang TJ and Luo KJ: CT and MRI findings of intracranial solitary
fibrous tumor. J Clin Radiol. 30:1265–1268. 2011.(In Chinese).
|
|
15
|
Chen H, Zeng XW, Wu JS, Dou YF, Wang Y,
Zhong P, Xu R, Jiang CC and Wang XQ: Solitary fibrous tumor of the
central nervous system: A clinicopathologic study of 24 cases. Acta
Neurochir (Wien). 154:237–248. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhanlong M, Haibin S, Xiangshan F,
Jiacheng S and Yicheng N: Variable solitary fibrous tumor
locations: CT and MR imaging features. Medicine (Baltimore).
95:e30312016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Smith AB, Horkanyne-Szakaly I, Schroeder
JW and Rushing EJ: From the radiologic pathology archives: Mass
lesions of the dura: Beyond meningioma-radiologic-pathologic
correlation. Radiographics. 34:295–312. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Savastano S, d'Amore ES, Beghetto M, Borgo
DD, Franceschetti I and Capalbo M: A presacral solitary fibrous
tumor with extramedullary hematopoiesis: Radiologic and pathologic
findings. Rare Tumors. 5:e612013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bisceglia M, Galliani C, Giannatempo G,
Lauriola W, Bianco M, D'angelo V, Pizzolitto S, Vita G, Pasquinelli
G, Magro G and Dor DB: Solitary fibrous tumor of the central
nervous system: A 15-year literature survey of 220 cases (August
1996-July 2011). Adv Anat Pathol. 18:356–392. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tihan T, Viglione M, Rosenblum MK, Olivi A
and Burger PC: Solitary fibrous tumors in the central nervous
system. A clinicopathologic review of 18 cases and comparison to
meningeal hemangiopericytomas. Arch Pathol Lab Med. 127:432–439.
2003.PubMed/NCBI
|
|
21
|
Ke DB, Liu WK, Zhang S, Yu Y, Zhang YK and
Hui XH: MRI manifestations of intracranial solitary fibrous tumor.
West China Med J. 32:46–50. 2017.(In Chinese).
|
|
22
|
Fargen KM, Opalach KJ, Wakefield D, Jacob
RP, Yachnis AT and Lister JR: The central nervous system solitary
fibrous tumour: A review of clinical, imaging and pathologic
findings among all reported cases from 1996 to 2010. Clin Neurol
Neurosurg. 113:703–710. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Wang H, Wang S, Miao J, Piao Z
and Dong Y: Clinicopathological analysis of solitary fibrous tumor.
Chinese-German J Clin Oncol. 11:282–284. 2012. View Article : Google Scholar
|
|
24
|
Wang C, Manucha V, Faro S, Weaver M and
Mukherjee AL: Fourth ventricular solitary fibrous tumor: A case
report and review of the literature. J Med Case Rep. 6:2052012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Robert T, Duc C, San Millán Ruíz D and
Morard M: Solitary fibrous tumour with intramedullary component:
Case report and review of the literature. Neurol Neurochir Pol.
48:144–149. 2014.PubMed/NCBI
|
|
26
|
Treglia G, Oragano L, Fadda G, Raffaelli
M, Lombardi CP, Castaldi P and Rufini V: A rare case of solitary
fibrous tumor of the adrenal gland detected by (18)F-FDG PET/CT.
Clin Nucl Med. 39:475–477. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong A, Zuo C, Wang Y and Cui Y: Enhanced
CT and FDG PET/CT in malignant solitary fibrous tumor of the lung.
Clin Nucl Med. 39:488–491. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dai Y, Long LL and Ye W: MR imaging
features of intracranial solitary fibrous tumors. Radiol Pract.
30:127–130. 2015.(In Chinese).
|
|
29
|
Nawashiro H, Nagakawa S, Osada H, Katoh H,
Ohnuki A, Tsuzuki N, Miyazawa T, Shima K, Ogata S and Aida S:
Solitary fibrous tumor of the meninges in the posterior cranial
fossa: Magnetic resonance imaging and histological correlation-case
report. Neurol Med Chir (Tokyo). 40:432–434. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Caroli E, Salvati M, Orlando ER, Lenzi J,
Santoro A and Giangaspero F: Solitary fibrous tumors of the
meninges: Report of four cases and literature review. Neurosrug
Rev. 27:246–251. 2004.
|
|
31
|
Tihan T, Viglione M, Rosenblum MK, Olivi A
and Burger PC: Solitary fibrous tumors in the central nervous
system. A clinicopathologic review of 18 cases and comparison to
meningeal hemangiopericytomas. Arch pathol Lab Med. 127:432–439.
2003.PubMed/NCBI
|
|
32
|
Metellus P, Bouvier C, Guyotat J, Fuentes
S, Jouvet A, Vasiljevic A, Giorgi R, Dufour H, Grisoli F and
Figarella-Branger D: Solitary fibrous tumors of the central nervous
system: Clinicopathological and therapeutic considerations of 18
cases. Neurosurgery. 60:715–722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang XQ, Zhou Q, Li ST, Liao CL, Zhang H
and Zhang BY: Solitary fibrous tumors of the central nervous
system: Clinical features and imaging findings in 22 patients. J
Comput Assist Tomogr. 37:658–665. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Clarençon F, Bonneville F, Rousseau A,
Galanaud D, Kujas M, Naggara O, Cornu P and Chiras J: Intracranial
solitary fibrous tumor: Imaging findings. Eur J Radiol. 82:387–394.
2011. View Article : Google Scholar
|
|
35
|
Chong S, Kim TS, Cho EY, Kim J and Kim H:
Benign localized fibrous tumour of the pleura: CT features with
histopathological correlations. Clin Radiol. 61:875–882. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang WD, Chen JY, Cao Y, Liu QY and Luo
RG: Computed tomography and magnetic resounance imaging findings of
solitary fibrous tumors in the pelvis: Correlation with
histopathological findings. Eur J Radiol. 78:65–70. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang BT, Song ZL, Wang YZ, Dong JY and
Wang ZC: Solitary fibrous tumor of the sinonasal cavity: CT and MR
imaging findings. AJNR Am J Neuroradiol. 34:1248–1251. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Glazer-Hockstein C, Syed NA, Warhol M and
Gausas RE: Malignant solitary fibrous tumor metastatic to the
orbit. Ophthal Plast Reconstr Surg. 20:471–473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Woo KI, Suh YL and Kim YD: Solitary
fibrous tumor of the lacrimal sac. Ophthal Plast Reconstr Surg.
15:450–453. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Han YP, Zhang YT, Liu JL, Zhang XL and
Zhou JL: Comparison of imaging and pathological findings of
intracranial hemangiopericytoma and solitary fibrous tumor. Chin J
Magn Reson Imaging. 6:917–924. 2015.(In Chinese).
|
|
41
|
Sun J, Yu XR, Shi BB, Zheng J and Wu JT:
CT features of retroperitoneal solitary fibrous tumor: Report of
three cases and review of the literature. World J Surg Oncol.
12:3242014. View Article : Google Scholar : PubMed/NCBI
|