Introduction
Cervical cancer (CC) is one of the most malignant
female reproduction cancers in human, as well as the fourth most
common cause of cancer-related morality in the world (1,2). Globally,
it was estimated that there were about 530,000 new diagnosed cases
each year (1). In the meantime,
approximately 135,000 causes were found in China, accounting for
about 25% of all the new CC causes each year (3). Despite advances in the diagnosis and
treatment methods for CC in recent years, unfortunately, the
prognosis of CC patients remains poor (4–6).
Therefore, exploring the molecular mechanisms related to the tumor
initiation and progression of CC is of vital importance and might
provide new biomarker to predict and improve the prognosis of CC
patients.
Increasing researches have demonstrated that
microRNAs (miRs) are a class of small, non-coding RNAs that play
critical roles in various biological cellular processes, including
cell proliferation, migration, differentiation, and apoptosis
(7–9).
In recent decades, dysregulation of miRs have been implicated in
the development and malignant progression of human cancers and
might be used as potential diagnostic or therapeutic targets for
cancers (10–12). To date, over 2,500 miRs have been
identified in human genome (13).
Among numerous cancer-related miRs, miR-153 was recently found to
be dysregulated in human cancers including breast cancer (14), ovarian cancer (15), osteosarcoma (16), esophageal squamous cell carcinoma
(17), gastric cancer (18), pancreatic cancer (19), non-small-cell lung cancer (20), and colorectal cancer (21). Significantly, miR-153 expression was
found upregulated in colorectal cancer but downregulated in the
rest cancer types mentioned above (14–21), which
implies that miR-153 plays a dual role in the progression of human
cancers. However, up to now, the role of miR-153 in regulating
human CC cells remains unexplored. Importantly, miR-153 was found
dysregulated in gynecological tumors including breast cancer and
ovarian cancer (14,15). Therefore, it is worthwhile to
investigate the clinical significance of miR-153 in CC.
In the present study, we found miR-153 was
significantly downregulated in CC tissues and cell lines. Ectopic
the expression of miR-153 inhibited cell proliferation in CC cell
lines. Moreover, the downregulation of miR-153 in CC patients was
associated with tumor size and lymph node metastasis. Furthermore,
we revealed that the downregulation of miR-153 predicts the poor
prognosis of CC patients. Taken together, our data revealed that
miR-153 plays a tumor suppressor role in the progression of CC and
may provide a novel potential therapeutic target for CC.
Materials and methods
Clinical tissue samples
This study was approved by the Ethics Committee of
The Central Hospital of Wuhan. A total of 93 pairs of CC tissues
and matched noncancerous tissues were collected at the Central
Hospital of Wuhan between March 2008 and September 2011. The
written informed consent has been obtained from all the enrolled
patients. These enrolled patients did not receive any anti-tumor
treatments prior to surgical resection. Tissues were immediately
snap-frozen in liquid nitrogen after surgical resection, and stored
in liquid nitrogen before usage. Clinicopathological parameters of
the enrolled patients were collected at the beginning of the
follow-up period and summarized in Table
I. The overall survival time was calculated as the time between
the date of surgery and the date of mortality or last follow-up for
60 months.
 | Table I.Associations between microRNA-153
expression and clinicopathological features. |
Table I.
Associations between microRNA-153
expression and clinicopathological features.
|
|
| miR-153 expression
level |
|
|---|
|
|
|
|
|
|---|
| Variable | Cases (n) | High (n=25) % | Low (n=68) % | P-value |
|---|
| Age (years) |
|
|
|
|
|
>50 | 45 | 12 (26.7) | 33 (72.3) | 0.662 |
|
<50 | 48 | 13 (27.1) | 35 (71.9) |
|
| Lymph node
metastasis |
|
|
|
|
|
Negative | 39 | 11 (28.2) | 28 (71.8) | 0.039 |
|
Positive | 54 | 14 (25.9) | 40 (74.1) |
|
| Tumor size |
|
|
|
|
| ≥4
cm | 55 | 16 (29.1) | 39 (70.9) | 0.018 |
| <4
cm | 38 | 9 (23.7) | 29 (76.3) |
|
| Clinical stage |
|
|
|
|
| I–II | 47 | 10 (21.3) | 37 (78.7) | 0.093 |
| III | 46 | 15 (32.6) | 31 (67.4) |
|
Cell lines and cell culture
The human CC cell lines including C-33A, HeLa, and
SiHa were purchased from Type Culture Collection of the Chinese
Academy of Sciences (Shanghai, China). These cells were cultured in
Dulbecco's modified Eagle's medium (DMEM; Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal
bovine serum (FBS; HyClone; GE Healthcare Life Sciences, Logan, UT,
USA), and 100 U/ml penicillin and 0.1 mg/ml streptomycin. The human
cervical epithelial cell line End1 was purchased from American Type
Culture Collection (ATCC, Manassas, VA, USA) and cultured in
Keratinocyte-Serum Free medium (K-SFM; Invitrogen; Thermo Fisher
Scientific, Inc.) with 0.1 ng/ml human recombinant EGF, 0.05 mg/ml
bovine pituitary extract, and additional calcium chloride 44.1 mg/l
(final concentration 0.4 mM). Cells were maintained in humidified
incubator at 37°C containing 5% of CO2.
Cell transfection
The miR-153 mimic (5′-UUGCAUAGUCACAAAAGUGAUC-3′),
miR-153 inhibitor (5′-AUCACUUUUGUGACUAUGCA-3′) and negative control
(miR-con) (5′-UAGCUUAUCAGACUGAUGUUGA-3′) were purchased from
Guangzhou RiboBio Co., Ltd., (Guangzhou, China). The cells were
seeded into 6-well plates at a density of 3×105
cells/well. The synthetic miRNAs were transfected to the cultured
cells using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the instructions provided by the
manufacturer. Further analyses were performed 24 h post
transfection.
Reverse transcriptase-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from the cultured cell lines was isolated
using TRIzol reagent (Beyotime Institute of Biotechnology, Haimen,
China). RNA was reverse transcribed to cDNA using TaqMan miRNA
reverse transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.). RT-PCR was performed using PrimeScript miRNA
RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China)
according to the manufacturer's instructions. The following
procedures were used: 1 cycle at 95°C for 2 min, 40 cycles at 95°C
for 30 sec and 58°C for 40 sec. U6 snRNA was used as an internal
control to normalize the expression of miR-153. The primers for
miR-153 and U6 snRNA used in this study were purchased from
Guangzhou RiboBio Co., Ltd. and the detailed sequences were as
follows: miR-153: Forward, 5′-TTGCATAGTCACAAAAGTGAT-3′, Reverse,
5′-CAGTGCGTGTCGTGGAGT-3′; U6 snRNA: Forward,
5′-CTCGCTTCGGCAGCACATATACT-3′, Reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. The relative expression levels were
calculated using the 2−∆∆Cq method (22).
Cell proliferation assay
To assess the cell proliferation rate, MTT method
was employed. Briefly, the cells were cultured in 96-well plate at
the density of 3×103 cells/well. At indicated time
points (0, 24, 48, 72 h), 10 µl MTT solution (5 mg/ml; Beyotime
Institute of Biotechnology) was added to each well and incubated
for additional 3 h. Then, the supernatant was discarded followed by
adding 200 µl DMSO to dissolve the violet formazan crystals. After
incubation for 2 h, the optical density was measured at 570 nm
using the Multiskan Spectrum equipment (Thermo Fisher Scientific,
Inc.).
Statistical analysis
SPSS v16.0 software (SPSS, Inc., Chicago, IL, USA)
was used for statistical analysis. To determine the significance of
two groups and multiple groups, Student's t test and one-way ANOVA
were conducted respectively. Chi-square test was used to analyze
the correlation between the expression of miR-153 and
clinicopathological features. The Kaplan-Meier curve and log-rank
test was used to analyze the overall survival of CC patients.
Univariate and multivariate analyses with Cox proportional hazards
model were used to identify the independent predictors for the
prognosis of CC patients. Data were presented as the mean ± SD.
P<0.05 was considered to indicate a statistically significant
difference.
Results
MiR-153 is downregulated in CC tissues
and cell lines
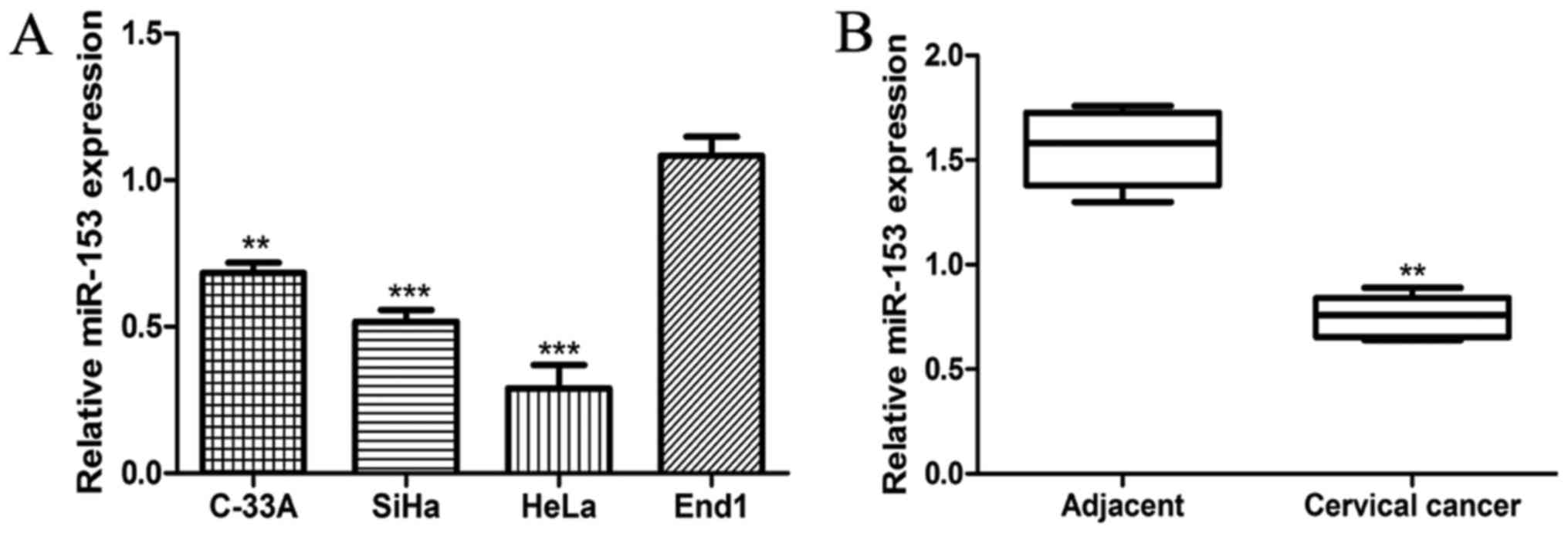
We measured the expression of miR-153 in CC cell
lines (C-33A, HeLa, and SiHa) and normal cervical epithelial cell
line End1 by RT-qPCR. As presented in Fig. 1A, the expression of miR-153 was
significantly reduced in CC cell lines investigated compared with
that in normal cervical epithelial cell line (all P<0.01). To
further confirm the significance of miR-153 expression in CC, the
expression level of miR-153 in 93 paired CC tissues and adjacent
normal tissues was measured by the same method. We found the
miR-153 expression was significantly lower in CC tissues compared
with in adjacent normal tissues (P<0.01; Fig. 1B), which was consistent with the
observations on CC cell lines and normal cervical epithelial cell
line. These 93 CC patients were then classified into two groups
based on the expression level of miR-153: Namely high miR-153
expression group and low miR-153 expression group. The 75th
percentile of 2−∆∆Cq was used as the cut-off point
(0.72) for patients with high or low miR-153 expression (23).
MiR-153 inhibits the proliferation of
CC cell lines in vitro
To explore the role of miR-153 in CC cells, miR-153
expression level was altered with miR-153 mimic and inhibitor.
RT-qPCR showed that miR-153 expression was significantly enhanced
in all the investigated CC cell lines transfected with miR-153
mimic compared with those transfected with miR-con (all P<0.01;
Fig. 2A). Conversely, the miR-153
inhibitor transfection could reduce the expression of miR-153 in
the CC cell lines investigated (all P<0.01; Fig. 2A). Cell proliferation rate was
measured using MTT assay, the cell proliferation of CC cell lines
was significantly higher than that of normal cervical epithelial
cell (all P<0.01; Fig. 2B).
Following, the cell proliferation of CC cell lines transfected with
miRNAs was also measured by the same method. As shown in Fig. 2C, we found the CC cells transfected
with miR-153 mimic showed obvious growth inhibition, while those
transfected with miR-153 inhibitor showed obvious growth
stimulation (all P<0.05).
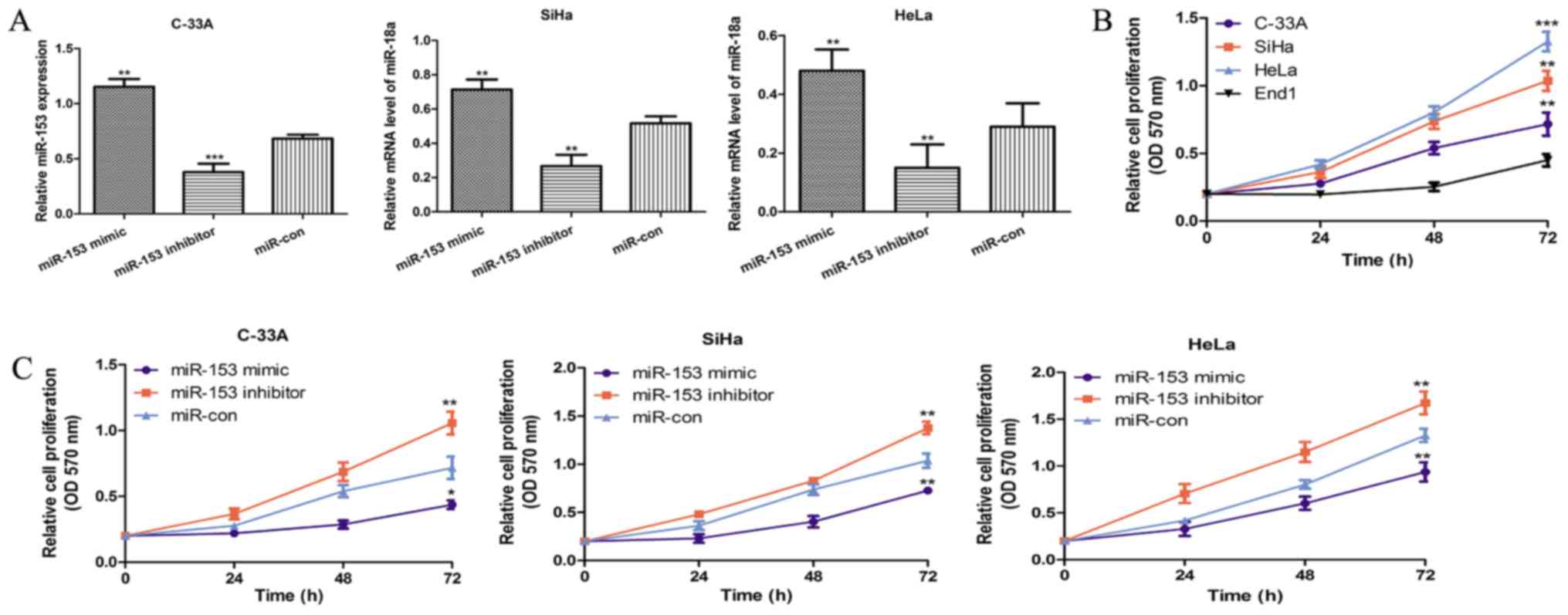 | Figure 2.miR-153 overexpression inhibits the
proliferation of CC cell lines. (A) Reverse
transcription-quantitative polymerase chain reaction was performed
to analyze the expression of miR-153 in the CC cell lines C-33A,
SiHa and HeLa, following transfection with miR-153 mimic, miR-153
inhibitor and negative control. (B) An MTT assay was performed to
analyze the cell proliferation of the CC cell lines (C-33A, SiHa
and HeLa) and the normal cervical epithelial cell line End1. (C) An
MTT assay was also performed to analyze the cell proliferation of
the CC cell lines (C-33A, SiHa and HeLa) following transfection
with miR-153 mimic, miR-153 inhibitor and negative control.
*P<0.05, **P<0.01 and ***P<0.001 vs. control
(End1/miR-con). miR-153, microRNA-153; CC, cervical cancer;
miR-con, negative control miRNA; OD 570 nm, optical density at 570
nm. |
Clinical significance of miR-153
expression in CC
To investigate the clinical significance of miR-153,
we assessed the association between miR-153 expression and
clinicalpathological features. The results revealed that low
miR-153 expression was associated with tumor size (P=0.018), lymph
node metastasis (P=0.039) but not related to age (P=0.662),
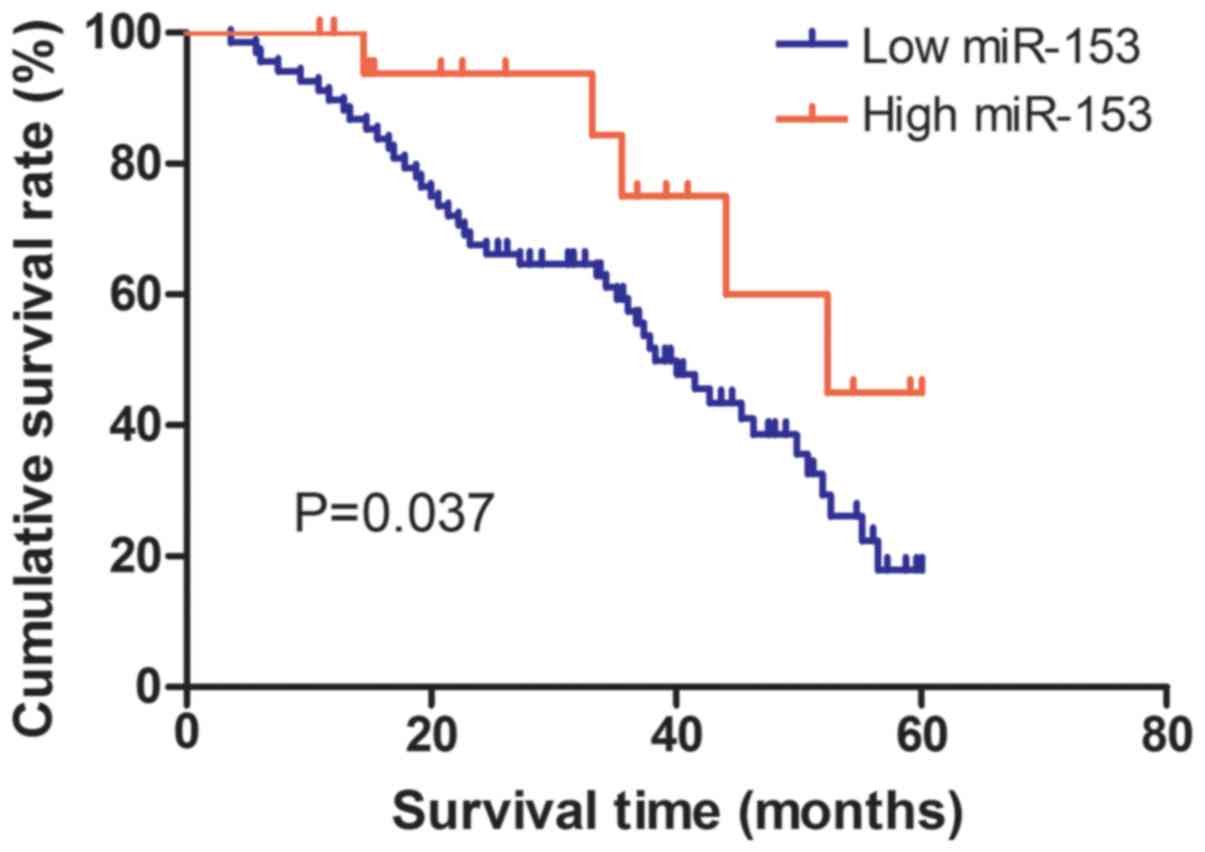
clinical stage (P=0.093) in CC patients (Table I). The Kaplan-Meier curve and log-rank
test was employed to analyze the effect of miR-153 expression on
5-year overall survival of the 93 enrolled CC patients. We found
the patients with high miR-153 expression (n=25) live longer than
those with low miR-153 expression (n=68) (P=0.037; Fig. 3). Additionally, multivariate analysis
indicated that miR-153 expression was an independent predictor for
the overall survival in CC patients (P=0.025; Table II). Taken together, there was a
strong correlation between low miR-153 expression and poor outcomes
in CC patients.
 | Table II.Univariate and multivariate analyses
of overall survival. |
Table II.
Univariate and multivariate analyses
of overall survival.
|
| Univariate
analysis |
| Multivariate
analysis |
|
|---|
|
|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| miR-153 | 2.062 | 1.080–3.936 | 0.028 | 2.080 | 1.096–3.949 | 0.025 |
| Age | 1.798 | 0.870–3.716 | 0.113 | – | – | – |
| Lymph node
metastasis | 1.981 | 1.103–3.873 | 0.046 | 2.001 | 1.030–3.889 | 0.041 |
| Tumor size | 2.021 | 1.047–3.904 | 0.036 | 2.043 | 1.065–3.922 | 0.032 |
| Clinical stage | 1.896 | 0.945–3.804 | 0.072 | – | – | – |
Discussion
miRNAs function mainly through complementary binding
to the 3′-UTR of target gene which can induce the degradation of
its mRNA, or suppress gene transcription to reduce the expression
levels of its target genes (24–26). Many
studies have confirmed that miRNAs were critical players in the
initiation and progression of human cancers including CC (27,28). For
CC patients, the overall survival has significantly improved in the
past decade due to the usage of HPV vaccination and improvement of
therapeutics methods but it is still undesirable (4,5).
Therefore, it is of great importance to understand the molecular
mechanisms underlying the progression of CC, which are important
for developing novel therapeutic strategies for patients with
CC.
Although documented evidence indicates that miR-153
can function as either oncogene or tumor-suppressor in cancers, the
role of miR-153 in regulating CC cells remains unexplored (14–21). In
the present study, the miR-153 expression was found to be
downregulated in CC tissues and cell lines. The cell proliferation
rate analysis results demonstrated that the cell proliferation rate
in CC cells was higher than in normal cervical epithelial cell
line. To further elucidate the effect of miR-153 on the progression
of CC, the synthetic miRNAs were introduced into the CC cell lines.
We discovered that the overexpression of miR-153 diminished but the
downregulate miR-153 expression increased the proliferation rate in
the CC cells investigated. The above findings indicated that
miR-153 functions as tumor-suppressor in CC and inhibits CC
progression partly through cell proliferation inhibition. The
progression of CC is a complex pathological process in which
proliferation and apoptosis of CC cells serves an important role
(29). The proliferation and
apoptosis of CC cells are regulated by multiple molecules,
including phosphatase and tension homolog (PTEN) (30), miR-21 (31), miR-940 (32), and DJ-1 (33). Therefore, the aberrant expressed
status of miR-153 and the involvement of miR-153 in CC
proliferation process highlighted the importance of miR-153 in
CC.
Then, we analyzed the 5-year overall survival rate
of the recruited CC patients. We found the low expression of
miR-153 indicates a poor 5-year survival. Following, we found the
low miR-153 expression was related to tumor size and lymph node
metastasis. The univariate and multivariate analyses demonstrated
that low miR-153 expression was an independent predictor for poor
overall survival of patients with CC.
In conclusion, we provided evidence that miR-153
exert its tumor suppressor potential by inhibiting cell
proliferation. Meanwhile, there was a strong correlation between
low miR-153 expression and poor outcomes in CC patients, indicating
miR-153 could potentially serve as a therapeutic target for CC.
Acknowledgements
The present study was supported by National Natural
Science Foundation of China (grant no. 31500840); Health and Family
Planning Commission of Wuhan Province (grant no. WZ17Z03).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
Statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang JM, Ju BH, Pan CJ, Gu Y, Li MQ, Sun
L, Xu YY and Yin LR: MiR-214 inhibits cell migration, invasion and
promotes the drug sensitivity in human cervical cancer by targeting
FOXM1. Am J Transl Res. 9:3541–3557. 2017.PubMed/NCBI
|
|
4
|
Ellenson LH and Wu TC: Focus on
endometrial and cervical cancer. Cancer Cell. 5:533–538. 2014.
View Article : Google Scholar
|
|
5
|
Yoshikawa H: Progress in the World and
challenges in Japan on HPV vaccination for cervical cancer
prevention. Gan To Kagaku Ryoho. 37:971–975. 2010.(In Japanese).
PubMed/NCBI
|
|
6
|
Ghebre RG, Grover S, Xu MJ, Chuang LT and
Simonds H: Cervical cancer control in HIV-infected women: Past,
present and future. Gynecol Oncol Rep. 21:101–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Calin GA and Croce CM: MicroRNA-cancer
connection: The beginning of a new tale. Cancer Res. 66:7390–7394.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Garzon R, Calin GA and Croce CM: MicroRNAs
in cancer. Annu Rev Med. 60:167–179. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak Rh, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Iorio MV and Croce CM: MicroRNA
dysregulation in cancer: Diagnostics, monitoring and therapeutics.
A comprehensive review. EMBO Mol Med. 9:8522017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kozomara A and Griffths-Jones S: miRBase:
Annotating high confdence microRNAs using deep sequencing data.
Nucleic Acids Res. 42:D68–D73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu XW, Li L, Li Y and Liu ZH: MiR-153
promotes breast cancer cell apoptosis by targeting HECTD3. Am J
Cancer Res. 6:1563–1571. 2016.PubMed/NCBI
|
|
15
|
Zhou J, Xie M, Shi Y, Luo B, Gong G, Li J,
Wang J, Zhao W, Zi Y, Wu X and Wen J: MicroRNA-153 functions as a
tumor suppressor by targeting SET6 and ZEB2 in ovarian cancer
cells. Oncol Rep. 34:111–120. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niu GF, Li B, Sun L and An CG:
MicroRNA-153 inhibits osteosarcoma cells proliferation and invasion
by targeting TGF-β2. PLoS One. 10:e01192252015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zuo J, Wang D, Shen H, Liu F, Han J and
Zhang X: MicroRNA-153 inhibits tumor progression in esophageal
squamous cell carcinoma by targeting SNAI1. Tumor Biol. 2016.(Epub
ahead of print). View Article : Google Scholar
|
|
18
|
Wang Z and Liu C: MiR-153 regulates
metastasis of gastric cancer through Snail1. Tumor Biol. 2015.(Epub
ahead of print).
|
|
19
|
Liu F, Liu B, Qian J, Wu G, Li J and Ma Z:
miR-153 enhances the therapeutic effect of gemcitabine by targeting
SnaiI in pancreatic cancer. Acta Biochim Biophys Sin (Shanghai).
49:520–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shan N, Shen L, Wang J, He D and Duan C:
MiR-153 inhibits migration and invasion of human non-small-cell
lung cancer by targeting ADAM19. Biochem Biophys Res Commun.
456:385–391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang L, Pickard K, Jenei V, Bullock MD,
Bruce A, Mitter R, Kelly G, Paraskeva C, Strefford J, Primrose J,
et al: miR-153 supports colorectal cancer progression via
pleiotropic effects that enhance invasion and chemotherapeutic
resistance. Cancer Res. 73:6345–6347. 2013.
|
|
22
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen ZL, Zhao XH, Wang JW, Li BZ, Wang Z,
Sun J, Tan FW, Ding DP, Xu XH, Zhou F, et al: microRNA-92a promotes
lymph node metastasis of human esophageal squamous cell carcinoma
via E-cadherin. J Biol Chem. 286:10725–10734. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wongfieng W, Jumnainsong A, Chamgramol Y,
Sripa B and Leelayuwat C: 5′-UTR and 3′-UTR regulation of MICB
expression in human cancer cells by novel microRNAs. Gene (Basel).
8:E2132017. View Article : Google Scholar
|
|
25
|
Biegel JM, Henderson E, Cox EM, Bonenfant
G, Netzband R, Kahn S, Eager R and Pager CT: Cellular DEAD-box RNA
helicase DDX6 modulates interaction of miR-122 with the 5′
untranslated region of hepatitis C virus RNA. Virology.
507:231–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xiao Y, Li X, Wang H, Wen R, He J and Tang
J: Epigenetic regulation of miR-129-2 and its effects on the
proliferation and invasion in lung cancer cells. J Cell Mol Med.
19:2172–2180. 2015.PubMed/NCBI
|
|
27
|
Gregory RI and Shiekhattar R: MicroRNA
biogenesis and cancer. Cancer Res. 65:3509–3512. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu L, Xu Q, Li X and Zhang X: MicroRNA-21
regulates the proliferation and apoptosis of cervical cancer cells
via tumor necrosis factor-α. Mol Med Rep. 16:4659–4663. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng Y, Zou W, Hu C, Li G, Zhou S, He Y,
Ma F, Deng C and Sun L: Modulation of CASC2/miR-21/PTEN pathway
sensitizes cervical cancer to cisplatin. Arch Biochem Biophys.
623–624:20–30. 2017. View Article : Google Scholar
|
|
31
|
Du G, Gao D and Meng L: miR-21 inhibitor
suppresses cell proliferation and colony formation regulating the
PTEN/AKT pathway and improves paclitaxel sensitivity in cervical
cancer cells. Mol Med Rep. 15:2713–2719. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Su K, Wang CF, Zhang Y, Cai YJ, Zhang YY
and Zhao Q: miR-940 upregulation contributes to human cervical
cancer progression through p27 and PTEN inhibition. Int J Oncol.
2017.(Epub ahead of print). View Article : Google Scholar
|
|
33
|
Wang H and Gao W: DJ-1 expression in
cervical carcinoma and its effects on cell viability and apoptosis.
Med Sci Monit. 22:2943–2949. 2016. View Article : Google Scholar : PubMed/NCBI
|