Introduction
Lung cancer is responsible for ~26% of all cancer
mortalities worldwide (1). Despite
the continuous increase in survival rates for most types of cancer,
the 5-year survival rate of lung cancer has remained low, at only
18% (1). Lung cancer can be divided
pathologically into two types. Most lung cancer cases (>80%)
correspond to non-small cell lung cancer (NSCLC), with the
remainder being small-cell lung cancer (2). The pathogenesis of NSCLC is not fully
understood, although studies about the molecular mechanisms of lung
cancer continue to provide new insights into the disease.
Long noncoding RNAs (lncRNAs) are attracting
increasing attention for their potential roles in numerous cancer
types (3,4). For example, in NSCLC, two
lncRNAs-plasmacytoma variant translocation 1 (PVT1) and urothelial
carcinoma-associated 1 (UCA1)-were found to promote oncogenesis and
tumor growth, respectively (5,6). LncRNAs
may participate in tumorigenesis through the competitive endogenous
RNA (ceRNA) network (7,8) by interacting with microRNAs (miRNAs)
(9). Differential miRNA expression
has been detected in precancerous and cancerous tissues (10,11).
Therefore, it is possible that the ceRNA network might be important
in carcinogenesis and cancer development. Furthermore, knowledge
about ceRNA interactions might provide insights into the potential
mechanisms and biological functions of lncRNAs in cancer. One study
demonstrated that the carcinogen H19 acted as a ceRNA to alter
expression levels of certain miRNAs (miR-138 and miR-200a) and
genes [zinc finger E-box binding homeobox 1 (ZEB)1/ZEB2] in
colorectal cancer (8). In addition,
ceRNA networks have been identified in other cancer types,
including hepatocellular, breast, pancreatic and gastric cancer
(12–15).
Using bioinformatic analysis, it was previously
demonstrated by the authors that the lncRNA LINC00968 was
differentially expressed between normal lung and tumor tissues
(16). In the present study,
microarray analysis was employed to identify which miRNAs were
differentially expressed with LINC00968 overexpression. Among the
five identified differentially expressed miRNAs, miR-9-3p was
selected for further analysis. In total, nine prediction algorithms
were applied to identify target genes of miR-9-3p, which were then
subjected to functional enrichment analysis (17–20).
Finally, the lncRNA-miRNA-mRNA regulatory axis of LINC00968 was
validated by bioinformatic and correlation analyses.
Materials and methods
miRNA microarray profiling of NSCLC
cells
The NSCLC A549 cell line was obtained from the Cell
Bank of the Chinese Academy of Sciences (Shanghai, China). A549
cells are adenocarcinoma human alveolar basal epithelial cells,
maintained in F-12 culture medium (Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA), trypsin-EDTA solution (Gibco; Thermo
Fisher Scientific, Inc.) and D-Hank's solution (Shanghai GenePharma
Co., Ltd., Shanghai, China). A549 cells were transduced with
lentiviruses encoding LINC00968 for 24–72 h, then transduction
efficiency was examined by an inverted fluorescent microscope and
subsequent experimentation was performed. Considering the effects
of green fluorescent protein (GFP)-containing lentiviral
transfection on subsequent experiments such as apoptosis detection,
a GFP-free lentivirus was selected. Optimal transfection conditions
(achieving 95% efficiency) were when the lentivirus titer reached
1×108 TU/ml, diluted to 1:5. miRNA profiles were
determined by using miRCURY LNA Array (version 18.0; Exiqon, Inc.,
Woburn, MA, USA) on an Axon GenePix 4000B microarray scanner (Axon
Instruments; Molecular Devices, LLC, Sunnyvale, CA, USA). Results
of the microarray analysis indicated significant downregulation of
miR-9-3p (log2|fold change (FC)| >0.585, P<0.05).
Comparison with Gene Expression
Omnibus (GEO) dataset
Data for miR-9-3p were searched and assembled in the
GEO database (https://www.ncbi.nlm.nih.gov/gds/; date accessed: 4th
June 2017), using the following search terms: (Lung OR pulmonary OR
respiratory OR bronchi OR bronchioles OR alveoli OR pneumocytes OR
‘air way’) AND (cancer OR carcinoma OR tumor OR neoplas* OR
malignan* OR adenocarcinoma) AND (microRNA OR miRNA OR ‘micro RNA’
OR ‘small temporal RNA’ OR ‘noncoding RNA’ OR ncRNA OR ‘small
RNA’). For microarrays, the following inclusion criteria were used:
i) Lung adenocarcinoma and adjacent noncancerous tissues (or normal
lung tissues) were included in each dataset; ii) sample organism
was Homo sapiens; and iii) expression levels of miR-9-3p
(hsa-miR-9* or hsa-miR-9-3p) in the experimental and control groups
were provided or could be calculated.
The mean and standard deviations of expression
values were extracted to estimate miR-9-3p levels in the test and
control groups using STATA (version 12.0; StataCorp LP, College
Station, TX, USA). P<0.05 or I2 value >50%
indicated significant heterogeneity. Standard mean differences
(SMDs) with 95% confidence intervals (CIs) were used to evaluate
continuous outcomes. Funnel plots were generated to evaluate
publication bias. P<0.05 was considered to indicate statistical
significance.
Bioinformatic prediction of miRNA
targets and functional enrichment analysis
The putative target genes of miR-9-3p were predicted
using nine miRNA prediction algorithms: TargetScan (http://www.targetscan.org/), miRDB (http://www.mirdb.org/), RNA22 (https://cm.Jefferson.edu/rna22/), miRMap, mirTarBase
(http://mirtarbase.mbc.nctu.edu.tw/),
DIANA-microT (http://diana.imis.athena-innovation.gr/DianaTools/index.php?r=microT_CDS/iinde),
GeneCards (http://www.genecards.org), TarBase
(http://diana.imis.athenainnovation.gr/DianaTools/index.php?r=tarbase/index)
and TargetMiner (https://www.isical.ac.in/~bioinfo_miu/final_html_targetminer/hsa-miR-9-3p.html).
Only target genes found in at least three databases were considered
for further analysis. To clarify potential roles of the target
genes, the Database for Annotation, Visualization, and Integrated
Discovery (DAVID; http://david.abcc.ncifcrf.gov/) was used to conduct
Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes
(KEGG) pathway analyses. The method used to identify significant
regulatory functions and enrichment pathways was based on the
P-value as previously described (21).
Bioinformatic analysis of the
association between LINC00968 and miR-9-3p using The Cancer Genome
Atlas (TCGA) database
Level 3 RNAseq and miRNAseq data of lung
adenocarcinoma were downloaded from TCGA (http://cancergenome.nih.gov/). An R package was used
to distinguish differentially expressed RNAs and miRNAs between
lung cancer and adjacent non-tumor lung tissues. For all P-values,
the false discovery rate (FDR) was applied to correct for multiple
hypothesis testing. The threshold of log2|FC|≥1 and FDR <0.05
was considered significant. Genes with an absolute correlation
coefficient >0.1 were regarded as correlated genes.
Construction of protein-protein
interaction (PPI) network
Next, how closely connected the predicted target
genes were with LINC00968 and miR-9-3p was determined. The
predicted genes were integrated with the correlated genes to obtain
prospective objective genes. To analyze interactions of the
objective genes, the Search Tool for the Retrieval of Interacting
Genes (STRING, http://string-db.org) was used to
construct the PPI network (22),
using a medium CI of 0.4 as a threshold.
Statistical analysis
The SPSS (version 22.0; SPSS Inc., Chicago, IL, USA)
and R (version 3.4.0) (23) software
packages were used to analyze all experimental data. Begg's test
and Egger's test were used to detect publication bias in
meta-analysis. Receiver operating characteristic (ROC) curves were
constructed to evaluate the sensitivity and specificity of the
molecular biological indicator in diagnostic tests. Differences in
overall survival between two groups were estimated and compared
using the Kaplan-Meier method with the log-rank test. FDR was used
to correct the P-value for multiple testing. Pearson's correlation
analysis was used to identify the association between LINC00968,
miR-9-3p and CCNA2 in TCGA database. P<0.05 was considered to
indicate a statistically significant difference.
Results
Downregulation of miR-9-3p expression
in LINC00968-overexpressing A549 cells
The miRNAs that were differentially expressed in
LINC00968-overexpressing A549 cells were profiled using miRNA
microarray analysis. A total of five differentially expressed
miRNAs were identified: One miRNA (miR-3675-3p) was upregulated and
four miRNAs (miR-9-3p, miR-22-5p, miR-668-3p and miR-4536-3p) were
downregulated in LINC00968-overexpressing A549 cells. miR-9-3p
(FC=0.569, P=0.044) was selected for further analysis.
Confirmation of miR-9-3p
downregulation in the GEO database
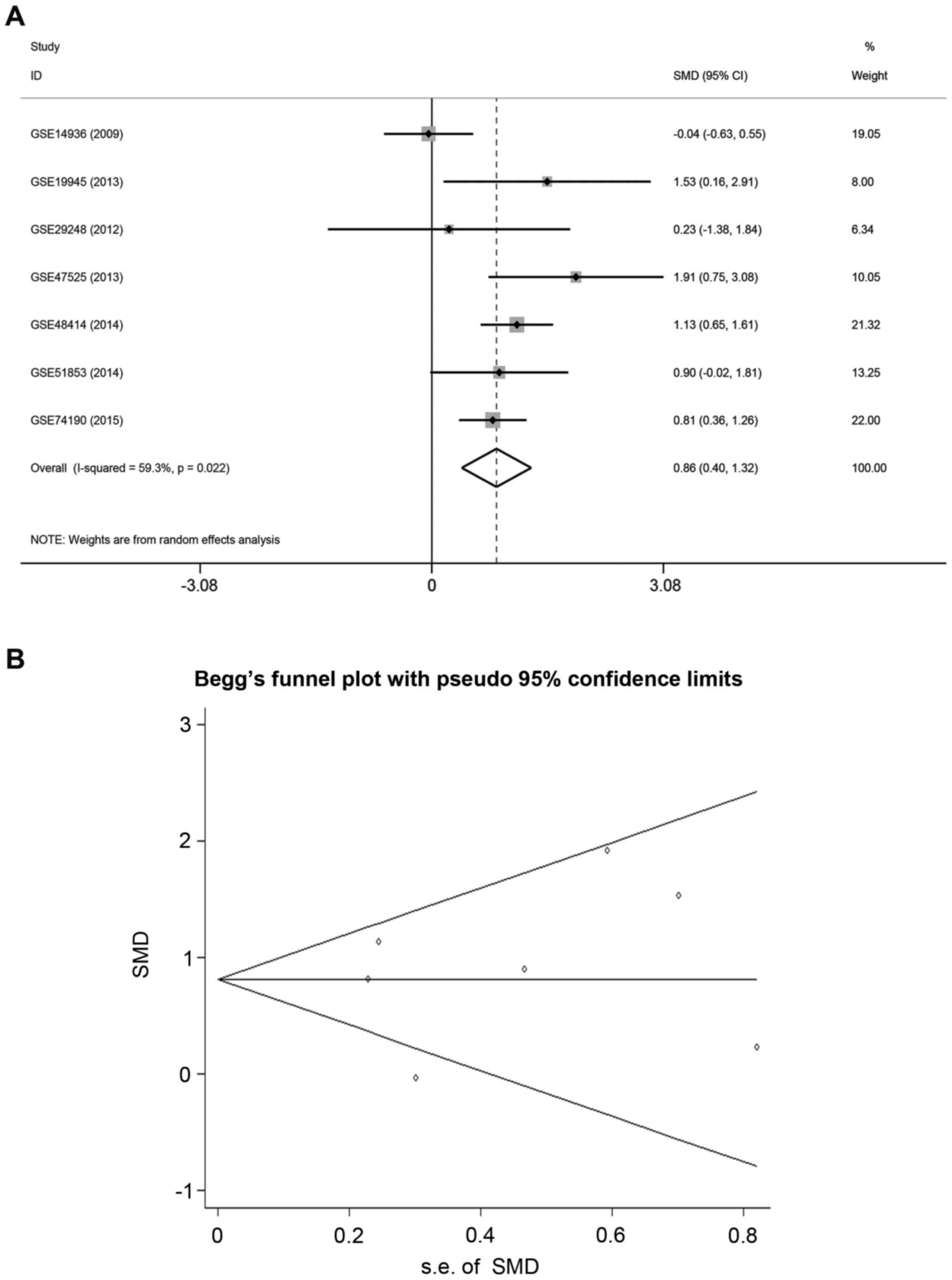
A meta-analysis was performed to verify miR-9-3p
expression in other lung adenocarcinoma tissue microarrays. A total
of seven eligible microarrays were identified. As significant
heterogeneity was detected among the microarrays
(I2=59.3%, P<0.05), the random-effects model was used
to assess the pooled SMD and 95% CIs. Pooled SMD was 0.86 (95% CI,
0.40–1.32, P=0.022; Fig. 1A), which
suggested that the expression of miR-9-3p in lung adenocarcinoma
tissues was higher compared with normal lung tissues. Funnel plots
revealed no significant publication bias in the meta-analysis
(Begg's test, P=0.681; Egger's test: P=0.681; Fig. 1B).
Functional enrichment analysis of
miR-9-3p target genes
A total of nine miRNA target prediction algorithms
were used to identify target genes of miR-9-3p. Of the 7856
predicted target genes, 2047 genes overlapped in at least three
datasets. To elucidate the biological function of miR-9-3p,
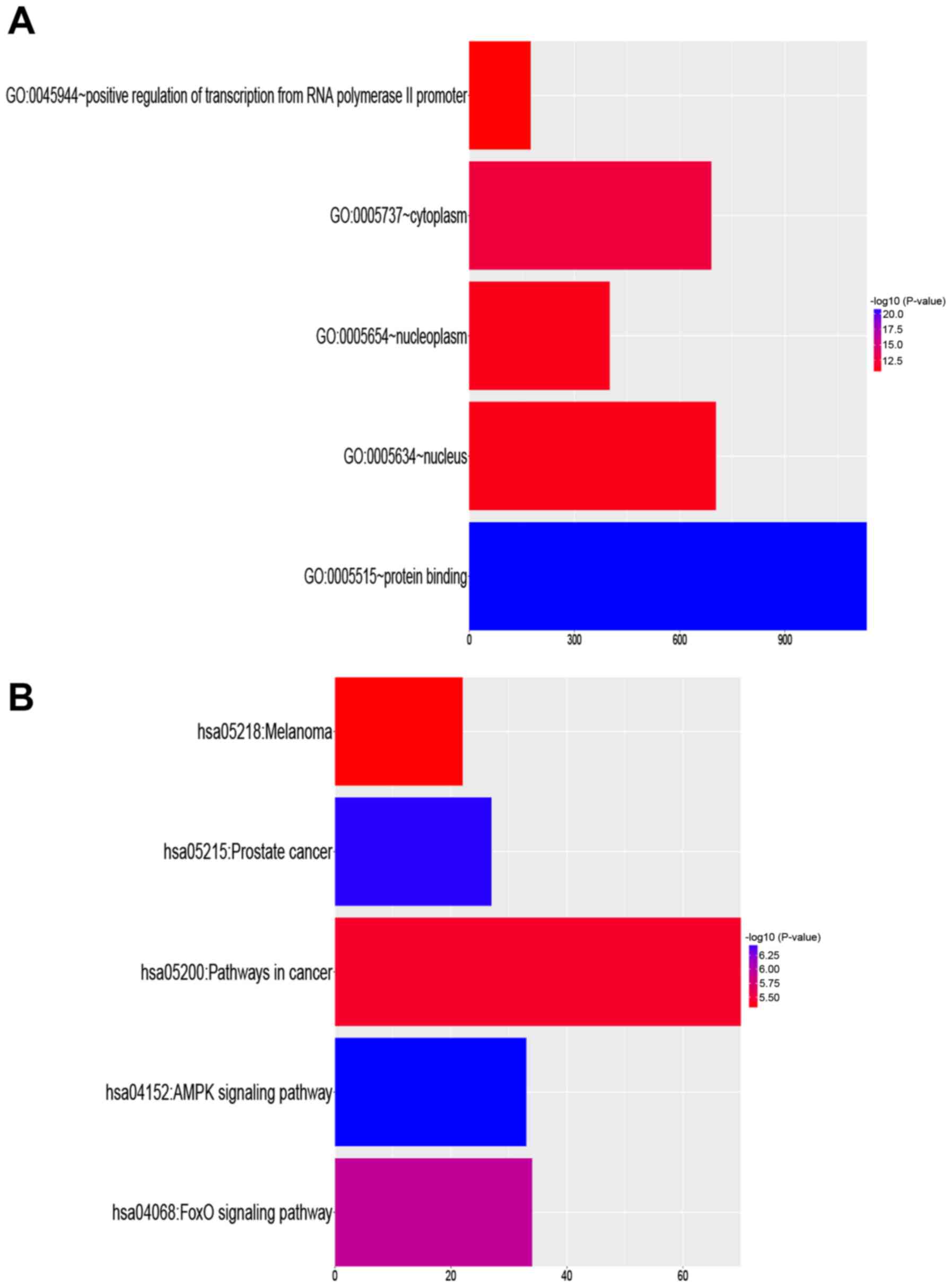
functional enrichment analysis was implemented using these 2,047
target genes. Positive regulation of transcription from RNA
polymerase II promoter (GO:0045944) was the most significant
biological process term. Cytoplasm (GO:0005737) was the most
significant cellular component term, and protein binding
(GO:0005515) was the most significant molecular function term
(Table I). A schematic of the top
five most significant GO processes are presented in Fig. 2A.
 | Table I.Significant GO terms most strongly
enriched by target genes of miR-9-3p. |
Table I.
Significant GO terms most strongly
enriched by target genes of miR-9-3p.
| GO term | Count | P-value | FDR |
|---|
| Biological
processes |
|
|
|
|
GO:0045944: Positive
regulation of transcription from RNA polymerase II promoter | 175 |
1.90×10−11 |
3.62×10−8 |
|
GO:0045893: Positive
regulation of transcription, DNA-templated | 97 |
6.97×10−8 |
1.33×10−4 |
|
GO:0000122: Negative
regulation of transcription from RNA polymerase II promoter | 123 |
3.09×10−7 |
5.87×10−4 |
|
GO:0006366: Transcription from
RNA polymerase II promoter | 93 |
7.81×10−7 |
1.48×10−3 |
|
GO:0006351: Transcription,
DNA-templated | 277 |
1.03×10−6 |
1.95×10−3 |
| Cellular
components |
|
|
|
|
GO:0005737: Cytoplasm | 690 |
3.07×10−13 |
4.72×10−10 |
|
GO:0005654: Nucleoplasm | 400 |
6.87×10−12 |
1.05×10−8 |
|
GO:0005634: Nucleus | 703 |
7.26×10−12 |
1.11×10−8 |
|
GO:0005923: Bicellular tight
junction | 29 |
1.26×10−5 |
1.93×10−2 |
|
GO:0016020: Membrane | 291 |
1.78×10−5 |
2.73×10−2 |
| Molecular
function |
|
|
|
|
GO:0005515: Protein
binding | 1133 |
1.65×10−21 |
2.72×10−18 |
|
GO:0003730: mRNA 3′-UTR
binding | 22 |
8.11×10−9 |
1.34×10−5 |
|
GO:0043565: Sequence-specific
DNA binding | 97 |
5.74×10−8 |
9.48×10−5 |
|
GO:0003700: Transcription
factor activity, sequence-specific DNA binding | 156 |
1.03×10−7 |
1.71×10−4 |
|
GO:0001077: Transcriptional
activator activity, RNA polymerase II core promoter proximal region
sequence-specific binding | 54 |
1.36×10−7 |
2.25×10−4 |
The biological relevance of KEGG pathways that were
enriched in these target genes was analyzed. The targets were
involved in many cancer-associated pathways (Table II). The top five KEGG pathways that
were enriched in the target genes are listed in Fig. 2B. KEGG pathway analysis identified
many important signaling pathways [e.g., AMP-activated protein
kinase (AMPK) and forkhead box O1 signaling pathways] known to be
involved in the development, invasion, and metastasis of cancer.
These findings indicate the biological relevance of miR-9-3p.
 | Table II.Kyoto Encyclopedia of Genes and
Genomes pathways most strongly enriched by target genes of
miR-9-3p. |
Table II.
Kyoto Encyclopedia of Genes and
Genomes pathways most strongly enriched by target genes of
miR-9-3p.
| Terms | Count | P-value | FDR |
|---|
| hsa04152: AMPK
signaling pathway | 33 |
3.61×10−7 |
4.74×10−4 |
| hsa05215: Prostate
cancer | 27 |
3.74×10−7 |
4.91×10−4 |
| hsa04068: FoxO
signaling pathway | 34 |
1.14×10−6 |
1.49×10−3 |
| hsa05200: Pathways
in cancer | 70 |
3.77×10−6 |
4.95×10−3 |
| hsa05218:
Melanoma | 22 |
4.87×10−6 |
6.39×10−3 |
| hsa04390: Hippo
signaling pathway | 35 |
6.78×10−6 |
8.91×10−3 |
| hsa04550: Signaling
pathways regulating pluripotency of stem cells | 32 |
2.45×10−5 |
3.22×10−2 |
| hsa05214:
Glioma | 18 |
2.10×10−4 |
2.75×10−1 |
| hsa03015: mRNA
surveillance pathway | 22 |
2.70×10−4 |
3.54×10−1 |
| hsa04151: PI3K-Akt
signaling pathway | 57 |
2.87×10−4 |
3.77×10−1 |
PPI network
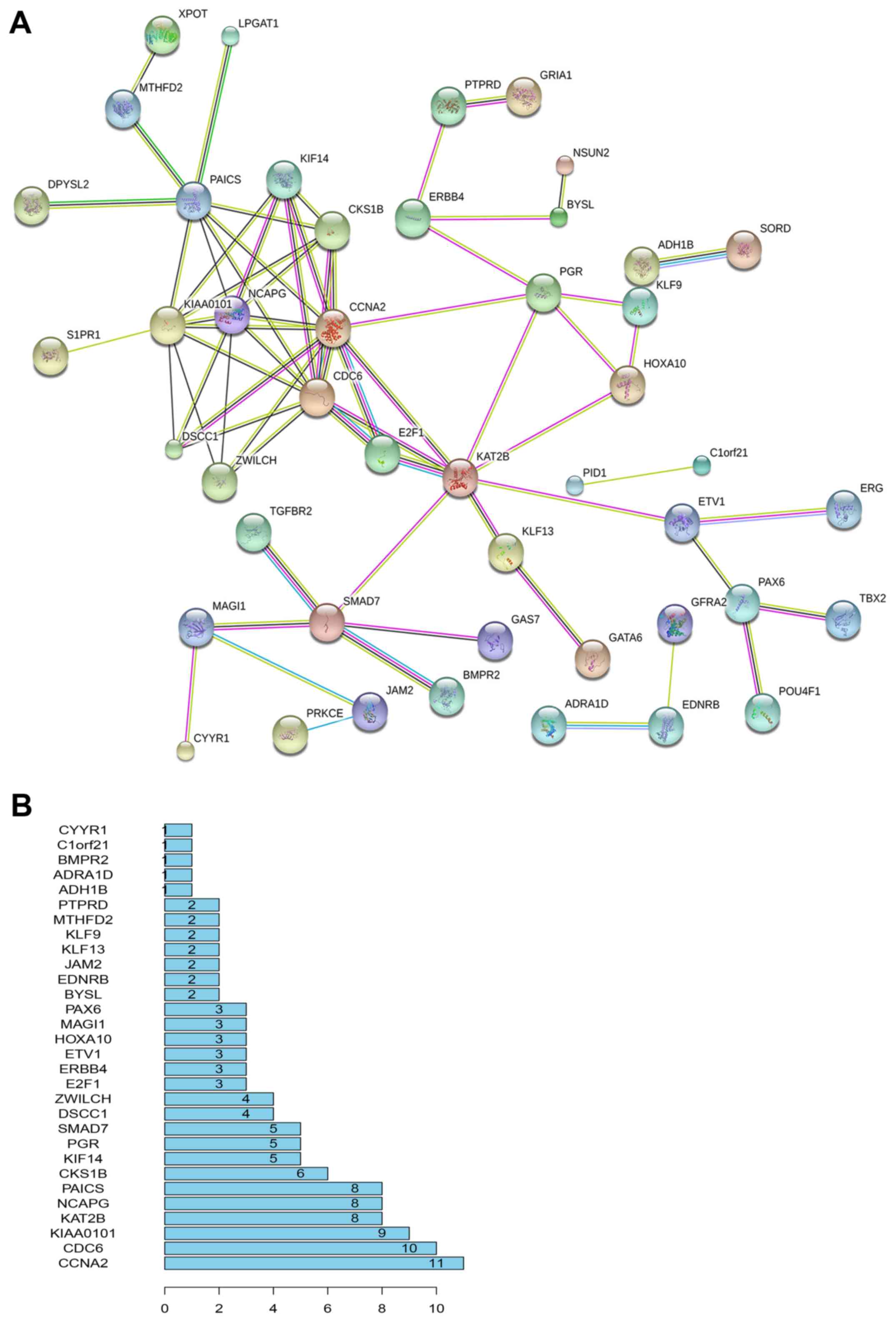
To understand the interactions between target genes
and their associations with LINC00968 and miR-9-3p, the predicted
genes were integrated with the correlated genes. A total of 6,515
and 2,677 genes were correlated with LINC00968 and miR-9-3p,
respectively, from the TCGA database. Using a Venn diagram, the
2,047 predicted target genes were integrated with the 6515
LINC00968-associated genes and the 2677 miR-9-3p-associated genes,
uncovering 120 objective genes. To depict the complex PPI
relationships among these objective genes, the PPI network of the
top 30 genes were visualized by STRING (Fig. 3A). The number of links between genes
was calculated, and a list of the top 30 most connected genes was
obtained. Among them, cyclin A2 (CCNA2) had the greatest
connectivity (Fig. 3B) and was
subjected to further validation.
Validation of regulatory axis of
LINC00968 in TCGA database
Predictive performances of LINC00968, miR-9-3p and
CCNA2 in lung adenocarcinoma were estimated by ROC curve analysis.
Areas under the ROC curve (AUCs) of components in this regulatory
axis among lung adenocarcinoma patients from TCGA database were as
follows: LINC00968, AUC=0.988 (95% CI, 0.977–0.998, P=0.000),
miR-9-1, AUC=0.983 (95% CI, 0.967–0.999, P=0.000), miR-9-2
AUC=0.982 (95% CI, 0.968–0.997, P=0.000), miR-9-3 AUC=0.981 (95%
CI, 0.968–0.995, P=0.000), and CCNA2, AUC=0.960 (95% CI,
0.921–0.999, P=0.000). The data suggested that the three signatures
had good predictive performance in lung adenocarcinoma
patients.
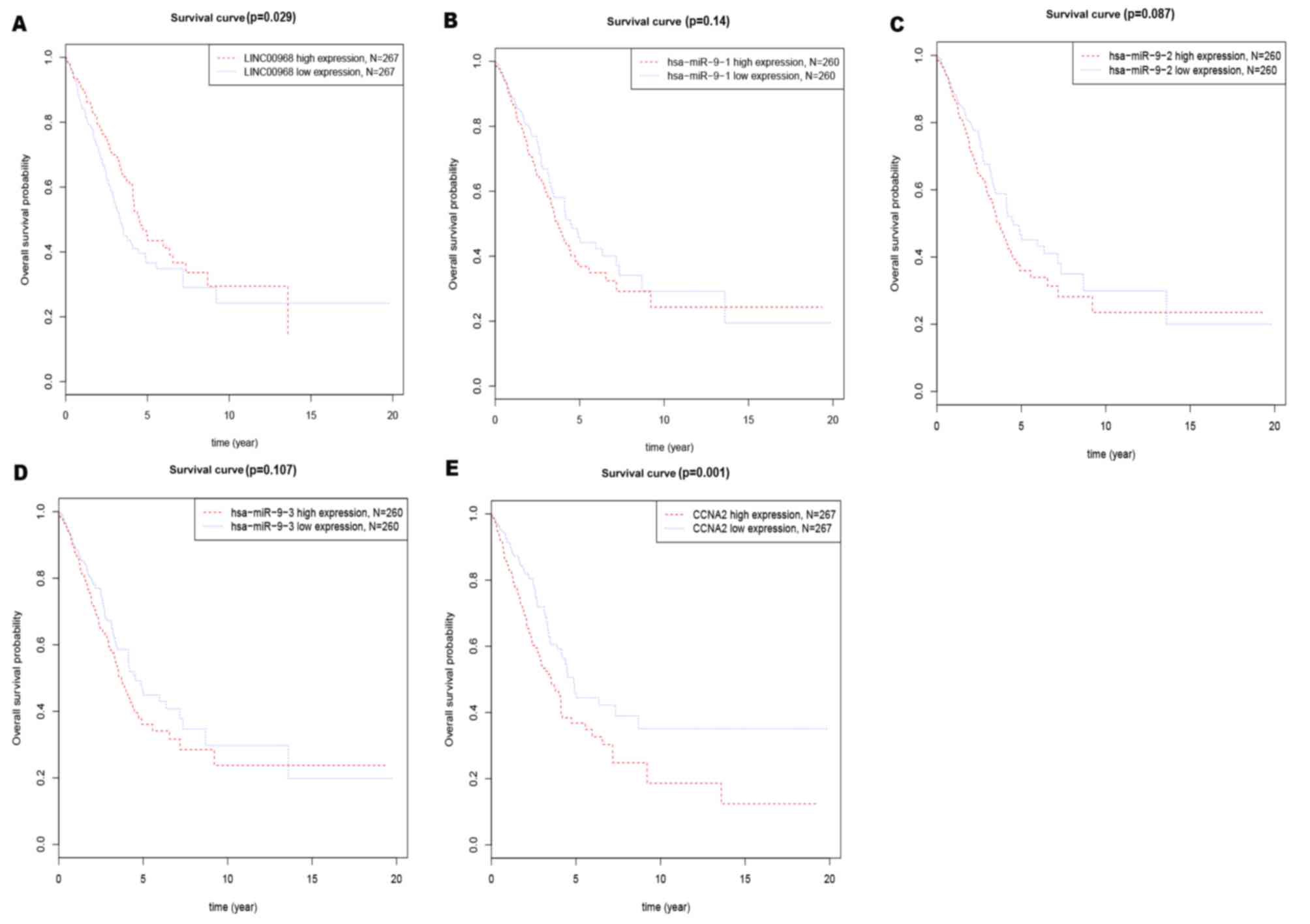
A Kaplan-Meier survival analysis was performed to
determine the effects of the three components on predicting
clinical outcome (Fig. 4). The median
gene expression (which, for LINC00968, CCNA2, miR-9-1, miR-9-2 and
miR-9-3 were 20.70, 694.90, 1009.58, 986.66 and 1010.02,
respectively) was used to separate the patients into high and low
expression groups in TCGA datasets. The number of patients
expressing LINC00968 and CCNA2 in the high-expression and
low-expression groups in the present study was 267. The number of
patients expressing miR-9-3p in the high-expression and
low-expression groups was 260. Low expression of LINC00968
(Fig. 4A) or high expression of CCNA2
(Fig. 4E) was a significant predictor
of poor prognosis for overall survival in lung adenocarcinoma
patients. These findings suggested that LINC00968 and CCNA2 had
good potential as diagnostic and prognostic indicators.
Finally, the underlying mechanisms of the LINC00968
regulatory axis in lung adenocarcinoma were investigated. In our
previous GEO results, miR-9-3p was detected to be significantly
upregulated in tumor tissues and had oncogenic potential in lung
adenocarcinoma. Therefore, the correlation between LINC00968 and
miR-9-3p (including miR-9-1, miR-9-2, and miR-9-3 in TCGA database)
was examined. Negative correlations were identified between
LINC00968 and miR-9-3p (Fig. 5A-C)
and between LINC00968 and CCNA2 (Fig.
5D), whereas a positive correlation between miR-9-3p and CCNA2
(Fig. 5E-G) was identified in
patients with lung adenocarcinoma. Therefore, it is concluded that
the regulatory role of LINC00968 in lung adenocarcinoma might
involve the LINC00968/miR-9-3p/CCNA2 axis.
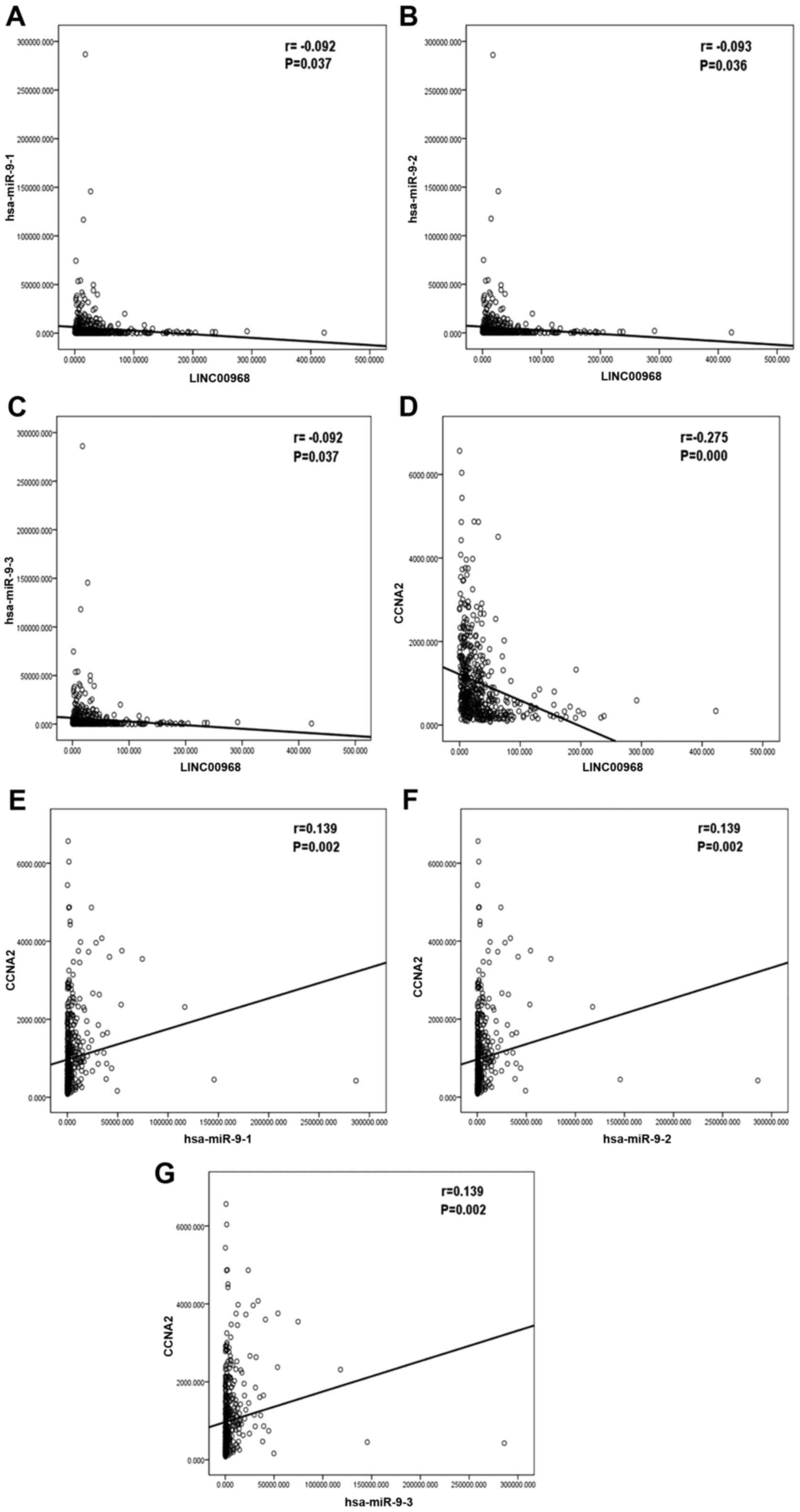 | Figure 5.Correlation analysis among LINC00968,
miR-9-3p, and CCNA2 expression in lung adenocarcinoma based on TCGA
database. Correlations (A) between LINC00968 and miR-9-1, (B)
between LINC00968 and miR-9-2, (C) between LINC00968 and miR-9-3,
(D) between LINC00968 and CCNA2, (E) between miR-9-1 and CCNA2, (F)
between miR-9-2 and CCNA2, (G) between miR-9-3 and CCNA2. CCNA2,
cyclin A2; miR, microRNA; TCGA, The Cancer Genome Atlas. |
Discussion
In recent years, microarray studies, RNA sequencing,
and quantitative reverse-transcription PCR have been used to
investigate the differential expression profiles of lncRNAs in
various types of cancer. LncRNAs regulate diverse biological
processes and act as oncogenes or tumor suppressors in many cancer
types (24–28). As such, lncRNAs have great potential
for use as molecular biomarkers in cancer diagnosis and prognosis.
For example, the lncRNA, metastasis associated in lung
adenocarcinoma transcript 1, was found to promote the proliferation
and metastasis of lung cancer (29).
The authors previously reported that the lncRNA LINC00968 was
differentially expressed in normal vs. lung tumor tissues in the
GEO database (16). However, the
regulatory mechanisms of LINC00968 in lung cancer have been
unclear.
LncRNAs and miRNAs may have important interactions
that contribute to cancer development. Evidence for this ceRNA
hypothesis has been provided by several reports. For instance,
lncRNA cancer susceptibility candidate 2 (CASC2) was shown to
inhibit cell proliferation and tumor growth by regulating miR-18a
in colorectal cancer (30). C032469
regulated telomerase reverse transcriptase expression by binding to
miR-1207-5p in gastric cancer (31).
Growth Arrest Specific Transcript 5 suppressed carcinogenesis in
NSCLC by repressing miR-135b expression (32). In light of this research, the present
authors postulated that LIN00968 may act as a ceRNA in NSCLC. The
expression profile of miR-9-3p in NSCLC A549 cells that
overexpressed LINC00968 was detected. Finally, bioinformatic
analysis was used to reveal the potential regulatory role of
LINC00968 in lung adenocarcinoma.
miR-9-3p has been demonstrated to regulate
biological functions in multiple types of cancer. For example, Yang
et al (33) reported that
H2O2-induced apoptosis of glioma cells was
facilitated by miR-9-3p through downregulation of Herpud1
expression. In another study, miR-9-3p inhibited cell proliferation
by targeting the PDZ-binding motif TAZ in hepatocellular carcinoma
(34). miR-9-3p was detected to
suppress tumor biological behaviors in gastric cancer (35). Other studies have suggested regulatory
roles for miR-9-3p in tumor initiation, growth and progression in
NSCLC (36–38).
Results of the present analysis of miR-9-3p target
genes indicated that the most enriched GO term was protein binding,
a key process in carcinogenesis. For example, adiponectin was
detected to inhibit proliferation of A549 cells through its
downregulation of binding to cyclic AMP response element (39). Another study described a fusion
protein that could be used to improve antitumor activities in NSCLC
by targeting epidermal growth factor receptor (EGFR) and
insulin-like growth factor 1 receptor (IGFR1) (40). In the present study, KEGG pathway
analysis indicated that the most enriched pathway was the AMPK
signaling pathway. Phosphoinositide-3-kinase/Akt/mechanistic target
of rapamycin kinase and Raf/Mek/extracellular signal-regulated
kinase, which are regulated by AMPK, are crucial signaling pathways
related to cell growth, survival and molecular expression (41). Furthermore, numerous studies have
demonstrated that AMPK is an important mediator of NSCLC
progression (42–44). Taken together, all of these results
indicate that miR-9-3p participates in the tumorigenesis process
and acts as an efficient regulator of NSCLC development.
As an important regulator of the cell cycle and cell
proliferation, CCNA2 has been consistently demonstrated to be
closely associated with the pathogenesis of lung cancer. For
instance, coiled-coil domain containing 106 promoted proliferation
of A549 and H1299 cells by upregulating CCNA2 expression (45). In another report, miR-137 and miR-30a
inhibited tumor growth by inducing cell-cycle arrest and
downregulating cell cycle-associated regulators, including CCNA2
(46,47). In addition, the gene E2F1 is an
important transcription factor and is one of the most important
pro-metastatic genes. Mei et al (48) observed that E2F1 had a significant
effect on the prognosis of patients and was closely associated with
patient survival, suggesting that E2F1 has an important role in the
progression of clear cell renal cell carcinoma and hepatocellular
carcinoma.
In the present study, the correlation analysis
results suggested the existence of tight connections among
LINC00968, miR-9-3p and CCNA2 in lung adenocarcinoma. Additionally,
results of the Kaplan-Meier survival analysis indicated that
LINC00968 may be a prospective biomarker for the diagnosis and
prognosis of lung adenocarcinoma. Overall, these findings indicate
that the LINC00968/miR-9-3p/CCNA2 regulatory axis deserves further
research and investigation. The continuous development of novel
research tools for identifying signal transduction pathways and
discovering new therapeutic targets was inspired by the current
investigation of molecular tumor profiles. Using tools to identify
the prognostic and diagnostic markers in the present study presents
an analysis concept based on existing tools, a number of which are
available online, including R packages, Cytoscape, GO and the KEGG
(49). Furthermore, the combination
of the application of these tools and experimental verification
will have a more important role in the identification of miRNAs.
Therefore, the regulatory mechanisms of LINC00968 should be
experimentally verified.
In conclusion, LINC00968 might have important
functions in NSCLC via the LINC00968/miR-9-3p/CCNA2 regulatory
axis. Results of the present study describe a novel approach to
research the underlying mechanisms of LINC00968 in the
tumorigenesis and development of NSCLC.
Acknowledgements
Not applicable.
Funding
The present study was supported by Guangxi Key
Project of Science and Technology (grant no. 1598012-30), the Fund
of the Guangxi Provincial Health Bureau Scientific Research Project
(grant no. Z2013201), the Fund of the National Natural Science
Foundation of China (grant nos. NSFC81660488, NSFC81360327 and
NSFC81560469), and the Natural Science Foundation of Guangxi, China
(grant no. 2015GXNSFCA139009). The funders had no role in the study
design, the data collection and analysis, the decision to publish,
or the preparation of the manuscript.
Availability of data and materials
The datasets generated and analyzed during the
current study are available in GEO (https://www.ncbi.nlm.nih.gov/gds) and TCGA (http://cancergenome.nih.gov/).
Authors' contributions
SKL and GC conceived the idea for the study. DYL,
WJC and JS analyzed and interpreted the data. DYL was a major
contributor in writing the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Travis WD: The 2015 WHO classification of
lung tumors. Pathologe. 35 Suppl 2:S1882014. View Article : Google Scholar
|
|
3
|
Yang R, Li P, Zhang G, Lu C, Wang H and
Zhao G: Long non-coding RNA XLOC_008466 functions as an oncogene in
human non-small cell lung cancer by targeting miR-874. Cell Physiol
Biochem. 42:126–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
He Y, Meng XM, Huang C, Wu BM, Zhang L, Lv
XW and Li J: Long noncoding RNAs: Novel insights into hepatocelluar
carcinoma. Cancer Lett. 344:20–27. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wan L, Sun M, Liu GJ, Wei CC, Zhang EB,
Kong R, Xu TP, Huang MD and Wang ZX: Long noncoding RNA PVT1
promotes non-small cell lung cancer cell proliferation through
epigenetically regulating LATS2 expression. Mol Cancer Ther.
15:1082–1094. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nie W, Ge HJ, Yang XQ, Sun X, Huang H, Tao
X, Chen WS and Li B: LncRNA-UCA1 exerts oncogenic functions in
non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett.
371:99–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liz J and Esteller M: lncRNAs and
microRNAs with a role in cancer development. Biochim Biophys Acta.
1859:169–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liang WC, Fu WM, Wong CW, Wang Y, Wang WM,
Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF and Waye MM: The lncRNA
H19 promotes epithelial to mesenchymal transition by functioning as
miRNA sponges in colorectal cancer. Oncotarget. 6:22513–22525.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tay Y, Rinn J and Pandolfi PP: The
multilayered complexity of ceRNA crosstalk and competition. Nature.
505:344–352. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arima C, Kajino T, Tamada Y, Imoto S,
Shimada Y, Nakatochi M, Suzuki M, Isomura H, Yatabe Y, Yamaguchi T,
et al: Lung adenocarcinoma subtypes definable by lung
development-related miRNA expression profiles in association with
clinicopathologic features. Carcinogenesis. 35:2224–2231. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujita Y, Yagishita S, Hagiwara K,
Yoshioka Y, Kosaka N, Takeshita F, Fujiwara T, Tsuta K, Nokihara H,
Tamura T, et al: The clinical relevance of the
miR-197/CKS1B/STAT3-mediated PD-L1 network in chemoresistant
non-small-cell lung cancer. Mol Ther. 23:717–727. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xia T, Liao Q, Jiang X, Shao Y, Xiao B, Xi
Y and Guo J: Long noncoding RNA associated-competing endogenous
RNAs in gastric cancer. Sci Rep. 4:60882014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Fan D, Jian Z, Chen GG and Lai
PB: Cancer specific long noncoding RNAs show differential
expression patterns and competing endogenous RNA potential in
hepatocellular carcinoma. PLoS One. 10:e01410422015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhou X, Liu J and Wang W: Construction and
investigation of breast-cancer-specific ceRNA network based on the
mRNA and miRNA expression data. IET Syst Biol. 8:96–103. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou M, Diao Z, Yue X, Chen Y, Zhao H,
Cheng L and Sun J: Construction and analysis of dysregulated
lncRNA-associated ceRNA network identified novel lncRNA biomarkers
for early diagnosis of human pancreatic cancer. Oncotarget.
7:56383–56394. 2016.PubMed/NCBI
|
|
16
|
Yang J, Lin J, Liu T, Chen T, Pan S, Huang
W and Li S: Analysis of lncRNA expression profiles in non-small
cell lung cancers (NSCLC) and their clinical subtypes. Lung Cancer.
85:110–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu X, Wang X, Fu B, Meng L and Lang B:
Differentially expressed genes and microRNAs in bladder carcinoma
cell line 5637 and T24 detected by RNA sequencing. Int J Clin Exp
Pathol. 8:12678–12687. 2015.PubMed/NCBI
|
|
18
|
Li Q, Ge X, Xu X, Zhong Y and Qie Z:
Comparison of the gene expression profiles between gallstones and
gallbladder polyps. Int J Clin Exp Pathol. 7:8016–8023.
2014.PubMed/NCBI
|
|
19
|
Jiang CM, Wang XH, Shu J, Yang WX, Fu P,
Zhuang LL and Zhou GP: Analysis of differentially expressed genes
based on microarray data of glioma. Int J Clin Exp Med.
8:17321–17332. 2015.PubMed/NCBI
|
|
20
|
Chen L, Zhuo D, Chen J and Yuan H:
Screening feature genes of lung carcinoma with DNA microarray
analysis. Int J Clin Exp Med. 8:12161–12171. 2015.PubMed/NCBI
|
|
21
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43:(Database Issue). D447–D452. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
R Core Team: R: A language and environment
for statistical computing, R foundation for statistical computing.
Vienna, Austria: 2013, https://www.R-project.org
|
|
24
|
Naemura M, Murasaki C, Inoue Y, Okamoto H
and Kotake Y: Long noncoding RNA ANRIL regulates proliferation of
non-small cell lung cancer and cervical cancer cells. Anticancer
Res. 35:5377–5382. 2015.PubMed/NCBI
|
|
25
|
Liu YY, Chen ZH, Peng JJ, Wu JL, Yuan YJ,
Zhai ET, Cai SR, He YL and Song W: Up-regulation of long non-coding
RNA XLOC_010235 regulates epithelial-to-mesenchymal transition to
promote metastasis by associating with Snail1 in gastric cancer.
Sci Rep. 7:24612017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hua F, Li CH, Chen XG and Liu XP: Long
noncoding RNA CCAT2 knockdown suppresses tumorous progression by
sponging miR-424 in epithelial ovarian cancer. Oncol Res.
26:241–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng Q, Ren M, Li Y and Song X:
LncRNA-RMRP acts as an oncogene in lung cancer. PLoS One.
11:e01648452016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cao S, Wang Y, Li J, Lv M, Niu H and Tian
Y: Tumor-suppressive function of long noncoding RNA MALAT1 in
glioma cells by suppressing miR-155 expression and activating FBXW7
function. Am J Cancer Res. 6:2561–2574. 2016.PubMed/NCBI
|
|
29
|
Chou J, Wang B, Zheng T, Li X, Zheng L, Hu
J, Zhang Y, Xing Y and Xi T: MALAT1 induced migration and invasion
of human breast cancer cells by competitively binding miR-1 with
cdc42. Biochem Biophys Res Commun. 472:262–269. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang G, Wu X, Li S, Xu X, Zhu H and Chen
X: The long noncoding RNA CASC2 functions as a competing endogenous
RNA by sponging miR-18a in colorectal cancer. Sci Rep. 6:265242016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang CG, Yin DD, Sun SY and Han L: The
use of lncRNA analysis for stratification management of prognostic
risk in patients with NSCLC. Eur Rev Med Pharmacol Sci. 21:115–119.
2017.PubMed/NCBI
|
|
32
|
Xue Y, Ni T, Jiang Y and Li Y: Long
noncoding RNA GAS5 inhibits tumorigenesis and enhances
radiosensitivity by suppressing miR-135b expression in non-small
cell lung cancer. Oncol Res. 25:1305–1316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang L, Mu Y, Cui H, Liang Y and Su X:
MiR-9-3p augments apoptosis induced by H2O2 through down regulation
of Herpud1 in glioma. PLoS One. 12:e01748392017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Higashi T, Hayashi H, Ishimoto T, Takeyama
H, Kaida T, Arima K, Taki K, Sakamoto K, Kuroki H, Okabe H, et al:
miR-9-3p plays a tumour-suppressor role by targeting TAZ (WWTR1) in
hepatocellular carcinoma cells. Br J Cancer. 113:252–258. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meng Q, Xiang L, Fu J, Chu X, Wang C and
Yan B: Transcriptome profiling reveals miR-9-3p as a novel tumor
suppressor in gastric cancer. Oncotarget. 8:37321–37331. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yuva-Aydemir Y, Simkin A, Gascon E and Gao
FB: MicroRNA-9: Functional evolution of a conserved small
regulatory RNA. RNA Biol. 8:557–564. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen X, Zhu L, Ma Z, Sun G, Luo X, Li M,
Zhai S, Li P and Wang X: Oncogenic miR-9 is a target of erlotinib
in NSCLCs. Sci Rep. 5:170312015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mitra R, Edmonds MD, Sun J, Zhao M, Yu H,
Eischen CM and Zhao Z: Reproducible combinatorial regulatory
networks elucidate novel oncogenic microRNAs in non-small cell lung
cancer. RNA. 20:1356–1368. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Illiano M, Nigro E, Sapio L, Caiafa I,
Spina A, Scudiero O, Bianco A, Esposito S, Mazzeo F, Pedone PV, et
al: Adiponectin down-regulates CREB and inhibits proliferation of
A549 lung cancer cells. Pulm Pharmacol Ther. 45:114–120. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Guo XF, Zhu XF, Cao HY, Zhong GS, Li L,
Deng BG, Chen P, Wang PZ, Miao QF and Zhen YS: A bispecific
enediyne-energized fusion protein targeting both epidermal growth
factor receptor and insulin-like growth factor 1 receptor showing
enhanced antitumor efficacy against non-small cell lung cancer.
Oncotarget. 8:27286–27299. 2017.PubMed/NCBI
|
|
41
|
Troncone M, Cargnelli SM, Villani LA,
Isfahanian N, Broadfield LA, Zychla L, Wright J, Pond G, Steinberg
GR and Tsakiridis T: Targeting metabolism and AMP-activated kinase
with metformin to sensitize non-small cell lung cancer (NSCLC) to
cytotoxic therapy: Translational biology and rationale for current
clinical trials. Oncotarget. 8:57733–57754. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yim NH, Hwang YH, Liang C and Ma JY: A
platycoside-rich fraction from the root of Platycodon grandiflorum
enhances cell death in A549 human lung carcinoma cells via mainly
AMPK/mTOR/AKT signal-mediated autophagy induction. J
Ethnopharmacol. 194:1060–1068. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Li ZY, Hou XX, Wang X, Luo YH,
Ying YP and Chen G: Clinical significance and effect of AEG-1 on
the proliferation, invasion, and migration of NSCLC: A study based
on immunohistochemistry, TCGA, bioinformatics, in vitro and in vivo
verification. Oncotarget. 8:16531–16552. 2017.PubMed/NCBI
|
|
44
|
Praveen P, Hülsmann H, Sültmann H, Kuner R
and Fröhlich H: Cross-talk between AMPK and EGFR dependent
signaling in non-small cell lung cancer. Sci Rep. 6:275142016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang X, Zheng Q, Wang C, Zhou H, Jiang G,
Miao Y, Zhang Y, Liu Y, Li Q, Qiu X and Wang E: CCDC106 promotes
non-small cell lung cancer cell proliferation. Oncotarget.
8:26662–26670. 2017.PubMed/NCBI
|
|
46
|
Chen R, Zhang Y, Zhang C, Wu H and Yang S:
miR-137 inhibits the proliferation of human non-small cell lung
cancer cells by targeting SRC3. Oncol Lett. 13:3905–3911. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wen XP, Ma HL, Zhao LY, Zhang W and Dang
CX: MiR-30a suppresses non-small cell lung cancer progression
through AKT signaling pathway by targeting IGF1R. Cell Mol Biol
(Noisy-le-grand). 61:78–85. 2015.PubMed/NCBI
|
|
48
|
Mei Y, Yang JP and Qian CN: For robust big
data analyses: A collection of 150 important pro-metastatic genes.
Chin J Cancer. 36:162017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Van Laere S, Dirix L and Vermeulen P:
Molecular profiles to biology and pathways: A systems biology
approach. Chin J Cancer. 35:532016. View Article : Google Scholar : PubMed/NCBI
|