Introduction
The mortality rate of lung cancer has increased
6.9-fold in China from 1973 to 2014 (1). In 2015, there were 4,292,000 new cancer
cases and 2,814,000 incidences of cancer-associated mortality in
China, with lung cancer the principal cause of this mortality
(2). Non-small cell lung cancer
(NSCLC) accounts for ~85% of all cases of lung cancer (3). NSCLC is a heterogeneous disease
comprised of common cancer subtypes, including squamous cell
carcinoma (SCC), adenocarcinoma and large cell carcinoma. Although
novel therapeutics have alleviated the disease burden and enhanced
the overall quality of life in patients with NSCLC, the 5-year
survival rate of patients with NSCLC remains low, at 15.9%
(4). The poor rate of early detection
and limited efficacy of presently available therapies for
advanced-stage NSCLC are the cause of the low 5-year survival rate
of patients with NSCLC (5). Current
diagnostic methods for NSCLC include chest radiography, sputum
cytology, computed tomography (CT) or assessment of a combination
of clinical protein markers of tumors. These markers include
carcinoembryonic antigen (CEA), neuron-specific enolase,
cytokeratin fragment 21-1 and tissue polypeptide-specific antigen;
these protein markers have previously been evaluated in large-scale
clinical trials (1–3). However, these current diagnostic and
prognostic methods have a number of undesirable side effects,
Examples of these side effects include the high erroneous
diagnostic rate, the low sensitivity and specificity (5).
MicroRNAs (miRNAs/miRs) represent an appealing
alternative for assessing the diagnosis and prognosis of NSCLC as
they can be detected in the blood in a non-invasive manner. miRNAs
are endogenous, noncoding RNA molecules ~20 nucleotides in length
that have a marked influence on the biological functions of single
cells and complete organisms (6). A
number of prior studies have validated that miRNAs can be readily
detected in the blood, and that miRNAs are released by three
different mechanisms: Energy-free passive leakage from lysed cells,
active release through microvesicles and active secretion in the
microvesicles free form. For example, serum miRNAs remain stable
under harsh conditions, including boiling, acidic (pH 1.0) or
alkaline (pH 13.0) solutions, long-term storage and undergoing
multiple freeze-thaw cycles. Furthermore, serum miRNAs in healthy
subjects are also consistent, with a Pearson correlation
coefficient close to 1 (6).
Therefore, the present review focuses on miRNAs derived from blood
samples.
There are three principal membrane vesicle types: i)
Microvesicles, ii) exosome vesicles, and iii) apoptotic vesicles
(7). Microvesicles, also termed
shedding vesicles, are large (>200 nm diameter), dense, and
include phosphatidylserine, integrins and CD40 ligand (8). Exosomes are spherical nano-sized
vesicles, with a round cup-shaped morphology, a density of
1.13–1.19 g/ml and a diameter of 40–100 nm (9). Exosomes contain large amounts of
molecular cargo, including mRNAs, miRNAs, proteins and DNA, which
are protected by a lipid bilayer. In 2007, Valadi et al
(10) identified exosomal miRNAs,
which were subsequently identified to be obtained readily, had the
potential to be used as diagnostic markers and could serve as an
alternative for biopsy profiling. They are suitable for profiling
as the miRNA profile in exosomes exhibit similarities to primary
tumor profile (11); therefore, this
feature may be a powerful tool for early diagnosis, prognosis,
metastasis and targeted therapy in NSCLC.
Clinical significance of miRNAs derived from
blood of NSCLC
Blood-based miRNAs and clinical
diagnosis of NSCLC
According to clinical analysis of stage I to IV
cases of NSCLC, the mean risk score and high risk-score rate
progressively increased as the stage increased (Table I). Conversely, the false-positive rate
progressively decreased (3). The
Tumor-Node-Metastasis (TNM) stage (12) of patients with NSCLC was also
associated with the expression level of blood-based miRNAs. A
previous study demonstrated that twelve plasma miRNAs could
significantly discriminate between stages I–III of patients with
NSCLC and controls (13). Levels of
blood-based miRNAs could also be used to distinguish early stages
(stages I and II) from advanced stages (stages III and IV) of
NSCLC. In the present review, blood-based miRNAs are divided into
three categories: Serum-based miRNAs, plasma-based miRNAs and
miRNAs derived from peripheral blood mononuclear cells (PBMCs).
 | Table I.Blood-based miRNA and clinical stage
of NSCLC. |
Table I.
Blood-based miRNA and clinical stage
of NSCLC.
| Author, year | Stage | Resource | miRNA
profiling | Year | Country | Cases | Controls | Sensitivity, % | Specificity, % | (Refs.) |
|---|
| Sanfiorenzo et al,
2013 | TNM | Plasma | 11 miRNAs | 2013 | France | 52 | 20 | 81.1 | 82.9 | (13) |
| Foss et al,
2011 | Early | Serum | miR-1254,
−574-5p | 2011 | USA | 22 | 31 | 73 | 71 | (14) |
| Wang et al,
2015 |
| Serum | miR-125a-5p, −25,
−126 | 2015 | China | 142 | 111 | 88 | 82.6 | (15) |
| Bianchi et al,
2011 |
| Serum | 34 miRNA panel | 2011 | Italy | 34 | 30 | 71 | 90 | (16) |
| Chen et al,
2012 |
| Serum | miR-20a, −24, −25,
−145, −152, −199a-5p, −221,-222, 223, −320 | 2012 | China | 200 | 110 | 92.5 | 90 | (17) |
| Shen et al,
2011 |
| Plasma | miR-21, −126, −210,
−486-5P | 2011 | USA | 58 | 29 | 86.2 | 96.6 | (18) |
| Ulivi et al,
2013 | Advanced | PBMC | miR-328 | 2013 | Italy | 86 | 24 | 70 | 89 | (19) |
| Yuxia et al,
2012 |
| Serum | miR-125b | 2012 | China | 193 | 110 | 78.2 | 66.4 | (21) |
| Sozzi et al,
2014 |
| Plasma | miR signature
classifier | 2014 | Italy | 69 | 870 | 87 | 76.9 | (22) |
Blood-based miRNAs and early stage of
NSCLC
Early detection is particularly crucial for the
initial stage diagnosis of patients with NSCLC who present without
clinical symptoms. The properties of blood-based miRNAs could
predict disease probability irrespective of whether the patient is
asymptomatic or symptomatic, and also is able to distinguish benign
from malignant lesions. Furthermore, miRNAs could determine the
onset of the malignant disease in individual patients over time
(14–16). Serum-based miRNAs had been identified
differentially between patients with early-stage NSCLC and
controls: hsa-miR-1254 and hsa-miR-574-5p were evidently increased
in early-stage NSCLC samples (compared with the control group) in
the identification and validation cohort of the study by Foss et
al (14). The combination of
miR-125a-5p, miR-25 and miR-126 could also discriminate between
NSCLC patients and controls (15).
Bianchi et al detected 34 miRNAs in the serum that were able
to distinguish patients with early stage NSCLCs from asymptomatic
high-risk individuals with 80% accuracy (16). In a retrospective study, 10-serum
miRNA for the early detection of NSCLC could precisely classify
serum samples which were collected 33 months ago (17). Similarly, the four miRNAs (miRNA-21,
−126, −210, and 486-5p) derived from plasma were also identified as
stable and reliable in discriminating patients with NSCLC from
controls (18). Furthermore, using
miRNAs also produced high sensitivities and specificities in
validating the presence of stage I NSCLC. A further study revealed
that miR-328 derived from the PBMCs was the most effective
diagnostic discriminator in the group of early stage tumors
(19).
Blood-based miRNAs and advanced stage
of NSCLC
Patients with stage III/IV NSCLC usually have a poor
prognosis: The majority of these patients have a median survival of
~1 year (20). Clinicopathological
criteria, including age, histological type, TNM stage and treatment
method, are frequently used prediction factors for the prognosis of
NSCLC. Serum miR-125b was markedly associated with poor prognosis
in NSCLC in a prior study (21). A
study concerning a large number of plasma samples indicated that
the miRNAs signature classifier possessed predictive, diagnostic
and prognostic significance, and could reduce the false-positive
rate of low-dose CT (22). Low
miR-10b expression in patients with stage I–II carcinoma was a
positive indicator for the clinical prognosis of NSCLC, whereas
high miRNA-10b expression in stage III–IV carcinoma was a negative
indicator of the clinical prognosis of NSCLC. Furthermore, the
5-year survival rate of the low miR-10b expression group was
markedly higher than the high miR-10b expression group (23).
Blood-based miRNAs and clinical
protein markers CEA, Cyfra21-1 and CA125 in NSCLC
Recently, serum-based miRNAs were identified to have
a higher diagnostic efficacy than the clinical protein markers CEA,
CA125 and Cyfra21-1 in patients with NSCLC when compared with
controls. A large number of samples were used to validate the
authenticity of the results obtained and reproducibility of the
experiments. A total of 70 patients with stage I NSCLC and 48
controls were collected in order to analyze the expression levels
of miR-29c, miR-93 and miR-429 with CEA. miR-29c, miR-429 and CEA
had an area under the curve (AUC; 0.833) higher than single serum
miR-29c (0.727) and miR-429 (0.723), all of which were higher than
CEA (0.534) (24). Serum specimens
from 112 patients with NSCLC and 104 controls were subjected to
research the levels of miR-182, miR-183, miR-126 and miR-210. These
four miRNAs in conjunction with CEA were further validated by the
AUC of 0.965, with a high sensitivity (88.5%) and specificity
(92.5%) (25). Zhou et al
(26) screened 396 serum samples from
252 patients with NSCLC and 144 healthy individuals, finding that
miR-652 together with miR-660 had a markedly higher diagnostic
value than CEA and CA125 for distinguishing between patients with
NSCLC/adenocarcinoma from controls in the training and test cohort.
The miR652+ miR-660+ Cyfra21-1 model had the highest clinical value
for distinguishing patients with NSCLC from controls, which
displayed higher early diagnostic value compared with the clinical
value of the double miRNA models and Cyfra21-1 alone, in the
training and test cohort (26).
Table II surmises the findings of
studies with respect to blood-based miRNAs and clinical protein
markers CEA, Cyfra21-1 and CA125.
 | Table II.Blood-based miRNAs and clinical
protein markers CEA, Cyfra21-1 and CA125 in NSCLC. |
Table II.
Blood-based miRNAs and clinical
protein markers CEA, Cyfra21-1 and CA125 in NSCLC.
| Author, year | miRNA
profiling | Year | Cases | Controls | Marker (AUC) | miRNA (AUC) | Marker/miRNA
combination (AUC) | (Refs.) |
|---|
| Zhu et al,
2014 | Serum miR-29c, −93,
−429 | 2014 | 70 | 48 | CEA (0.534) | miR-29c (0.727),
miR-429 (0.723) | The combination of
miR-29c and CEA (0.757), the combination of miR-429 and CEA
(0.659), the combination of miR-29c, miR-429 and CEA (0.833) | (24) |
| Zhu et al,
2016 | Serum miR-182,
−183, −210, −126a | 2016 | 112 | 104 | CEA (0.648) | miR-182 (0.781),
miR-183 (0.638) miR-210 (0.650), miR-126 (0.845) | The combination of
four miRNAs and CEA (0.975) | (25) |
| Zhou et al,
2015 | Serum miR-194,
−652, −660 | 2015 | 252 | 144 | Training cohort:
CEA (0.745) Cyfra21-1 (0.830), CA125 (0.802) | Training cohort:
miR-194 (0.576), miR-652 (0.819), miR-660 (0.735), | Training cohort:
The combination of miR-652 and miR-660 (0.896), the combination of
miR-652, miR-660 and Cyfra21-1 (0.953) | (26) |
|
|
|
|
|
| Test cohort: CEA
(0.678), Cyfra21-1 (0.819), CA-125 (0.746) | Test cohort:
miR-194 (0.660), miR-652 (0.819) miR-660 (0.714) miR-660
(0.714) | Test cohort: The
combination of miR-652 and miR-660 (0.858), the combination of
miR-652, miR-660 and Cyfra21-1 (0.943) |
|
Blood-based miRNAs and metastasis of
NSCLC
miRNA expression levels may be associated with
distant metastasis, prognosis and TNM stage of the tumor.
Metastasis is the principal cause of mortality in patients with
tumors (27). A large number of
miRNAs had been demonstrated to be involved in cancer metastasis.
For example, studies had demonstrated that miR155, miR-222 and
miR-107 serve a role in pancreatic cancer (27,28).
miR-200 was involved in gastric cancer (29) and miR-133b acted in colorectal cancer
(30). Therefore, an improved
understanding of the role of miRNA expression in the metastasis of
NSCLC may result in an improved understanding of disease
development and an improvement in the detection and therapy of
NSCLC.
Metastasis is a multistep procedure, in which, tumor
cells lose their adhesion to the stroma, pass through the basal
membrane, then move into the blood vessels, alive in the blood
circulation, attached to the blood stream, extravasate and
proliferation in the host organ. Tumor cells either enter a dormant
state or proliferate a form that shaping from multicellular
epithelial-mesenchymal transition (EMT) of NSCLC cells (31–33).
miR-152 regulated the proliferation and invasion of NSCLC cells by
downregulating basic fibroblast growth factor (FGF2) (34). miR-194 suppressed the proliferation,
migration, invasion and metastasis by EMT of NSCLC cells that form
lung metastases in vivo (35).
miRNA-449a induced G1 arrest, apoptosis and cellular
senescence (36). An in vitro
study demonstrated that miR-449 inhibited cell migration and
invasion in NSCLC, in part by targeting c-Met (36). A total of 74 patients with NSCLC were
selected for two groups (high and low expression of miRNA10b), and
the low expression of miRNA10b, metastasis, with stage III–IV
carcinoma was identified to indicate poor prognosis in patients
with NSCLC (22).
Blood-based miRNAs and targeted
therapies of NSCLC
Chemotherapy and molecular targeted therapies are
generally applied either alone or in conjunction with surgery and
radiotherapy to treat patients with NSCLC (Table III) (37). miRNAs may act as molecular tumor
biomarkers, predicting the incidence of multidrug resistance in
NSCLC. Epidermal growth factor receptor (EGFR)-mutated or
anaplastic lymphoma kinase (ALK)-rearranged tumors of patients with
NSCLC could be treated with tyrosine kinase inhibitors (TKIs)
including, erlotinib, gefitinib, crizotinib and ceritinib (37,38). For
instance, one mechanism of action of erlotinib was the regulation
of miR-9-Foxo1 in NSCLC (39). It is
hypothesized that miR-21 could maintain the acquired resistance of
EGFR-TKI in NSCLC by downregulation of phosphatase and tensin
homolog and programmed cell death 4, and activation of the
phosphoinositide 3-kinase/protein kinase B pathway (40). miR-20a regulated expression of the
iron exporter ferroportin in NSCLC (41). miR-512-5p and miR-373 expression
increased cisplatin-induced apoptosis in lung cancer and the
re-expression of miRNAs did benefit from epigenetic cancer therapy
(42). The miR-17 and miR-92 families
were involved in cisplatin resistance and were regulated by
cyclin-dependent kinase 1A and double-strand-break repair protein
RAD21 homolog in NSCLC. These major factors contributed to
cisplatin resistance, potentially by suppressing DNA synthesis and
the repair of DNA damage (43).
miR-221 and miR-222 increased the chemosensitivity to
S-phase-targeting drugs, cisplatin and gemcitabine by inducing
growth suppression in NSCLC cells (44). These two miRNAs were also regulated by
p27kip1 and tumor necrosis factor-related apoptosis-inducing
ligand-induced caspase mechanism (45). A previous study demonstrated that
miRNA-155 was upregulated in the doxorubicin-resistant lung cancer
A549/dox cell line (46).
 | Table III.Blood-based miRNA and targeted
therapies of NSCLC. |
Table III.
Blood-based miRNA and targeted
therapies of NSCLC.
| Author, year | miRNA
profiling | Year | Targeted
therapies |
Mechanism/description | (Refs.) |
|---|
| Chen et al,
2015 | miR-9 | 2015 | EGFR-targeted
therapy (erlotinib) | Erlotinib is an
EGFR inhibitor, miR-9 regulate FoxO1 expression is a target of
erlotinib in NSCLCs | (39) |
| Li et al, 2014 | miR-21 | 2014 | Acquired resistance
of EGFR-TKI | miR-21 is involved
in acquired resistance of EGFR-TKI in NSCLC, which is mediated by
downregulating PTEN and PDCD4 and activating PI3K/Akt pathway | (40) |
| Babu and
Muckenthaler, 2016 | miR-20a | 2016 | – | Expression of
miR-20a is inversely correlated to FPN in NSCLC | (41) |
| Adi Harel et al,
2016 | miR-512-5p and
miR-373 | 2016 | Epigenetic
therapy | miR-512-5p and
miR-373 augment cisplatin-induced apoptosis in lung cancer | (42) |
| Zhao et al,
2015 | miR-17 and miR-92
families | 2015 | Platinum-based
chemotherapy | miR-17 and miR-92
families can maintain cisplatin resistance regulated by CDKN1A and
RAD21 | (43) |
| Yamashita et al,
2015; | miR-221 and
222 | 2015 | S-phase
targeting drugs | miR-221 and miR-222
induce growth | (44,45) |
| Garofalo et al,
2008 |
| 2008 | and
TRAIL-resistant | suppression in
NSCLC cells. These two also modulated by p27kip1 expression and
TRAIL-induced caspase mechanism |
|
| Lv et al, 2016 | miR-155 | 2016 |
Doxorubicin-resistant | MicroRNA-155
upregulated in the doxorubicin-resistant lung cancer | (46) |
Clinical significance of exosomal miRNAs
derived from the blood of patients with NSCLC
Blood-based exosomal miRNAs and
clinical diagnosis of NSCLC
Tumor cells produce an increased number of exosomes
compared with healthy organs and cells (47). There are approximately >3 billion
exosomes/ml in the blood of cancer patients, which is almost twice
the concentration than in the blood of healthy controls (48). Exosomal miRNAs are similar to the
miRNAs of parental cancer cells, resulting in an increased amount
of research into exosomal miRNAs for cancer diagnosis. A number of
examples of potential clinical applications for identification of
high levels of exosomal miRNAs have been identified in melanoma,
glioblastoma, esophageal squamous cell carcinoma, pancreatic and
prostate cancer (Table IV) (47–50).
 | Table IV.Blood-based exosomal miRNA and
clinical diagnosis of NSCLC. |
Table IV.
Blood-based exosomal miRNA and
clinical diagnosis of NSCLC.
| Author, year | Stage | Resource | miRNA
profiling | Year | Country | Cases | Controls | (Refs.) |
|---|
| Rabinowits et al,
2009 | Early | Plasma | 12 special
miRNA | 2009 | USA | 27 | 9 | (51) |
| Cazzoli et al,
2013 |
| Plasma | Screening test: 4
microRNAs (miR-378a, miR-379, miR-139-5p and miR-200b-5p);
Diagnostic test: 6 microRNAs (miR-151a-5p, 6 microRNAs
(miR-151a-5p, miR-30a-3p, miR-200b-5p, miR-629, miR-100 and
miR-154-3p) | 2013 | Italy | Screening test: 20
Diagnostic test: 80 | Screening test: 10;
Diagnostic test: 25 | (52) |
| Rodriguez et al,
2014 |
| Plasma | miR-28,29c, 141,
144, 146, 195 | 2014 | Spain | 30 | 75 | (53) |
| Zhou et al,
2017 |
| Plasma | miR-19b-3p,
miR-21-5p, miR-221-3p, miR-409-3p, miR-425-5p and miR-584-5p | 2017 | China | 18 | 14 | (54) |
| Silva et al,
2011 | Advanced | Plasma | 5 miRNAs (let-7f,
miR-20b, miR-30e-3p, miR-223 and miR-301) | 2011 | Spain | 28 | 20 | (55) |
| Aushev et al,
2013 |
| Plasma | miR-205, −19a,
−19b, −30b, and −20a | 2013 | France | 50 | 6 | (56) |
| Liu et al,
2017 |
| Plasma | miR-23b-3p,
miR-10b-5p and miR-21-5p | 2017 | China | 196 | 0 | (57) |
Blood-based exosomal miRNAs and early
stage of NSCLC
A number of studies have previously attempted to
validate that exosomal miRNAs may be used as a biomarker for the
detection, diagnosis and metastatic spread of cancer. A study
involving 27 patients with lung adenocarcinoma and 9 controls were
selected to validate the special 12 miRNAs; the presence of the 12
miRNAs were also mirrored in the circulating exosomes (51). Four miRNAs (miR-378a, miR-379,
miR-139-5p and miR-200b-5p) were enrolled for screening tests and
six miRNAs (miR-151a-5p, miR-30a-3p, miR-200b-5p, miR-629, miR-100
and miR-154-3p) were enrolled for diagnostic tests, all of which
have demonstrated high sensitivities and specificities of patients
with lung adenocarcinomas and controls (52). A prospective analysis by Rodriguez
et al (53) regarding blood
and bronchoalveolar lavage (BAL) samples derived from 30 patients
with NSCLC and 75 controls demonstrated that exosomes and miRNAs
levels were higher in the plasma and BAL from patients with NSCLC.
Zhou et al (54) identified
six upregulated plasma miRNAs (miR-19b3p, miR-21-5p, miR-221-3p,
miR-409-3p, miR-425-5p and miR-584-5p). These miRNAs were able to
distinguish between patients with lung adenocarcinoma and healthy
controls, with a receiver operating characteristic curve (ROC) of
0.72, 0.74 and 0.84 for the training, testing and the external
validation stage, respectively. Additionally, levels of miR-19-3p,
miR-21-5p and miR-221-3p were significantly upregulated in exosomes
derived from peripheral plasma samples of patients with lung
adenocarcinoma (54).
Blood-based exosomal miRNAs and
advanced stage of NSCLC
The vesicle-associated miRNAs let-7f and miR30e-3p
could be used to discriminate between two groups of patients for
different tumor stages and therefore the surgical options available
to the patients. Notably, the two miRNAs examined in NSCLC have
also been associated with a poor clinical outcome (55). A follow-up survey revealed that the
levels of miR-205, miR-19a, miR-19b, miR-30b and miR-20a from the
plasma of patients was evidently decreased following SCC surgery
(resection). Assessment of miRNA levels in patients with lung SCC
revealed the presence of high levels of exosomal miRNAs expression
in tumor (56). The presence of
exosomal miR-23b-3p, miR-10b-5p and miR-21-5p were identified as
prognostic biomarkers for patients with NSCLC. The median follow-up
time was 14.40 months (range, 3.43–36.87 months), with 71 (36.22%)
patients succumbing to disease by the end of the experiment
(57). These data indicate that the
novel biomarkers and the technology for the detection of exosomal
miRNAs for NSCLC are vital to enhance the sensitivity and
specificity, which may be essential for improving the overall
survival of patients.
Blood-based exosomal miRNAs and
metastasis of NSCLC
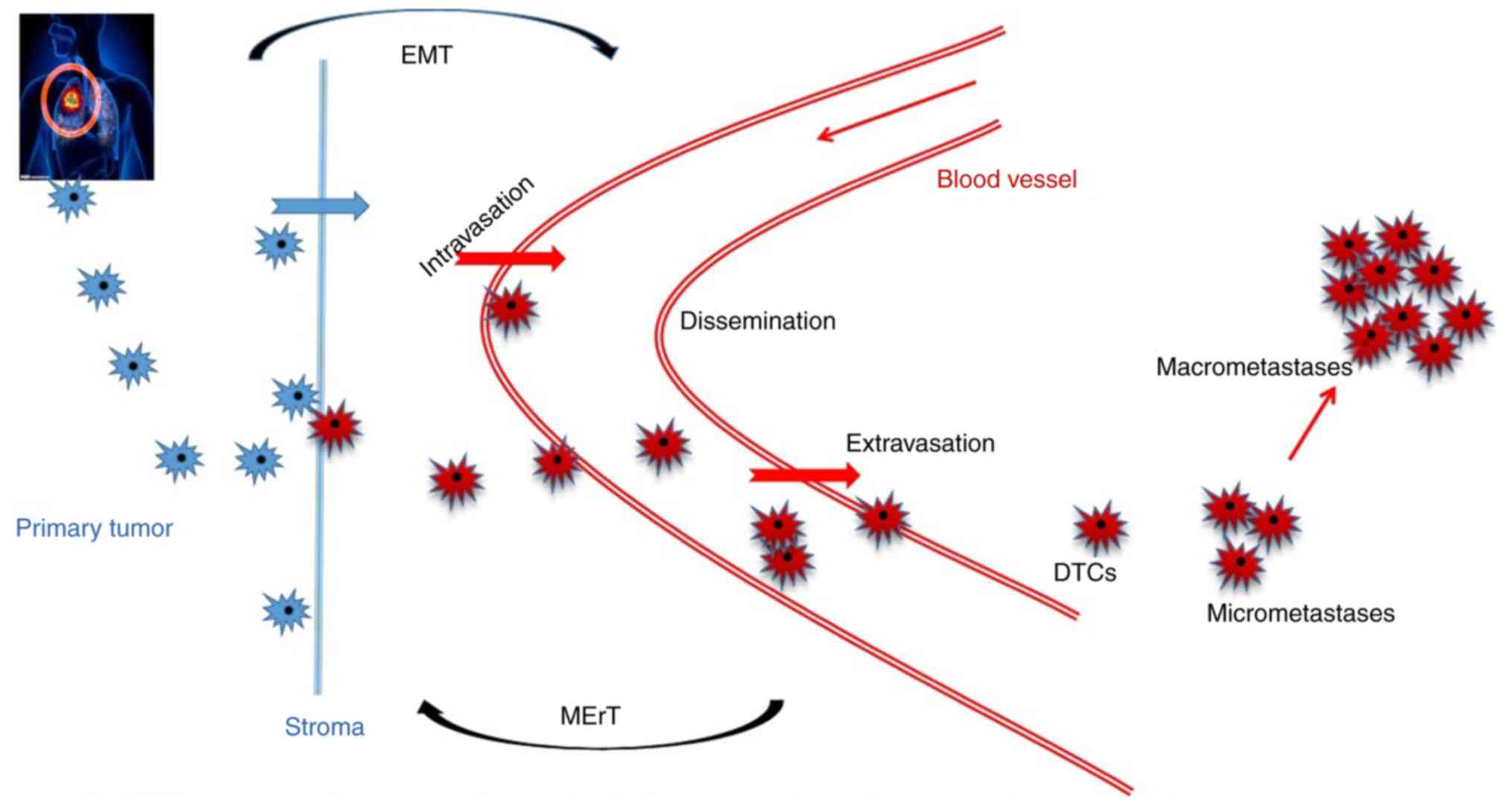
(Fig. 1) Extracellular
vehicles (EVs) deliver nucleic acids to target cells, allowing for
the exchange of genetic information between cells. EVs are also
capable of altering the phenotype of neighboring cells (58). Therefore, exosomes can not only
promote the biological processes of tumors, but also influence the
metastatic signatures of malignant tumors. Additionally, the
regulatory signatures of tumor-derived exosomes are important for
shaping the tumor microenvironment (31).
Exosomes, as mediators of EMT, are involved in the
migration and invasion of metastasis (31). EMT continually initiates the process
of metastasis, where tumor cells lose their polarity and cell-cell
junctions and acquire migratory and invasive capabilities with a
low proliferation state (32). If the
tumor cells reach the distant pre-metastatic niche, the reverse
process occurs (33). Moreover, when
EMT cells arrive at the metastatic side, these EMT cells undergo
epithelial to mesenchymal transition. (31). For example, exosomal miR-23a sustained
the EMT-promoting effect of transforming growth factor-β1 by
suppressing E-cadherin synthesis in lung carcinoma (59,60).
Tumor-derived exosomes can lead cancer cells to acquire a
mesenchymal phenotype and can convert mesenchymal stem cells into
cancer-associated fibroblasts following an increase in the
expression of α-smooth muscle actin (61). Exosome-derived miR-302b was also
demonstrated to inhibit cell proliferation and migration in lung
cancer (62).
Exosomal blood-based miRNAs and
targeted therapies of NSCLC
As in artificial loaders of signaling molecules,
exosomes possess signatures, including biocompatibility, stability,
biological barrier permeability, low immunogenicity and low
toxicity (58). These features make
exosomes attractive for therapeutic use. Additionally, exosomes are
able to escape rapid clearance by the mononuclear phagocyte system
owing to their small size (63). Xiao
et al (64) reported that A549
cell-derived exosomes reduced the sensitivity of non-treated A549
cells to cisplatin, following cisplatin exposure. Notably, the
phospholipid constituents of EV were markedly distinguished in
gefitinib-resistant NSCLC. Sophisticated mass-spectrometry-based
shotgun lipidomic assays were performed for in-depth analysis.
Lipid matrix-assisted laser desorption/ionization analysis revealed
that EV phospholipid composition was significantly more distinct in
PC9R cells compared with PC9 cells (65). There are three techniques to load an
exosome with a therapeutic miRNA mimic or antagonist:
Co-transfection with two plasmids, or co-transduction with two
viruses; electroporation; or the transient transfection of miRNAs
(66,67).
Several trials have been conducted to assess the
safety of EV-based antitumor and antibacterial vaccines. This
included a phase I trial that administered EVs from autologous DCs
pulsed with melanoma antigen gene peptides to patients with NSCLC
(68). Furthermore, a previous phase
II trial was also conducted in order to evaluate
interferon-γ-dendritic cell-derived exosomes loaded with major
histocompatibility complex I/II-restricted cancer antigens
following chemotherapeutic induction in patients with inoperable
NSCLC without tumor progression (69). These results indicate that DC-derived
exosomes vaccines may be safe and may promote T-cell and natural
killer cell responses in patients. The extracorporeal
hemofiltration of circulating EVs is a novel potential strategy,
and has been previously proposed to be a therapeutic strategy for
patients with cancer (70,71).
Clinical significance of miRNAs derived from
tissue of NSCLC
In addition to blood samples, studies have
demonstrated that miRNAs are also obtained from a large number of
tissue specimens of patients with NSCLC and controls to validate
the potential clinical significance of miRNAs in NSCLC. For
example, miR-30d-5p was demonstrated to be downregulated in NSCLC
tissues, with a 2-fold difference in expression between tumor
tissues and the corresponding para-tumorous tissues (72). In 2006 a study describing the
diagnostic miRNA signatures of NSCLC revealed that 12 specific
miRNAs were overexpressed when compared with normal lung tissue
(73). miRNAs extracted from tissues
have been identified to be a more direct and intuitive method;
however, tissue as another sample resource, are not the primary
focus of the present review.
Conclusion
Currently, circulating tumor cells, circulating
tumor DNA and exosomes, are all covered in the concept ‘liquid
biopsies’. A liquid biopsy is a liquid biomarker that may be easily
isolated from numerous body fluids (blood, saliva, urine, ascites
and pleural effusion and a tissue biopsy, a representative of the
tissue from which it is obtained (74). Exosomes, as a non-invasive means of
performing ‘liquid biopsies’, may be used for early diagnosis,
obtaining prognostic information, metastasis development, real-time
monitoring of tumor stages and understanding of therapeutic targets
in patients with NSCLC.
The present review focuses on the clinical
significance of blood-based exosomal miRNAs in the detection,
metastasis and therapies of NSCLC. As a novel means of
intercellular communication, exosomes can stimulate the growth,
invasiveness and metastasis of NSCLC (75). Compared with the conventional methods
used for clinical diagnosis of NSCLC, exosomes have numerous
advantages: Exosomes contain an increased amount of genetic
information, which can spread widely throughout the bodily fluids;
exosomes have a relatively long circulating half-life with drugs
in vivo; exosomes secreted by cells exhibit target
selection; exosomes can enhance their cell-specific targeting
function by altering their membrane; and exosomes can carry drugs
in vivo and in vitro (76,77).
According to recent findings in NSCLC, the significant difference
in miRNAs and exosomal miRNAs derived from blood between patients
with NSCLC and controls, and the similarities between exosomal
miRNAs and miRNAs all indicate that exosomal miRNAs may be useful
as a biomarker for clinical diagnosis in NSCLC (76).
A number of problems surrounding the use of exosomes
require addressing, which include: A lack of a reliable and
cost-effective detection platforms for exosomal miRNAs in routine
clinical settings; the complication of procedures, including
specificity screening, detection methods suitable for the selection
of the reference genes; the apparent shortcomings, which include
time-consuming experiments, expensive instruments and a heavy
reliance on the sample-handling skills; and a lack of research into
the mechanism of action of exosomes.
Although research into exosomal miRNAs and NSCLC in
its infancy, with the development of detection methods and the
maturity of the research, in the future blood-based exosomal miRNAs
may have prospects for application in terms of prediction,
diagnosis, metastasis, prognostic evaluation and individualized
treatment in lung cancer. Therefore, identifying novel therapeutic
strategies and acquiring a better understanding of the biological
functions of exosomal miRNAs as biomarkers in NSCLC will be of the
utmost relevance in modern molecular oncology.
Acknowledgements
Not applicable.
Funding
The presents study was supported by the National
Nature Science Foundation of China (grant no. 81471308).
Availability of data and materials
The datasets generated and analysed in the present
study are included in this published article.
Authors' contributions
JL devised the study topic. YS and LW revised the
paper. MF provided additional clinical knowledge. LL wrote this
manuscript.
Ethics and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shen X, Wang L and Zhu L: Spatial analysis
of regional factors and lung cancer mortality in China, 1973–2013.
Cancer Epidemiol Biomarkers Prev. 26:569–577. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fujita Y, Kosaka N, Araya J, Kuwano K and
Ochiya T: Extracellular vesicles in lung microenvironment and
pathogenesis. Trends Mol Med. 21:533–542. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen X, Ba Y, Ma L, Cai X, Yin Y, Wang K,
Guo J, Zhang Y, Chen J, Guo X, et al: Characterization of microRNAs
in serum: A novel class of biomarkers for diagnosis of cancer and
other diseases. Cell Res. 18:997–1006. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Théry C, Ostrowski M and Segura E:
Membrane vesicles as conveyors of immune responses. Nat Rev
Immunol. 9:581–593. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cocucci E, Racchetti G and Meldolesi J:
Shedding microvesicles: Artefacts no more. Trends Cell Biol.
19:43–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Simons M and Raposo G: Exosomes-vesicular
carriers for intercellular communication. Curr Opin Cell Biol.
21:575–581. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Valadi H, Ekström K, Bossios A, Sjöstrand
M, Lee JJ and Lötvall JO: Exosome-mediated transfer of mRNAs and
microRNAs is a novel mechanism of genetic exchange between cells.
Nat Cell Biol. 9:654–659. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jaiswal R, Gong J, Sambasivam S, Combes V,
Mathys JM, Davey R, Grau GE and Bebawy M: Microparticle-associated
nucleic acids mediate trait dominance in cancer. FASEB J.
26:420–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sanfiorenzo C, Ilie MI, Belaid A, Barlési
F, Mouroux J, Marquette CH, Brest P and Hofman P: Two panels of
plasma microRNAs as non-invasive biomarkers for prediction of
recurrence in resectable NSCLC. PLoS One. 8:e545962013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Foss KM, Sima C, Ugolini D, Neri M, Allen
KE and Weiss GJ: miR-1254 and miR-574-5p: Serum-based microRNA
biomarkers for early-stage non-small cell lung cancer. J Thorac
Oncol. 6:482–488. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang P, Yang D, Zhang H, Wei X, Ma T,
Cheng Z, Hong Q, Hu J, Zhuo H, Song Y, et al: Early detection of
lung cancer in serum by a panel of MicroRNA biomarkers. Clin Lung
Cancer. 16:313–319.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bianchi F, Nicassio F, Marzi M, Belloni E,
Dall'olio V, Bernard L, Pelosi G, Maisonneuve P, Veronesi G and Di
Fiore PP: A serum circulating miRNA diagnostic test to identify
asymptomatic high-risk individuals with early stage lung cancer.
EMBO Mol Med. 3:495–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen X, Hu Z, Wang W, Ba Y, Ma L, Zhang C,
Wang C, Ren Z, Zhao Y, Wu S, et al: Identification of ten serum
microRNAs from a genome-wide serum microRNA expression profile as
novel noninvasive biomarkers for nonsmall cell lung cancer
diagnosis. Int J Cancer. 130:1620–1628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen J, Todd NW, Zhang H, Yu L, Lingxiao
X, Mei Y, Guarnera M, Liao J, Chou A, Lu CL, et al: Plasma
microRNAs as potential biomarkers for non-small-cell lung cancer.
Lab Invest. 91:579–587. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ulivi P, Foschi G, Mengozzi M, Scarpi E,
Silvestrini R, Amadori D and Zoli W: Peripheral blood miR-328
expression as a potential biomarker for the early diagnosis of
NSCLC. Int J Mol Sci. 14:10332–10342. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scagliotti G, Hanna N, Fossella F,
Sugarman K, Blatter J, Peterson P, Simms L and Shepherd FA: The
differential efficacy of pemetrexed according to NSCLC histology: A
review of two Phase III studies. Oncologist. 14:253–263. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuxia M, Zhennan T and Wei Z: Circulating
miR-125b is a novel biomarker for screening non-small-cell lung
cancer and predicts poor prognosis. J Cancer Res Clin Oncol.
138:2045–2050. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sozzi G, Boeri M, Rossi M, Verri C,
Suatoni P, Bravi F, Roz L, Conte D, Grassi M, Sverzellati N, et al:
Clinical utility of a plasma-based miRNA signature classifier
within computed tomography lung cancer screening: A correlative
MILD trial study. J Clin Oncol. 32:768–773. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang YL, Xu LP, Zhuo FL and Wang TY:
Prognostic value of microRNA-10b overexpression in peripheral blood
mononuclear cells of nonsmall-cell lung cancer patients. Tumour
Biol. 36:7069–7075. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu W, He J, Chen D, Zhang B, Xu L, Ma H,
Liu X, Zhang Y and Le H: Expression of miR-29c, miR-93 and miR-429
as potential biomarkers for detection of early stage non-small lung
cancer. PLoS One. 9:e877802014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu W, Zhou K, Zha Y, Chen D, He J, Ma H,
Liu X, Le H and Zhang Y: Diagnostic value of serum miR-182,
miR-183, miR-210, and miR-126 levels in patients with early-stage
non-small cell lung cancer. PLoS One. 11:e01530462016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou C, Chen Z, Dong J, Li J, Shi X, Sun
N, Luo M, Zhou F, Tan F and He J: Combination of serum miRNAs with
Cyfra21-1 for the diagnosis of non-small cell lung cancer. Cancer
Lett. 367:138–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Greither T, Grochola LF, Udelnow A,
Lautenschläger C, Würl P and Taubert H: Elevated expression of
microRNAs 155, 203, 210 and 222 in pancreatic tumors is associated
with poorer survival. Int J Cancer. 126:73–80. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee KH, Lotterman C, Karikari C, Omura N,
Feldmann G, Habbe N, Goggins MG, Mendell JT and Maitra A:
Epigenetic silencing of MicroRNA miR-107 regulates cyclin-dependent
kinase 6 expression in pancreatic cancer. Pancreatology. 9:293–301.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ahn SM, Cha JY, Kim J, Kim D, Trang HT,
Kim YM, Cho YH, Park D and Hong S: Smad3 regulates E-cadherin via
miRNA-200 pathway. Oncogene. 31:3051–3059. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Duan FT, Qian F, Fang K, Lin KY, Wang WT
and Chen YQ: miR-133b, a muscle-specific microRNA, is a novel
prognostic marker that participates in the progression of human
colorectal cancer via regulation of CXCR4 expression. Mol Cancer.
12:1642013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Diepenbruck M and Christofori G:
Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no,
maybe? Curr Opin Cell Biol. 43:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Steinbichler TB, Dudás J, Riechelmann H
and Skvortsova II: The role of exosomes in cancer metastasis. Semin
Cancer Biol. 44:170–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li J, Tan Q, Yan M, Liu L, Lin H, Zhao F,
Bao G, Kong H, Ge C, Zhang F, et al: miRNA-200c inhibits invasion
and metastasis of human non-small cell lung cancer by directly
targeting ubiquitin specific peptidase 25. Mol Cancer. 13:1662014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cheng Z, Ma R, Tan W and Zhang L: MiR-152
suppresses the proliferation and invasion of NSCLC cells by
inhibiting FGF2. Exp Mol Med. 46:e1122014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu X, Li D, Yu F, Jia C, Xie J, Ma Y, Fan
S, Cai H, Luo Q, Lv Z and Fan L: miR-194 inhibits the
proliferation, invasion, migration, and enhances the
chemosensitivity of non-small cell lung cancer cells by targeting
forkhead box A1 protein. Oncotarget. 7:13139–13152. 2016.PubMed/NCBI
|
|
36
|
Luo W, Huang B, Li Z, Li H, Sun L, Zhang
Q, Qiu X and Wang E: MicroRNA-449a is downregulated in non-small
cell lung cancer and inhibits migration and invasion by targeting
c-Met. PLoS One. 8:e647592013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wheeler DL, Dunn EF and Harari PM:
Understanding resistance to EGFR inhibitors-impact on future
treatment strategies. Nat Rev Clin Oncol. 7:493–507. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sangodkar J, Dhawan NS, Melville H, Singh
VJ, Yuan E, Rana H, Izadmehr S, Farrington C, Mazhar S, Katz S, et
al: Targeting the FOXO1/KLF6 axis regulates EGFR signaling and
treatment response. J Clin Invest. 122:2637–2651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen X, Zhu L, Ma Z, Sun G, Luo X, Li M,
Zhai S, Li P and Wang X: Oncogenic miR-9 is a target of erlotinib
in NSCLCs. Sci Rep. 5:170312015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li B, Ren S, Li X, Wang Y, Garfield D,
Zhou S, Chen X, Su C, Chen M, Kuang P, et al: MiR-21 overexpression
is associated with acquired resistance of EGFR-TKI in non-small
cell lung cancer. Lung Cancer. 83:146–153. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Babu KR and Muckenthaler MU: miR-20a
regulates expression of the iron exporter ferroportin in lung
cancer. J Mol Med (Berl). 94:347–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Harel Adi S, Bossel Ben-Moshe N, Aylon Y,
Bublik DR, Moskovits N, Toperoff G, Azaiza D, Biagoni F, Fuchs G,
Wilder S, et al: Reactivation of epigenetically silenced miR-512
and miR-373 sensitizes lung cancer cells to cisplatin and restricts
tumor growth. Cell Death Differ. 22:1328–1340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhao J, Fu W, Liao H, Dai L, Jiang Z, Pan
Y, Huang H, Mo Y, Li S, Yang G and Yin J: The regulatory and
predictive functions of miR-17 and miR-92 families on cisplatin
resistance of non-small cell lung cancer. BMC Cancer. 15:7312015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yamashita R, Sato M, Kakumu T, Hase T,
Yogo N, Maruyama E, Sekido Y, Kondo M and Hasegawa Y: Growth
inhibitory effects of miR-221 and miR-222 in non-small cell lung
cancer cells. Cancer Med. 4:551–564. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Garofalo M, Quintavalle C, Di Leva G,
Zanca C, Romano G, Taccioli C, Liu CG, Croce CM and Condorelli G:
MicroRNA signatures of TRAIL resistance in human non-small cell
lung cancer. Oncogene. 27:3845–3855. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lv L, An X, Li H and Ma L: Effect of
miR-155 knockdown on the reversal of doxorubicin resistance in
human lung cancer A549/dox cells. Oncol Lett. 11:1161–1166. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kalluri R: The biology and function of
exosomes in cancer. J Clin Invest. 126:1208–1215. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jaiswal R, Gong J, Sambasivam S, Combes V,
Mathys JM, Davey R, Grau GE and Bebawy M: Microparticle-associated
nucleic acids mediate trait dominance in cancer. FASEB J.
26:420–429. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pigati L, Yaddanapudi SC, Iyengar R, Kim
DJ, Hearn SA, Danforth D, Hastings ML and Duelli DM: Selective
release of microRNA species from normal and malignant mammary
epithelial cells. PLoS One. 5:e135152010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rabinowits G, Gercel-Taylor C, Day JM,
Taylor DD and Kloecker GH: Exosomal microRNA: A diagnostic marker
for lung cancer. Clin Lung Cancer. 10:42–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cazzoli R, Buttitta F, Di Nicola M,
Malatesta S, Marchetti A, Rom WN and Pass HI: microRNAs derived
from circulating exosomes as noninvasive biomarkers for screening
and diagnosing lung cancer. J Thorac Oncol. 8:1156–1162. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Rodriguez M, Silva J, López-Alfonso A,
López-Muñiz MB, Peña C, Domínguez G, García JM, López-Gónzalez A,
Méndez M, Provencio M, et al: Different exosome cargo from
plasma/bronchoalveolar lavage in non-small-cell lung cancer. Genes
Chromosomes Cancer. 53:713–724. 2014.PubMed/NCBI
|
|
54
|
Zhou X, Wen W, Shan X, Zhu W, Xu J, Guo R,
Cheng W, Wang F, Qi LW, Chen Y, et al: A six-microRNA panel in
plasma was identified as a potential biomarker for lung
adenocarcinoma diagnosis. Oncotarget. 8:6513–6525. 2017.PubMed/NCBI
|
|
55
|
Silva J, García V, Zaballos Á, Provencio
M, Lombardía L, Almonacid L, García JM, Domínguez G, Peña C, Diaz
R, et al: Vesicle-related microRNAs in plasma of nonsmall cell lung
cancer patients and correlation with survival. Eur Respir J.
37:617–623. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Aushev VN, Zborovskaya IB, Laktionov KK,
Girard N, Cros MP, Herceg Z and Krutovskikh V: Comparisons of
microRNA patterns in plasma before and after tumor removal reveal
new biomarkers of lung squamous cell carcinoma. PLoS One.
8:e786492013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu Q, Yu Z, Yuan S, Xie W, Li C, Hu Z,
Xiang Y, Wu N, Wu L, Bai L and Li Y: Circulating exosomal microRNAs
as prognostic biomarkers for non-small-cell lung cancer.
Oncotarget. 8:13048–13058. 2017.PubMed/NCBI
|
|
58
|
Barile L and Vassalli G: Exosomes: Therapy
delivery tools and biomarkers of diseases. Pharmacol Ther.
174:63–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cao M, Seike M, Soeno C, Mizutani H,
Kitamura K, Minegishi Y, Noro R, Yoshimura A, Cai L and Gemma A:
MiR-23a regulates TGF-β-induced epithelial-mesenchymal transition
by targeting E-cadherin in lung cancer cells. Int J Oncol.
41:869–875. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Kim J, Kim TY, Lee MS, Mun JY, Ihm C and
Kim SA: Exosome cargo reflects TGF-β1-mediated
epithelial-to-mesenchymal transition (EMT) status in A549 human
lung adenocarcinoma cells. Biochem Biophys Res Commun. 478:643–648.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Webber J, Steadman R, Mason MD, Tabi Z and
Clayton A: Cancer exosomes trigger fibroblast to myofibroblast
differentiation. Cancer Res. 70:9621–9630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li J, Yu J, Zhang H, Wang B, Guo H, Bai J,
Wang J, Dong Y, Zhao Y and Wang Y: Exosomes-derived MiR-302b
suppresses lung cancer cell proliferation and migration via TGFβRII
inhibition. Cell Physiol Biochem. 38:1715–1726. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
van den Boorn JG, Schlee M, Coch C and
Hartmann G: SiRNA delivery with exosome nanoparticles. Nat
Biotechnol. 29:325–326. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Xiao X, Yu S, Li S, Wu J, Ma R, Cao H, Zhu
Y and Feng J: Exosomes: Decreased sensitivity of lung cancer A549
cells to cisplatin. PLoS One. 9:e895342014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jung JH, Lee MY, Choi DY, Lee JW, You S,
Lee KY, Kim J and Kim KP: Phospholipids of tumor extracellular
vesicles stratify gefitinib-resistant nonsmall cell lung cancer
cells from gefitinib-sensitive cells. Proteomics. 15:824–835. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Alvarez-Erviti L, Seow Y, Yin H, Betts C,
Lakhal S and Wood MJ: Delivery of siRNA to the mouse brain by
systemic injection of targeted exosomes. Nat Biotechnol.
29:341–345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cooper JM, Wiklander PB, Nordin JZ,
Al-Shawi R, Wood MJ, Vithlani M, Schapira AH, Simons JP,
El-Andaloussi S and Alvarez-Erviti L: Systemic exosomal siRNA
delivery reduced alpha-synuclein aggregates in brains of transgenic
mice. Mov Disord. 29:1476–1485. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Morse MA, Garst J, Osada T, Khan S,
Hobeika A, Clay TM, Valente N, Shreeniwas R, Sutton MA, Delcayre A,
et al: A phase I study of dexosome immunotherapy in patients with
advanced non-small cell lung cancer. J Transl Med. 3:92005.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Besse B, Charrier M, Lapierre V, Dansin E,
Lantz O, Planchard D, Le Chevalier T, Livartoski A, Barlesi F,
Laplanche A, et al: Dendritic cell-derived exosomes as maintenance
immunotherapy after first line chemotherapy in NSCLC.
OncoImmunology. 5:e10710082015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pitt JM, André F, Amigorena S, Soria JC,
Eggermont A, Kroemer G and Zitvogel L: Dendritic cell-derived
exosomes for cancer therapy. J Clin Invest. 126:1224–1232. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Marleau AM, Chen CS, Joyce JA and Tullis
RH: Exosome removal as a therapeutic adjuvant in cancer. J Transl
Med. 10:1342012. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Chen D, Guo W, Qiu Z, Wang Q, Li Y, Liang
L, Liu L, Huang S, Zhao Y and He X: MicroRNA-30d-5p inhibits tumour
cell proliferation and motility by directly targeting CCNE2 in
non-small cell lung cancer. Cancer Lett. 362:208–217. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Yanaihara N, Caplen N, Bowman E, Seike M,
Kumamoto K, Yi M, Stephens RM, Okamoto A, Yokota J, Tanaka T, et
al: Unique microRNA molecular profiles in lung cancer diagnosis and
prognosis. Cancer Cell. 9:189–198. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Rolfo C, Castiglia M, Hong D, Alessandro
R, Mertens I, Baggerman G, Zwaenepoel K, Gil-Bazo I, Passiglia F,
Carreca AP, et al: Liquid biopsies in lung cancer: The new ambrosia
of researchers. Biochim Biophys Acta. 1846:539–546. 2014.PubMed/NCBI
|
|
75
|
Xu W, Yang Z and Lu N: From pathogenesis
to clinical application: Insights into exosomes as transfer vectors
in cancer. J Exp Clin Cancer Res. 35:1562016. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
György B, Hung ME, Breakefield XO and
Leonard JN: Therapeutic applications of extracellular vesicles:
Clinical promise and open questions. Annu Rev Pharmacol Toxicol.
55:439–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kourembanas S: Exosomes: Vehicles of
intercellular signaling, biomarkers, and vectors of cell therapy.
Annu Rev Physiol. 77:13–27. 2015. View Article : Google Scholar : PubMed/NCBI
|