Introduction
Cervical cancer is the third most common
gynecological malignancy and one of leading causes of
cancer-associated mortality in the world (1). Although current treatment strategies for
cervical cancer utilize chemotherapy alongside surgical resection
or radiotherapy, the survival rate of patients with late-stage
cervical cancer remains poor (2). The
tumor stage at diagnosis and tumor volume are the most notable
prognostic factors for patients. However, regional lymph node
involvement, distant metastasis and loco-regional recurrence occur
frequently, despite the use of radical surgery and multimodal
therapies (3). Metastasis is the
primary cause of mortality from cervical cancer; however, the
mechanism of metastasis in patients with cervical cancer is
complicated and remains incompletely understood (4,5).
Therefore, novel well-characterized biomarkers would aid clinicians
in predicting metastatic progression and the prognosis of patients
with cervical cancer.
Centrosomal protein 55 (CEP55) is a
microtubule-bundling protein (6).
CEP55 was originally identified as a novel coiled-coil protein
during bioinformatic screening for genes involved in mitosis
(7). The CEP55 gene is located on
chromosome 10q23.33 and encodes three central coiled-coil domains.
As a mitotic phosphoprotein, CEP55 is recruited to the midbody
during cytokinesis and is essential for the completion of cell
division (8). The upregulation or
downregulation of CEP55 can result in cytokinesis defects and an
increase in the number of multinucleated cells. Evidence indicates
that CEP55 is specifically expressed in the normal human testes and
in various malignancies; it can regulate membrane fission and
fusion events near the midbody ring at the last stage of cancer
cell division (9,10). Overexpression of CEP55 increased the
growth of cancer cells, indicating that the suppression of
CEP55-mediated signal transduction may be a novel therapeutic
approach for a range of human tumor types (11). In addition, elevated CEP55 expression
in mammalian cells can enhance cellular migration and invasion,
again indicating its potential as a therapeutic target for treating
patients with cancer (12). The
results of functional assays indicated that CEP55 displays a number
of characteristics associated with oncogenic behavior, including
anchorage-independent growth, enhanced proliferation at low serum
levels and the in vivo induction of tumorigenesis (13). However, the functional role of CEP55
in cervical cancer remains largely unknown.
The present study aimed to investigate the
expression pattern of CEP55 in cervical cancer, and its
clinicopathological implications. The current study provides
evidence that CEP55 expression was significantly increased in
cervical cancer tissues, compared with adjacent non-cancerous
tissues. High CEP55 expression was observed in 62.0% of patients
(77/124) with cervical cancer, and was significantly associated
with lymph node metastasis and advanced tumor stage. Furthermore,
high CEP55 expression was a significant, independent predictive
factor for survival of patients with cervical cancer.
Materials and methods
Patients and samples
For the present retrospective study, archived
formalin-fixed, paraffin-embedded tissue specimens from 124
patients with primary cervical cancer who underwent radical
hysterectomy at Luoyang Central Hospital affiliated to Zhengzhou
University between December 2004 and December 2007 were recruited.
For the reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) assay, fresh cervical cancer and paired adjacent
non-cancerous tissue samples were obtained from eight patients with
cervical cancer who underwent surgical resection. Tumor resection
was performed by experienced surgeons, and the surgical procedures,
which followed the International Federation of Gynecology and
Obstetrics (FIGO) guidelines (14),
were similar in all patients who underwent radical resection. No
patients had received chemotherapy or radiotherapy prior to
surgery. The median age of the cohort was 44 years (range, 25–68
years). All patients were staged using the FIGO staging system.
Tissue samples from non-cancerous cervical lesions were also
collected during the study period and served as the normal
controls. Written informed consent was obtained from each patient
for the use of the tissue samples for research purposes. The
present study was approved by the Institutional Ethics Committee of
the Luoyang Central Hospital affiliated to Zhengzhou University
(Zhengzhou, China).
RT-qPCR
Total RNA was extracted from cervical cancer tissues
and adjacent non-cancerous tissues using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
according to the manufacturer's protocol. RNAse-free DNAase I was
used to eliminate DNA contamination. Following reverse
transcription of the total RNA using cDNA synthesis kit (Thermo
Fisher Scientific, Inc.), the first-strand cDNA was then used as
the template for detection of CEP55 expression by RT-qPCR with the
SYBR-Green I chemistry using SYBR-Green PCR kit (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Thermocycling begins
with 95°C for 5 min before 40 cycles of amplification at 95°C for
15 sec, and 60°C for 60 sec. GAPDH was used as internal control.
The primers were as follows: CEP55 forward,
5′-TGAAGAGAAAGACGTATTGAAACAA-3′ and reverse,
5′-GCAGTTTGGAGCCACAGTCT-3′; and GAPDH forward,
5′-AGCCACATCGCTCAGACAC-3′ and reverse, 5′-GCCCAATACGACCAAATCC-3′.
The relative expression level was determined using the
2−ΔΔCq method (15). Data
are presented as the expression level relative to the calibrator
(control sample).
Immunohistochemistry
Immunohistochemical analysis was performed to
investigate CEP55 expression in 124 paraffin-embedded tissues,
which had been processed into 5-mm serial sections. Briefly, the
sections were de-waxed in xylene and rehydrated in a graded alcohol
series (95, 80, 70 and 50%, v/v), then they were boiled for 3 min
in 10 mmol/l of citrate buffer (pH 6.0) for antigen retrieval.
Following the inhibition of endogenous peroxidase activities for 15
min at room temperature by 3% hydrogen peroxide in methanol, slides
were treated with 1% bovine serum albumin (Gibco; Thermo Fisher
Scientific, Inc.) for 30 min at room temperature to block
non-specific binding. Next, the slides were incubated with a rabbit
monoclonal anti-CEP55 antibody (1:100; Abcam, Cambridge, UK; cat.
no. ab214302) overnight at 4°C. Following washing with 0.01 M
phosphate buffer, the tissue sections were then incubated with the
biotinylated secondary antibody (goat anti-rabbit; dilution,
1:1,000; Abcam; cat. no. ab6702) at room temperature for 30 min,
followed by further incubation with streptavidin-horseradish
peroxidase complex (OriGene Technologies, Inc., Rockville, MD, USA)
for 20 min at room temperature. Negative control slides were
prepared by omitting the primary antibody under the same
experimental conditions, and the absence of non-specific
immunoreactive staining was confirmed.
Semi-quantitative estimation of expression was made
using a composite score obtained by multiplying the values of
staining intensity and relative abundance of positive cells by two
independent pathologists from Luoyang Central Hospital affiliated
to Zhengzhou University using a transmitted light microscope
(×400). The intensity of staining was graded as follows: 0, no
staining; 1, weak staining; 2, moderate staining; or 3, strong
staining. The abundance of positive cells was graded from 0 to 4,
as follows: 0, <5% positive cells; 1, 5–25%; 2, 26–50%; 3,
51–80%; and 4, >80%). The composite score was defined as low
expression for a score of 0–4 and as high expression for a score of
6–12.
Statistical analysis
Several clinicopathological factors were evaluated,
including age, FIGO stage, tumor differentiation and lymph node
status. The χ2 test was used to evaluate the association
between clinicopathological variables and expression of CEP55.
P<0.05 was considered to indicate a statistically significant
difference. Clinicopathological variables and CEP55 expression were
taken into account for the survival analysis, based on the
Kaplan-Meier method, with statistical significance assessed using
the log-rank test. To determine the effect of particular prognostic
factors on survival, a multivariate analysis was performed
according to the Cox regression model.
Results
CEP55 was overexpressed in cervical
cancer specimens
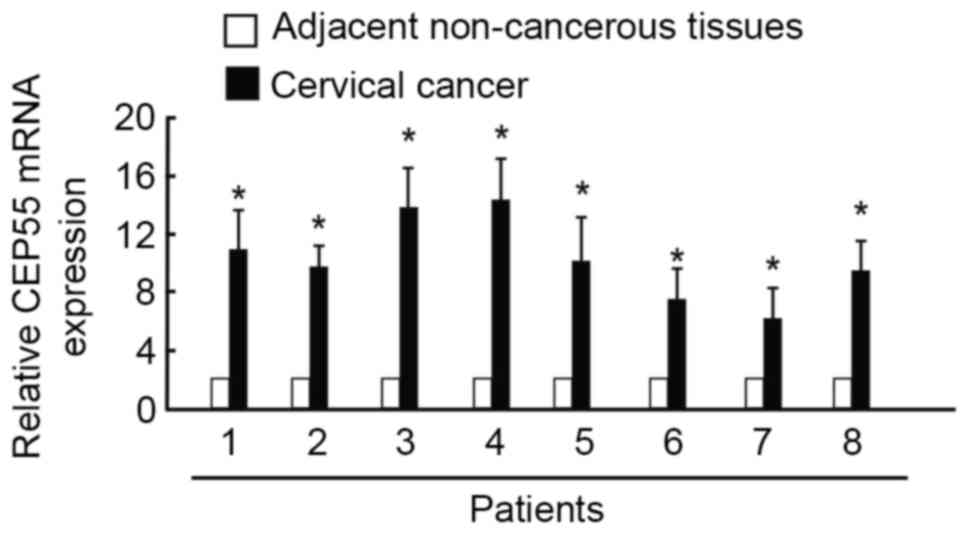
To determine whether CEP55 participates in the
pathogenesis of cervical carcinoma, RT-qPCR was performed on eight
pairs of cervical cancer and adjacent non-cancerous tissue samples.
CEP55 expression was higher in the tumor specimens than in the
normal tissues. The mean expression level of CEP55 was 9-fold
higher in cervical cancer samples than in normal tissue samples
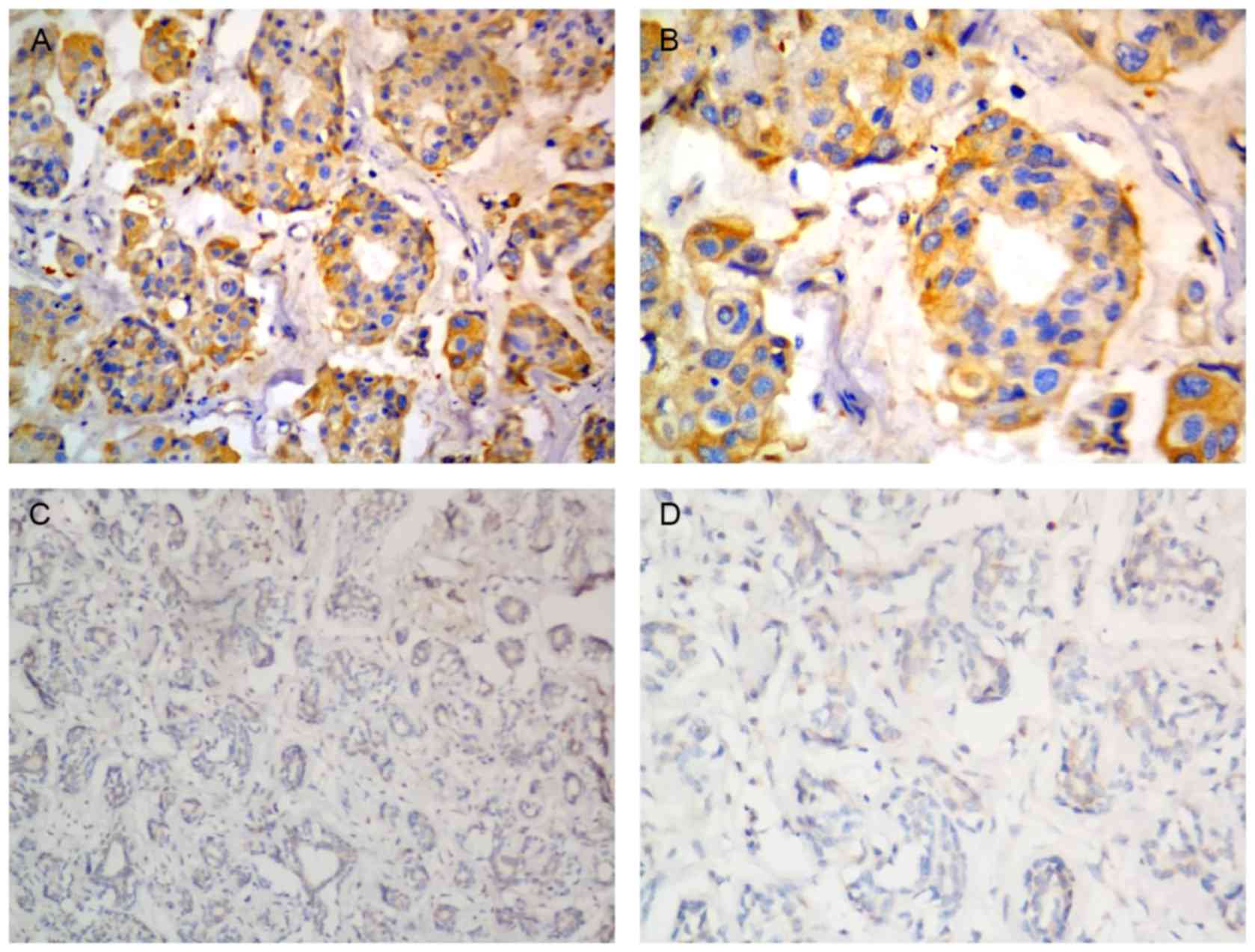
(Fig. 1). Representative
immunostaining for CEP55 in cervical cancer tissues is depicted in
Fig. 2.
Clinicopathological factors and
survival rates of patients with cervical cancer expressing
CEP55
To investigate whether an increase in the expression
of CEP55 was associated with various prognostic factors, patients
were classified into groups based on the semi-quantitative results
of immunohistochemical staining (low vs. high CEP55 expression). As
depicted in Table I, patients with an
advanced tumor stage and a higher rate of lymph node metastasis
expressed significantly higher levels of CEP55 than patients with
early stage tumors (P=0.010) or no lymph node metastasis (P=0.008).
No significant difference between levels of CEP55 expression was
observed in patients with different ages or histological disease
subtypes at the time of diagnosis.
 | Table I.Association between CEP55 expression
and clinicopathological variables of 124 patients with cervical
cancer. |
Table I.
Association between CEP55 expression
and clinicopathological variables of 124 patients with cervical
cancer.
|
|
| CEP55 |
|
|---|
|
|
|
|
|
|---|
| Parameter | Total | Low | High | P-value |
|---|
| Age, years |
|
|
| 0.194 |
| ≤50 | 75 | 25 | 50 |
|
|
>50 | 49 | 22 | 27 |
|
| FIGO stage |
|
|
| 0.010 |
| IB | 83 | 38 | 45 |
|
|
>IB | 41 | 9 | 32 |
|
| Tumor size, cm |
|
|
| 0.713 |
| ≤4 | 90 | 35 | 55 |
|
|
>4 | 34 | 12 | 22 |
|
| Differentiation |
|
|
| 0.731 |
| 1/2 | 97 | 36 | 61 |
|
| 3 | 27 | 11 | 16 |
|
| Histological
type |
|
|
| 0.310 |
| SCC | 85 | 41 | 44 |
|
| AC | 39 | 15 | 24 |
|
| LN metastasis |
|
|
| 0.008 |
| No | 95 | 53 | 42 |
|
| Yes | 29 | 8 | 21 |
|
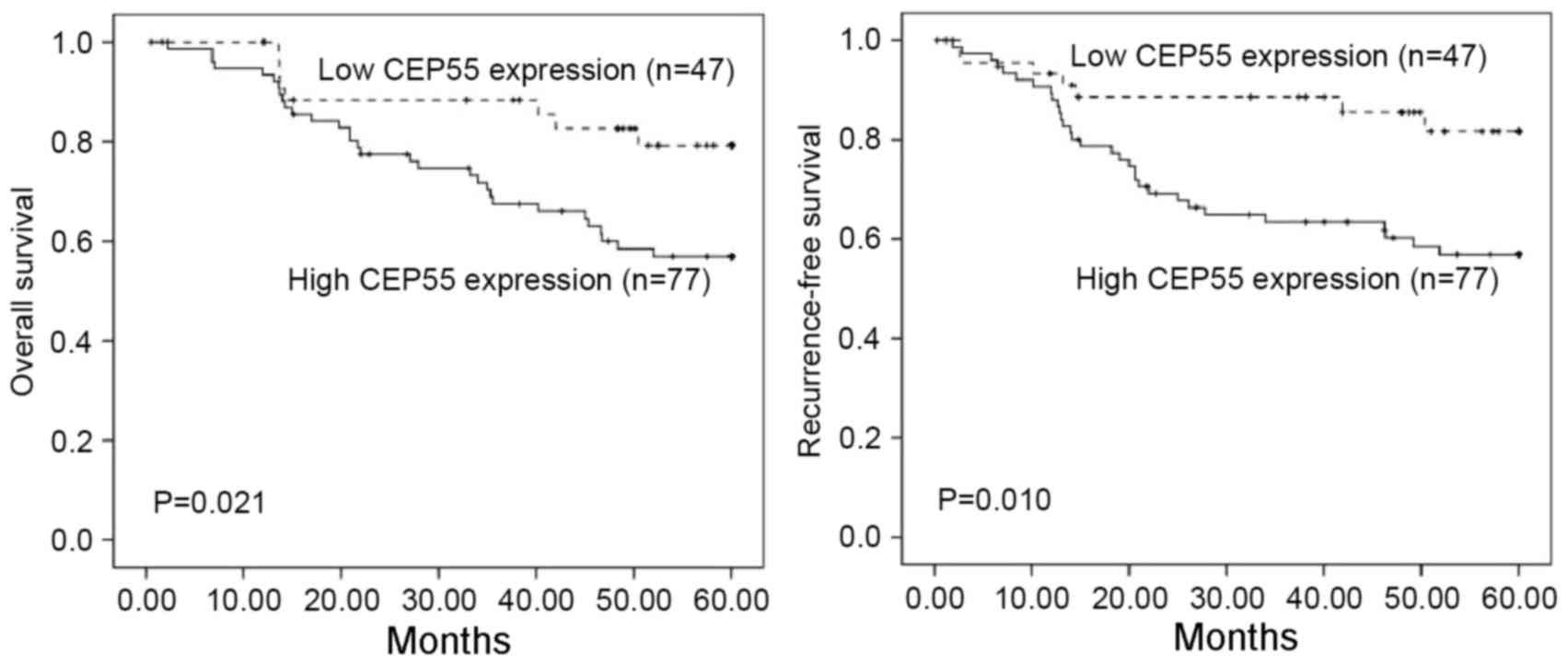
Survival analysis using the Kaplan-Meier method
revealed that the prognosis of patients with tumors expressing high
levels of CEP55 was significantly poorer than those with tumors
expressing low levels of CEP55 (P=0.021 and P=0.010 for overall and
recurrence-free survival, respectively) (Fig. 3). Multivariate analysis revealed that
CEP55 expression was a significant independent prognostic factor
for the overall survival rate of patients (P=0.035), as depicted in
Table II. The results of the present
study clearly indicated that the prognosis of patients with
cervical cancer was associated with CEP55 expression.
 | Table II.Cox regression analyses for predictors
of outcome. |
Table II.
Cox regression analyses for predictors
of outcome.
|
| Overall survival | Recurrence-free
survival |
|---|
|
|
|
|
|---|
| Prognostic
variable | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age (>50 vs. ≤50
years) | 1.580
(0.149–4.977) | 0.413 | 1.385
(0.109–4.111) | 0.502 |
| Tumor stage (>IB
vs. IB) | 2.492
(0.995–5.114) | 0.004 | 2.247
(0.713–6.410) | 0.042 |
| Tumor size (>4 vs.
≤4 cm) | 2.256
(0.612–5.102) | 0.074 | 2.197
(0.710–5.016) | 0.052 |
| Tumor grade (3 vs.
1/2) | 1.247
(0.210–2.515) | 0.401 | 1.295
(0.265–2.258) | 0.550 |
| Histological type
(SCC vs. AC) | 1.813
(1.555–4.211) | 0.214 | 1.368
(1.118–4.764) | 0.613 |
| LN metastasis (yes
vs. no) | 4.258
(1.819–7.314) | 0.026 | 3.349
(1.201–7.594) | 0.014 |
| CEP55 expression
(high vs. low) | 3.057
(1.404–7.482) | 0.035 | 3.267
(1.406–4.594) | 0.065 |
Discussion
Cervical cancer is one of the most common
malignancies in females worldwide, culminating in ~265,700 deaths
in 2012 (16). EP55 is involved in
tumorigenesis in a number of types of cancer. However, the
expression pattern of CEP55 has, to the best of our knowledge, not
been investigated in cervical cancer. The current study revealed
that CEP55 expression was significantly higher in cervical cancer
tissues than in adjacent non-cancerous tissues, as evidenced by the
results of RT-qPCR. High CEP55 expression was observed in 62.0%
(77/124) tissues from patients with cervical cancer, and was
significantly associated with lymph node metastasis and FIGO stage.
Furthermore, the data from the present study indicated that CEP55
expression was of significant, independent predictive value for the
survival rates of patients with cervical cancer. Thus, the results
of the current study also indicated that CEP55 could be a putative
biomarker for tumor metastasis and patient prognosis in cervical
cancer.
The centrosome is an organelle that has several key
functions in the vertebrate cell cycle as the primary
microtubule-organizing center. In tumor cells, a number of
structural changes, including increases to the volume and number of
centrosomes, the presence of supernumerary centrioles, the
accumulation of excess pericentriolar material and the
non-phosphorylation of centrosomal proteins (17). The centrosome-associated protein CEP55
is included in the pericentriolar material and, when overexpressed,
can cause ectopic microtubule nucleation (18). Previous studies have revealed that
CEP55 is overexpressed in several types of cancer, including
hepatocarcinoma, colon carcinoma, ovarian carcinoma and lung cancer
(11–13,19). To
the best of our knowledge, the current study is the first to
demonstrate CEP55 was upregulated in cervical cancer tissues
compared with the adjacent non-cancerous tissues. Taken together,
the results of the current study indicated that CEP55 may have an
oncogenic role in the development and progression of tumors.
Notably, CEP55 has been demonstrated to be involved
in growth-associated signaling pathways in tumor cells. Chen et
al (13) revealed that the
cellular transformation driven by CEP55 is mediated by activation
of the phosphoinositide 3-kinase/protein kinase B (AKT) pathway in
hepatocellular carcinoma cells. Additionally, CEP55 expression
levels were positively associated with increased cellular
proliferation and that tumor protein p53 (hereafter p53) is able to
negatively regulate the activity of the CEP55 promoter and protein,
and that the existence of a p53/polo-like kinase 1/CEP55 axis in
which p53 negatively regulates expression of CEP55, is a positive
regulator of CEP55 protein stability (20). Additionally, Tao et al
(21) demonstrated that the ectopic
overexpression of CEP55 enhanced the cellular proliferation, colony
formation and tumorigenicity of gastric cancer cells, which was
mediated by the AKT signaling pathway and contributes to
carcinogenesis and progression of gastric carcinogenesis. In the
cohort assessed in the present study, CEP55 protein expression was
significantly associated with the FIGO stage of patients with
cervical cancer, further indicating that CEP55 expression was
positively associated with the growth of cancer cells.
Several studies have assessed the role of CEP55 in
tumor progression. Chen et al (22) observed that CEP55 expression was
associated with increased aggressiveness of oral cavity squamous
cell carcinoma by inducing the migratory and invasive behavior of
cells via increased forkhead box M1 and matrix metalloproteinase-2
activity. Further study by the same group revealed that CEP55
expression was associated with tumor progression in nasopharyngeal
carcinoma and contributed to nasopharyngeal cell proliferation and
metastasis via the osteopontin/cluster of differentiation 44
pathway (23). Hwang et al
(24) demonstrated that fibulin-5
promoted nasopharyngeal carcinoma cell metastasis via the CEP55/AKT
pathway and was associated with poor patient prognosis. In
concordance with the findings of these prior studies, the current
study demonstrated that CEP55 expression was significantly
associated with lymph node metastasis in patients with cervical
cancer. Notably, high CEP55 expression was an indicator of poor
overall and recurrence-free survival rates in patients with
cervical cancer.
In conclusion, higher expression of CEP55 in
cervical carcinoma tissues was associated with lymph node
metastasis and poor patients prognosis. CEP55 has the potential to
be used as a therapeutic target for patients with cervical
carcinoma and should be investigated further.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Innovation
Fund of Luoyang Central Hospital affiliated to Zhengzhou University
(grant no. 20120420).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JQ participated in the data collecting and drafted
the manuscript. GL performed the statistical analysis. JQ and FW
participated in the design of the study. All authors analyzed and
interpreted the patient data.
Ethics approval and consent to
purticipate
The present study was approved by the Institutional
Ethics Committee of the Luoyang Central Hospital affiliated to
Zhengzhou University (Zhengzhou, China).
Consent for publication
Written informed consent was obtained from each
patient for the use of the tissue samples for paper
publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Colombo N, Carinelli S, Colombo A, Marini
C, Rollo D and Sessa C: ESMO Guidelines Working Group: Cervical
cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment
and follow-up. Ann Oncol. 23 Suppl 7:vii27–vii32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tao X, Hu W, Ramirez PT and Kavanagh JJ:
Chemotherapy for recurrent and metastatic cervical cancer. Gynecol
Oncol. 110 3 Suppl 2:S67–S71. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bellmunt J, Orsola A, Leow JJ, Wiegel T,
De Santis M and Horwich A: ESMO Guidelines Working Group: Bladder
cancer: ESMO Practice Guidelines for diagnosis, treatment and
follow-up. Ann Oncol. 25 Suppl 3:iii40–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galloway TJ and Ridge JA: Management of
squamous cancer metastatic to cervical nodes with an unknown
primary site. J Clin Oncol. 33:3328–3337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Grossman JS: Management of metastatic
cervical cancer: Should we ignore lessons learned from treating
other advanced squamous cell cancers? J Clin Oncol. 33:965–966.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Doxsey S, McCollum D and Theurkauf W:
Centrosomes in cellular regulation. Annu Rev Cell Dev Biol.
21:411–434. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Doxsey SJ: Molecular links between
centrosome and midbody. Mol Cell. 20:170–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fabbro M, Zhou BB, Takahashi M, Sarcevic
B, Lal P, Graham ME, Gabrielli BG, Robinson PJ, Nigg EA, Ono Y and
Khanna KK: Cdk1/Erk2- and Plk1-dependent phosphorylation of a
centrosome protein, Cep55, is required for its recruitment to
midbody and cytokinesis. Dev Cell. 9:477–488. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Morita E, Sandrin V, Chung HY, Morham SG,
Gygi SP, Rodesch CK and Sundquist WI: Human ESCRT and ALIX proteins
interact with proteins of the midbody and function in cytokinesis.
EMBO J. 26:4215–4227. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carlton JG and Martin-Serrano J: Parallels
between cytokinesis and retroviral budding: A role for the ESCRT
machinery. Science. 316:1908–1912. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sakai M, Shimokawa T, Kobayashi T,
Matsushima S, Yamada Y, Nakamura Y and Furukawa Y: Elevated
expression of C10orf3 (chromosome 10 open reading frame 3) is
involved in the growth of human colon tumor. Oncogene. 25:480–486.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen CH, Lai JM, Chou TY, Chen CY, Su LJ,
Lee YC, Cheng TS, Hong YR, Chou CK, Whang-Peng J, et al: VEGFA
upregulates FLJ10540 and modulates migration and invasion of lung
cancer via PI3K/AKT pathway. PLoS One. 4:e50522009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen CH, Lu PJ, Chen YC, Fu SL, Wu KJ,
Tsou AP, Lee YC, Lin TC, Hsu SL, Lin WJ, et al: FLJ10540-elicited
cell transformation is through the activation of PI3-kinase/AKT
pathway. Oncogene. 26:4272–4283. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
FIGO Committee on Gynecologic Oncology:
FIGO staging for carcinoma of the vulva, cervix, and corpus uteri.
Int J Gynaecol Obstet. 125:97–98. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Marth C, Landoni F, Mahner S, McCormack M,
Gonzalez-Martin A and Colombo N: ESMO Guidelines Committee:
Cervical cancer: ESMO clinical practice guidelines for diagnosis,
treatment and follow-up. Ann Oncol. 28:iv72–iv83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nigg EA and Raff JW: Centrioles,
centrosomes, and cilia in health and disease. Cell. 139:663–678.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nakamura M, Masuda H, Horii J, Kuma Ki,
Yokoyama N, Ohba T, Nishitani H, Miyata T, Tanaka M and Nishimoto
T: When overexpressed, a novel centrosomal protein, RanBPM, causes
ectopic microtubule nucleation similar to gamma-tubulin. J Cell
Biol. 143:1041–1052. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang W, Niu C, He W, Hou T, Sun X, Xu L
and Zhang Y: Upregulation of centrosomal protein 55 is associated
with unfavorable prognosis and tumor invasion in epithelial ovarian
carcinoma. Tumour Biol. 37:6239–6254. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang YC, Wu CH, Yen TC and Ouyang P:
Centrosomal protein 55 (Cep55) stability is negatively regulated by
p53 protein through Polo-like kinase 1 (Plk1). J Biol Chem.
287:4376–4385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tao J, Zhi X, Tian Y, Li Z, Zhu Y, Wang W,
Xie K, Tang J, Zhang X, Wang L and Xu Z: CEP55 contributes to human
gastric carcinoma by regulating cell proliferation. Tumour Biol.
35:4389–4399. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen CH, Chien CY, Huang CC, Hwang CF,
Chuang HC, Fang FM, Huang HY, Chen CM, Liu HL and Huang CY:
Expression of FLJ10540 is correlated with aggressiveness of oral
cavity squamous cell carcinoma by stimulating cell migration and
invasion through increased FOXM1 and MMP-2 activity. Oncogene.
28:2723–2737. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen CH, Shiu LY, Su LJ, Huang CY, Huang
SC, Huang CC, Yin YF, Wang WS, Tsai HT, Fang FM, et al: FLJ10540 is
associated with tumor progression in nasopharyngeal carcinomas and
contributes to nasopharyngeal cell proliferation, and metastasis
via osteopontin/CD44 pathway. J Transl Med. 10:932012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hwang CF, Shiu LY, Su LJ, Yu-Fang Yin,
Wang WS, Huang SC, Chiu TJ, Huang CC, Zhen YY, Tsai HT, et al:
Oncogenic fibulin-5 promotes nasopharyngeal carcinoma cell
metastasis through the FLJ10540/AKT pathway and correlates with
poor prognosis. PLoS One. 8:e842182013. View Article : Google Scholar : PubMed/NCBI
|