Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most aggressive and fatal types of malignancy, ranking the
fourth highest cause of disease-associated mortality worldwide
(1). Statistical studies have
revealed that the 5-year survival of PDAC is <5%, and ~50% of
patients are diagnosed at the advanced stage (2,3). Only
<20% of patients are eligible for curative resection, and among
these patients, the majority experience recurrence within a year.
The identification of reliable clinical markers to predict the
prognosis is required for the management of patients with PDAC. It
is necessary to identify novel molecular markers for early
diagnosis and prediction of prognosis.
Ubiquitin specific peptidase 9X (USP9X), a member of
the deubiquitinating protease family, is encoded on the X
chromosome and is widely expressed in all tissues (4). Previous studies have demonstrated that
the aberrant expression of USP9X is associated with multiple human
cancer types, including lung cancer (5), breast cancer (6,7),
esophageal carcinoma (8), colorectal
carcinoma (9,10), prostate cancer (11), cervical cancer (12), chronic myelogenous leukemia (13), lymphoma and multiple myeloma (14), suggesting the role of USP9X in
tumorigenesis, and progression. It has been revealed that USP9X
primarily affects proteins that are meant to undergo proteasomal
degradation by removing ubiquitin components. Consistently, in a
xenograft model with the KRAS wild-type PDAC cell line, tumor
volume decreased when USP9X was knockdown (14). However, a report noted that the
knockout of USP9X enhances the transformation and protects cancer
cells from anoikis in a murine model of PDAC, indicating that USP9X
behaves as a tumor suppressor (15).
The data of another research argued that USP9X promoted cell growth
for established PDAC tumor cells and served a context-dependent
role in PDAC (16).
In the present study, the expression level of USP9X
was analyzed in Chinese patients with PDAC using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
immunohistochemistry (IHC). Then, the association between
clinicopathological parameters and USP9X expression of PDAC was
explored, and the prognostic value of USP9X expression for the
overall survival of Chinese patients with PDAC was evaluated.
Materials and methods
Patients and tissue specimens
The clinicopathological data of 205 patients with
PDAC with surgical resection were retrospectively analyzed at the
Biliary-Pancreatic Department of Ren Ji Hospital, School of
Medicine, Shanghai Jiao Tong University (Shanghai, China) between
January 2002 and June 2014. In this cohort, there were 88 females
and 117 males, age ranging from 38 to 90 years old, with a median
age of 65 years. The diagnosis was confirmed by pathological
examination. Patients who received preoperative chemotherapy,
radiotherapy or other anticancer therapies were excluded from this
study. An additional 30 fresh frozen cancerous and corresponding
non-cancerous tissues of PDAC were also obtained from the same
department. The present study was reviewed and approved by the
Ethics Committee of the Ren Ji Hospital, School of Medicine,
Shanghai Jiao Tong University. Written informed consent was
obtained from all participating patients.
RNA extraction and RT-qPCR. According to the
manufacturers' protocols, total RNA from cancerous and
corresponding non-cancerous tissue samples was extracted with
TRIzol® reagent (Takara Bio, Inc., Otsu Japan), and
reverse transcribed using a PrimeScript RT-PCR kit (Takara Bio,
Inc.). 1 ug total RNAs were added to each reaction. qPCR reactions
were then performed on a 7500 Real-time PCR system (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) using
the SYBR Premix Ex Taq II (Takara, Japan) in a 10 ul system. The
reactions were incubated at 95°C for 30 sec, followed by 40 cycles
of 95°C for 5 sec, and 60°C for 34 sec. All quantitative PCR
reactions were performed in triplicate. The primer sequences used
for USP9X detection were as follows: Forward,
5′-GTAATCCTGAGGAGGAAGAG-3′ and reverse, 5′-ACCACAGGCAGCGAAACAT-3′.
The relative levels of USP9X expression were normalized to GAPDH
RNA (forward, 5′-GACATCAAGAAGGTGGTGAA-3′ and reverse,
5′-TGTCATACCACGAAATGAGC-3′) using the 2−ΔΔCt method
(17).
Tissue microarray (TMA)
construction
TMAs were assembled using 1.5-mm diameter cores. In
all 205 cases, corresponding non-cancerous tissue specimen cores
were used as internal controls. After marking the representative
area of each tissue, the samples were punched out and fitted into
the paraffin array blocks.
IHC staining and scoring
The tissue microarray sections were rehydrated and
treated with 3% hydrogen peroxide, followed by antigen retrieval.
After being blocked with 10% normal goat serum (Shanghai Long
Island Biotec, Co., Ltd., Shanghai, China) at room temperature for
30 min, the sections were incubated with primary antibodies at 4°C
overnight, followed by incubation, with a peroxidase-labeled
secondary antibody for 30 min at room temperature. Finally,
Diaminobenzidine tetrahydrochloride (DAB; Fuzhou Maixin Biotech
Co., Ltd., Fuzhou, China) was used for the color-reaction followed
by nucleus counterstaining with hematoxylin. The following
antibodies were used: rabbit anti-USP9X (1:100, Abcam, Cambridge,
UK); Elivision plus Polyer HRP (Mouse/Rabbit) IHC Kit (Fuzhou
Maixin Biotech Co., Ltd.). IHC staining was performed on the TMA
containing 205 paired PDAC samples by two senior pathologists
independently as previously described (18–20). The
percentage of positively stained cells were scored using the
following scale: 0, 0–5%; 1, 6–35%; 2, 36–70%; and 3, >70% of
cells stained. The staining intensity was graded as following:
Negative, 0; weakly graded, 1; moderately graded, 2; and strongly
graded, 3. The final score was the product of the scores for the
positive-staining rate and intensity as follows: ‘−’ for a score of
0–1, ‘+’ for a score of 2–3, ‘++’ for a score of 4–6 and ‘+++’ for
a score of >6. A total score <4 in USP9X expression was
considered to exhibit low expression and ≥4 as high expression.
Follow-up
The results of clinical and laboratory examinations
were followed-up periodically until patients succumbed. Overall
survival was defined as the duration between the date of surgery
and the date of mortality or the last follow-up.
Statistical analysis
All statistical analyses were performed using SPSS
20.0 (IBM Corp., Armonk, NY, USA) and GraphPad Prism 5.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Paired-samples t-tests were
used to compare the mRNA levels of USP9X in the tissue samples. The
chi-squared test for proportion was used to analyze the comparison
of IHC grades in PDAC and normal pancreatic tissues, and the
association between clinicopathological characteristics and USP9X
expression. The Kaplan-Meier method and the log-rank test were
applied in the comparison of the survival curves. Univariate and
multivariate Cox proportional hazard analysis was used to
investigate the association between clinicopathological variables
and USP9X expression on survival. Two-tailed P<0.05 was
considered to indicate a statistically significant difference.
Results
USP9X expression in PDAC
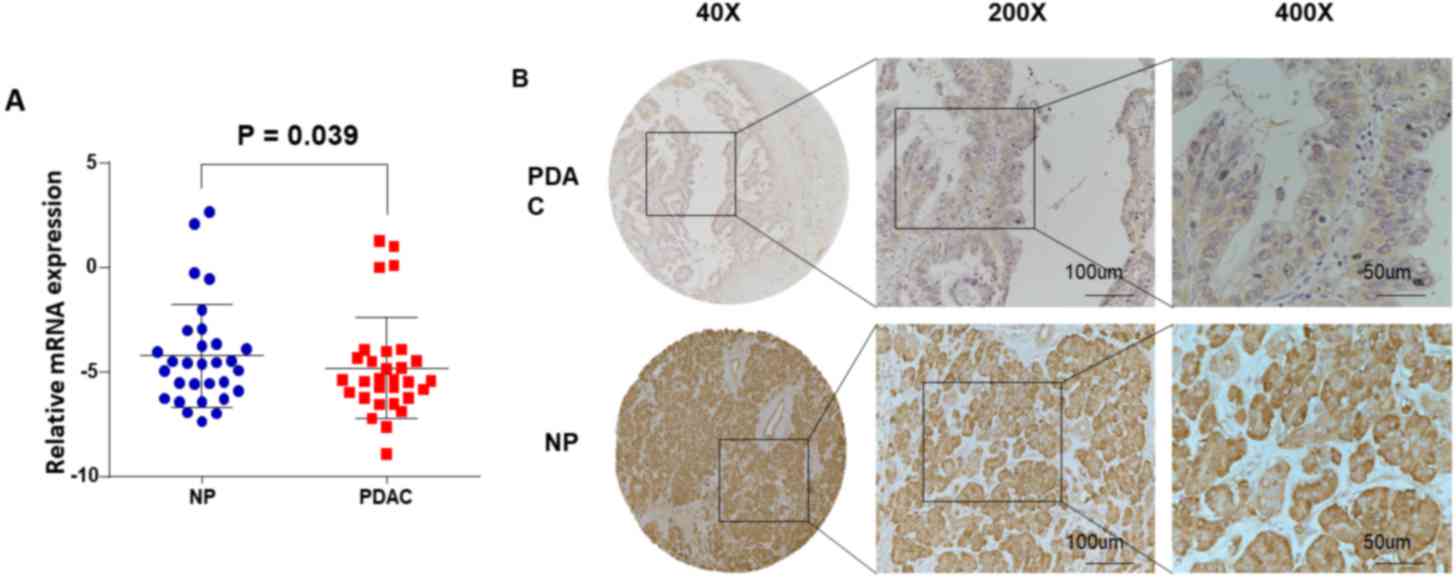
RT-qPCR assays were performed using 30 pairs of
fresh specimens from patients with PDAC to determine the mRNA
levels of USP9X. The USP9X mRNA levels were significantly decreased
in PDAC tissue samples (18/30, 60.0%) compared with the matched
surrounding non-tumor tissue samples (P=0.039; Fig. 1A). Based on the aforementioned scoring
criteria mentioned, the IHC data from the 205 PDAC samples revealed
that 73 (35.6%) exhibited high USP9X expression (USP9X ++ or USP9X
+++), whereas the remaining 132 cases (64.4%) exhibited low USP9X
expression (USP9X- or USP9X +) (Fig.
1B; Tables I and II).
 | Table I.Associations between USP9X expression
and clinicopathological features in patients with PDAC. |
Table I.
Associations between USP9X expression
and clinicopathological features in patients with PDAC.
|
| USP9X expression |
|---|
|
|
|
|---|
| Characteristics | Total | Low (n=132) (%) | High (n=73) (%) | P-value
(χ2 test) |
|---|
| Age, years |
|
|
| 0.153 |
|
<65 | 98 | 68 (69.4) | 30 (30.6) |
|
| ≥65 | 107 | 64 (59.8) | 43 (40.2) |
|
| Sex |
|
|
| 0.491 |
| Male | 117 | 73 (62.4) | 44 (37.6) |
|
|
Female | 88 | 59 (67.0) | 29 (33.0) |
|
| Tumor location |
|
|
| 0.875 |
| Head | 139 | 89 (64.0) | 50 (36.0) |
|
|
Body/tail | 66 | 43 (66.2) | 23 (34.8) |
|
| Size, cm |
|
|
| 0.662 |
| ≤2 | 28 | 17 (60.7) | 11 (39.3) |
|
|
>2 | 177 | 115 (65.0) | 62 (35.0) |
|
| Tumor
differentiation |
|
|
| 0.244 |
| Well | 14 | 7 (50.0) | 7 (50.0) |
|
|
Moderate/poor | 191 | 125 (65.4) | 66 (34.6) |
|
| AJCC stage |
|
|
| 0.238 |
| Stage
I–II | 150 | 93 (62.0) | 57 (38.0) |
|
| Stage
III–IV | 55 | 39 (70.9) | 16 (29.1) |
|
| Lymph node
metastasis |
|
|
| 0.328 |
|
Absent | 143 | 89 (62.2) | 54 (37.8) |
|
|
Present | 62 | 43 (69.4) | 19 (30.6) |
|
| Liver metastasis |
|
|
| 0.032a |
|
Absent | 184 | 114 (62.0) | 70 (38.0) |
|
|
Present | 21 | 18 (85.7) | 3 (14.3) |
|
| Vascular
invasion |
|
|
| 0.055 |
|
Absent | 176 | 107 (60.8) | 69 (39.2) |
|
|
Present | 29 | 23 (79.3) | 6 (20.7) |
|
 | Table II.Comparison of high and low USP9X
immunohistochemical expression in PDAC and normal pancreatic
tissues. |
Table II.
Comparison of high and low USP9X
immunohistochemical expression in PDAC and normal pancreatic
tissues.
|
| Tissue |
|
|---|
|
|
|
|
|---|
| Immunohistochemical
grade | PDAC (n=205,%) | NP (n=205,%) | P-value
(χ2 test) |
|---|
| High USP9X
expression | 73 (35.6) | 98 (47.8) | 0.012a |
| Low USP9X
expression | 132 (64.4) | 107 (52.2) |
|
Association between USP9X expression
and PDAC clinicopathological parameters
The IHC staining scoring of USP9X level was
statistically analyzed to explore the association between USP9X
expression and clinicopathological parameters in PDAC. The
clinicopathological parameters included age, sex, tumor location,
tumor size, differentiation status, clinical stage, lymph node
metastasis, liver metastasis and vascular invasion. Although the
number of patients with PDAC with low USP9X expression was more
than that with high expression, USP9X expression was only
significantly associated with liver metastasis (P=0.032; Table I).
Prognostic significance of USP9X
expression in PDAC
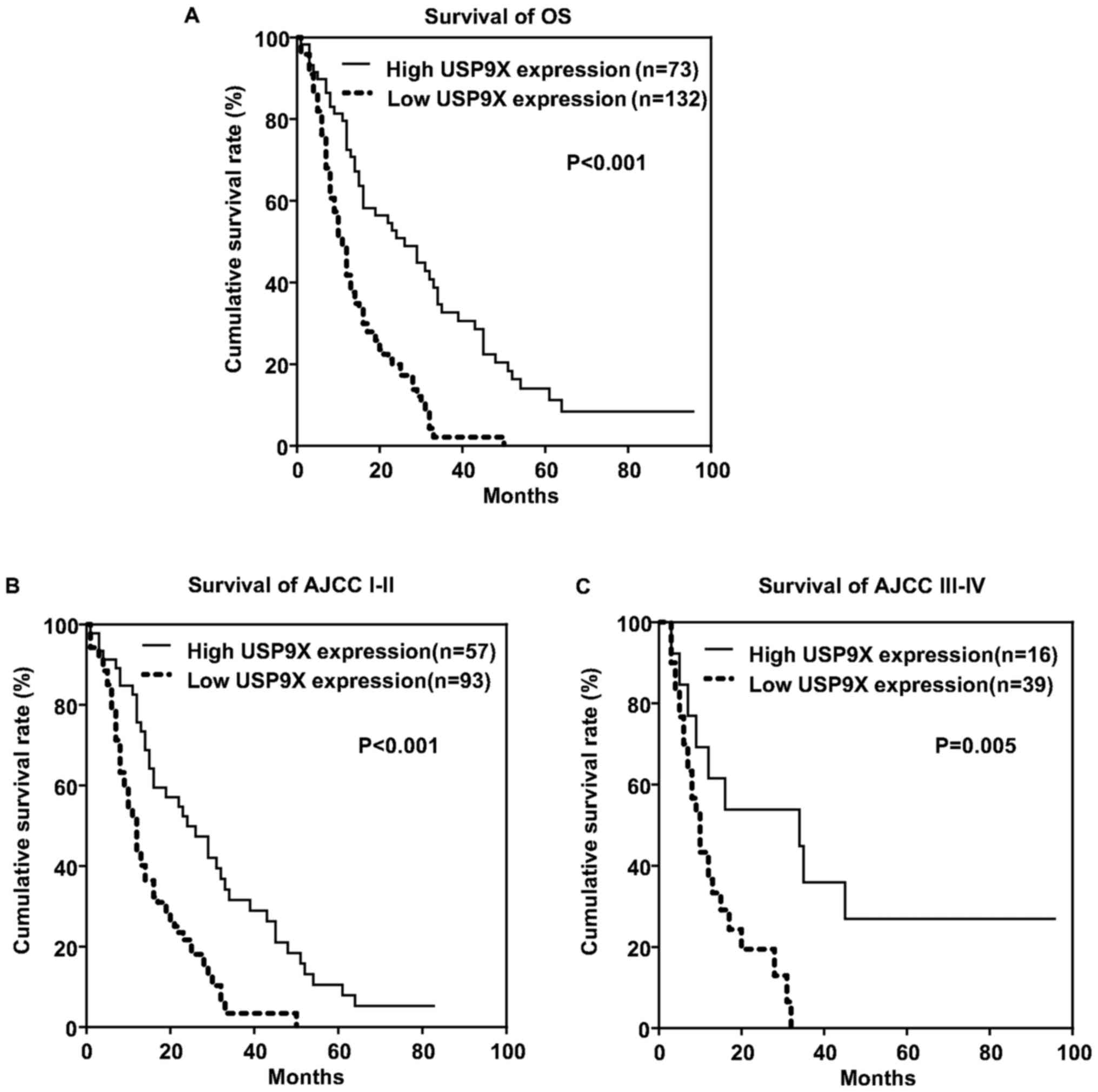
Survival analysis of patients was conducted using
the Kaplan-Meier curve and log-rank test. As shown in Fig. 2A, patients with a high expression of
USP9X exhibited a longer clinical overall survival time compared
with those with low USP9X expression (P<0.001). The association
between overall survival and USP9X expression level was evaluated
according to American Joint Committee on Cancer (AJCC) stage 7th
edition (Fig. 2B and C) (21). Similarly patients with stage I–II and
stage III–IV with high USP9X expression exhibited a longer overall
survival time compared with their low USP9X expression
counterparts.
Furthermore, univariate Cox regression analysis was
performed in the 205 PDAC cases (Table
III). Overall survival was significantly associated with USP9X
expression, age, sex, tumor size, tumor differentiation and liver
metastasis (P<0.05). Furthermore, following multivariate Cox
regression analysis, all six parameters remained significantly
associated with overall survival and were identified as independent
prognostic factors for PDAC (Table
III).
 | Table III.Univariate and multivariate analyses
of prognostic parameters for survival in patients with PDAC. |
Table III.
Univariate and multivariate analyses
of prognostic parameters for survival in patients with PDAC.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Prognostic
parameter | RR | 95% CI | P-value | RR | 95% CI | P-value |
|---|
| USP9X (low vs.
high) | 0.332 | 0.232–0.477 |
<0.001a | 0.375 | 0.263–0.534 |
<0.001a |
| Age (<65 vs. ≥65
years) | 1.486 | 1.111–1.988 | 0.008a | 1.601 | 1.183–2.166 | 0.002a |
| Sex (male vs.
female) | 0.723 | 0.534–0.978 | 0.035a | 0.708 | 0.515–0.974 | 0.034a |
| Tumor location
(head vs. body/tail) | 1.045 | 0.767–1.424 | 0.781 |
|
|
|
| Tumor size (≤2 vs.
>2 cm) | 1.589 | 1.038–2.430 | 0.033a | 1.735 | 1.115–2.698 | 0.014a |
| Tumor
differentiation (Well vs. moderate/poor) | 3.907 | 1.950–7.826 |
<0.001a | 3.470 | 1.715–7.020 | 0.001a |
| AJCC stage (I–II
vs. III–IV) | 1.042 | 0.739–1.470 | 0.815 |
|
|
|
| Lymph node
metastasis (present vs. absent) | 1.288 | 0.939–1.765 | 0.116 |
|
|
|
| Liver metastasis
(present vs. absent) | 3.474 | 1.887–6.394 |
<0.001a | 4.457 | 2.373–8.371 |
<0.001a |
| Vascular invasion
(present vs. absent) | 1.300 | 0.843–2.004 | 0.235 |
|
|
|
Discussion
Pancreatic cancer is one of the most aggressive and
fatal types of malignant tumor in solid tumor oncology in the
world. One of the reasons for the poor prognosis of pancreatic
cancer is that it is difficult to diagnose at the early stage. In
the present study, USP9X expression, and its association with
clinicopathological features and clinical prognosis were
investigated in Chinese patients with PDAC. It was demonstrated
that USP9X may serve as a candidate tumor suppressor and prognostic
biomarker in PDAC.
First, USP9X expression was demonstrated to be
decreased at the protein level by IHC analysis in 205-paired PDAC
sample TMA (132/205, 64.4%). USP9X expression was also
significantly decreased in PDAC tissues compared with the matched
surrounding non-tumor tissue samples at the mRNA level (P=0.039).
Furthermore, liver metastasis is an important negative prognostic
indicator of PDAC. In the current study, a potential association
was identified between USP9X expression and liver metastasis by
statistical analysis with various clinicopathological parameters,
which indicated that low USP9X expression suppressed liver
metastasis in PDAC (P=0.032). Nevertheless, no statistical
significance was identified between USP9X expression and tumor
differentiation (P=0.244) or AJCC stage (P=0.238) in PDAC, which
may be attributed to the variability in the distribution of
different stages across the cohort.
In the further study, it was demonstrated that
longer clinical overall survival times occurred in patients with
high expression of USP9X when compared with those with low USP9X
expression, which verified the suppressor role of USP9X.
Perez-Mancera et al (15)
reported that knockdown the USP9X expression increased the colony
of formation rate of pancreatic cancer cells and protected
pancreatic cancer cells from anoikis. The present study revealed
that the level of USP9X was significantly reduced in Chinese PDAC
tissues compared with the matched surrounding non-tumor tissues. It
may be possible that USP9X serves a tumor suppressor role in PDAC.
Several previous reports presented that USP9X was a cancer promoter
in other malignant solid tumors, including lung cancer (5), breast cancer (6,7),
esophageal carcinoma (8), colorectal
carcinoma (9,10), prostate cancer (11) and pancreatic cancer (14). Cox et al (16) downregulated the USP9X expression using
short hairpin RNA, which resulted in a reduction in the growth of
human PDAC cells, indicating that USP9X serves as an oncogene.
Notably, it was also reported that the growth of PDAC cell lines
was impaired by deubiquitinating protease inhibitor WP1130, which
revealed that USP9X may promote PDAC cell growth (16). We hypothesized that USP9X contributes
to PDAC by a relatively complicated mechanism involving other
participants, including an association with a Kras gene mutation
(15), which requires further studies
to verify, although Cox et al (16) argued that there is a context-dependent
function of USP9X in different stages of PDAC.
In conclusion, the present study demonstrated that
USP9X may serve as a candidate tumor suppressor and prognostic
biomarker in Chinese patients with PDAC. Further studies are
necessary to explore the probable mechanism pathway regarding the
regulation of USP9X, which may be a potential target for
therapeutic intervention in PDAC.
Acknowledgements
The present study was supported by the National
Basic Research Program of the National Natural Science Foundation
(grant nos. 81702726, 81702739 and 81702844) and the Cross Research
Fund of Biomedical Engineering of Shanghai Jiao Tong University
(grant nos. YG2017QN48).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Stathis A and Moore MJ: Advanced
pancreatic carcinoma: Current treatment and future challenges. Nat
Rev Clin Oncol. 7:163–172. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: The impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharma C, Eltawil KM, Renfrew PD, Walsh MJ
and Molinari M: Advances in diagnosis, treatment and palliation of
pancreatic carcinoma: 1990–2010. World J Gastroenterol. 17:867–897.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jones MH, Furlong RA, Burkin H, Chalmers
J, Brown GM, Khwaja O and Affara NA: The Drosophila developmental
gene fat facets has a human homologue in Xp11.4 which escapes
X-inactivation and has related sequences on Yq11.2. Hum Mol Genet.
5:1695–1701. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dasgupta S, Jang JS, Shao C, Mukhopadhyay
ND, Sokhi UK, Das SK, Brait M, Talbot C, Yung RC, Begum S, et al:
SH3GL2 is frequently deleted in non-small cell lung cancer and
downregulates tumor growth by modulating EGFR signaling. J Mol Med
(Berl). 91:381–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng S, Zhou H, Xiong R, Lu Y, Yan D, Xing
T, Dong L, Tang E and Yang H: Over-expression of genes and proteins
of ubiquitin specific peptidases (USPs) and proteasome subunits
(PSs) in breast cancer tissue observed by the methods of RFDD-PCR
and proteomics. Breast Cancer Res Treat. 104:21–30. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xie Y, Avello M, Schirle M, McWhinnie E,
Feng Y, Bric-Furlong E, Wilson C, Nathans R, Zhang J, Kirschner MW,
et al: Deubiquitinase FAM/USP9X interacts with the E3 ubiquitin
ligase SMURF1 protein and protects it from ligase
activity-dependent self-degradation. J Biol Chem. 288:2976–2985.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng J, Hu Q, Liu W, He X, Cui L, Chen X,
Yang M, Liu H, Wei W, Liu S and Wang H: USP9X expression correlates
with tumor progression and poor prognosis in esophageal squamous
cell carcinoma. Diagn Pathol. 8:1772013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peddaboina C, Jupiter D, Fletcher S, Yap
JL, Rai A, Tobin RP, Jiang W, Rascoe P, Rogers MK, Smythe WR and
Cao X: The downregulation of Mcl-1 via USP9X inhibition sensitizes
solid tumors to Bcl-xl inhibition. Bmc Cancer. 12:5412012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harris DR, Mims A and Bunz F: Genetic
disruption of USP9X sensitizes colorectal cancer cells to
5-fluorouracil. Cancer Biol Ther. 13:1319–1324. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang S, Kollipara RK, Srivastava N, Li R,
Ravindranathan P, Hernandez E, Freeman E, Humphries CG, Kapur P,
Lotan Y, et al: Ablation of the oncogenic transcription factor ERG
by deubiquitinase inhibition in prostate cancer. Proc Natl Acad Sci
USA. 111:4251–4256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rolen U, Kobzeva V, Gasparjan N, Ovaa H,
Winberg G, Kisseljov F and Masucci MG: Activity profiling of
deubiquitinating enzymes in cervical carcinoma biopsies and cell
lines. Mol Carcinog. 45:260–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun H, Kapuria V, Peterson LF, Fang D,
Bornmann WG, Bartholomeusz G, Talpaz M and Donato NJ: Bcr-Abl
ubiquitination and Usp9x inhibition block kinase signaling and
promote CML cell apoptosis. Blood. 117:3151–3162. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schwickart M, Huang X, Lill JR, Liu J,
Ferrando R, French DM, Maecker H, O'Rourke K, Bazan F,
Eastham-Anderson J, et al: Deubiquitinase USP9X stabilizes MCL1 and
promotes tumour cell survival. Nature. 463:103–107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pérez-Mancera PA, Rust AG, van der Weyden
L, Kristiansen G, Li A, Sarver AL, Silverstein KA, Grützmann R,
Aust D, Rümmele P, et al: The deubiquitinase USP9X suppresses
pancreatic ductal adenocarcinoma. Nature. 486:266–270.
2012.PubMed/NCBI
|
|
16
|
Cox JL, Wilder PJ, Wuebben EL, Ouellette
MM, Hollingsworth MA and Rizzino A: Context-dependent function of
the deubiquitinating enzyme USP9X in pancreatic ductal
adenocarcinoma. Cancer Biol Ther. 15:1042–1052. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang H, Li Q, He C, Li F, Sheng H, Shen
X, Zhang X, Zhu S, Chen H, Chen X, et al: Activation of the Wnt
pathway through Wnt2 promotes metastasis in pancreatic cancer. Am J
Cancer Res. 4:537–544. 2014.PubMed/NCBI
|
|
19
|
Yang JY, Jiang SH, Liu DJ, Yang XM, Huo
YM, Li J, Hua R, Zhang ZG and Sun YW: Decreased LKB1 predicts poor
prognosis in Pancreatic Ductal Adenocarcinoma. Sci Rep.
5:158692015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huo Y, Yang M, Liu W, Yang J, Fu X, Liu D,
Li J, Zhang J, Hua R and Sun Y: High expression of DDR1 is
associated with the poor prognosis in Chinese patients with
pancreatic ductal adenocarcinoma. J Exp Clin Cancer Res. 34:882015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saka B, Balci S, Basturk O, Bagci P,
Postlewait LM, Maithel S, Knight J, El-Rayes B, Kooby D, Sarmiento
J, et al: Pancreatic ductal adenocarcinoma is spread to the
peripancreatic soft tissue in the majority of resected cases,
rendering the AJCC T-stage protocol (7th Edition) inapplicable and
insignificant: A size-based staging system (pT1: ≤na pT2: & gt;
2- & c pT3: & gt; 4 cm) is more valid and clinically
relevant. Ann Surg Oncol. 23:2010–2018. 2016. View Article : Google Scholar : PubMed/NCBI
|