Introduction
Breast cancer is the leading cause of
cancer-associated mortality among women worldwide (1). Although there are a range of therapeutic
methods available to treat breast cancer, including surgery,
chemotherapy, endocrine therapy, radiation and targeted therapy,
there remains a large proportion therapeutic failures, subsequently
resulting in cancer recurrence and metastasis (2).
Cisplatin, a platinum-containing antineoplastic
drug, is a chemotherapeutic agent used to treat a range of solid
malignant tumors, including ovarian cancer, breast cancer, gastric
carcinoma and rectal carcinoma (3,4). Previous
studies have revealed that cisplatin exerts its anticancer effects
by activating the apoptotic pathway and inducing cell death
(5–7).
Apoptosis is the main response induced in tumor cells upon
treatment chemotherapeutic agents (8). Cisplatin is an antitumor agent with a
high rate of success in treating tumors; however, resistance to
this therapy can develop. Evidence indicates the involvement of the
tumor microenvironment (TME) in acquisition of chemoresistance
(9,10). The TME influences the growth of tumors
and affects the outcome of chemotherapy (11,12).
Whether the TME has a role in cisplatin-triggered chemoresistance
in breast cancer is unclear.
The TME is a dynamic system that consists of complex
non-malignant cells, extracellular matrix (ECM) and signaling
molecules that communicate with cancer cells. The non-malignant
cells include fibroblasts, endothelial cells and immune cells that,
together with the surrounding ECM, affect tumor activity.
Mesenchymal stem cells (MSCs), one of the pivotal components of the
TME, are a focus of research in the progression of tumors (13,14).
Notably, there is evidence to suggest that MSCs release cytokines
and growth factors to influence the behavior of tumor in a
paracrine manner (15–17). In particular, owing to the influence
on the tumor itself and the inflammatory TME, tumor-tissue-derived
MSCs are widely studied (14,18). Previous studies have demonstrated that
tumor progression, enhancement of metastatic potential, and
resistance to chemo- and radiotherapy may all be attributed to MSCs
(19–21). Whether breast-cancer-tissue-derived
mesenchymal stem cells (BC-MSCs) are able to promote
chemoresistance of cisplatin requires further investigation.
In the present study, it was revealed that BC-MSCs
enhanced the proliferation of breast cancer cells and decreased
cisplatin triggered apoptosis in MCF-7 cell. Interleukin-6 (IL-6)
was released by BC-MSCs, which mediated a reduction in apoptosis
induced by cisplatin by activating the signal transducer and
activator of transcription 3 (STAT3) signaling pathway. This study
revealed a novel mechanism of drug resistance of cisplatin.
Materials and methods
Cells and reagents
The human breast cancer MCF-7 cell line was obtained
from the American Type Culture Collection (Manassas, VA, USA) and
cultured in Dulbecco's modified Eagle medium (DMEM) (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 15%
fetal bovine serum (FBS) (Gibco; Thermo Fisher Scientific, Inc.) at
37°C with 5% CO2 atmosphere in a humidified incubator.
Breast cancer tissues were obtained from female patients (n=12)
aged 40–60 years (mean 52 years), who underwent surgical operation
in the First People's Hospital of Lianyungang from April 2015 to
July 2015 (Jiangsu, China), all patients provided written informed
consent to participate in the study and all experiment protocols
were approved by the Medical Ethics Committee of First People's
Hospital of Lianyungang. The cell isolation procedure was typically
undertaken within 4 h of tumor removal. In brief, fresh tissue
specimens were collected, washed with PBS, cut into 1
mm3-sized pieces and put into culture dishes for 30 min
at 37°C. Then, tissue samples were maintained in DMEM supplemented
with 10% FBS, 1% penicillin and streptomycin at 37°C with 5%
CO2. Medium was replaced every three days after the
initial plating. When adherent fibroblast-like cells confluence
reached ~80%, the cells were passaged into new flasks for further
expansion. Cells at passage 3–4 were used for the evaluation of the
experiments. Bone marrow-mesenchymal stem cells (BM-MSCs) from
patients (n=12) aged 3–60 years (mean, 22 years) with suspected
blood system diseases who were diagnosed with no hematological
disease or other cancers at the First People's Hospital of
Lianyungang from April 2015 to July 2015 (Jiangsu, China) were
chosen as the MSC controls.
Flow cytometry analysis
To investigate the surface antigen markers of
different passages of BC-MSCs and BM-MSCs, flow cytometric analysis
was performed using fluorescein isothiocyanate (FITC)-conjugated or
phycoerythrin (PE)-conjugated antibodies: CD14 (cat. no. 561712;
dilution, 1:50), CD34 (cat. no. 555822; dilution, 1:50) and CD45
(cat. no. 560976; dilution, 1:50) were FITC-conjugated, CD29 (cat.
no. 561795; dilution, 1:50), CD44 (cat. no. 561858; dilution,
1:50), CD90 (cat. no. 561970; dilution, 1:50) and CD105 (cat. no.
562380; dilution, 1:50) were PE-conjugated. Mouse PE-IgG1 (cat. no.
349043; dilution, 1:50) and FITC-IgG1 (cat. no. 349041; dilution,
1:50) isotypic immunoglobulins were used as controls (BD
Biosciences, San Jose, CA, USA). BC-MSCs and BM-MSCs cells
(1.0×106) were trypsinized, washed twice in PBS,
incubated with monoclonal antibodies for 30 min on ice at 4°C and
then washed with PBS. Labeled cells were analyzed using a FACSCanto
II flow cytometer (BD Biosciences).
Multidifferentiation capacity
To determine the ability of BC-MSCs to undergo
osteogenic and adipogenic differentiation, BC-MSCs were seeded in
35-mm plates at 3×104 cells/cm2 and cultured
in L-DMEM containing 15% FBS. The next day, the medium was changed
to osteogenic medium or adipogenic medium (both from Cyagen
Biosciences, Sunnyvale, CA, USA) in accordance with the culture
protocol. After 2 weeks, the osteogenic differentiation of BC-MSCs
was examined by alkaline phosphatase staining at room temperature
for 15 min. At 3 weeks later, adipocytes were stained with
Oil-Red-O at room temperature for 15 min to confirm the ability of
adipogenic differentiation of BC-MSCs. A fluorescence-inverted
microscope (Olympus Corporation, Tokyo, Japan) was used to observe
the staining results at ×100 and ×400 magnification.
Preparation of MSC-conditioned
medium
A total of (1.0×106) BC-MSC cells were
seeded in 35-mm plates and cultured in L-DMEM with 15% FBS. The
following day, the media was removed and the cells were washed with
PBS, re-incubated with fresh medium for 48 h. Next,
BC-MSC-conditioned medium (BC-MSC-CM) and BM-MSC-conditioned medium
(BM-MSC-CM) were collected, and centrifuged at room temperature to
remove possible cell debris (1,000 × g for 10 min).
MTT assay
To investigate the effect of cisplatin, BC-MSC-CM
combination cisplatin on the viability of MCF-7 cells, an MTT assay
was performed. Briefly, MCF-7 cells were plated at a density of
1×104 cells/well in a 96-well rounded bottom plate.
After incubation for 12 h, cells were incubated with cisplatin for
another 48 h at 37°C by a continuous induction from 2.5 to 80 µM
(2.5, 5, 10, 20, 40 and 80 µM) in a stepwise increasing
concentration manner in the presence or absence of BC-MSC-CM.
Control medium was used as a control. All the cells were cultured
for a further 48 h. Subsequently, MTT reagent was added into each
well and the cells were incubated for an additional 4 h at 37°C.
Following incubation and removal of the supernatant, 150 µl DMSO
was added to dissolve the dye and the absorbance was measured at
490 nm with a microplate reader. IC50 was defined as the
drug concentration causing a 50% apoptosis relative to the negative
control. In this experiment, the IC50 was 20 µM. The
experiment was repeated three times; six parallel samples were
detected each time.
Measurement of cell apoptosis and
vitality
In brief, a total of 5.0×104 MCF-7 cells
were seeded into 6-well plates. After 48 h, cells were treated with
20 µM cisplatin diluted in complete medium or BC-MSC-CM. Control
cells were treated with medium without the addition of cisplatin.
After culturing for 48 h, cells were suspended in PBS and incubated
with reagents from the Annexin V & Dead Cell kit and Count
& Viability kit (both from Merck KGaA, Darmstadt, Germany)
according to manufacturer's protocol. Data was processed using
Muse™ smart touch FACS (Merck KGaA) to generate dot
plots. The values were exported to GraphPad Prism software (version
6.0; GraphPad Inc., La Jolla, CA, USA) for further analysis.
Luminex immunoassay
To investigate which component of the BC-MSC-CM
decreased the level cisplatin-induced apoptosis and promote the
viability of MCF-7 cells, the concentration of 6 cytokines and
chemokines (IL-17A, IL-7, IL-6, VEGF, EGF, FGF) in BC-MSC-CM were
quantified using a Luminex immunoassay. MILLIPLEXH human cytokine
96-well plate assays (cat. no. HCYTOMAG-60K, EMD Millipore,
Billerica, MA, USA) were performed according to the manufacturer's
protocol.
Antibody blocking assay
To evaluate the effect of IL-6 on cisplatin-induced
apoptosis in MCF-7 cells, 10 µg/ml IL-6 neutralization antibody
(R&D Systems, Inc., Minneapolis, MN, USA, MAB206.) was added to
a total of 5.0×104 MCF-7 cells cultured with BC-MSC-CM.
Following incubation at 37°C for 48 h, cells were collected for
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) and western blot analyses.
RT-qPCR
Cells were treated as aforementioned and total RNA
was extracted using TRIzol reagent (Thermo Fisher Scientific,
Inc.). Reverse transcription was performed using Superscript II
reverse transcriptase, Oligo(dT) primer (Roche Diagnostics,
Indianapolis, IN, USA) in a 40-µl reaction volume. qPCR was
performed using the Veriti 96 Well Thermal Cycler (Applied
Biosystems, Foster City, CA, USA). The thermocycling conditions
were as follows: 94°C for 30 sec, 60°C (primer) for 30 sec and 72°C
for 30 sec, with a final extension at 72°C for 10 min, performed
for 35 cycles. Primers of human IL-6 and β-actin were designed
using the Primer 5.0 Software (Biosoft International, Palo Alto,
CA, USA) as shown: IL-6 forward, 5′-GAGGAGACTTGCCTGGTGAA-3′ and
reverse, 5′-GCGCAGAATGAGATGAGTTG-3′; β-actin forward,
5′-TGGACTTCGAGCAAGAGATG-3′ and reverse, 5′-GGATGTCCACGTCACACTTC-3′.
Data was quantified by the 2−ΔΔCq method (22).
Western blotting analysis
Cells were treated as aforementioned, MCF-7 cells
(1.0×106) were lysed in radioimmunoprecipitation assay
buffer containing 1 mM phenylmethanesulfonyl fluoride (Beyotime
Institute of Biotechnology, Nanjing, China). The protein
concentrations were determined using a NanoDrop 2000 Micro-volume
spectrophotometer (Thermo Fisher Scientific, Inc., Wilmington, DE,
USA). A total of 200 µg protein in each lane was electrophoresed
using 12% SDS-PAGE, and the gels were transferred onto a
polyvinylidene fluoride membrane. Subsequently, membranes were
blocked with 5% skim milk at room temperature for 1 h, and
incubated with primary antibodies at 4°C overnight. Then the
antibody-bound membranes were washed three times, each time for 10
min. The HRP conjugated goat anti-rabbit secondary antibody (cat.
no. A0208; dilution, 1:1,000; Beyotime Institute of Biotechnology)
incubated with membranes at 37°C for 1 h. After washed three times,
the membranes were visualized by using LuminataTM Crescendo Western
HRP substrate (EMD Millipore, USA). Primary antibodies were as
follows: Anti-STAT3 (cat. no. MAB1799; dilution, 1:500),
anti-phosphorylated (p)-STAT3 (cat. no. MAB4607; dilution, 1:500;
R&D Systems, Minneapolis, MN, USA), anti-β-actin (cat. no.
sc47778; dilution, 1:1,000) and anti-B-cell lymphoma-associated X
(Bax) (cat. no. sc493; dilution, 1:1,000; Santa Cruz Biotechnology,
Santa Cruz, CA, USA).
Statistical analysis
All values were expressed as the mean ± standard
deviation. Statistically significant differences between groups
were assessed by two-way analysis of variance or Student's t-test
using GraphPad Prism software (version 6.0; GraphPad, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Characterization and identification of
BC-MSCs
After 10–14 days of primary culture of tissue
samples, a small population of fibroblastic cells was observed.
When the confluence of adherent fibroblast-like cells reached ~80%,
the cells were passaged into new flasks, and spindle-shaped
morphologies were observed on BC-MSCs and BM-MSCs (Fig. 1A). Flow cytometric analysis
demonstrated that BC-MSCs and BM-MSCs expressed high levels of
CD29, CD44, CD90, and CD105, but were negative for CD14, CD34 and
CD45 (Fig. 1B and C). In addition, in
order to evaluate the pluripotent differentiation potential of
BC-MSCs, cells were cultured in osteoblastic-induction medium for
two weeks and numerous Alkaline phosphatase staining-positive cells
were observed. In addition, a number of BC-MSCs were positive for
Oil-Red-O intracellular staining following three weeks of
incubation indicating adipogenic induction. Control cells cultured
in the complete medium were negative for Oil-Red-O staining
(Fig. 1D).
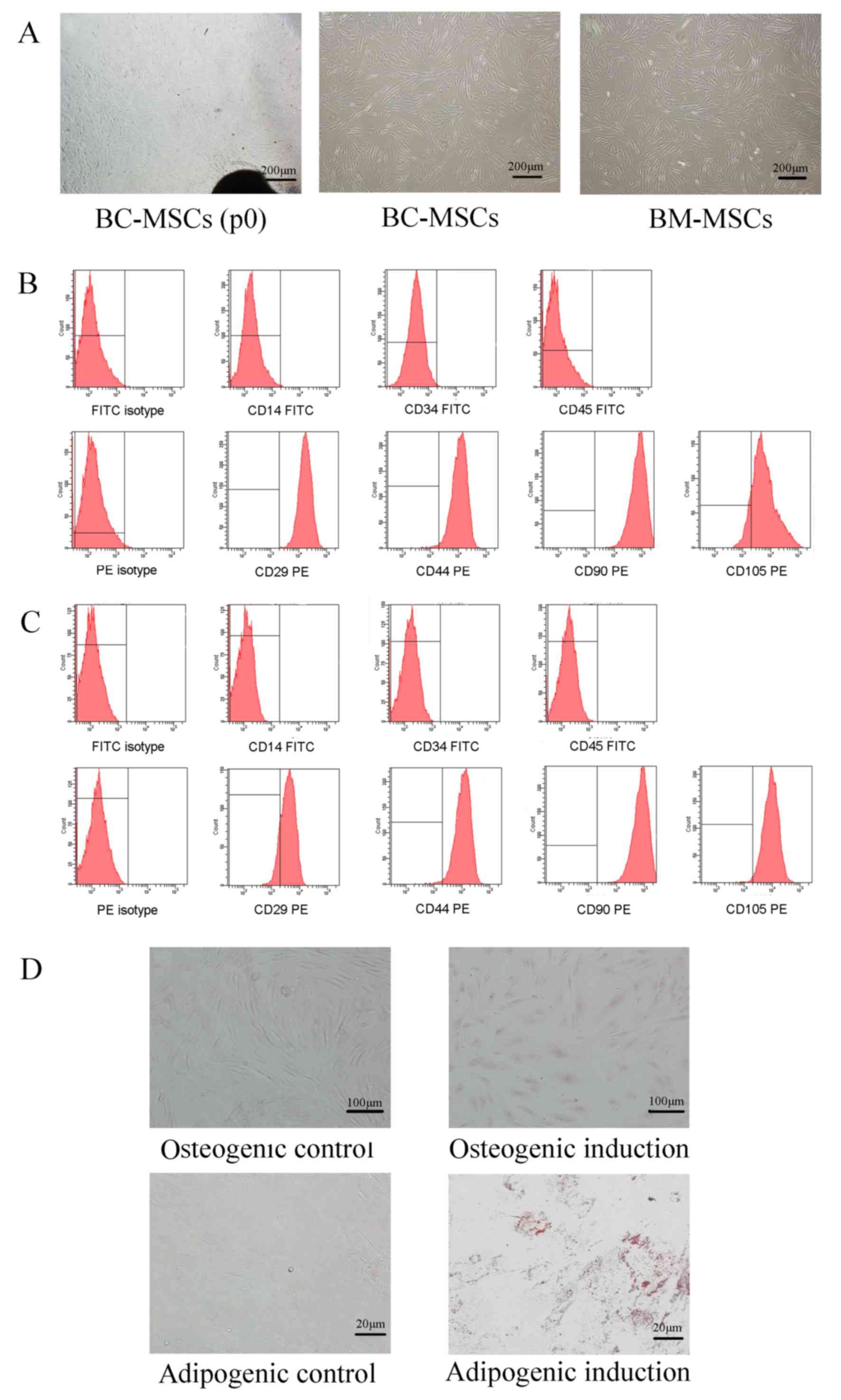 | Figure 1.Characterization of human
breast-cancer-derived MSCs. (A) Spindle-shaped cells migrated from
breast cancer tissues after 10–14 days of primary culture (BC-MSCs
P0), BC-MSCs and BM-MSCs were spindle-shaped and fibroblastic in
appearance of passage 3 (magnification, ×40). (B) Flow cytometric
characterization of BC-MSCs. BC-MSCs were positive for CD29, CD44,
CD90, CD105, but were negative for CD14, CD34 and CD45. (C) Flow
cytometric characterization of BC-MSCs. BM-MSCs were positive for
CD29, CD44, CD90 and CD105, but were negative for CD14, CD34 and
CD45. (D) Adipogenic and osteogenic differentiation of BC-MSCs.
Osteogenic differentiation of BC-MSCs was shown by alkaline
phosphatase staining (magnification, ×100). Adipogenic
differentiation was analyzed by Oil-Red-O staining (magnification,
×400). BC-MSC, breast-cancer-derived mesenchymal stem cell; BM-MSC,
bone-marrow-derived MSC; CD, cluster of differentiation; FITC,
fluorescein isothiocyanate; PE, phycoerythrin. |
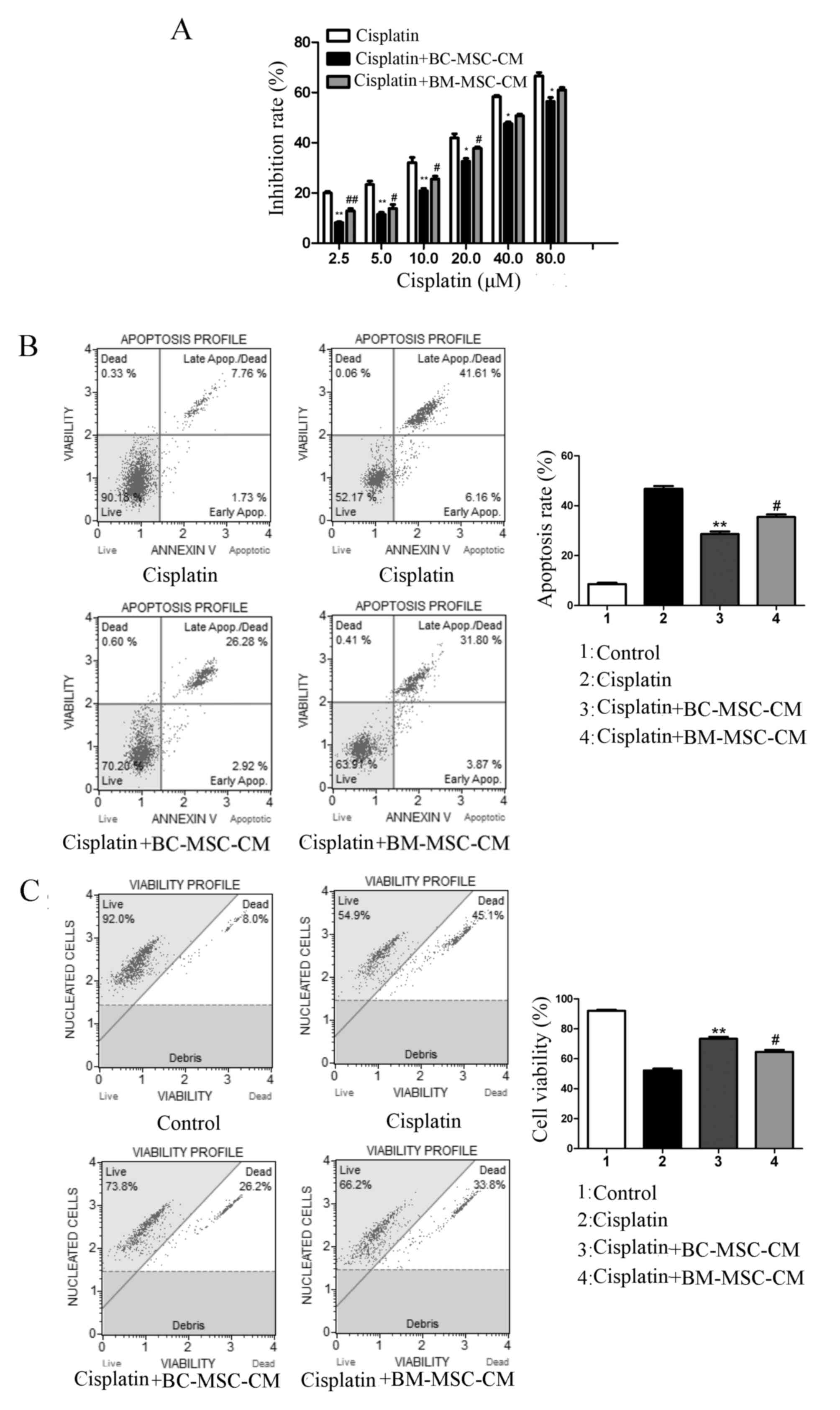
BC-MSCs decrease MCF-7 cell apoptosis
and promote MCF-7 cell survival from cisplatin
To evaluate the effects of BC-MSCs in the
cisplatin-induced MCF-7 cell response, cells were seeded in 96-well
plates or 6-well plates for different experiments. As presented in
Fig. 2A, the results of an MTT assay
revealed that MCF-7 cells exposed to cisplatin in the presence of
BC-MSC-CM exhibited significantly higher growth rates compared with
those treated with cisplatin alone (P<0.01) and cisplatin plus
BM-MSC-CM-treated cells (P<0.05). FACS analysis revealed that
the proportion of annexin-V-bound MCF-7 cells cultured with control
medium increased significantly in response to cisplatin treatment
compared with those cultured with BC-MSC-CM (P<0.01; Fig. 2B). The anti-apoptosis effect of
BC-MSC-CM was significantly increased compared with that in the
BM-MSC-CM group (P <0.05; Fig.
2B). Co-treatment with BC-MSC-CM and cisplatin revealed that
the vitality of MCF-7 cells increased significantly when compared
with cells treated with cisplatin alone (Fig. 2C; P<0.01). Furthermore, BC-MSCs had
a significantly higher potential to decrease MCF-7 cell apoptosis
and promote cell proliferation compared with BM-MSCs (P<0.05;
Fig. 2). These results suggested that
BC-MSCs weakened the antitumor effect of cisplatin more effectively
than BM-MSCs.
Secretion of IL-6 was higher in
BC-MSCs compared with BM-MSCs
BM-MSCs exhibit marked tropism for tumor sites and
have the ability to transition to cancer-associated stromal cells
(23). In other words,
cancer-associated stromal cells may originate from BM-MSCs.
Detecting the different expression of paracrine factors between
BC-MSCs and BM-MSCs may reveal which component of the BC-MSC-CM
enhanced the survival of MCF-7 cells. The expression of important
cytokines was therefore assessed using a Luminex immunoassay, which
demonstrated that IL-6 secretion was significantly higher in the
BC-MSC-CM group compared with that in the BM-MSC-CM group
(P<0.01; Fig. 3A). RT-qPCR
confirmed that the level of IL-6 mRNA was significantly increased
in MCF-7 cells treated with cisplatin and BC-MSC-CM compared with
the control and cisplatin group. IL-6 neutralizing antibody was
added to cisplatin+BC-MSC-CM, IL-6 mRNA expression significantly
decreased when compared with the cisplatin+BC-MSC-CM group
(P<0.01; Fig. 3B). IL-6 secreted
by BC-MSCs may therefore have a pivotal role in mediating the
enhanced proliferation of MCF-7 cells.
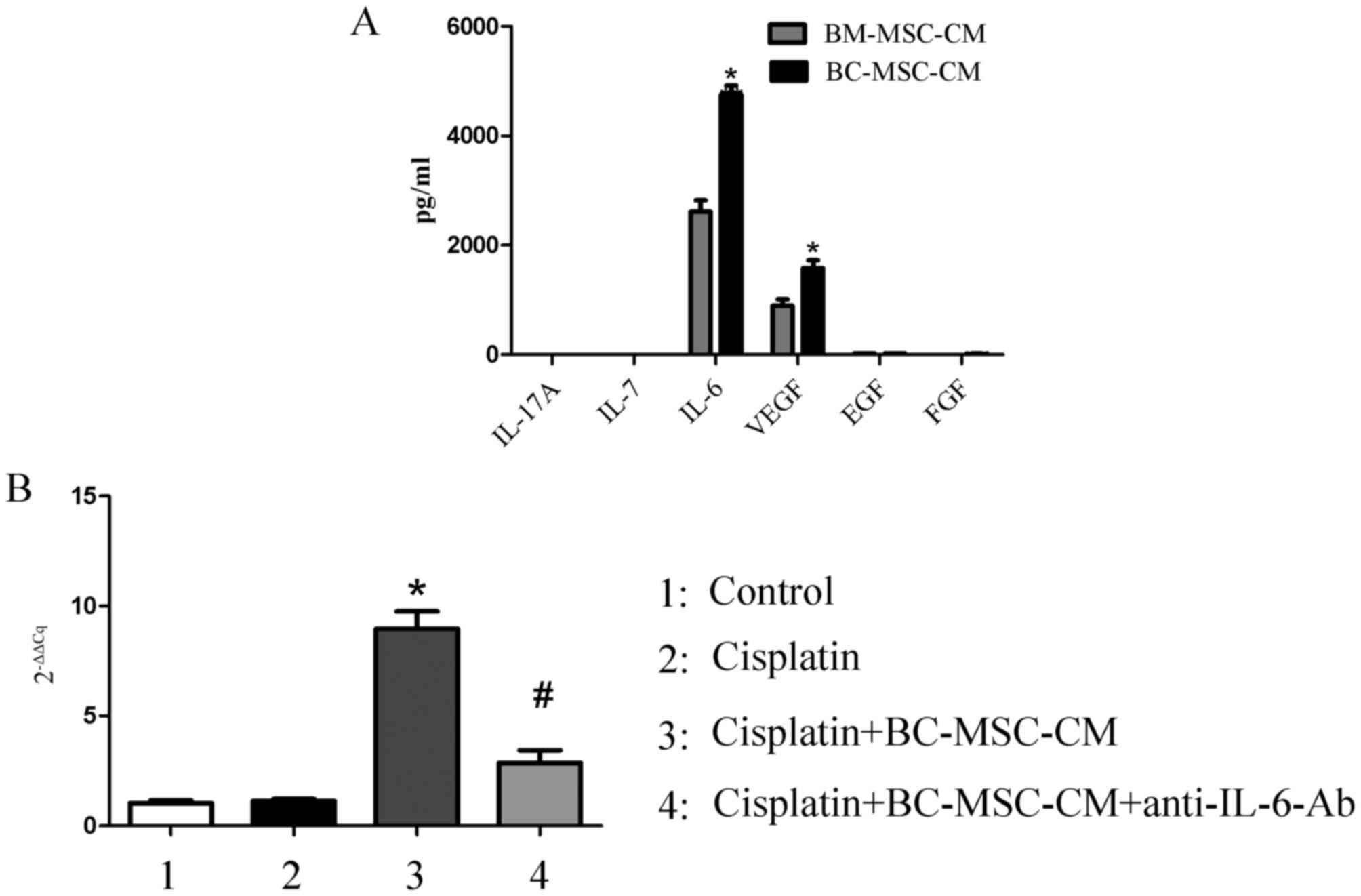 | Figure 3.BC-MSC-secreted IL-6 is higher with
increased mRNA expression of IL-6 in MCF-7 cells. (A) Cytokine
profile analysis of BC-MSCs and BM-MSCs using a Luminex
immunoassay. *P<0.01, compared with the BM-MSCs group. (B)
Expression of IL-6 in MCF-7 with different treatment. *P<0.01,
compared with the cisplatin group; #P<0.01, compared
with the cisplatin/BC-MSC-CM group. IL-6, interleukin-6; BC-MSC-CM,
breast-cancer-derived mesenchymal-stem-cell-conditioned medium;
BM-MSC, bone-marrow-derived MSC; VEGF, vascular endothelial growth
factor; EGF, endothelial growth factor; FGF, follicular growth
factor; Ab, antibody. |
Neutralizing IL-6 attenuates the
action of BC-MSCs on inhibition of MCF-7 cells induced by
cisplatin
To investigate whether the effect of BC-MSCs
secreted IL-6 promoted MCF-7 cell survival from cisplatin, IL-6
neutralization antibody was added to BC-MSC-CM. The resultant
depletion of IL-6 recovered cisplatin-induced apoptosis (Fig. 4A). Furthermore, MCF-7 cell viability
evidently decreased following IL-6 neutralization antibody
treatment (Fig. 4B). To assess
whether the change in response of MCF-7 cells to cisplatin
treatment caused by BC-MSCs was mediated through activation of the
IL-6/STAT3 signaling pathway, western blot analysis was performed
(Fig. 4C). Data indicated that in the
presence of BC-MSC-CM, levels of p-STAT3 were raised markedly in
MCF-7 cells, which could be reversed by incubation with
IL-6-neutralizing antibody. The expression of the
apoptosis-associated protein Bax was reduced in MCF-7 cells on
incubation with BC-MSC-CM compared with cisplatin alone treated
cells. The aforementioned data indicated that IL-6 is crucial in
the BC-MSC-mediated reduction in apoptosis induced by cisplatin in
breast cancer cells.
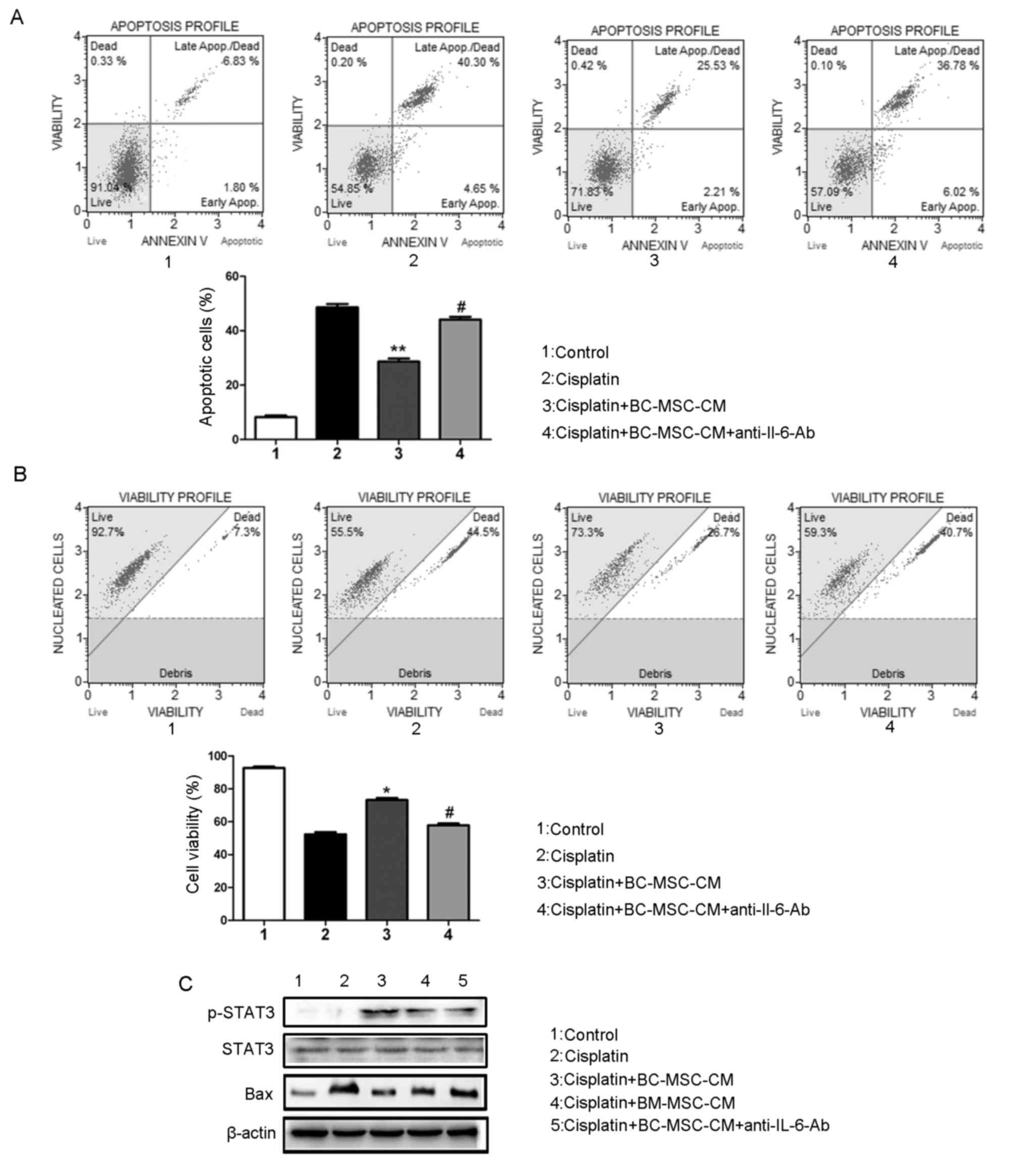 | Figure 4.Neutralizing IL-6 attenuates the
action of BC-MSCs on inhibition of MCF-7 cells induced by
cisplatin. (A) IL-6-neutralization antibody recovered
cisplatin-induced apoptosis in MCF-7 cells incubated with
BC-MSC-CM. **P<0.01, compared with the cisplatin group;
#P<0.05, compared with the BC-MSC-CM group. (B) IL-6
neutralization antibody decreased the MCF-7 cell vitality.
*P<0.05, compared with the cisplatin group;
#P<0.05, compared with the BC-MSC-CM group. (C)
Western blot analysis of protein levels of Bax, STAT3, p-STAT3,
IL-6 in MCF-7 cells in response to the indicated treatment. IL-6,
interleukin-6; BC-MSC, breast-cancer-derived
mesenchymal-stem-cell-conditioned medium; p-STAT3, phosphorylated
signal transducer and activator of transcription 3; Bax, B-cell
lymphoma-associated X. |
Discussion
The interactions between the stromal
microenvironment and tumor cells have a central role in patient
survival, and the response to chemotherapy. MSCs are commonly used
as stromal cells for in vitro studies on multiple tumor
types, including gastric, breast and ovarian cancer (24–26).
Tumor-tissue-derived MSCs have been widely studied owing to their
proximity to tumors and the influence they exert on tumors
(16,27–30). MSCs
act as regulators of apoptosis, proliferation, angiogenesis and
immune regulation, and, when in contact with tumor cells, produce a
variety of cytokines that affect proliferation, survival and the
acquisition of chemoresistance (31,32). The
present study focused on the paracrine effects of BC-MSCs on the
behavior of MCF-7 cells during cisplatin treatment. MSCs were
isolated from human breast cancer tissues and revealed to exhibit a
heterogeneous immunophenotype with fibroblastic morphology and the
potential to differentiated into multiple cell types. First of all,
BC-MSC-CM was prepared for the subsequent experiments. BC-MSC-CM
significantly decreased the inhibitory effect of cisplatin
treatment on MCF-7 cell growth and promoted MCF-7 cell survival.
The results of flow cytometric analysis revealed that in the
presence of BC-MSC-CM, the degree of cisplatin-triggered apoptosis
was evidently decreased and the proportion of apoptotic cells was
more evidently reduced in the presence of BC-MSC-CM compared with
in the control medium. A prior study revealed that MSCs protect
tumor cells exposed to chemotherapeutic drugs from apoptosis more
potently than BM-MSC-CM (33).
To investigate the underlying mechanism by which
BC-MSC-CM enhances the survival of the MCF-7 cells and protects
them from drug-induced apoptosis, cytokine levels in BC-MSC-CM were
examined by a Luminex immunoassay. The present study demonstrated
that the level of IL-6 was markedly higher in the BC-MSC-CM
compared with in BM-MSC-CM, suggesting that IL-6 may act as a key
mediator of the tumor-promoting activity of BC-MSCs. IL-6, as a key
mediator of the inflammatory response, has a pathological role in
the development of several neoplasms, including malignant
mesothelioma, breast tumor, endometrial cancer and lung cancer
(16,34,35). It
has been reported that bone-marrow- and glioma-derived MSCs enhance
cancer cell proliferation via the IL-6/STAT3 signaling pathway
(15,36). It is well established that the
IL-6/glycoprotein 130/STAT3 signaling pathway further enhances the
growth of cancer cells and reduces the sensitivity of cancer cells
to antitumor drugs (37,38). The present study was designed to
determine the role of BC-MSCs on cisplatin treatment of tumor cells
via the IL-6/STAT3 signaling pathway. The current study
demonstrated that the degree of proliferation, viability and
apoptosis of MCF-7 cells in response to cisplatin treatment
regulated by BC-MSCs may be attenuated by incubation with an IL-6
neutralizing antibody. Western blot analysis revealed that
incubation of MCF-7 cells with BC-MSC-CM activated the IL-6/STAT3
signaling pathway in MCF-7 cells, markedly decreasing Bax
expression. In addition, this effect was partially abolished in the
presence of IL-6 neutralizing antibody. The data of the present
study indicated that BC-MSC-secreted IL-6 attenuated the function
of cisplatin on MCF-7 cells, preventing apoptosis and thus
promoting breast cancer growth and survival. A more marked
promotion was observed in breast cancer growth and survival when
MCF-7 cells were incubated with BC-MSC-CM compared with BM-MSC-CM,
suggesting that BC-MSCs have a greater potential to promote breast
cancer growth and decrease apoptosis upon exposure to cisplatin
than BM-MSCs.
In summary, BC-MSCs significantly enhanced the
survival of MCF-7 cells that were exposed to cisplatin, one of the
reasons behind the development of drug resistance. Furthermore,
IL-6 was demonstrated to contribute to the BC-MSC-induced
protection of MCF-7 cells from apoptosis. Therefore,
BC-MSC-secreted IL-6 should be considered as a novel therapeutic
target to aid the improvement of patient responses to
cisplatin.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Haosen Fund
Project of Youth Excellence, the First People's Hospital of
Lianyungang (grant no. QN140302).
Availability of data and materials
The datasets generated/analyzed during the present
study are available on reasonable request from the corresponding
author.
Author's contributions
HX and YZ participated in design of the study,
performed most of the experiments, analyzed the data, and wrote the
paper; WL and BZ contributed to MSCs isolations. HZ, SZ and PZ
characterized MSCs and contributed to the molecular analysis. HW
and JY designed the experiments, analyzed the data, critically
revised the paper and approved the final manuscript.
Ethics approval and consent to
participate
The experiment protocols and study were approved by
the Medical Ethics Committee of First People's Hospital of
Lianyungang (Lianyungang, China). All study participants provided
written informed consent.
Consent for publication
All participants provided informed consent for the
publication of the present data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Samavat H and Kurzer MS: Estrogen
metabolism and breast cancer. Cancer Lett. 356:231–243. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mao Y, Keller ET, Garfield DH, Shen K and
Wang J: Stromal cells in tumor microenvironment and breast cancer.
Cancer Metastasis Rev. 32:303–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shirmanova MV, Druzhkova IN, Lukina MM,
Dudenkova VV, Ignatova NI, Snopova LB, Shcheslavskiy VI, Belousov
VV and Zagaynova EV: Chemotherapy with cisplatin: Insights into
intracellular pH and metabolic landscape of cancer cells in vitro
and in vivo. Sci Rep. 7:89112017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu WK, Wang Z, Fong CC, Liu D, Yip TC, Au
SK, Zhu G and Yang M: Chemoresistant lung cancer stem cells display
high DNA repair capability to remove cisplatin-induced DNA damage.
Br J Pharmacol. 174:302–313. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shi S, Tan P, Yan B, Gao R, Zhao J, Wang
J, Guo J, Li N and Ma Z: ER stress and autophagy are involved in
the apoptosis induced by cisplatin in human lung cancer cells.
Oncol Rep. 35:2606–2614. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ethiraj P, Veerappan K, Samuel S and
Sivapatham S: Interferon β improves the efficacy of low dose
cisplatin by inhibiting NF-κB/p-Akt signaling on HeLa cells. Biomed
Pharmacother. 82:124–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matsumoto M, Nakajima W, Seike M, Gemma A
and Tanaka N: Cisplatin-induced apoptosis in non-small-cell lung
cancer cells is dependent on Bax- and Bak-induction pathway and
synergistically activated by BH3-mimetic ABT-263 in p53 wild-type
and mutant cells. Biochem Biophys Res Commun. 473:490–496. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Blanc C, Deveraux QL, Krajewski S, Jänicke
RU, Porter AG, Reed JC, Jaggi R and Marti A: Caspase-3 is essential
for procaspase-9 processing and cisplatin-induced apoptosis of
MCF-7 breast cancer cells. Cancer Res. 60:4386–4390.
2000.PubMed/NCBI
|
|
9
|
Cadamuro M, Brivio S, Spirli C, Joplin RE,
Strazzabosco M and Fabris L: Autocrine and paracrine mechanisms
promoting chemoresistance in cholangiocarcinoma. Int J Mol Sci.
18:pii: E149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li M, Li M, Yin T, Shi H, Wen Y, Zhang B,
Chen M, Xu G, Ren K and Wei Y: Targeting of cancer-associated
fibroblasts enhances the efficacy of cancer chemotherapy by
regulating the tumor microenvironment. Mol Med Rep. 13:2476–2484.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Borriello L and DeClerck YA: Tumor
microenvironment and therapeutic resistance process. Med Sci
(Paris). 30:445–451. 2014.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flister MJ, Endres BT, Rudemiller N,
Sarkis AB, Santarriaga S, Roy I, Lemke A, Geurts AM, Moreno C, Ran
S, et al: CXM: A new tool for mapping breast cancer risk in the
tumor microenvironment. Cancer Res. 74:6419–6429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gould CM and Courtneidge SA: Regulation of
invadopodia by the tumor microenvironment. Cell Adh Migr.
8:226–235. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun Z, Wang S and Zhao RC: The roles of
mesenchymal stem cells in tumor inflammatory microenvironment. J
Hematol Oncol. 7:142014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Scherzad A, Steber M, Gehrke T, Rak K,
Froelich K, Schendzielorz P, Hagen R, Kleinsasser N and Hackenberg
S: Human mesenchymal stem cells enhance cancer cell proliferation
via IL-6 secretion and activation of ERK1/2. Int J Oncol.
47:391–397. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Zhou Y, Yang J, Zhang X, Zhang H,
Zhang T, Zhao S, Zheng P, Huo J and Wu H: Gastric cancer-derived
mesenchymal stem cells prompt gastric cancer progression through
secretion of interleukin-8. J Exp Clin Cancer Res. 34:522015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ooi YY, Dheen ST and Tay SS: Paracrine
effects of mesenchymal stem cells-conditioned medium on microglial
cytokines expression and nitric oxide production.
Neuroimmunomodulation. 22:233–242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
El-Khattouti A, Sheehan NT, Monico J,
Drummond HA, Haikel Y, Brodell RT, Megahed M and Hassan M: CD133+
melanoma subpopulation acquired resistance to caffeic acid
phenethyl ester-induced apoptosis is attributed to the elevated
expression of ABCB5: Significance for melanoma treatment. Cancer
Lett. 357:83–104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Velletri T, Xie N, Wang Y, Huang Y, Yang
Q, Chen X, Chen Q, Shou P, Gan Y, Cao G, et al: P53 functional
abnormality in mesenchymal stem cells promotes osteosarcoma
development. Cell Death Dis. 7:e20152016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou K, Xia M, Tang B, Yang D, Liu N, Tang
D, Xie H, Wang X, Zhu H, Liu C and Zuo C: Isolation and comparison
of mesenchymal stem cell-like cells derived from human gastric
cancer tissues and corresponding ovarian metastases. Mol Med Rep.
13:1788–1794. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hendijani F, Javanmard ShH, Rafiee L and
Sadeghi-Aliabadi H: Effect of human Wharton's jelly mesenchymal
stem cell secretome on proliferation, apoptosis and drug resistance
of lung cancer cells. Res Pharm Sci. 10:134–142. 2015.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bergfeld SA and DeClerck YA: Bone
marrow-derived mesenchymal stem cells and the tumor
microenvironment. Cancer Metastasis Rev. 29:249–261. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song B, Kim B, Choi SH, Song KY, Chung YG,
Lee YS and Park G: Mesenchymal stromal cells promote tumor
progression in fibrosarcoma and gastric cancer cells. Korean J
Pathol. 48:217–224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim SH, Bang SH, Kang SY, Park KD, Eom JH,
Oh IU, Yoo SH, Kim CW and Baek SY: Human amniotic membrane-derived
stromal cells (hAMSC) interact depending on breast cancer cell type
through secreted molecules. Tissue Cell. 47:10–16. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Castells M, Milhas D, Gandy C, Thibault B,
Rafii A, Delord JP and Couderc B: Microenvironment mesenchymal
cells protect ovarian cancer cell lines from apoptosis by
inhibiting XIAP inactivation. Cell Death Dis. 4:e8872013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chang WW, Hu FW, Yu CC, Wang HH, Feng HP,
Lan C, Tsai LL and Chang YC: Quercetin in elimination of tumor
initiating stem-like and mesenchymal transformation property in
head and neck cancer. Head Neck. 35:413–419. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
López J, Poitevin A, Mendoza-Martínez V,
Pérez-Plasencia C and García-Carrancá A: Cancer-initiating cells
derived from established cervical cell lines exhibit stem-cell
markers and increased radioresistance. BMC Cancer. 12:482012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kansy BA, Dißmann PA, Hemeda H, Bruderek
K, Westerkamp AM, Jagalski V, Schuler P, Kansy K, Lang S, Dumitru
CA and Brandau S: The bidirectional tumor-mesenchymal stromal cell
interaction promotes the progression of head and neck cancer. Stem
Cell Res Ther. 5:952014. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Behnan J, Isakson P, Joel M, Cilio C,
Langmoen IA, Vik-Mo EO and Badn W: Recruited brain tumor-derived
mesenchymal stem cells contribute to brain tumor progression. Stem
Cells. 32:1110–1123. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ji R, Zhang B, Zhang X, Xue J, Yuan X, Yan
Y, Wang M, Zhu W, Qian H and Xu W: Exosomes derived from human
mesenchymal stem cells confer drug resistance in gastric cancer.
Cell Cycle. 14:2473–2483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lou G, Song X, Yang F, Wu S, Wang J, Chen
Z and Liu Y: Exosomes derived from miR-122-modified adipose
tissue-derived MSCs increase chemosensitivity of hepatocellular
carcinoma. J Hematol Oncol. 8:1222015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kurtova AV, Balakrishnan K, Chen R, Ding
W, Schnabl S, Quiroga MP, Sivina M, Wierda WG, Estrov Z, Keating
MJ, et al: Diverse marrow stromal cells protect CLL cells from
spontaneous and drug-induced apoptosis: Development of a reliable
and reproducible system to assess stromal cell adhesion-mediated
drug resistance. Blood. 114:4441–4450. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
van der Zee M, Sacchetti A, Cansoy M,
Joosten R, Teeuwssen M, Heijmans-Antonissen C, Ewing-Graham PC,
Burger CW, Blok LJ and Fodde R: IL6/JAK1/STAT3 signaling blockade
in endometrial cancer affects the ALDHhi/CD126+ stem-like component
and reduces tumor burden. Cancer Res. 75:3608–3622. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hossain A, Gumin J, Gao F, Figueroa J,
Shinojima N, Takezaki T, Priebe W, Villarreal D, Kang SG, Joyce C,
et al: Mesenchymal stem cells isolated from human gliomas increase
proliferation and maintain stemness of glioma stem cells through
the IL-6/gp130/STAT3 pathway. Stem Cells. 33:2400–2415. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mochizuki D, Adams A, Warner KA, Zhang Z,
Pearson AT, Misawa K, McLean SA, Wolf GT and Nör JE: Anti-tumor
effect of inhibition of IL-6 signaling in mucoepidermoid carcinoma.
Oncotarget. 6:22822–22835. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rodriguez-Barrueco R, Yu J, Saucedo-Cuevas
LP, Olivan M, Llobet-Navas D, Putcha P, Castro V, Murga-Penas EM,
Collaz-Lorduy A, Castillo-Martin M, et al: Inhibition of the
autocrine IL-6-JAK2-STAT3-calprotectin axis as targeted therapy for
HR-/HER2+ breast cancers. Genes Dev. 29:1631–1648. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren T, Shan J, Qing Y, Qian C, Li Q, Lu G,
Li M, Li C, Peng Y, Luo H, et al: Sequential treatment with AT-101
enhances cisplatin chemosensitivity in human non-small cell lung
cancer cells through inhibition of apurinic/apyrimidinic
endonuclease 1-activated IL-6/STAT3 signaling pathway. Drug Des
Devel Ther. 8:2517–2529. 2014. View Article : Google Scholar : PubMed/NCBI
|