Introduction
Breast cancer accounts for 25% of all cancer cases
around the world. In 2012, breast cancer was reported to cause 15%
of all cancer-associated mortality among women (1). Furthermore, cancer metastasis is the
major cause of cancer-associated mortality. Metastatic breast
cancer is one of the most life-threatening types of cancer in
women, with a mortality rate of >400,000 each year worldwide
(2). A series of molecular mechanisms
are associated with the migration and invasion of breast cancer
cells, including the upregulated expression of matrix
metalloproteinases, the promotion of the epithelial-to-mesenchymal
transition (EMT), and the activation of nuclear factor-κB,
cancer-induced angiogenesis, vascular endothelial growth factor,
and mammalian target of rapamycin signaling pathways (3,4). However,
cancer metastasis is a complex process and a detailed understanding
of its mechanism requires further investigation.
B-cell lymphoma/leukemia gene-2 (Bcl-2) is the first
proto-oncogene to be demonstrated to function by inhibiting
programmed cell death/apoptosis (5).
Bcl-2 integrates multiple survival- and death-related signals,
which are generated within the cell (5–7). However,
the mechanisms underlying the non-apoptotic functions of Bcl-2
remain to be fully elucidated. Previously, Bcl-2 was found to be
involved in cancer cell invasion and metastasis (8). At the cellular level, the overexpression
of Bcl-2 was reported to increase invasion and migration in glioma
(9,10), lung (11) and breast cancer (12,13). In
animal models, Kang et al showed that the overexpression of
Bcl-2 mediated the metastasis of the MDA-MB-231 human mammary
epithelial cell line to the bone (14). Bcl-2 has subsequently been shown to
induce cellular metastasis to the lung with in mouse EpH4 mammary
epithelial cell line (15). Efforts
made to develop chemical inhibitors of BCL-2 have been reported
extensively (16). In primary breast
cancer, ~75% of cases express elevated levels of Bcl-2, of which
85% are estrogen receptor (ER)-positive and 50% are human epidermal
growth factor receptor 2 (HER-2)-positive tumors (17,18). In
breast cancer, there appears to be a correlation between a high
level of Bcl-2 and a poorer clinical outcome (19,20). The
analysis of patient samples has shown that the expression of Bcl-2
in cancer cells was associated with liver metastasis in breast
(21) and colorectal (22) cancer. Additionally, upregulation in
the expression of Bcl-2 was found during the progression from
pre-invasive lesions to invasive carcinoma in lung cancer samples
(11). The overexpression of
Bcl-extra large (Bcl-XL), another member of the Bcl-2 family,
induces invasion and metastasis (lymph node or distal metastasis)
in patients with breast cancer (22).
This evidence led the present study to focus on the function of
Bcl-2 in the invasion and migration of breast cancer.
The EMT is one of the key events in cancer invasion
and metastasis. It has been reported that reversing EMT may
suppress the metastasis of breast cancer (23). The overexpression of N-cadherin in
MCF-7 breast cancer cells also induces cell migration, invasion and
metastasis, without altering the endogenous expression or
adhesiveness of E-cadherin (24).
Furthermore, it was reported that Bcl-2 regulated Twist-targeted
genes, including the E-cadherin gene (13,25). The
overexpression of Bcl-2 may inhibit the expression of E-cadherin
and promote EMT in different types of cell (13,26,27).
Therefore, Bcl-2 may be involved directly or indirectly in the
transcriptional regulation of EMT.
In the present study, it was shown that the
overexpression of the anti-apoptotic protein Bcl-2 increased the
migration of BCap37 cells, an ER-negative, HER2-positive human
medullary breast cancer cell line (28) and induced cancer metastasis in
vivo. The results also indicated that this Bcl-2-induced
metastasis may result from the progression of EMT.
Materials and methods
Mice and cell lines
Female Balb/c mice were purchased from Vital River
Laboratories, Co., Ltd. (Beijing, China). The BCap37 human breast
cancer cell line was purchased from the Cell Bank of the Chinese
Scientific Academy (Shanghai, China). The BCap37 cells were
cultured in Roswell Park Memorial Institute (RPMI)-1640 medium
(cat. no. 31800105; Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) supplemented with 10% fetal bovine serum (FBS; cat. no.
04-0101-1; Biological Industries USA, Inc., Cromwell, CT, USA). The
cell culture medium was replaced every 2–3 days, and cells were
passaged using 0.25% trypsin-EDTA (cat. no. 25200056; Gibco; Thermo
Fisher Scientific, Inc.), once they reached 90% confluence. The
cultures were maintained at 37°C, 5% CO2 in a
water-jacketed incubator (Thermo Fisher Scientific, Inc.).
Knock down of Bcl-2 in BCap37
cells
The Bcl-2 short hairpin (sh)RNA
5′-CCGGCGTGATGAAGTACATACATTACTCGAGTAATGTATGTACTTCATCACGTTTTTG-3′
(BC-shRNA-Bcl2 #1) or
5′-CCGGGCACACCTGGATCCAGGATAACTCGAGTTATCCTGGATCCAGGTGTGCTTTTTG-3′
(BC-shRNA-Bcl2 #2) sequences were cloned into the pLKO.1-TRC
cloning vector. The plasmids were transfected into 293T cells (Cell
Bank of the Chinese Academy of Sciences, Beijing, China) using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and
the supernatant containing lentiviral plasmids was collected to
infect BCap37 cells. RNA silencing was analyzed using reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
western blot analyses.
Vector construction, lentivirus
production and cell transfection
A Bcl-2 overexpression vector was constructed by
introducing the Bcl-2 coding sequence into the pCDH-CMV-EF1-Puro
vector (pLVX-Bcl2). The Bcl-2 cDNA sequence was amplified from
BCap37 cDNA using the forward
5′-ATAGCTAGCGCCACCATGGCGCACGCTGGGAGAACA-3′ and reverse
5′-ATAGCGGCCGCTCACTTGTGGCCCAGATAGGCAC-3′ primers (the thermocycling
steps are provided in Table I). The
pLVX-Bcl2 vector was mixed with the psPAX2 and PMD2.G packaging
plasmids, and cotransfected into the 293T cells using Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The virus particles were harvested 48 h
following transfection. The BCap37 cells were infected with the
harvested recombinant lentivirus and were isolated using puromycin
to establish stable cell lines constitutively overexpressing Bcl-2
(BC-Bcl2). BCap37 cells transfected with an empty lentiviral vector
served as a control (BC-Mock).
 | Table I.Thermocycling steps for B-cell
lymphoma 2 gene amplification. |
Table I.
Thermocycling steps for B-cell
lymphoma 2 gene amplification.
| 98°C | 2 min (Ramp rate:
4.4°C/sec) | 1 cycle |
| 98°C | 10 sec (Ramp rate:
4.4°C/sec) | 35 cycles |
| 55°C | 15 sec (Ramp rate:
2.2°C/sec) |
|
| 72°C | 15 sec (Ramp rate:
2.2°C/sec) |
|
| 72°C | 10 min (Ramp rate:
2.2°C/sec) | 1 cycle |
| 4°C | 1 min (Ramp rate:
2.2°C/sec) | 1 cycle |
RNA isolation and RT-qPCR
analysis
Total RNA was isolated using RNAiso Plus (Takara
Biotechnology Co., Ltd., Dalian, China) and transcribed into cDNA
using the PrimeScript RT Reagent kit (Takara Biotechnology Co.,
Ltd.). The RT-qPCR was performed using an ABI 7500 FAST Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.) and
a SYBR Green PCR kit (cat. no. RR420A, Takara Biotechnology Co.,
Ltd.). The thermocycling steps were according to the manufacturer's
instruction and the qPCR primers were shown in Table II. The relative expression levels of
mRNA were quantified with the 2−ΔΔCq method (29), following normalization using GAPDH
endogenous reference.
 | Table II.Primers used for reverse
transcription-quantitative polymerase chain reaction. |
Table II.
Primers used for reverse
transcription-quantitative polymerase chain reaction.
| Primer | Sequence
(5′-3′) |
|---|
| BCL-2-F |
GTCTTCGCTGCGGAGATCAT |
| BCL-2-R |
CATTCCGATATACGCTGGGAC |
| GAPDH-F |
GGAGCGAGATCCCTCCAAAAT |
| GAPDH-R |
GGCTGTTGTCATACTTCTCATGG |
Wound-healing assays
Following transfection, the cells were grown to 100%
confluence in six-well plates. A micropipette tip was used to
introduce a cross wound, and wound healing was observed 48 h later.
Images were captured under phase-contrast microscopy (Olympus,
Tokyo, Japan), immediately or 48 h following wounding.
Cell proliferation assay
Cell proliferation was analyzed using the Cell
Counting kit-8 assay (CCK8; Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan). The BCap37, BC-shRNA-NC, BC-shRNA-Bcl2, BC-Mock
or BC-Bcl2 cells were seeded into 96-well plates at a density of
1,000 cells per well and the growth rates were subsequently
evaluated. Each assay was performed in triplicate.
Cell migration and invasion assay
The cell migration and invasion assays were
performed using Transwell chambers (EMD Millipore, Boston, MA,
USA). For the invasion assay, the inserts were coated with Matrigel
(BD Biosciences, Franklin Lakes, NJ, USA) on the upper surface.
Following transfection, 6×104 BCap37 cells were
suspended in 0.2 ml serum-free medium and added to the inserts.
Subsequently, 0.6 ml RPMI-1640 medium supplemented with 10% FBS was
added to the lower compartment, as a chemoattractant. Following
incubation at 37°C for 24 h, the cells on the upper surface of the
membrane were carefully removed using a cotton swab, and the cells
on the lower surface were fixed with 100% methanol and stained with
0.1% crystal violet. Five visual fields (magnification, ×200) of
each insert were randomly selected and counted using light
microscopy.
Western blot analysis
Cellular proteins were isolated in a protein
extraction buffer (Beyotime Institute of Biotechnology, Haimen,
China). Equal quantities (40 µg/lane) of proteins were fractionated
on 6–10% SDS-PAGE gels and transferred onto polyvinylidene
difluoride membranes. The membranes were incubated with anti-Bcl2
(1:1,000 dilution; cat. no. sc-130307), anti-E-cadherin (1:1,000
dilution; cat. no. sc-59780) and anti-vimentin (1:1,000 dilution;
cat. no. sc-80975) primary antibodies (Santa Cruz Biotechnology,
Inc., Santa Cruz, CA, USA) at 4°C overnight. Then, the blots were
incubated for 2 h at room temperature in a 1:10,000 dilution of HRP
goat anti-rabbit IgG antibody (cat. no. ab6721; Abcam, Cambridge,
UK) after 3 washes with PBST. After extensive washing, antibody
detection was accomplished with a sensitive substrate
(Immun-Star™ Western C™ kit, 170-5070;
Bio-Rad Laboratories, Inc., Hercules, CA, USA). β-actin (1:1,000
dilution; cat. no. ab20272; Abcam) was used for the normalization
of protein loading. The image densitometry was performed using
Image Lab software (version 5.2.1 Bio-Rad Laboratories).
Examination of tumor growth and
metastasis in vivo
To develop human breast xenografts, in vitro
grown BCap37, BC-shRNA-C, BC-shRNA-Bcl2, BC-Mock and BC-Bcl2 cells
(1×106 cells in 0.2 ml PBS) were implanted into the
right flanks of homozygous female nude athymic mice (5–6 weeks old
abd weighing 17–21 g). The mice were housed in a
temperature-controlled environment with 12 h light and dark cycles
and received a standard food and water diet ad libitum. Two
perpendicular diameters (width and length) of the mouse tumors were
measured three times a week until the animals were sacrificed after
30 days. In addition, a tumor growth curve was drawn based on the
tumor volume and the corresponding time (days) post-injection. When
the animals were sacrificed, the lungs were harvested and fixed in
10% neutral formalin and embedded in paraffin. 4 µm sections were
prepared and stained with hematoxylin and eosin (H&E), followed
by examination under a microscope and image capture. The area of
metastasis (pixels) of each lung metastasis from different
injection groups was calculated using Adobe Photoshop (version CS5;
Adobe Systems, Inc., San Jose, CA, USA). Each dot represented the
sum of pixels in the lung metastases in the paraffin-embedded
section for each mouse (magnification, ×200). Data are
representative of at least three separate experiments.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism Software (GraphPad Software, Inc., La Jolla, CA, USA).
Briefly, data are presented as the mean ± standard error of the
mean or mean ± standard deviation of three independent experiments.
Two-way analysis of variance (ANOVA) followed by Bonferroni's
post-hoc test was used to compare the means of groups affected by
two independent factors, whereas a one-way ANOVA followed by
Tukey's post-hoc test was used to compare the means of three
independent groups. Student's t test was used to compare the means
of two independent groups. P<0.05 was considered to indicate a
statistically significant difference.
Ethics statement
All animal experiments in the present study were
approved by Zhejiang University Animal Care Committee (Hangzhou,
China). All animal manipulations were performed in accordance with
the National Institutes of Health Guide for the Care and Use of
Laboratory Animals (NIH, eighth edition), revised in 2012. The mice
were sacrificed using carbon dioxide inhalation. All surgery was
performed under sodium pentobarbital anesthesia, and all efforts
were made by the attending skilled technician to minimize
suffering.
Results
Proliferation of BCap37 cells is not
affected by the expression of Bcl-2 in vivo or in vitro
To investigate the effects of Bcl-2 in breast cancer
cells in the present study, the expression of Bcl-2 in the BCap37
human breast cancer line was increased or silenced. To increase the
expression of Bcl-2, BCap37 cells were transfected either with a
CMV promoter-driven Bcl-2 expression vector (BC-Bcl2 1# and BC-Bcl2
2#) or with an empty vector (BC-Mock). Stable cell lines were
established and expanded. To inhibit the expression of Bcl-2, the
cells were transduced with a lentiviral vector expressing
shRNA-Bcl2 (BC-shRNA-Bcl2 1# and BC-shRNA-Bcl2 2#) or with an empty
vector (BC-shRNA-NC). The expression level of Bcl-2 in the
different cell lines was verified by RT-qPCR and western blot
analyses. Bcl-2 was expressed at high levels in the BC-Bcl2 cells,
compared with its expression in the BC-Mock cells (Fig. 1A and B). Furthermore, Bcl-2 was
effectively silenced in the BC-shRNA-Bcl2 cells, when compared with
its level in the BC-shRNA-NC cells. BC-Bcl2 2# and BC-shRNA-Bcl2 1#
were selected for the following experiments.
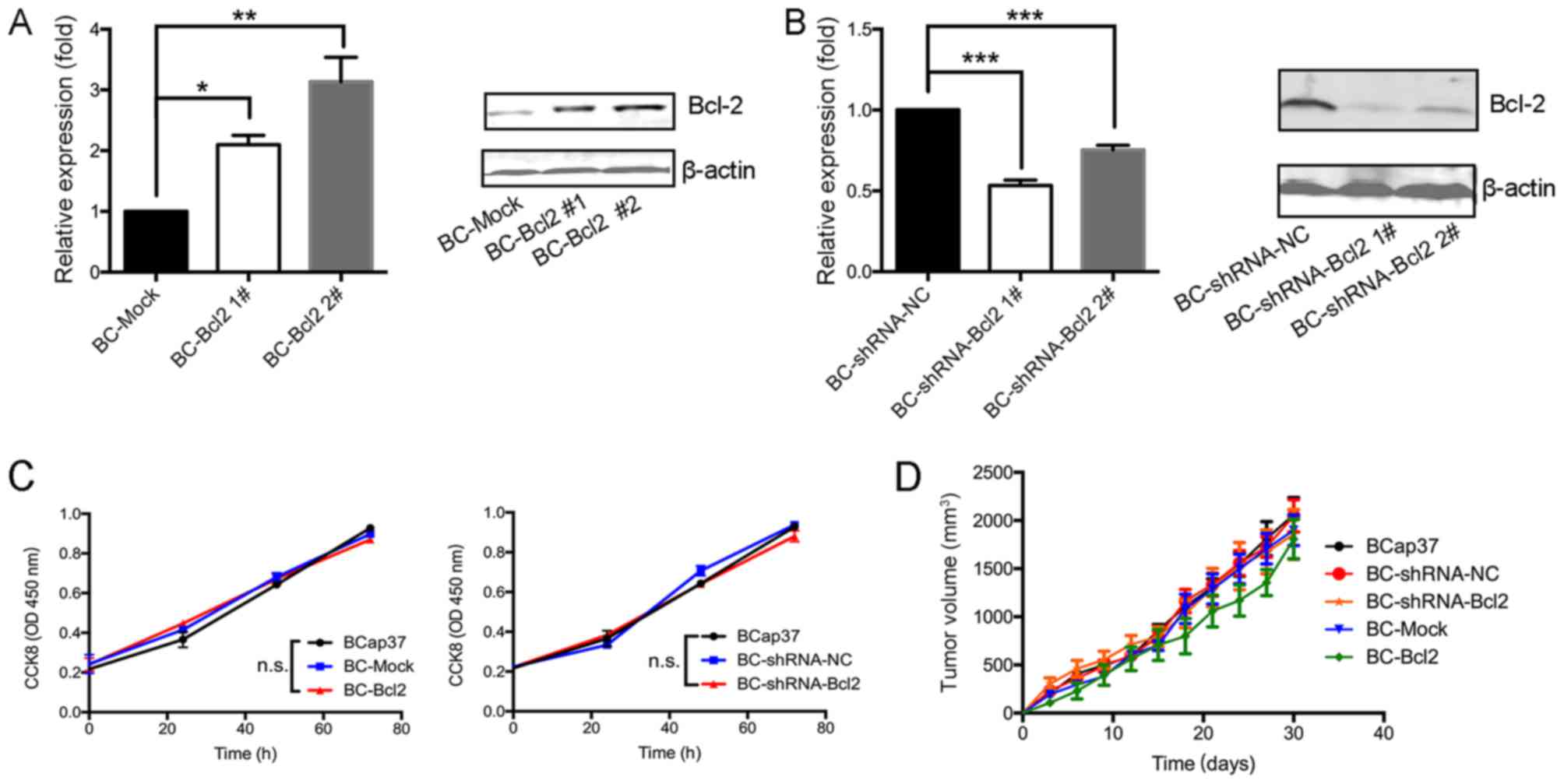 | Figure 1.Proliferation of BCap37 cells is not
affected by the expression of Bcl-2 in vivo or in
vitro. Results of reverse transcription-quantitative polymerase
chain reaction (left) and western blot (right) analyses showed that
(A) Bcl-2 was expressed at high levels in BC-Bcl2 cells, compared
with its levels in BC-Mock cells, and (B) Bcl-2 was effectively
silenced in the BC-shRNA-Bcl2 cells, compared with the BC-shRNA-NC
cells. Protein levels were quantitated by densitometry. (C) CCK8
assay revealed no statistically significant difference in relative
cellular viability of BCap37 cells upon Bcl-2 overexpression or
silencing, compared with the control. (D) Tumor growth was not
affected by Bcl-2 in BCap37 tumor xenografts. Nude athymic mice
were implanted with BCap37, BC-shRNA-NC, BC-shRNA-Bcl2, BC-Mock,
and BC-Bcl2, respectively (n=5 for each group). The tumor growth
curves in each group are shown. The data are presented as the mean
± standard deviation of three independent experiments. *P<0.05,
**P<0.01, ***P<0.001. BC, BCap37 cell; Bcl-2, B-cell lymphoma
2; shRNA, short hairpin RNA; NC, negative control; CCK8, Cell
Counting kit-8; n.s., not significant. |
To examine the effect of Bcl-2 on the proliferation
of BCap37 cells in vitro, the growth rate of BCap37 cells
was compared following varying of the expression of Bcl-2. Using
the CCK8 assay, no significant differences were observed in the
growth rates of the BCap37, BC-Mock, BC-Bcl2, BC-shRNA-NC or
BC-shRNA-Bcl2 cells (Fig. 1C).
Subsequently, to assess the effect of Bcl-2 on the
proliferation kinetics of BCap37 cells in vivo, the
above-mentioned cells were implanted into the right flanks of
homozygous nude athymic mice. The tumor volumes following
implantation of the different cells were measured every 3 days.
After 30 days, when the animals were sacrificed, the mean tumor
volumes in the BCap37, BC-Mock, BC-Bcl2, BC-shRNA-NC and
BC-shRNA-Bcl2 groups were similar at ~2,059.7±311.1, 2,048.3±290.6,
1,855.5±447.4, 1,898.3±277.2 and 1,806.5±355.1 mm3,
respectively (n=5 for each group, Fig.
1D). There were no significant statistical differences among
tumor volumes for the different cell groups. In conclusion, the
proliferation of BCap37 cells was not affected by the expression of
Bcl-2, neither in vitro nor in vivo.
Bcl-2-overexpressing BCap37 cells
exhibit a higher migratory and invasive capacity, compared with
control cells in vitro
Subsequently, the present study examined whether
Bcl-2 affected the migration and invasion capacity of BCap37 cells.
Using a wound-healing assay, it was observed that the inhibition of
Bcl-2 in BCap37 cells was associated with the inhibition of wound
closure, compared with that in the control. Additionally, the
overexpression of Bcl-2 in BCap37 cells accelerated wound closure
(Fig. 2A and B). A migration chamber
assay was used to verify the role of Bcl-2 in cell motility. Bcl-2
significantly promoted the migration of BCap37 cells, whereas the
knockdown of Bcl-2 in BCap37 cells inhibited their migration
(Fig. 2C upper panel, and D). The
invasion chamber assay showed that Bcl-2 increased the invasive
capacity of the BCap37 cells. Taken together, these results
suggested that Bcl-2-overexpressing BCap37 cells exhibit a higher
migratory and invasive capacity, compared with control cells in
vitro.
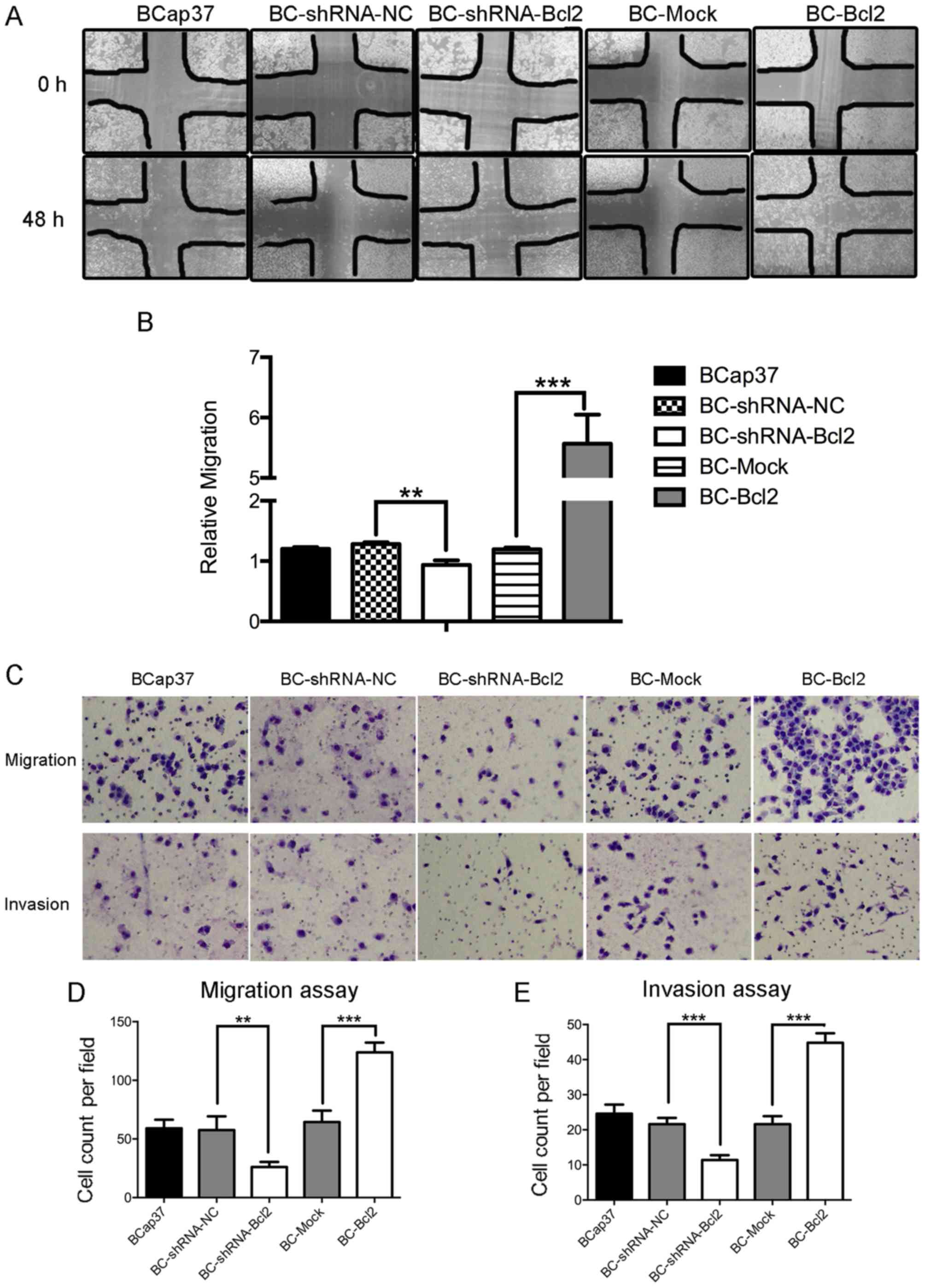 | Figure 2.Bcl-2-overexpressing BCap37 cells
exhibit a higher migratory and invasive capacity, compared with
control cells in vitro. (A) Representative micrographs
(magnification, ×100) and results of (B) wound-healing assay in
cells expressing BC-shRNA-NC, BC-shRNA-Bcl2, BC-Mock and BC-Bcl2,
performed with a 48-h recovery period. Relative migration was
calculated as follows: (Cell covered areas (48 h)/Cell covered
areas (0 h)). (C) Representative micrographs of Transwell (D)
migration and (E) invasion assays (magnification, ×200). Bcl-2
enhanced the migratory and invasive capacity of BCap37 cells in
vitro. The data are presented as the mean ± standard deviation
of three independent experiments. **P<0.01, ***P<0.001. BC,
BCap37 cell; Bcl-2, B-cell lymphoma 2; shRNA, short hairpin RNA;
NC, negative control. |
Bcl-2 induces lung metastasis of
BCap37 cells in vivo
To investigate the effect of Bcl-2 on the kinetics
of metastasis of BCap37 cells in vivo, BCap37, BC-Mock,
BC-Bcl2, BC-shRNA-NC or BC-shRNA-Bcl2 cells were implanted into the
right flanks of homozygous nude athymic mice. Following sacrifice
of the animals 30 days post-implantation, the lungs were harvested
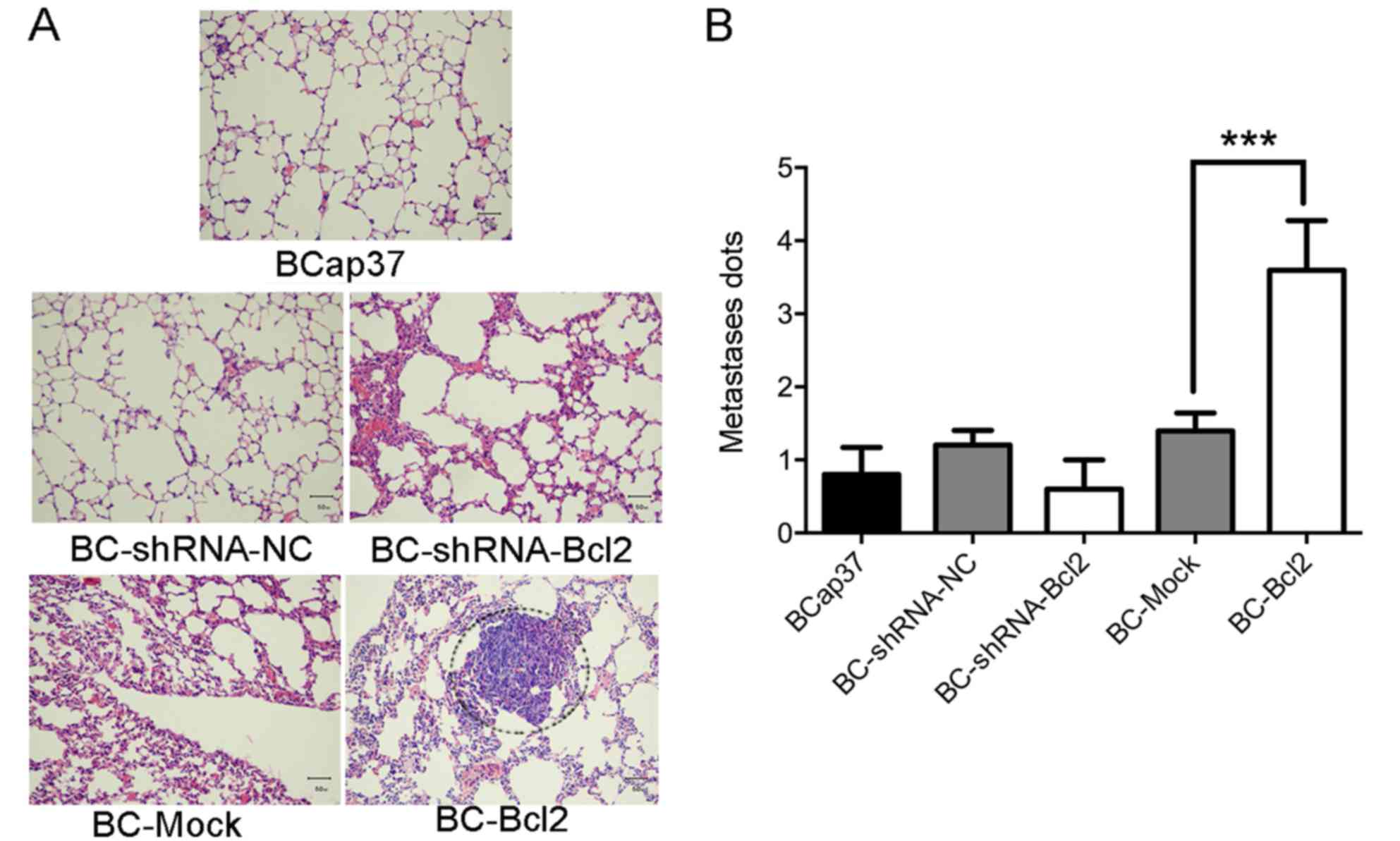
and stained with H&E. As shown in Fig. 3A and B, the overexpression of Bcl-2
significantly induced the migration of BCap37 cells to the lung.
Therefore, Bcl-2 induced the metastasis of BCap37 cells in
vivo.
Overexpression of Bcl-2 in BCap37
cells induces the progression of EMT
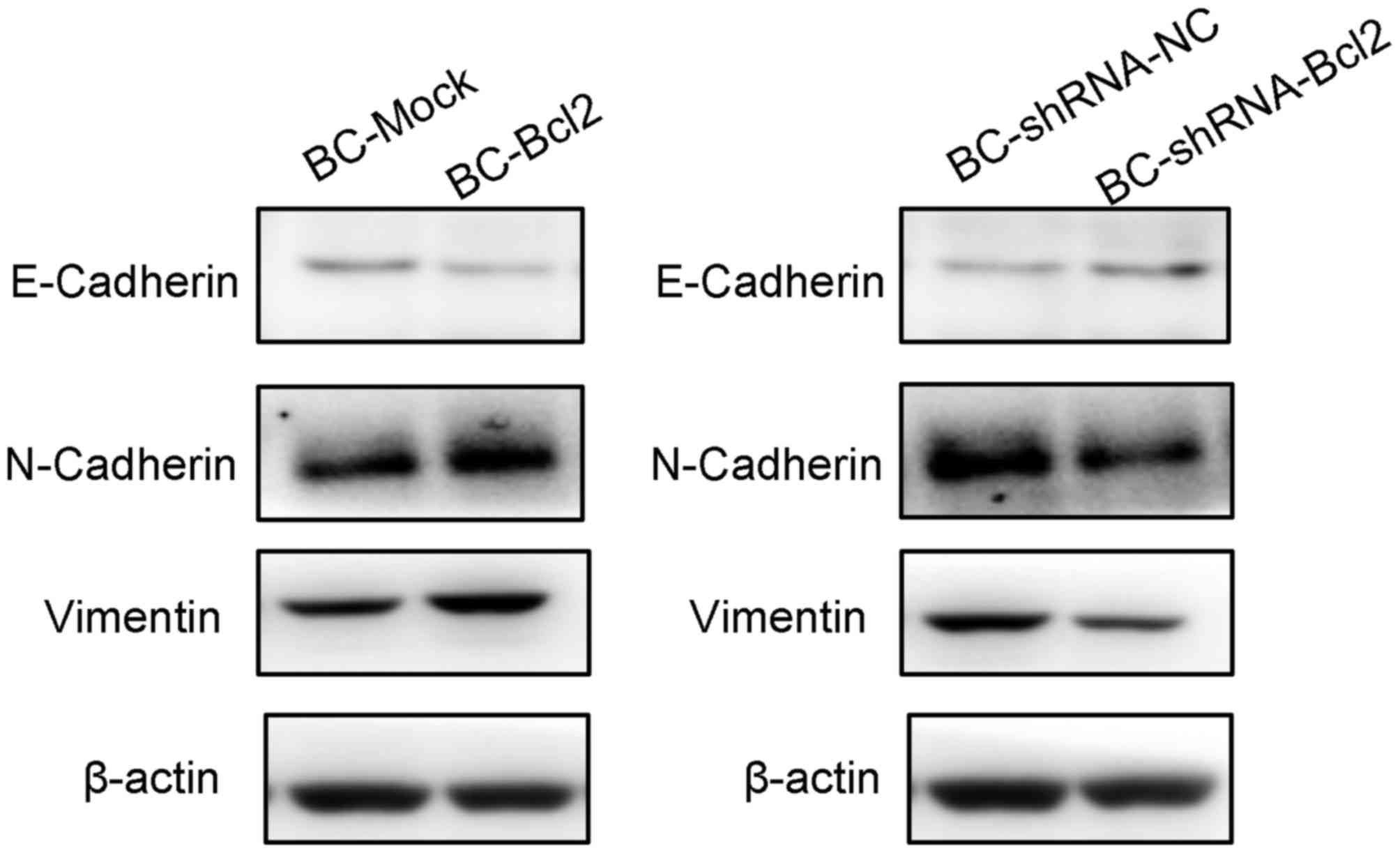
To investigate the effect of Bcl-2 on the
progression of EMT, a western blot assay was used to detect the
protein levels of EMT markers in cells expressing BC-Mock BC-Bcl2,
BC-shRNA-NC or BC-shRNA-Bcl2. The western blot results showed that
the overexpression of Bcl-2 inhibited the expression of E-cadherin
(an epithelial marker), but increased the levels of N-cadherin and
vimentin (mesenchymal markers). In addition, the inhibition of
Bcl-2 led to an increase in the expression of E-cadherin, but
inhibited the expression of N-cadherin and vimentin (Fig. 4). These results are in agreement with
previous reports (18,21), suggesting that the overexpression of
Bcl-2 increases breast cancer invasion and metastasis by inducing
EMT.
Discussion
Bcl-2 is known as the first proto-oncogene by
inhibiting apoptosis, however, the role of Bcl-2 in breast cancer
migration and metastasis remains to be fully elucidated. In the
present study, it was first found that the proliferation of BCap37
cells was not affected by the expression of Bcl-2, neither in
vitro nor in vivo. It has been reported that the
knockdown of Bcl-2 has no anti-proliferative effects on colorectal
cancer cells (30). Presumably, this
may be due to the antagonistic effect of other members of the Bcl-2
family, of which there are several members and a number of which
have an anti-proliferative function, with others having the
opposite effect. For example, Bcl-2-associated X factor can have
negative effects on the anti-apoptotic function of Bcl-2 by forming
two dimers with Bcl-2 (31). The
present study found that Bcl-2 promoted the migration and
metastasis of BCap37 cells in vitro and Bcl-2-overexpressing
BCap37 cells were more likely to transfer to the lungs in mice. In
line with previous findings, the present study showed the ability
of Bcl-2 to regulate cancer invasion and metastasis, and is the
first, to the best of our knowledge, to report its function
specifically in breast cancer. EMT is one of the key and common
events occur in the early age of cancer development. In the present
study, it was observed that Bcl-2 induced EMT progression in BCap37
cells, indicating the early and extensive involvement of Bcl-2 in
regulating cancer invasion.
As Bcl-2 is vital in tumorigenesis and neoplastic
progression, it may be a promising candidate for anticancer
therapy. Previous findings confirmed that Bcl-2 offers potential as
a prospective drug target. Several small molecular inhibitors of
Bcl-2, including ABT-737 and ABT-199, have been investigated
extensively (16,32). ABT-737, designed to neutralize the
prosurvival proteins BCL-2, BCL-XL and BCL-W, was reported to
improve anti-estrogen tamoxifen susceptibility in the treatment of
ER-positive breast cancer and triple negative breast tumor
xenografts (18,33,34). In
addition, it has been reported that herceptin-resistant breast
cancer cells show increased sensitivity to ABT-737, suggesting that
ABT-737 offers potential as a therapeutic agent (35). ABT-199 (venetoclax), a potent
Bcl-2-selective inhibitor previously approved by the Food and Drug
Administration for the treatment of chronic lymphocytic leukemia,
was reported to be effective in breast cancer treatment, with
satisfactory safety and efficacy (36,37). The
combination of Bcl-2/Bcl-xl inhibitor ABT-263/ABT-199 with
cyclin-dependent kinase 7 inhibitor thz1 can further promote
anti-proliferation and induce apoptosis in human triple-negative
breast tumor cells (38), showing the
compatibility of Bcl-2 chemical inhibitors with other
chemotherapeutic drugs.
In conclusion, the present study comprehensively
investigated the effect of Bcl-2 on the motility of the BCap37 cell
line. It was found that Bcl-2 did not affect the proliferation of
BCap37 cells, but promoted cell invasion in vitro and in
vivo. In addition, a lung metastasis mouse model of breast
cancer was developed, which may be used to evaluate the
effectiveness of certain novel medications for metastatic breast
cancer in further investigations. For example, one first-in-class
CDK4/6 inhibitor, palbociclib, was approved for the treatment of
postmenopausal women with ER(+), HER2(−) advanced breast cancer in
combination with letrozole, and achieved a good outcome (39–41). In
addition, utidelone, an analog of epothilone, has been tested in a
phase II trial as a monotherapy or in combination with capecitabine
in patients with heavily pre-treated metastatic breast cancer, and
the results showed marked advantages of this novel medication with
higher efficacy and tolerability (42). The mouse model established in the
present study may be used by others to assess these novel agents
in vivo. Upon implantation of breast cancer cells and
successful model establishment, these agents can be transferred
into the body by intravenous injection to determine their effect on
tumor growth featured by the number and size of lung metastases. In
addition, the combination of agents and cancer cell lines can vary
according to the investigation design. For example, as a
supplement, the MCF-7 cell line, which expresses ERs, but not HER2,
may be used in the model to assess the effect of palbociclib
mentioned above. However, the molecular mechanisms underlying the
different effects of Bcl-2 on proliferation, migration and invasion
require further investigation.
Acknowledgements
The authors would like to thank Professor Youfa Zhu,
Ms Yanwei Li and Ms Li Liu of the Public Platform of Zhejiang
University of Medicine, for providing technical support for the
flow cytometry and immunohistochemical assays.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author, on reasonable
request.
Authors' contributions
PF conceived and designed the experiments. CD
performed the majority of the experiments, including the in
vivo work, the and in vitro migration and invasion
assays, and contributed to the western blotting. XZ, MY and KL
performed the CCK-8 assay and analyzed the majority of the data.
JW, LC and SW performed RT-qPCR and helped analyze the HE
staining.
Ethics approval and consent to
participate
The present study was approved by the Zhejiang
University Animal Care Committee (Hangzhou, China). All animal
manipulations were performed in accordance with the National
Institutes of Health Guide for the Care and Use of Laboratory
Animals (8th edition), revised in 2012.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tevaarwerk AJ, Gray RJ, Schneider BP,
Smith ML, Wagner LI, Fetting JH, Davidson N, Goldstein LJ, Miller
KD and Sparano JA: Survival in patients with metastatic recurrent
breast cancer after adjuvant chemotherapy: Little evidence of
improvement over the past 30 years. Cancer. 119:1140–1148. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang YL and Liu ZP: Natural products as
anti-invasive and anti-metastatic agents. Curr Med Chem.
18:808–829. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ci Y, Qiao J and Han M: Molecular
mechanisms and metabolomics of natural polyphenols interfering with
breast cancer metastasis. Molecules. 21:E16342016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Borner C: The Bcl-2 protein family:
Sensors and checkpoints for life-or-death decisions. Mol Immunol.
39:615–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chipuk JE, Moldoveanu T, Llambi F, Parsons
MJ and Green DR: The BCL-2 family reunion. Mol Cell. 37:299–310.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Adams JM and Cory S: Life-or-death
decisions by the Bcl-2 protein family. Trends Biochem Sci.
26:61–66. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Um HD: Bcl-2 family proteins as regulators
of cancer cell invasion and metastasis: A review focusing on
mitochondrial respiration and reactive oxygen species. Oncotarget.
7:5193–5203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wick W, Wagner S, Kerkau S, Dichgans J,
Tonn JC and Weller M: BCL-2 promotes migration and invasiveness of
human glioma cells. FEBS Lett. 440:419–424. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wick W, Wild-Bode C, Frank B and Weller M:
BCL-2-induced glioma cell invasiveness depends on furin-like
proteases. J Neurochem. 91:1275–1283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choi J, Choi K, Benveniste EN, Rho SB,
Hong YS, Lee JH, Kim J and Park K: Bcl-2 promotes invasion and lung
metastasis by inducing matrix metalloproteinase-2. Cancer Res.
65:5554–5560. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Del Bufalo D, Biroccio A, Leonetti C and
Zupi G: Bcl-2 overexpression enhances the metastatic potential of a
human breast cancer line. FASEB J. 11:947–953. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
An J, Lv J, Li A, Qiao J, Fang L, Li Z, Li
B, Zhao W, Chen H and Wang L: Constitutive expression of Bcl-2
induces epithelial-Mesenchymal transition in mammary epithelial
cells. BMC Cancer. 15:4762015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kang Y, Siegel PM, Shu W, Drobnjak M,
Kakonen SM, Cordón-Cardo C, Guise TA and Massagué J: A multigenic
program mediating breast cancer metastasis to bone. Cancer Cell.
3:537–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pinkas J, Martin SS and Leder P:
Bcl-2-mediated cell survival promotes metastasis of EpH4 betaMEKDD
mammary epithelial cells. Mol Cancer Res. 2:551–556.
2004.PubMed/NCBI
|
|
16
|
Cang S, Iragavarapu C, Savooji J, Song Y
and Liu D: ABT-199 (venetoclax) and BCL-2 inhibitors in clinical
development. J Hematol Oncol. 8:1292015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dawson SJ, Makretsov N, Blows FM, Driver
KE, Provenzano E, Le Quesne J, Baglietto L, Severi G, Giles GG,
McLean CA, et al: BCL2 in breast cancer: A favourable prognostic
marker across molecular subtypes and independent of adjuvant
therapy received. Br J Cancer. 103:668–675. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Oakes SR, Vaillant F, Lim E, Lee L,
Breslin K, Feleppa F, Deb S, Ritchie ME, Takano E, Ward T, et al:
Sensitization of BCL-2-expressing breast tumors to chemotherapy by
the BH3 mimetic ABT-737. Proc Natl Acad Sci USA. 109:2766–2771.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kerr DA II and Wittliff JL: A five-gene
model predicts clinical outcome in ER+/PR+, early-stage breast
cancers treated with adjuvant tamoxifen. Horm Cancer. 2:261–271.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi JE, Kang SH, Lee SJ and Bae YK:
Prognostic significance of Bcl-2 expression in non-basal
triple-negative breast cancer patients treated with
anthracycline-based chemotherapy. Tumour Biol. 35:12255–12263.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Neri A, Marrelli D, Roviello F, DeMarco G,
Mariani F, DeStefano A, Megha T, Caruso S, Corso G, Cioppa T and
Pinto E: Bcl-2 expression correlates with lymphovascular invasion
and long-term prognosis in breast cancer. Breast Cancer Res Treat.
99:77–83. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ishijima N, Miki C, Ishida T, Kinoshita T
and Suzuki H: The immunohistochemical expression of BCL-2
oncoprotein in colorectal adenocarcinoma. Surg Today. 29:682–684.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin D, Kuang G, Wan J, Zhang X and Li H,
Gong X and Li H: Luteolin suppresses the metastasis of
triple-negative breast cancer by reversing
epithelial-to-mesenchymal transition via downregulation of
β-catenin expression. Oncol Rep. 37:895–902. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hazan RB, Phillips GR, Qiao RF, Norton L
and Aaronson SA: Exogenous expression of N-cadherin in breast
cancer cells induces cell migration, invasion, and metastasis. J
Cell Biol. 148:779–790. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun T, Sun BC, Zhao XL, Zhao N, Dong XY,
Che N, Yao Z, Ma YM, Gu Q, Zong WK and Liu ZY: Promotion of tumor
cell metastasis and vasculogenic mimicry by way of transcription
coactivation by Bcl-2 and Twist1: A study of hepatocellular
carcinoma. Hepatology. 54:1690–1706. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zuo J, Ishikawa T, Boutros S, Xiao Z,
Humtsoe JO and Kramer RH: Bcl-2 overexpression induces a partial
epithelial to mesenchymal transition and promotes squamous
carcinoma cell invasion and metastasis. Mol Cancer Res. 8:170–182.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu PJ, Lu QL, Rughetti A and
Taylor-Papadimitriou J: Bcl-2 overexpression inhibits cell death
and promotes the morphogenesis, but not tumorigenesis of human
mammary epithelial cells. J Cell Biol. 129:1363–1378. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jiang D, Sui M, Zhong W, Huang Y and Fan
W: Different administration strategies with paclitaxel induce
distinct phenotypes of multidrug resistance in breast cancer cells.
Cancer Lett. 335:404–411. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Koehler BC, Scherr AL, Lorenz S, Urbanik
T, Kautz N, Elssner C, Welte S, Bermejo JL, Jäger D and
Schulze-Bergkamen H: Beyond cell death-antiapoptotic Bcl-2 proteins
regulate migration and invasion of colorectal cancer cells in
vitro. PLoS One. 8:e764462013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bonnefoy-Berard N, Aouacheria A,
Verschelde C, Quemeneur L, Marçais A and Marvel J: Control of
proliferation by Bcl-2 family members. Biochim Biophys Acta.
1644:159–168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pan R, Ruvolo VR, Wei J, Konopleva M, Reed
JC, Pellecchia M, Andreeff M and Ruvolo PP: Inhibition of Mcl-1
with the pan-Bcl-2 family inhibitor (−)BI97D6 overcomes ABT-737
resistance in acute myeloid leukemia. Blood. 126:363–372. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Oltersdorf T, Elmore SW, Shoemaker AR,
Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges
J, Hajduk PJ, et al: An inhibitor of Bcl-2 family proteins induces
regression of solid tumours. Nature. 435:677–681. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen J, Jin S, Abraham V, Huang X, Liu B,
Mitten MJ, Nimmer P, Lin X, Smith M, Shen Y, et al: The
Bcl-2/Bcl-X(L)/Bcl-w inhibitor, navitoclax, enhances the activity
of chemotherapeutic agents in vitro and in vivo. Mol Cancer Ther.
10:2340–2349. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Crawford A and Nahta R: Targeting Bcl-2 in
herceptin-resistant breast cancer cell lines. Curr Pharmacogenomics
Persona Med. 9:184–190. 2011. View Article : Google Scholar
|
|
36
|
Vaillant F, Merino D, Lee L, Breslin K,
Pal B, Ritchie ME, Smyth GK, Christie M, Phillipson LJ, Burns CJ,
et al: Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen
receptor-positive breast cancer. Cancer Cell. 24:120–129. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Deng J and Letai A: Priming BCL-2 to kill:
The combination therapy of tamoxifen and ABT-199 in ER+ breast
cancer. Breast Cancer Res. 15:3172013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Li B, Chonghaile Ni T, Fan Y, Madden SF,
Klinger R, O'Connor AE, Walsh L, O'Hurley G, Udupi Mallya G, Joseph
J, et al: Therapeutic rationale to target highly expressed CDK7
conferring poor outcomes in triple-negative breast cancer. Cancer
Res. 77:3834–3845. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Dukelow T, Kishan D, Khasraw M and Murphy
CG: CDK4/6 inhibitors in breast cancer. Anticancer Drugs.
26:797–806. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xu H, Yu S, Liu Q, Yuan X, Mani S, Pestell
RG and Wu K: Recent advances of highly selective CDK4/6 inhibitors
in breast cancer. J Hematol Oncol. 10:972017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu J: Palbociclib: A first-in-class
CDK4/CDK6 inhibitor for the treatment of hormone-receptor positive
advanced breast cancer. J Hematol Oncol. 8:982015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang P, Tong Z, Tian F, Wang Y, Yang J,
Li W, Di L, Liu W, Tang L, Qiu R and Xu B: Phase II trial of
utidelone as monotherapy or in combination with capecitabine in
heavily pretreated metastatic breast cancer patients. J Hematol
Oncol. 9:682016. View Article : Google Scholar : PubMed/NCBI
|