Introduction
Breast cancer is one of the most commonly diagnosed
malignant tumors in women, and, based on data from 2011, its
incidence is increasing (1). Triple
negative breast cancer (TNBC) is characterized by the negative
expression of the estrogen receptor, progesterone receptor and
human epidermal growth factor receptor 2, and accounts for 10–20%
breast cancer cases worldwide (1,2).
Characteristically, TNBC is associated with early age of onset,
high degree of malignancy, high recurrence rate, early metastasis
and poor prognosis (2,3). Receptor interacting protein 140 (RIP140)
is a nuclear receptor transcriptional co-regulator that controls
the transcription of target genes in several tissues, including
adipose, skeletal muscle, cardiac muscle, liver and tumor tissues
(4,5).
It has been demonstrated to serve notable roles in metabolism,
inflammation and tumor development. RIP140 is a metabolic switch
that regulates a number of metabolic pathways. As a co-repressor,
RIP140 facilitates high-fat diet-induced obesity and induces
insulin resistance. As a co-activator, RIP140 activates nuclear
factor (NF)-κB and promotes the expression of pro-inflammatory
cytokines, including tumor necrosis factor (TNF)-α and interleukin
(IL)-6 in immune cells (4–6). RIP140 also affects tumorigenesis and
tumor metastasis via E2F transcription factor and Wnt/adenomatous
polyposis coli (APC)/β-catenin signaling pathways (5,7). Aziz
et al (8) demonstrated that
inhibition of RIP140 inhibited the growth of breast cancer cells
in vivo and in vitro. However, to the best of our
knowledge, no previous research has investigated the association
between the expression of RIP140 in TNBC and the postoperative
prognosis of patients with TNBC. Thus, the aim of the present study
was to investigate the expression of RIP140 in TNBC, and the
association between RIP140 expression and postoperative prognosis
of patients with the disease.
Materials and methods
Cell culture and lentivirus
transfection
The TNBC cell line, MDA-MB-231, was purchased from
the Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences (Shanghai, China) and maintained in Dulbecco's Modified
Eagle's Medium (DMEM; HyClone; GE Healthcare, Chicago, IL, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone; GE
Healthcare Life Sciences, Logan, UT, USA) and 1% penicillin and
streptomycin (HyClone; GE Healthcare Life Sciences) at 37°C in 5%
CO2.
The short hairpin RNA (shRNA)-RIP140 lentivirus and
empty lentivirus were purchased from Shanghai GeneChem Co., Ltd.
(Shanghai, China). Cells transfected with an empty lentivirus
served as the negative control (NC) group, and untreated cells
served as the control group. Lentiviral cell transduction was
performed as previously described by Ho et al (6).
Clinical breast cancer samples
Tissue samples were collected from 179 patients with
breast cancer who had undergone modified radical mastectomies at
903 Hospital (Jiangyou, China) between February 2005 and February
2008. Patient age ranged between 23 and 81 years old (median age,
54.2±19.7 years). Postoperative pathological examination defined 41
cases of TNBC and 138 cases of non-TNBC. All tissues were used for
immunohistochemistry and western blot analysis, with 10 samples of
TNBC paracancerous tissue and 10 samples of non-TNBC paracancerous
tissue from these patients also analyzed as controls. The
clinicopathological information of the two groups of patients is
provided in Table I. The present
retrospective study was approved by the Research Ethics Committee
of 903 Hospital (Jiangyou, China).
 | Table I.RIP140 expression exhibited by TNBC
and non-TNBC breast cancer tissues and basic clinicopathological
patient information. |
Table I.
RIP140 expression exhibited by TNBC
and non-TNBC breast cancer tissues and basic clinicopathological
patient information.
|
|
| RIP140
expression |
|
|---|
|
|
|
|
|
|---|
| Parameter | Patients, n | Weak, n | Strong, n | P-value |
|---|
| Total | 179 | 115 | 64 |
|
| TNBC | 41 | 6 | 35 | <0.001 |
| Non-TNBC | 138 | 109 | 29 |
|
| Age, years |
|
|
|
|
| ≥50 | 141 | 78 | 63 | 0.995 |
|
<50 | 38 | 21 | 17 |
|
| Tumor diameter,
cm |
|
|
|
|
| ≥5 | 61 | 28 | 33 | 0.069 |
|
<5 | 118 | 71 | 47 |
|
| Lymphatic
metastasis |
|
|
|
|
| Yes | 82 | 36 | 46 | 0.196 |
| No | 97 | 52 | 45 |
|
Western blot analysis
After 72 h of transfection of MDA-MB-231 cells,
total protein was extracted using the Whole Protein Extraction kit
(Nanjing KeyGen Biotech Co., Ltd., Nanjing, China), according to
the manufacturer's protocol. The same method was used to extract
protein from paracancerous, non-TNBC and TNBC tissues. The protein
concentrations were determined using the BCA kit (Boster Biological
Technology, Pleasanton, CA, USA), according to the manufacturer's
protocols. Total cellular protein (80 µg) was mixed with loading
buffer (Boster Biological Technology) and boiled in 100°C for 5
min, prior to protein separation by 8% SDS-PAGE. Proteins were then
transferred onto polyvinylidene difluoride membranes (Merck KGaA,
Darmstadt, Germany). Subsequent to blocking in 5% skim-milk, the
membranes were incubated overnight at 4°C with anti-RIP140
(dilution, 1:1,000; catalog no. ab42126; Abcam, Cambridge, UK) or
anti-GAPDH (dilution, 1:1,000; catalog no. sc293335; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) primary antibodies in
Tris-buffered saline with 0.01% Tween-20 (TBST) with 5% bovine
serum albumin (BSA). The following day, the membranes were
incubated with the corresponding horseradish peroxidase
(HRP)-conjugated mouse anti-goat secondary antibody (dilution,
1:2,000; catalog no. sc-516246; Santa-Cruz Biotechnology, Inc.) at
37°C for 30 min prior to washing three times with TBST. Protein
signals were analyzed using Quantity One Software (version 4.62;
Bio-Rad Laboratories, Inc., Hercules, CA, USA) following incubation
with an enhanced chemiluminescence reagent (EMD Millipore,
Billerica, MA, USA), according to the manufacturer's protocol. The
PVDF membrane was purchased from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA).
Establishment of a subcutaneous breast
cancer model in BALB/c nude mice
A total of 30 4-week-old BALB/c nude mice (15 g)
were purchased from Chongqing Medical University Experimental
Animal Center (0005818; Chongqing, China). Mice were housed in a
temperature at 27°C, 50% relative humidity, alternately exposed to
light for 10 h and without light for 14 h pathogen-free
environment. All mice had free access to food and water.
Subcutaneous tumors were generated by subcutaneous injection of
5×106 MDA-MB-231 cells (shRNA-RIP140, NC or control)
under the right forelimb of each mouse. A total of 10 nude mice
were sacrificed each week the tumors were excised for volume
measurement and paraffin sectioning for nuclear proliferating
antigen (Ki-67) immunohistochemistry. All experimental procedures
were conducted in accordance with the Guide for the Care and Use of
Laboratory Animals and approved by the ethical guidelines for
animal experiments of 903 Hospital.
Immunohistochemistry
The expression of RIP140 in clinical breast cancer
tissues and the expression of Ki-67 in the subcutaneous tumor
tissues of nude mice were detected by immunohistochemical staining.
All tissue samples were fixed with 30% formalin at 28°C for 60 min
and then embedded with paraffin. The samples were sectioned to a
thickness of 5-mm. The sections were incubated at 60°C for 1 h,
then, placed in xylene I and xylene II for 30 min in turn. All the
slices were dehydrated in a graded alcohol series (100, 95, 90 and
85%). Slides were placed in 0.01 M potassium citrate solution and
microwaved at 90°C for 10 min prior to cooling at room temperature.
Slides were then rinsed in PBS 3 times. Endogenous peroxidase
activity was blocked with 3% H2O2 and goat
serum (Haoranbio, Shanghai, China) at 37°C for 30 min, then
immediately incubated with anti-RIP140 primary antibody (dilution,
1:500; catalog no., ab42126; Abcam) or anti-Ki-67 primary antibody
(dilution, 1:100; catalog no., sc56319; Santa Cruz Biotechnology,
Inc.) at 4°C overnight. The next day, the slides were washed with
PBS 3 times prior to incubation with corresponding HRP-conjugated
mouse anti-goat secondary antibody (dilution, 1:100; catalog no.,
sc-516246; Santa-Cruz Biotechnology, Inc.) at 37°C for 1 h. The
slides were then washed three more times in PBS. The slides were
stained with DAB (Hengyuan Bio, Shanghai, China) at room
temperature for 3–5 min, then rinsed 3 times with tap water, and
dyed again prior to sealing using mounting medium (Hengyuan Bio).
Images were captured using a Leica DMLA light microscope (Leica
Microsystems, Inc., Buffalo Grove, IL, USA). Brown nuclear staining
identified RIP140-positive cells. Tissues with <50% positive
staining were considered to exhibit weakly positive RIP140
expression, while tissues with >50% positive staining were
considered to exhibit strongly positive RIP140 expression (9).
Statistical analysis
Statistical analyses were performed using SPSS 16.0
software (SPSS, Inc., Chicago, IL, USA). Statistical differences
between two groups were determined by Student's t-test. To compare
≥3 groups, one-way analysis of variance followed by Bonferroni's
post-hoc test was used when all assumptions (equal variance and
normal distribution) were satisfied. All data are expressed as the
mean ± standard deviation. The Kaplan-Meier method and log-rank
test were used to perform survival analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
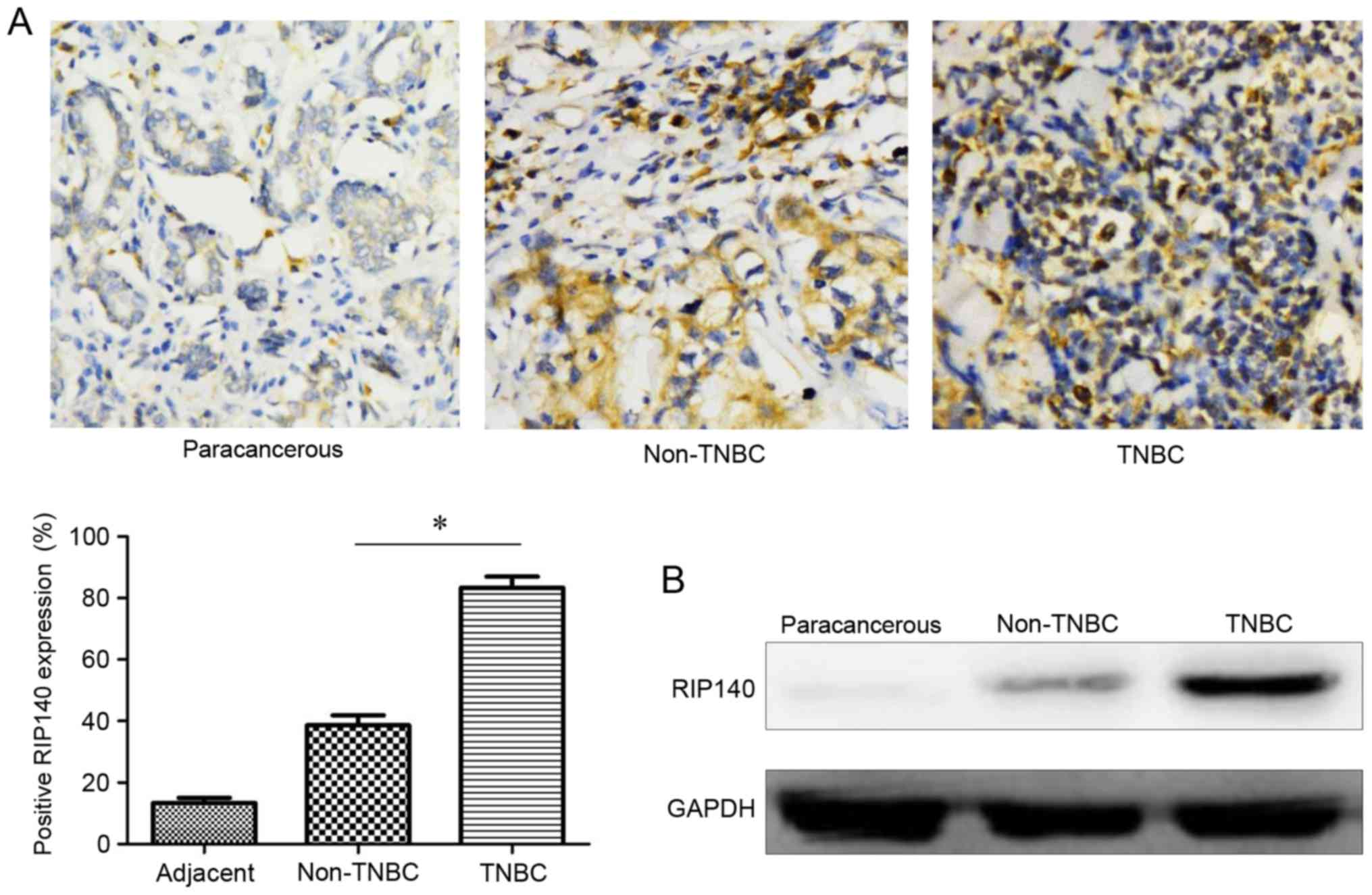
High expression of RIP140 in TNBC
As depicted in Fig.
1A, weakly positive RIP140 expression was exhibited by
paracancerous tissues (14.7±5.5% positive staining) and non-TNBC
tissues (39.6±10.1% positive staining), whereas TNBC tissues
exhibited strongly positive expression in TNBC tissues (83.5±11.4%
positive staining). The expression of RIP140 was significantly
increased in TNBC tissues compared with non-TNBC tissues
(P<0.05). To verify these results, western blotting was used to
determine the protein expression level of RIP140 in three groups of
tissue. As demonstrated in Fig. 1B,
the protein expression level of RIP140 in TNBC was increased
compared with non-TNBC tissues, and the expression of RIP140 was
lowest in paracancerous tissues.
The association of RIP140 expression
with clinicopathological features
As demonstrated in Table
I, there was a significant difference between the expression of
RIP140 in TNBC tissues and non-TNBC tissues (P<0.05). However,
there was no difference in age, tumor size or lymph node metastasis
between the two groups (P>0.05).
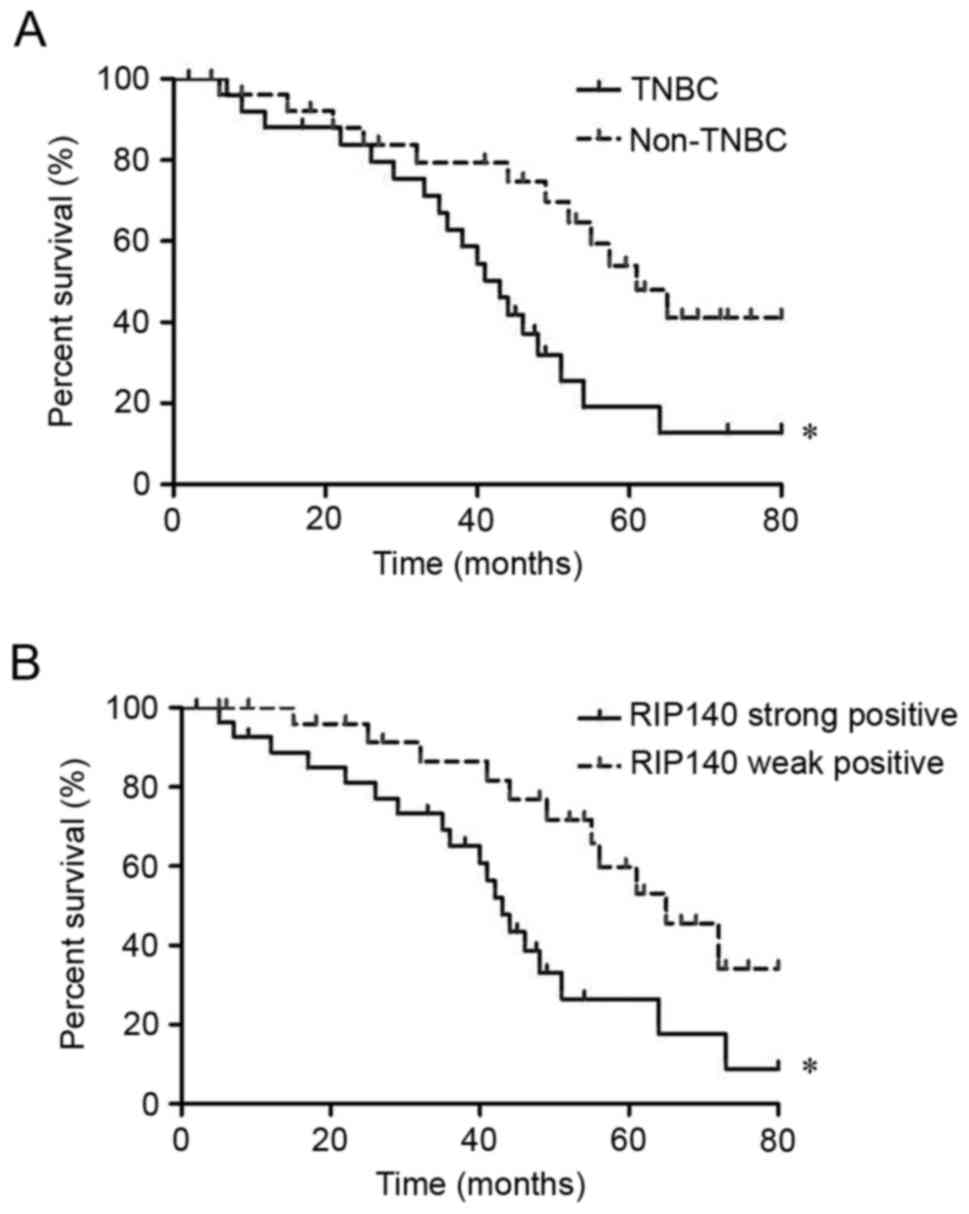
The association of RIP140 expression
with postoperative prognosis of patients with breast cancer
Patient follow-up data were used to analyze the
association of TNBC vs. non-TNBC, and weak vs. high RIP140
expression, with postoperative prognosis of the patients. As
depicted in Fig. 2A, the median
survival time of patients with TNBC was 34.2±5.1 months, whereas
the median survival time of patients with non-TNBC was 62.5±7.4
months. The prognosis of patients with TNBC was relatively poor
compared with that of non-TNBC patients (P=0.043). As depicted in
Fig. 2B, the median survival time was
33.8±6.3 months in breast cancer patients exhibiting strong RIP140
expression compared with 63.2±10.3 months in patients exhibiting
weak RIP140 expression. The overall survival rate of patients
exhibiting strong positive expression of RIP140 was low compared
with patients exhibiting weakly positive expression of RIP140
(P=0.046).
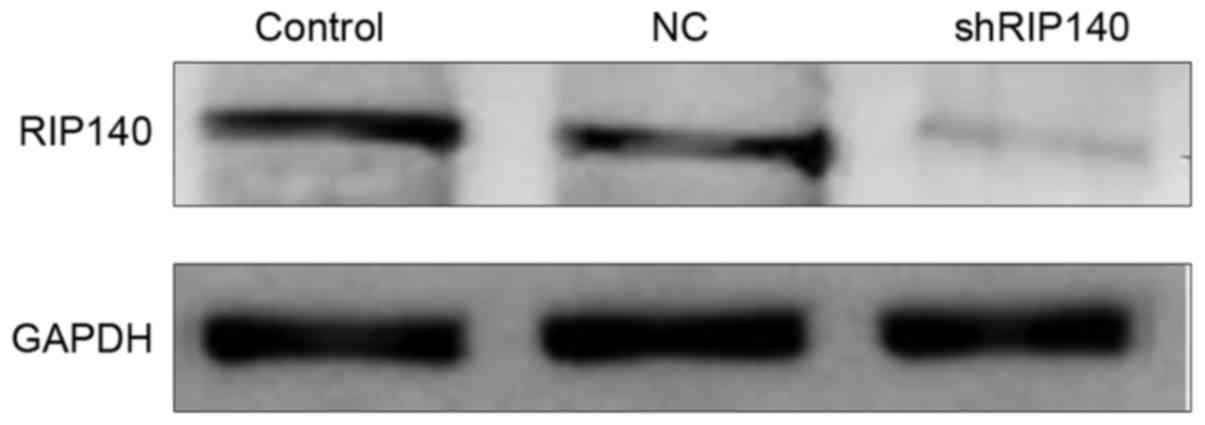
The expression of RIP140 in MDA-MB-231
cells significantly decreases following transfection with
shRNA-RIP140
After 72 h of transfection, western blotting was
used to analyze the protein expression level of RIP140 in cells of
the three groups. As depicted in Fig.
3, the protein expression level of RIP140 in the shRNA-RIP140
group was significantly lower than that in the NC and control
groups (P<0.05).
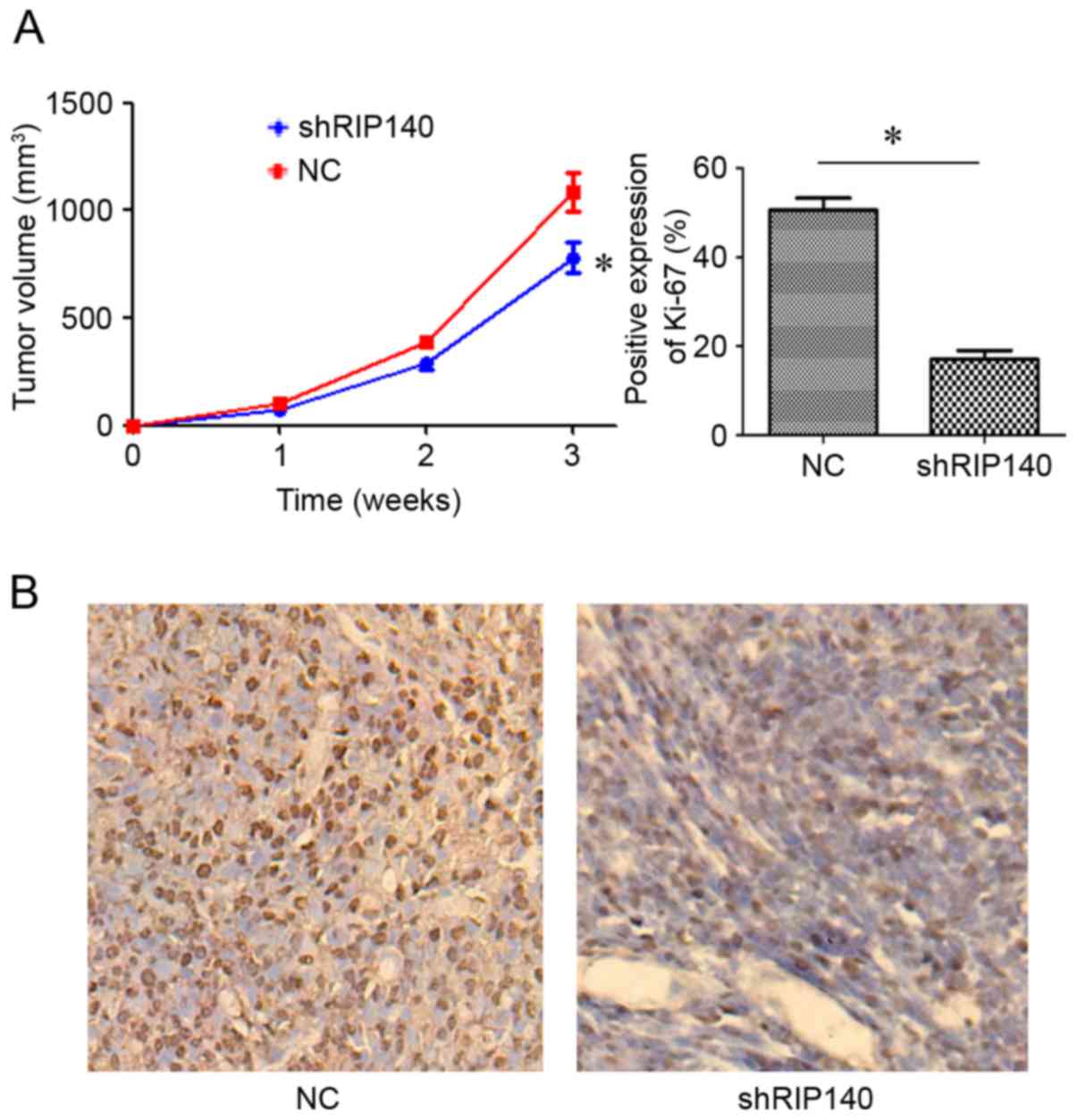
Downregulation of RIP140 expression in
MDA-MB-231 cells inhibits the growth and proliferation of TNBC
cells in BALB/c nude mice
The results of immunohistochemistry and western
blotting in clinical breast cancer samples demonstrated that RIP140
was highly expressed in TNBC tissues, and that RIP140 expression
was negatively associated with postoperative prognosis, indicating
that high expression of RIP140 accelerated the development of
breast cancer. To investigate whether silencing the expression of
RIP140 inhibits the occurrence and development of TNBC, RIP140 was
downregulated in MDA-MB-231 cells using a shRNA-RIP140 lentivirus,
and used to generate subcutaneous tumors in mice. The tumor volume
of the shRNA-RIP140 group was significantly lower than that of NC
group (P<0.05; Fig. 4A). The
expression intensity of Ki-67 in the shRNA-RIP140 group was lower
than that in the NC group (P<0.05; Fig. 4B). These results indicated that
downregulation of RIP140 in TNBC MDA-MB-231 cells may inhibit the
growth of TNBC.
Discussion
Breast cancer is among the most common types of
malignant tumor worldwide, and presents a serious threat to the
physical and mental health of women (1,2). In 2014,
the number of new cases of breast cancer in China accounted for
12.2% of the new cases worldwide, and the number of mortalities due
to breast cancer accounted for ~9.6% of the number mortalities due
to breast cancer worldwide (10). At
present, the major treatment modalities for breast cancer include
surgical resection, chemotherapy, radiotherapy and biological
therapy (11,12). Patients with non-TNBC have a better
prognosis when diagnosed and treated early (13); however, TNBC is characterized by an
early age of onset, high degree of malignancy, high recurrence
rate, early metastasis and poor prognosis (14). Previous research has demonstrated that
patients with TNBC have a median survival time of 4.2 years between
the date of surgery and the date of mortality, which is
significantly lower than the survival time of non-TNBC patients (6
years) (14,15). Thus, the identification of an
effective therapeutic target for TNBC is urgently required.
RIP140 serves notable roles in metabolism, the
inflammatory response and tumor development (4–6). Recent
studies have demonstrated that RIP140 expression, and the
associated pathological effects, vary between types of tumor. Zhang
et al (16) observed that
RIP140 was expressed at low expression in liver cancer, and that
downregulation of RIP140 expression in hepatocellular cells
promoted the development of liver cancer. Upregulation of RIP140
expression in hepatocellular carcinoma cells inhibited the growth
and proliferation of liver cancer. RIP140 was hypothesized to
negatively regulate the β-catenin/T-cell factor signaling pathway,
thus inhibiting the growth of hepatocellular carcinoma cells.
Lapierre et al (17) revealed
that expression of RIP140 in colon carcinoma was low, and that
overexpression of RIP140 in colon cancer cells promoted the
expression of adenomatous polyposis coli, which inhibited the
growth and metastasis of colon cancer by inhibiting the
Wnt/APC/β-catenin signaling pathway. However, Aziz et al
(8) demonstrated that RIP140 was
highly expressed in breast cancer tissues, and that inhibition of
RIP140 expression in breast cancer cells inhibited growth,
proliferation and invasion in vivo and in vitro.
In the present study, RIP140 expression was
investigated in TNBC tissues and non-TNBC tissues. The association
between RIP140 expression and the postoperative prognosis of
patients with breast cancer was also studied. The expression of
RIP140 in MDA-MB-231 cells was silenced using a shRNA, and the
cells were subcutaneously injected into BALB/c nude mice to
establish an in vivo TNBC model. The results demonstrated
that RIP140 expression in TNBC tissue was significantly higher than
that in non-TNBC tissue, and that RIP140 expression was higher in
the two types of breast cancer tissue than in paracancerous tissue.
Silencing the expression of RIP140 in MDA-MB-231 cells inhibited
the growth and proliferation of subcutaneous tumors in BALB/c nude
mice. Furthermore, the overall survival rate of patients exhibiting
strong RIP140 expression was lower than that of patients exhibiting
weak RIP140 expression.
In summary, data from the present and previous
studies indicated that, different types of tumor tissue exhibited
varying levels of expression of RIP140, with low expression in
liver cancer and colon cancer, and high expression in breast cancer
tissues. The abnormal expression of RIP140 in different tumor
tissues was associated with the risk of occurrence and development
of tumors (5,8). The present study confirmed that the
abnormal expression of RIP140 is associated with the malignant
biological behavior and prognosis of TNBC, serving a notable role
in the growth and proliferation of TNBC cells. The present study
also indicated that RIP140 represents a novel therapeutic target
for TNBC.
References
|
1
|
Warner E: Clinical practice, breast-cancer
screening. N Eng J Med. 365:1025–1032. 2011. View Article : Google Scholar
|
|
2
|
Zeichner SB, Terawaki H and Gogineni K: A
review of systemic treatment in metastatic triple-negative breast
cancer. Breast Cancer (Auckl). 10:25–36. 2016.PubMed/NCBI
|
|
3
|
Collignon J, Lousberg L, Schroeder H and
Jerusalem G: Triple-negative breast cancer: Treatment challenges
and solutions. Breast Cancer (Dove Med Press). 8:93–107.
2016.PubMed/NCBI
|
|
4
|
Rosell M, Jones MC and Parker MG: Role of
nuclear receptor corepressor RIP140 in metabolic syndrome. Biochim
Biophys Acta. 1812:919–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Docquier A, Harmand PO, Fritsch S,
Chanrion M, Darbon JM and Cavaillès V: The transcriptional
coregulator RIP140 represses E2F1 activity and discriminates breast
cancer subtypes. Clin Cancer Res. 16:2959–2970. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ho PC, Tsui YC, Feng X, Greaves DR and Wei
LN: NF-kB mediated degradation of the co-activator RIP140 regulates
inflammatory response and contributes to endotoxin tolerance. Nat
Immunol. 13:379–386. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Herbst EA, Bonen A and Holloway GP:
Changes in nuclear receptor corepressor RIP140 do not influence
mitochondrial content in the cortex. Appl Physiol Nutr Metab.
40:1086–1088. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aziz MH, Chen X, Zhang Q, DeFrain C,
Osland J, Luo Y, Shi X and Yuan R: Suppressing NRIP1 inhibits
growth of breast cancer cells in vitro and in vivo. Oncotarget.
6:39714–39724. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fu F, Xiao XI, Zhang T, Zou Q, Chen Z, Pei
L, Su J and Yi WJ: Expression of receptor protein tyrosine
phosphatase ζ is a risk factor for triple negative breast cancer
relapse. Biomed Rep. 4:167–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fan L, Strasser-Weippl K, Li JJ, St Louis
J, Finkelstein DM, Yu KD, Chen WQ, Shao ZM and Goss PE: Breast
cancer in China. Lancet Oncol. 15:e279–e289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Piroth MD, Petz D, Pinkawa M, Holy R and
Eble MJ: Usefulness of a thermoplastic breast bra for breast cancer
radiotherapy: A prospective analysis. Strahlenther Onkol.
192:609–616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moloney N, Sung JM, Kilbreath S and Dylke
E: Prevalence and risk factors associated with pain 21 months
following surgery for breast cancer. Support Care Cancer.
24:4533–4539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mangolini A, Ferracin M, Zanzi MV,
Saccenti E, Ebnaof SO, Poma VV, Sanz JM, Passaro A, Pedriali M,
Frassoldati A, et al: Diagnostic and prognostic microRNAs in the
serum of breast cancer patients measured by droplet digital PCR.
Biomark Res. 3:122015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dent R, Trudeau M, Pritchard KI, Hanna WM,
Kahn HK, Sawka CA, Lickley LA, Rawlinson E, Sun P and Narod SA:
Triple-negative breast cancer: Clinical features and patterns of
recurrence. Clin Cancer Res. 13:4429–4434. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
De P, Carlson JH, Wu H, Marcus A,
Leyland-Jones B and Dey N: Wnt-beta-catenin pathway signals
metastasis-associated tumor cell phenotypes in triple negative
breast cancers. Oncotarget. 7:43124–43149. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang D, Wang Y, Dai Y, Wang J, Suo T, Pan
H and Liu H, Shen S and Liu H: Downregulation of RIP140 in
hepatocellular carcinoma promoted the growth and migration of the
cancer cells. Tumour Biol. 36:2077–2085. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lapierre M, Bonnet S, Bascoul-Mollevi C,
Ait-Arsa I, Jalaguier S, Del Rio M, Plateroti M, Roepman P, Ychou
M, Pannequin J, et al: RIP140 increases APC expression and controls
intestinal homeostasis and tumorigenesis. J Clin Invest.
124:1899–1913. 2014. View
Article : Google Scholar : PubMed/NCBI
|